Issues and Prospects of Current Endoscopic Treatment Strategy for Superficial Non-Ampullary Duodenal Epithelial Tumors
Abstract
:1. Introduction
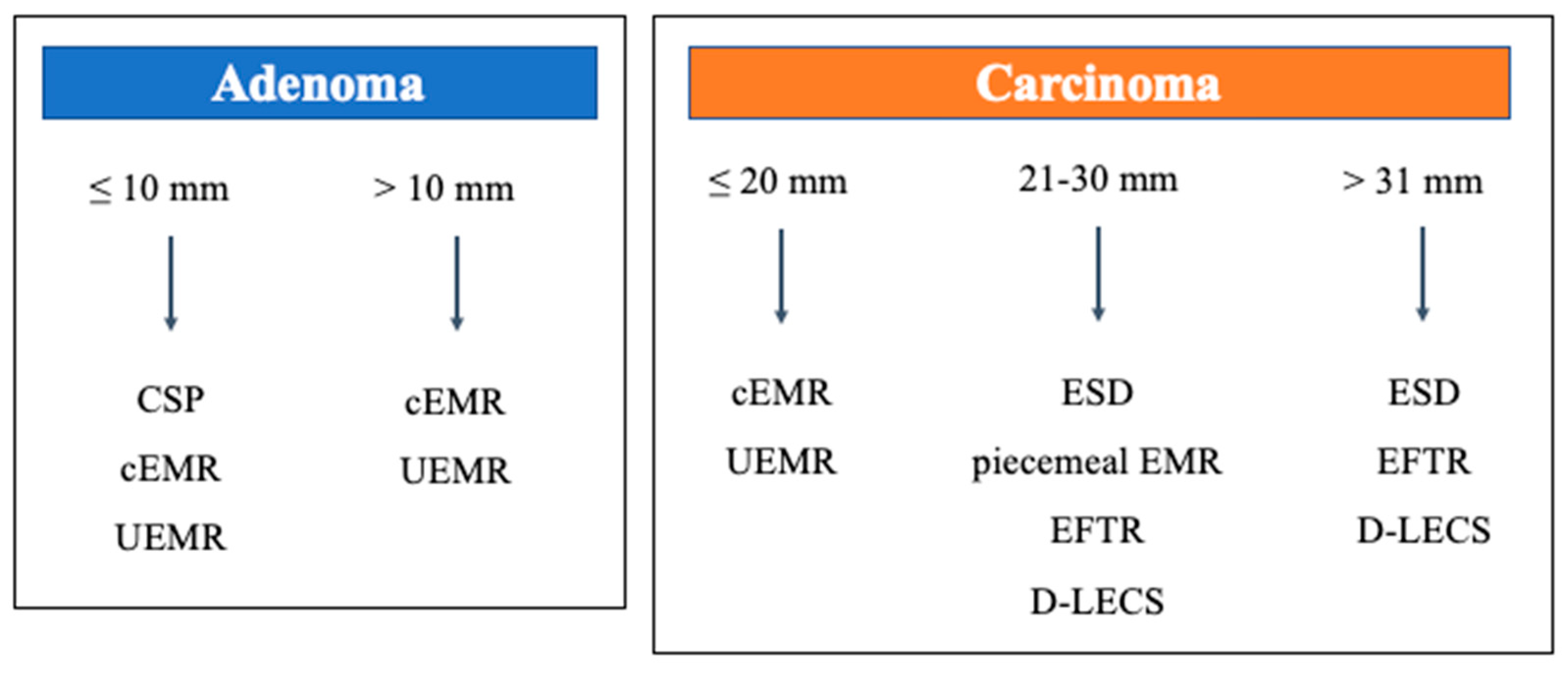
2. Overview of Clinical Practice Guidelines
3. Review of Endoscopic Treatment for SNADETs
3.1. Conventional Endoscopic Mucosal Resection (cEMR)
3.2. Endoscopic Submucosal Dissection (ESD)
3.3. Cold Snare Polypectomy (CSP)
3.4. Underwater Endoscopic Mucosal Resection (UEMR)
3.5. Endoscopic Full-Thickness Resection (EFTR)
3.6. Laparoscopic and Endoscopic Cooperative Surgery for Duodenal Tumors (D-LECS)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yoshida, M.; Yabuuchi, Y.; Kakushima, N.; Kato, M.; Iguchi, M.; Yamamoto, Y.; Kanetaka, K.; Uraoka, T.; Fujishiro, M.; Sho, M. The incidence of non-ampullary duodenal cancer in Japan: The first analysis of a national cancer registry. J. Gastroenterol. Hepatol. 2021, 36, 1216–1221. [Google Scholar] [CrossRef]
- Chathadi, K.V.; Khashab, M.A.; Acosta, R.D.; Chandrasekhara, V.; Eloubeidi, M.A.; Faulx, A.L.; Fonkalsrud, L.; Lightdale, J.R.; Salztman, J.R.; Shaukat, A.; et al. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest. Endosc. 2015, 82, 773–781. [Google Scholar] [CrossRef]
- Vanbiervliet, G.; Moss, A.; Arvanitakis, M.; Arnelo, U.; Beyna, T.; Busch, O.; Deprez, P.H.; Kunovsky, L.; Larghi, A.; Manes, G.; et al. Endoscopic management of superficial nonampullary duodenal tumors: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2021, 53, 522–534. [Google Scholar] [CrossRef]
- Japan Duodenal Cancer Guideline Committee. Clinical Practice Guidelines for Duodenal Cancer 2021; Kanehara Shuppan: Tokyo, Japan, 2021; pp. 22–31.
- Ono, H.; Kaise, M.; Nonaka, S.; Uedo, N.; Hirasawa, T.; Oyama, T. Clinical issues of duodenal endoscopic treatment. Stomach Intest. 2016, 51, 1585–1592. [Google Scholar] [CrossRef]
- Kato, M.; Takeuchi, Y.; Hoteya, S.; Oyama, T.; Nonaka, S.; Yoshimizu, S.; Kakushima, N.; Ohata, K.; Yamamoto, H.; Hara, Y.; et al. Outcomes of endoscopic resection for superficial duodenal tumors: 10 years’ experience in 18 Japanese high volume centers. Endoscopy 2022, 54, 663–670. [Google Scholar] [CrossRef]
- Nonaka, S.; Oda, I.; Tada, K.; Mori, G.; Sato, Y.; Abe, S.; Suzuki, H.; Yoshinaga, S.; Nakajima, T.; Matsuda, T.; et al. Clinical outcome of endoscopic resection for nonampullary duodenal tumors. Endoscopy 2015, 47, 129–135. [Google Scholar] [CrossRef]
- Valerii, G.; Tringali, A.; Landi, R.; Boškoski, I.; Familiari, P.; Bizzotto, A.; Perri, V.; Petruzziello, L.; Costamagna, G. Endoscopic mucosal resection of non-ampullary sporadic duodenal adenomas: A retrospective analysis with long-term follow-up. Scand. J. Gastroenterol. 2018, 53, 490–494. [Google Scholar] [CrossRef]
- Yahagi, N.; Kato, M.; Ochiai, Y.; Maehata, T.; Sasaki, M.; Kiguchi, Y.; Akimoto, T.; Nakayama, A.; Fujimoto, A.; Goto, O.; et al. Outcomes of endoscopic resection for superficial duodenal epithelial neoplasia. Gastrointest. Endosc. 2018, 88, 676–682. [Google Scholar] [CrossRef]
- Hoteya, S.; Furuhata, T.; Takahito, T.; Fukuma, Y.; Suzuki, Y.; Kikuchi, D.; Mitani, T.; Matsui, A.; Yamashita, S.; Nomura, K.; et al. Endoscopic submucosal dissection and endoscopic mucosal resection for non-ampullary superficial duodenal tumor. Digestion 2017, 95, 36–42. [Google Scholar] [CrossRef]
- Suwa, T.; Takizawa, K.; Kawata, N.; Yoshida, M.; Yabuuchi, Y.; Yamamoto, Y.; Ono, H. Current treatment strategy for superficial nonampullary duodenal epithelial tumors. Clin. Endosc. 2022, 55, 15–21. [Google Scholar] [CrossRef]
- Okimoto, K.; Maruoka, D.; Matsumura, T.; Shiratori, W.; Nagashima, A.; Ishikawa, T.; Tokunaga, M.; Kaneko, T.; Oura, H.; Kanayama, K.; et al. Long-term outcomes of cold snare polypectomy for superficial non-ampullary duodenal epithelial tumors. J. Gastroenterol. Hepatol. 2022, 37, 75–80. [Google Scholar] [CrossRef]
- Murakami, T.; Yoshida, N.; Yasuda, R.; Hirose, R.; Inoue, K.; Dohi, O.; Kamada, K.; Uchiyama, K.; Konishi, H.; Naito, Y.; et al. Local recurrence and its risk factors after cold snare polypectomy of colorectal polyps. Surg. Endosc. 2020, 34, 2918–2925. [Google Scholar] [CrossRef]
- Suzuki, S.; Gotoda, T.; Kusano, C.; Ikehara, H.; Sugita, A.; Yamauchi, M.; Moriyama, M. Width and depth of resection for small colorectal polyps: Hot versus cold snare polypectomy. Gastrointest. Endosc. 2018, 87, 1095–1103. [Google Scholar] [CrossRef]
- Kakushima, N.; Yoshida, M.; Iwai, T.; Kawata, N.; Tanaka, M.; Takizawa, K.; Ito, S.; Imai, K.; Hotta, K.; Ishiwatari, H.; et al. A simple endoscopic scoring system to differentiate between duodenal adenoma and carcinoma. Endosc. Int. Open 2017, 5, E763–E768. [Google Scholar] [CrossRef]
- Kikuchi, D.; Hoteya, S.; Iizuka, T.; Kimura, R.; Kaise, M. Diagnostic algorithm of magnifying endoscopy with narrow band imaging for superficial non-ampullary duodenal epithelial tumors. Dig. Endosc. 2014, 26 (Suppl. S2), 16–22. [Google Scholar] [CrossRef]
- Kinoshita, S.; Nishizawa, T.; Ochiai, Y.; Uraoka, T.; Akimoto, T.; Fujimoto, A.; Maehata, T.; Goto, O.; Kanai, T.; Yahagi, N. Accuracy of biopsy for the preoperative diagnosis of superficial nonampullary duodenal adenocarcinoma. Gastrointest. Endosc. 2017, 86, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Fujisaki, J.; Kasuga, A.; Omae, M.; Kubota, M.; Hirasawa, T.; Ishiyama, A.; Inamori, M.; Chino, A.; Yamamoto, Y.; et al. Sporadic nonampullary duodenal adenoma in the natural history of duodenal cancer: A study of follow-up surveillance. Am. J. Gastroenterol. 2011, 106, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Hamada, K.; Takeuchi, Y.; Ishikawa, H.; Tonai, Y.; Matsuura, N.; Ezoe, Y.; Ishihara, R.; Tomita, Y.; Iishi, H. Feasibility of cold snare polypectomy for multiple duodenal adenomas in patients with familial adenomatous polyposis: A pilot study. Dig. Dis. Sci. 2016, 61, 2755–2759. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Uedo, N.; Akamatsu, T.; Kagawa, T.; Higashi, R.; Dohi, O.; Furukawa, M.; Takahashi, Y.; Inoue, T.; Tanaka, S.; et al. Nonrecurrence Rate of Underwater EMR for ≤20-mm Nonampullary Duodenal Adenomas: A Multicenter Prospective Study (D-UEMR Study). Clin. Gastroenterol. Hepatol. 2022, 20, 1010–1018.e3. [Google Scholar] [CrossRef] [PubMed]
- Kiguchi, Y.; Kato, M.; Nakayama, A.; Sasaki, M.; Mizutani, M.; Tsutsumi, K.; Akimoto, T.; Takatori, Y.; Mutaguchi, M.; Takabayashi, K.; et al. Feasibility study comparing underwater endoscopic mucosal resection and conventional endoscopic mucosal resection for superficial non-ampullary duodenal epithelial tumor < 20 mm. Dig. Endosc. 2020, 32, 753–760. [Google Scholar] [CrossRef]
- Iwagami, H.; Takeuchi, Y.; Yamasaki, Y.; Nakagawa, K.; Ohmori, M.; Matsuno, K.; Inoue, S.; Iwatsubo, T.; Nakahira, H.; Matsuura, N.; et al. Feasibility of underwater endoscopic mucosal resection and management of residues for superficial non-ampullary duodenal epithelial neoplasms. Dig. Endosc. 2020, 32, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, Y.; Uedo, N.; Takeuchi, Y.; Ishihara, R.; Okada, H.; Iishi, H. Current status of endoscopic resection for superficial nonampullary duodenal epithelial tumors. Digestion 2018, 97, 45–51. [Google Scholar] [CrossRef]
- Ren, Z.; Lin, S.L.; Zhou, P.H.; Cai, S.L.; Qi, Z.P.; Li, J.; Yao, L.Q. Endoscopic full-thickness resection (EFTR) without laparoscopic assistance for nonampullary duodenal subepithelial lesions: Our clinical experience of 32 cases. Surg. Endosc. 2019, 33, 3605–3611. [Google Scholar] [CrossRef]
- Andrisani, G.; Di Matteo, F.M. Endoscopic full-thickness resection of duodenal lesions (with video). Surg. Endosc. 2020, 34, 1876–1881. [Google Scholar] [CrossRef]
- Tashima, T.; Ryozawa, S.; Tanisaka, Y.; Fujita, A.; Miyaguchi, K.; Ogawa, T.; Mizuide, M.; Mashimo, Y.; Kawasaki, T.; Yasuda, M. Endoscopic resection using an over-the-scope clip for duodenal neuroendocrine tumors. Endosc. Int. Open. 2021, 9, E659–E666. [Google Scholar] [CrossRef]
- Hiki, N.; Yamamoto, Y.; Fukunaga, T.; Yamaguchi, T.; Nunobe, S.; Tokunaga, M.; Miki, A.; Ohyama, S.; Seto, Y. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg. Endosc. 2008, 22, 1729–1735. [Google Scholar] [CrossRef]
- Ojima, T.; Nakamori, M.; Nakamura, M.; Hayata, K.; Katsuda, M.; Takifuji, K.; Yamaue, H. laparoscopic and endoscopic cooperative surgery versus endoscopic submucosal dissection for the treatment of low-risk tumors of the duodenum. J. Gastrointest. Surg. 2018, 22, 935–940. [Google Scholar] [CrossRef]
- Kanaji, S.; Morita, Y.; Yamazaki, Y.; Otowa, Y.; Takao, T.; Tanaka, S.; Urakawa, N.; Yamamoto, M.; Matsuda, T.; Oshikiri, T.; et al. Feasibility of laparoscopic endoscopic cooperative surgery for non-ampullary superficial duodenal neoplasms: Single-arm confirmatory trial. Dig. Endosc. 2021, 33, 373–380. [Google Scholar] [CrossRef]
- Nunobe, S.; Ri, M.; Yamazaki, K.; Uraoka, M.; Ohata, K.; Kitazono, I.; Terashima, M.; Yamagata, Y.; Tanabe, S.; Abe, N.; et al. Safety and feasibility of laparoscopic and endoscopic cooperative surgery for duodenal neoplasm: A retrospective multicenter study. Endoscopy 2021, 53, 1065–1068. [Google Scholar] [CrossRef]
- Masunaga, T.; Kato, M.; Takatori, Y. Spurting delayed bleeding on postoperative day six after cold snare polypectomy for small superficial duodenal epithelial tumor. Dig. Endosc. 2021, 33, 1198. [Google Scholar] [CrossRef]
- Akimoto, T.; Kato, M.; Yahagi, N. Severe acute pancreatitis following cold polypectomy of the minor duodenal papilla in a case with pancreas divisum. Dig. Endosc. 2020, 32, 151. [Google Scholar] [CrossRef]
| 47 Patients/53 Lesions | |
|---|---|
| Age, median (range), years | 67 (39–82) |
| Sex (male/female) | 37/10 |
| Location (1st/2nd/3rd) | 6/45/2 |
| Size (endoscopic), median (range), mm | 6 (2–12) |
| Macroscopic type (0–I/0–IIa/0–IIa + IIc/0–IIc) | 6/43/1/3 |
| Biopsy before CSP | 47% (25/53) |
| Closure after CSP | 58% (31/53) |
| en-bloc resection rate | 96% (51/53) |
| Histopathological assessment carcinoma/adenoma/nonneoplastic | 3/42/8 |
| R0 resection rate | 44% (20/45) |
| Horizontal margins—negative rate | 47% (21/45) |
| Vertical margins—negative rate | 91% (41/45) |
| Adverse event rate delayed bleeding/intraoperative perforation/delayed perforation | 0/0/0 |
| Residual recurrence rate a | 2.1% (1/47) b |
| 54 Patients/65 Lesions | |
|---|---|
| Age, median (range), years | 67 (28–89) |
| Sex (male/female) | 31/23 |
| Location (1st/2nd/3rd) | 9/52/4 |
| Size (endoscopic), median (range), mm | 12 (3–25) |
| Macroscopic type (0–I/0–IIa/0–IIa + IIc/0–IIc) | 8/36/17/4 |
| Biopsy before UEMR | 40% (26/65) |
| Closure after UEMR | 91% (59/65) |
| En-bloc resection rate | 86% (56/65) |
| Histopathological assessment carcinoma/adenoma/nonneoplastic | 15/46/4 |
| R0 resection rate | 51% (31/61) |
| Horizontal margins—negative rate | 52% (32/61) |
| Vertical margins—negative rate | 97% (59/61) |
| Adverse event rate delayed bleeding/intraoperative perforation/delayed perforation | 1/0/0 |
| Residual recurrence rate a | 4.2% (2/48) b |
| Kiguchi Y, et al. [21] | Iwagami H, et al. [22] | Yamasaki Y, et al. [23] | Yamasaki Y, et al. [20] | Kato M, et al. [6] | Suwa T, et al. [11] | |
|---|---|---|---|---|---|---|
| All En-bloc R0 HM0 Bleeding Perforation Recurrence | N = 104 87% (90/104) 67% (60/104) 67% (60/104) 2% (2/104) 0% (0/104) - | N = 162 68% (110/162) - 46% (74/162) 1.2% (2/162) 0.6% (1/162) 5% (7/157) | N = 79 77% (61/79) - - 3.8% (3/79) 0% (0/79) 3.8% (3/79) | N = 166 90% (149/166) 67% (111/166) - 1.2% (2/166) 0% (0/166) 2.6% (4/151) | N = 579 79% (455/579) 56% (316/579) - 2.1% (12/579) 0.2% (1/579) - | N = 65 86% (56/65) 51% (31/61) 52% (32/61) 1.5% (1/65) 0% 4% (2/48) |
| <10 mm En-bloc R0 HM0 Bleeding Perforation Recurrence | - | ≤10 mm N = 46 96% (44/46) - - 2.2% (1/46) 0% (0/46) 2.2% (1/46) | <10 mm N = 76 100% 75% (57/76) - 0% | < 10 mm 89.1–93.3% | N = 25 96% (24/25) 60% (15/23) 60% (15/23) 0% 0% 0% | |
| 10–20 mm En-bloc R0 HM0 Bleeding Perforation Recurrence | <20 mm N = 134 79% (106/134) - - - - 2.2% (3/134) | 11–20 mm N = 22 73% (16/22) - - 4.5% (1/22) 0% (0/22) 0% (0/22) | ≥ 10 mm N = 79 78% (62/79) 58% (46/79) - - 0% - | 10–19 mm 62–81.5% | N = 35 89% (31/35) 49% (15/33) 46% (16/33) 0% 0% 3.8% (1/26) | |
| >20 mm En-bloc R0 HM0 Bleeding Perforation Recurrence | ≥20 mm N = 28 14% (4/28) - - - - 14% (4/28) | >20 mm N = 11 9.1% (1/11) - - 50% (1/2) 0% (0/2) 18% (2/11) | ≥20 mm 30% | N = 5 20% (1/5) 20% (1/5) 20% (1/5) 20% (1/5) 0% 20% (1/5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suwa, T.; Yoshida, M.; Ono, H. Issues and Prospects of Current Endoscopic Treatment Strategy for Superficial Non-Ampullary Duodenal Epithelial Tumors. Curr. Oncol. 2022, 29, 6816-6825. https://doi.org/10.3390/curroncol29100537
Suwa T, Yoshida M, Ono H. Issues and Prospects of Current Endoscopic Treatment Strategy for Superficial Non-Ampullary Duodenal Epithelial Tumors. Current Oncology. 2022; 29(10):6816-6825. https://doi.org/10.3390/curroncol29100537
Chicago/Turabian StyleSuwa, Tetsuya, Masao Yoshida, and Hiroyuki Ono. 2022. "Issues and Prospects of Current Endoscopic Treatment Strategy for Superficial Non-Ampullary Duodenal Epithelial Tumors" Current Oncology 29, no. 10: 6816-6825. https://doi.org/10.3390/curroncol29100537
APA StyleSuwa, T., Yoshida, M., & Ono, H. (2022). Issues and Prospects of Current Endoscopic Treatment Strategy for Superficial Non-Ampullary Duodenal Epithelial Tumors. Current Oncology, 29(10), 6816-6825. https://doi.org/10.3390/curroncol29100537




