Botulinum Neurotoxin A in the Treatment of Pharyngocutaneous Fistula after Salvage Surgery in Head and Neck Cancer Patients: Our Preliminary Results
Abstract
:1. Introduction
2. Materials and Methods
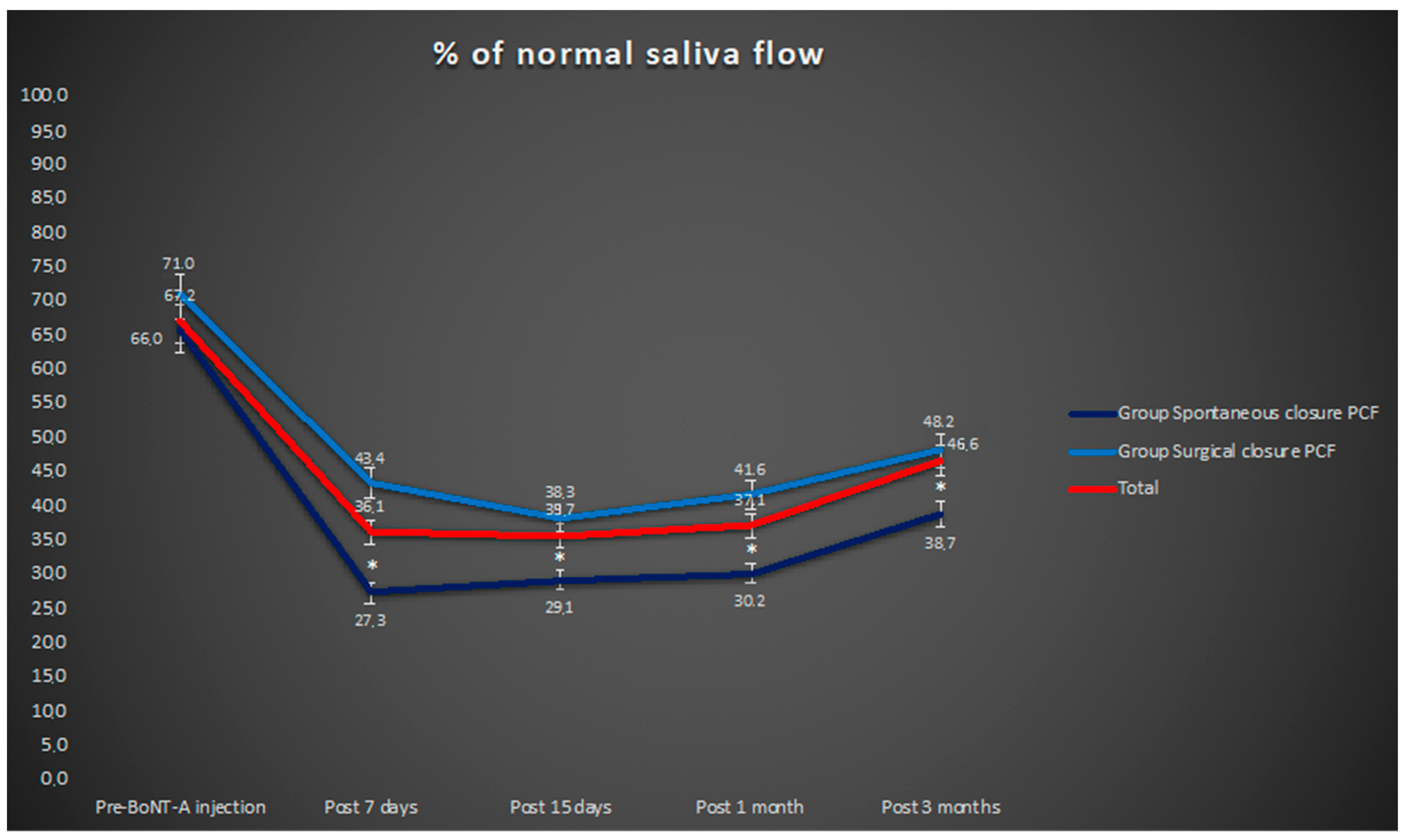
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makitie, A.A.; Irish, J.; Gullane, P.J. Pharyngocutaneous fistula. Curr. Opin. Otolaryngol. Head Neck Surg. 2003, 11, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, F.; Bettini, M.; Molteni, G.; Piccinini, A.; Valoriani, F.; Gabriele, S.; Presutti, L. Analysis of risk factors for pharyngocutaneous fistula after total laryngectomy with particular focus on nutritional status. Acta Otorhinolaryngol. Ital. 2015, 35, 243–248. [Google Scholar] [PubMed]
- Molteni, G.; Sacchetto, A.; Sacchetto, L.; Marchioni, D. Optimal Management of Post-Laryngectomy Pharyngo-Cutaneous Fistula. Open Access Surg. 2020, 13, 11–25. [Google Scholar] [CrossRef]
- Sayles, M.; Grant, D.G. Preventing pharyngo-cutaneous fistula in total laryngectomy: A systematic review and meta-analysis. Laryngoscope 2013, 124, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Virtaniemi, J.A.; Kumpulainen, E.J.; Hirvikoski, P.P.; Johansson, R.T.; Kosma, V.-M. The incidence and etiology of postlaryngectomy pharyngocutaneous fistulae. Head Neck 2000, 23, 29–33. [Google Scholar] [CrossRef]
- Almadori, G.; Di Cintio, G.; De Corso, E.; Mele, D.A.; Settimi, S.; Almadori, A.; Cina, A.; Visconti, G.; Paludetti, G.; Salgarello, M. The usefulness of the IMAP propeller flap for trachea and tracheostome reconstruction after resection of parastomal recurrence of squamous cell carcinoma following salvage total laryngectomy. Eur. Arch. Otorhinolaryngol. 2021, 278, 499–507. [Google Scholar] [CrossRef]
- Pellini, R.; Zocchi, J.; Pichi, B.; Manciocco, V.; Marchesi, P.; Sperduti, I.; Mercante, G.; Molteni, G.; Iocca, O.; Di Maio, P.; et al. Prevention of fistulas after salvage laryngectomy using temporoparietal fascia free flap. Acta Otorhinolaryngol. Ital. 2020, 40, 181–189. [Google Scholar] [CrossRef]
- Gendreau-Lefèvre, A.K.; Audet, N.; Maltais, S.; Thuot, F. Prophylactic pectoralis major muscle flap in prevention of pharyngocutaneous fistula in total laryngectomy after radiotherapy. Head Neck 2015, 37, 1233–1238. [Google Scholar] [CrossRef]
- Celikkanat, S.; Koc, C.; Ozdem, C. Effect of blood transfusion on tumor recurrence and postoperative pharyngocutaneous fistula formation in patients subjected to total laryngectomy. Acta Otolaryngol. 1995, 115, 566–568. [Google Scholar] [CrossRef]
- Natvig, K.; Boysen, M.; Tausjø, J. Fistulae following laryngectomy in patients treated with irradiation. J. Laryngol. Otol. 1993, 107, 1136–1139. [Google Scholar] [CrossRef]
- Galli, J.; De Corso, E.; Volante, M.; Almadori, G.; Paludetti, G. Postlaryngectomy pharyngocutaneous fistula: Incidence, predisposing factors, and therapy. Otolaryngol. Head Neck Surg. 2005, 133, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Magdy, E.A. Surgical closure of postlaryngectomy pharyngocutaneous fistula: A defect based approach. Eur. Arch. Otorhinolaryngol. 2008, 265, 97–104. [Google Scholar] [CrossRef]
- Marchese-Ragona, R.; Blotta, P.; Pastore, A.; Tugnoli, V.; Eleopra, R.; De Grandis, D. Management of parotid sialocele with botulinum toxin. Laryngoscope 1999, 109, 1344–1346. [Google Scholar] [CrossRef] [PubMed]
- Ellies, M.; Laskawi, R.; Rohrbach-Volland, S.; Arglebe, C.; Beuche, W. Botulinum toxin to reduce saliva flow: Selected indications for ultrasound-guided toxin application into salivary glands. Laryngoscope 2021, 112, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Marchese, M.R.; Almadori, G.; Giorgio, A.; Paludetti, G. Post-surgical role of botulinum toxin-A injection in patients with head and neck cancer: Personal experience. Acta Otorhinolaryngol. Ital. 2008, 28, 13–16. [Google Scholar]
- Hawkes, A.C.; Stell, P.M. Results of closure of pharyngocutaneous fistulae. Clin. Otolaryngol. Allied. Sci. 1980, 5, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Markou, K.D.; Vlachtsis, K.C.; Nikolaou, A.C.; Petridis, D.G.; Kouloulas, A.I.; Daniilidis, I.C. Incidence and predisposing factors of pharyngocutaneous fistula formation after total laryngectomy. Is there a relationship with tumor recurrence? Eur. Arch. Otorhinolaryngol. 2014, 261, 61–67. [Google Scholar] [CrossRef]
- Smith, T.J.; Burrage, K.J.; Ganguly, P. Prevention of postlaryngectomy pharyngocutaneous fistula: The Memorial University experience. J. Otolaryngol. 2003, 32, 222–225. [Google Scholar] [CrossRef]
- Tomkinson, A.; Shone, G.R.; Dingle, A. Pharyngocutaneous fistula following total laryngectomy and postoperative vomiting. Clin. Otolaryngol. 1996, 21, 369–730. [Google Scholar] [CrossRef]
- Giess, R.; Naumann, M.; Werner, E. Injections of botulinum toxin A into salivary glands improve sialorrhea in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2000, 69, 121–123. [Google Scholar] [CrossRef]
- Glickman, S.; Deaney, C.N. Treatment of relative sialorrhea with botulinum toxin type A: Description and rationale for an injection procedure with case report. Eur. J. Neurol. 2001, 8, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Beuche, W.; Arglebe, C.; Laskawi, R. Quantitative reduction of saliva production in two ALS patients with intraglandular injections of botulinum toxin. Neurol. Psychiatry Brain Res. 2000, 58, 1251–1256. [Google Scholar]
- Saki, N.; Nikakhlagh, S.; Kazemi, M. Pharyngocutaneous fistula after laryngectomy: Incidence, predisposing factors, and outcome. Arch. Iran. Med. 2008, 11, 314–317. [Google Scholar]
- Weber, R.S.; Berkey, B.A.; Forastiere, A.; Cooper, J.; Maor, M.; Goepfert, H.; Morrison, W.; Glisson, B.; Trotti, A.; Ridge, J.A.; et al. Outcome of Salvage Total Laryngectomy Following Organ Preservation Therapy. Arch. Otolaryngol. Head Neck Surg. 2003, 129, 44–49. [Google Scholar] [CrossRef]
- Svalestad, J.; Hellem, S.; Thorsen, E.; Johannessen, A.C. Effect of hyperbaric oxygen treatment on irradiated oral mucosa: Microvessel density. Int. J. Oral. Maxillofac. Surg. 2015, 44, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Busoni, M.; Deganello, A.; Gallo, O. Pharyngocutaneous fistula following total laryngectomy: Analysis of risk factors, prognosis and treatment modalities. Acta Otorhinolaryngol. Ital. 2015, 35, 400–405. [Google Scholar] [CrossRef]
- Soylu, L.; Kıroğlu, M.; Aydoğan, B.; Çetik, F.; Kıroğlu, F.; Özşahinoğlu, C. Pharyngocutaneous fistula following laryngectomy. Head Neck 1998, 20, 22–25. [Google Scholar] [CrossRef]
- Iteld, L.; Yu, P. Pharyngocutaneous fistula repair after radiotherapy and salvage total laryngectomy. J. Reconstr. Microsurg. 2007, 23, 339–345. [Google Scholar] [CrossRef]
- de Zinis, L.O.R.; Ferrari, L.; Tomenzoli, D.; Premoli, G.; Parrinello, G.; Nicolai, P. Postlaryngectomy pharyngocutaneous fistula: Incidence, predisposing factors, and therapy. Head Neck 1999, 21, 131–138. [Google Scholar] [CrossRef]
- Higashino, M.; Aihara, T.; Terada, T.; Kawata, R. Influence of Preoperative Radiation Therapy on the Occurrence of Pharyngocutaneous Fistula After Total Laryngectomy. Cureus 2021, 13, e13797. [Google Scholar] [CrossRef]
| Cases | Age | Sex | Primary Site | Primary Stage | Primary Therapy | Recurrent Therapy | Time to Closure (Days) | PCF Therapy |
|---|---|---|---|---|---|---|---|---|
| 1 | 75 | M | Supralgottic | IV | CT + RT | TL | 15 | BoNT-A |
| 2 | 78 | M | Glottic | IV | CT + RT | TL | 7 | BoNT-A |
| 3 | 68 | M | Glottic | IV | CT + RT | TL | 12 | BoNT-A |
| 4 | 72 | F | Transglottic | IV | CT + RT | TL | 18 | BoNT-A |
| 5 | 73 | M | Glottic | IV | CT + RT | TL | 14 | BoNT-A |
| 6 | 58 | M | Supraglottic | IV | CT + RT | TL | 16 | BoNT-A |
| 7 | 66 | M | Supraglottic | IV | CT + RT | TL | 13 | BoNT-A |
| 8 | 69 | F | Glottic | IV | CT + RT | TL | 26 | IMAP |
| 9 | 70 | M | Transglottic | IV | CT + RT | TL | 27 | IMAP |
| 10 | 71 | M | Supraglottic | III | CT + RT | PL | 28 | IMAP |
| 11 | 69 | M | Supraglottic | IV | CT + RT | TL | 29 | IMAP |
| 12 | 74 | M | Glottic | IV | CT + RT | TL | 32 | IMAP |
| 13 | 67 | M | Glottic | IV | CT + RT | TL | 25 | DS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchese, M.R.; Di Cesare, T.; De Corso, E.; Petracca, M.; Oliveto, G.; Almadori, G. Botulinum Neurotoxin A in the Treatment of Pharyngocutaneous Fistula after Salvage Surgery in Head and Neck Cancer Patients: Our Preliminary Results. Curr. Oncol. 2022, 29, 7099-7105. https://doi.org/10.3390/curroncol29100557
Marchese MR, Di Cesare T, De Corso E, Petracca M, Oliveto G, Almadori G. Botulinum Neurotoxin A in the Treatment of Pharyngocutaneous Fistula after Salvage Surgery in Head and Neck Cancer Patients: Our Preliminary Results. Current Oncology. 2022; 29(10):7099-7105. https://doi.org/10.3390/curroncol29100557
Chicago/Turabian StyleMarchese, Maria Raffaella, Tiziana Di Cesare, Eugenio De Corso, Martina Petracca, Giuseppe Oliveto, and Giovanni Almadori. 2022. "Botulinum Neurotoxin A in the Treatment of Pharyngocutaneous Fistula after Salvage Surgery in Head and Neck Cancer Patients: Our Preliminary Results" Current Oncology 29, no. 10: 7099-7105. https://doi.org/10.3390/curroncol29100557
APA StyleMarchese, M. R., Di Cesare, T., De Corso, E., Petracca, M., Oliveto, G., & Almadori, G. (2022). Botulinum Neurotoxin A in the Treatment of Pharyngocutaneous Fistula after Salvage Surgery in Head and Neck Cancer Patients: Our Preliminary Results. Current Oncology, 29(10), 7099-7105. https://doi.org/10.3390/curroncol29100557






