Abstract
Despite its decreasing incidence, gastric cancer remains an important global healthcare problem due to its overall high prevalence and high mortality rate. Since the MAGIC and FNLCC/FFCD trials, the neoadjuvant chemotherapy has been recommended throughout Europe in gastric cancer. Potential benefits of preoperative treatments include a higher rate of R0 resection achieved by downstaging the primary tumor, a likely effect on micrometastases and isolated tumor cells in the lymph nodes, and, as a result, improved cancer-related survival. Nevertheless, distortion of anatomical planes of dissection, interstitial fibrosis, and sclerotic tissue changes may increase surgical difficulty. The collection of at least twenty-five lymph nodes after neoadjuvant therapy would seem to ensure removal of undetectable node metastasis and reduce the likelihood of locoregional recurrence. It is not what you take but what you leave behind that defines survival. Therefore, para-aortic lymph node dissection is safe and effective after neoadjuvant chemotherapy, in both therapeutic and prophylactic settings. In this review, the efficacy of adequate lymph node dissection, also in a neoadjuvant setting, has been investigated in the key studies conducted to date on the topic.
1. Current Status and Clinical Significance of Lymph Node Dissection
1.1. D2 Lymphadenectomy
The lymphatic route is the main way that gastric cancer spreads; therefore, lymph node involvement represents the leading prognostic factor after surgery [1,2].
D2 lymphadenectomy currently represents the standard procedure for locally advanced gastric cancer [3,4]. According to the third and fourth edition of the Japanese guidelines, D2 lymphadenectomy for subtotal gastrectomy defines the surgical removal of stations 1, 3, 4sb, 4d, 5, 6, 7, 8a, 9, 11p, and 12a, while D2 lymphadenectomy for total gastrectomy includes stations 1–7, 8a, 9, 11p, 11d, 10, and 12a [5,6]. In the fifth Japanese guidelines [3], station 10 was excluded in D2 dissection for total gastrectomy, except for tumors located in the upper third of the stomach along the greater curvature. Current guidelines indicate D2 lymphadenectomy in all cN+ or cT2-4 tumors, while indicating D1 dissection (limited to stations 1–6) for cT1a tumors or for differentiated cT1b smaller than 1.5 cm in diameter [4,7,8].
The optimal extent of lymphadenectomy has been a topic of investigation in the past decades. Adequate lymphadenectomy resulted in four potential clinical benefits: (1) adequate disease staging due to the increased number of nodes retrieved; (2) the removal of potentially metastatic lymph nodes, resulting in increased surgical radicality; (3) a decrease in GC recurrence, especially locoregional; (4) prognostic benefit by potential improvement in long-term survival [9].
1.1.1. Adequate Disease Staging Due to the Increased Number of Nodes Retrieved
The radicality of the surgical procedure can be evaluated by the number of harvested lymph nodes. When the number of retrieved nodes is less than 10, it is extremely unlikely that we can adequately determine the truly negative incidence of regional lymph nodes [10,11,12]. For patients with T1-2 N0 stage, the risk of downstaging with an inadequate number of removed lymph nodes is especially high, therefore underestimating the need for a more radical lymphadenectomy [12,13,14,15,16]. Although the American Joint Committee on Cancer (AJCC) does not define a standard threshold for the number of nodes to be considered adequate in assigning an appropriate N stage, 16 has been recommended as the minimum number for adequate lymphadenectomy by the Japanese, Korean and European guidelines [12,17,18,19,20,21], while 15 has been suggested as the minimum number by British and North American societies [12,22,23,24]. The Italian Research Group for Gastric Cancer (GIRCG), based on data collected from a prospective database of 2822 patients treated in high-volume centers (78% and 53% of cases with more than 15 and 25 removed nodes, respectively), highlighted that overall postoperative mortality was 3.5%, even when including aged patients or advanced stages [9,25]. Additionally, an analysis of 40,281 patients, included in the American National Cancer Database for gastric cancer, showed a higher 30-day mortality after >29 nodes were removed (4.3%) compared to a resection of 15–28 nodes and <15 nodes (3.0% and 2.1%, respectively) [26,27]. In a recent update from the GIRCG database, which included 6230 patients, a strong correlation between the number of removed and metastatic lymph nodes was observed, with a plateau above 40–50 lymph nodes examined (Figure 1).
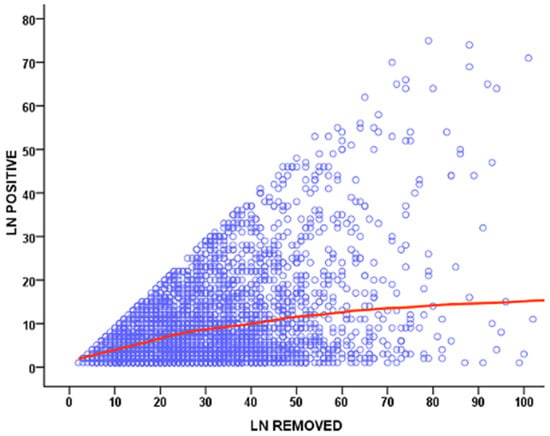
Figure 1.
Scatter plot showing the correlation between the number of removed and metastatic lymph nodes (LN) in GIRCG cohort.
1.1.2. Removal of Potentially Metastatic Lymph Nodes, Resulting in Increased Surgical Radicality
Removing the involved nodes could improve surgical radicality. The number of positive nodes is a well-established prognostic factor in gastric cancer (Figure 2). However, the GIRCG experience, similarly to other reports [28,29], indicates that the survival probability in patients with the same number of involved nodes is strongly affected by the number of removed nodes (Figure 3). For instance, the survival probability of patients with three involved nodes is 12% when less than 10 nodes are removed, 26% when 11–15 nodes are removed, and 60% when more than 25 nodes are examined. Similar behavior is observed for other lymph-node positive subgroups, with a plateau above 45 removed lymph nodes. These survival trends may be due to the stage migration, the “Will Rogers phenomenon”, but we strongly believe that the therapeutic effect of extended lymphadenectomy plays a crucial role in prognostic benefit. Furthermore, the removal of potential micrometastasis, even in node-negative patients, could also improve the oncological outcome [30,31]. In surgical practice, not only the total number of removed lymph nodes is important, but also the topographic sites of different nodal stations, according to the Japanese Gastric Cancer Association (JGCA) guidelines [32,33]. The term “contamination” was used to define over-treatment in a certain group (i.e., when a surgeon dissected two or more lymph node stations which he should not have), while the term “non-compliance” was used to define under-treatment (i.e., when a surgeon did not dissect two or more lymph node stations which he should have). The D2 non-compliance mainly involved nodal stations 10, 11d, and 12a in total gastrectomy and 4sb, 11p, and 12a in subtotal gastrectomy. Contamination mainly consisted of the removal of posterior stations (8p, 12b, 12p) in total gastrectomy and 8p, 11d, 12b, and 12p in subtotal gastrectomy. In previous literature, “standard” lymphadenectomy is usually referred to as the surgical dissection described by the JGCA, while “extended”, “more extended” and “super-extended” refer to D2, D2 plus and D3 dissections, respectively. As mentioned above, the D2 lymphadenectomy is routinely accepted in most international guidelines [4]. Despite a more extended procedure (D2plus) gaining acceptance among surgeons, D3 seems to fail in obscurity in recent years. In our opinion, more extended lymphadenectomy should be performed in selected cases at risk of metastasis to posterior or para-aortic lymph nodes (PAN), while proximal tumors or diffuse-type tumors are particularly prone to spread to distant nodes and may benefit from both posterior and PAN dissection [34].
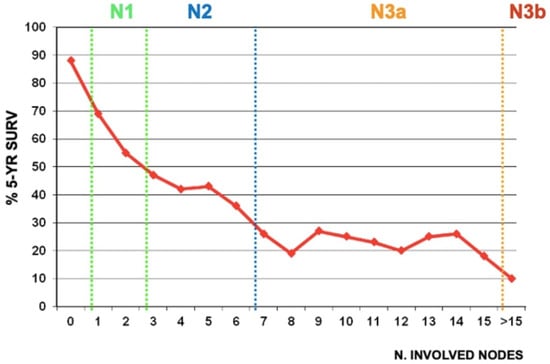
Figure 2.
Correlation between the number of metastatic nodes and 5-year overall survival in GIRCG cohort. “N” classification according to the 8th Edition of the AJCC is showed in subgroups.
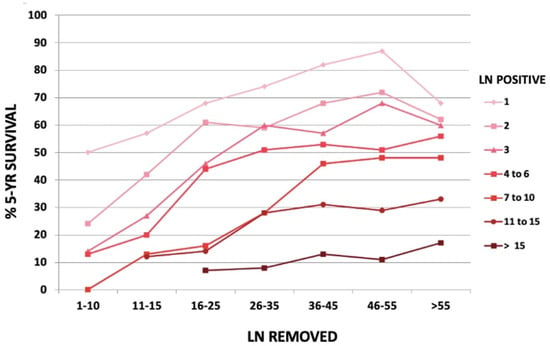
Figure 3.
Five-year overall survival according to the number of removed nodes in different node positive subgroups, in GIRCG cohort.
1.1.3. Decrease in GC Recurrence, Especially Locoregional
The increase in R0 obtained with extended lymphadenectomy has been associated with a decrease in locoregional tumor recurrence [9].
1.1.4. Prognostic Benefit by Potential Improvement in Long-Term Survival
The Dutch trial clearly observed lower loco-regional recurrences, as well as cancer-related mortality in the D2 arm compared to the D1 arm, when considering only long-term mortality without 30-day postoperative mortality [35,36]. An Italian trial noted a trend toward statistical significance in survival benefit, when compared lymph node-positive patients who underwent D2 and D1 gastrectomy [37,38,39,40]. The abovementioned evidence is confirmed in a Taiwanese randomized trial, which compared limited and extended lymph node dissection [41]. On the other hand, the surgical removal of the spleen and the pancreatic tail to achieve stations 10 and 11 strongly affects morbidity and mortality rates [42].
Key points:
- D2 lymphadenectomy is the standard surgical treatment with curative intent for advanced gastric cancer.
- Adequate D2 enables accurate disease staging, reduces the incidence of locoregional recurrences and contributes to an improved long-term survival.
- Undetectable node metastases are associated with high rates of locoregional recurrence.
1.2. D2 plus and D3 Lymphadenectomies
Radical surgery results from the balance of two elements: the need to guarantee optimal locoregional control of the disease, and the clinical cost of extensive surgery.
The extent of lymphadenectomy, defined by the amount of lymph nodes and the quality of the harvested stations, is a marker of quality of surgical resection. A Turkish anatomical study, comparing total gastrectomy with extended lymphadenectomy to station 16 on autopsy and in vivo cases, revealed higher numbers of lymph nodes harvested in autopsy cases at stations 10, 11, 12, and 16, but no difference at stations 1, 2, 3, 6, 7, 8, and 9 [43].
D2-plus lymphadenectomy is indicated by Japanese studies only in specific cases [3,6]: (i) D2+ station 14v consisting of dissection of superior mesenteric venous lymph nodes for cancer of the distal stomach with metastasis to the station 6 lymph nodes, (ii) D2+ station 13 with dissection of lymph nodes posterior to the pancreas head for cancer invading the duodenum, and (iii) D2+ station 16 with dissection of abdominal aortic lymph nodes for cancer with extensive lymph node involvement.
Historically, the so-called D3 dissection included both PAN (16a2 and 16b1) and “posterior” stations (8p, 12p and 13) [44]. However, as since the third edition of the Japanese guidelines, routine removal of lymph node stations beyond the standard D2 is no longer indicated; more extended procedures beyond the D2 are now called D2plus.
In a recent trial, the survival analysis indicated similar 3-year outcomes between D2 and D2+ arms, while, among patients with duodenum involvement, the 3-year disease-free survival rate of the D2+ group was higher than the D2 group (61.5% vs. 20%, respectively) [45].
A closer look at the literature on the definition of “extensive lymph node metastases (ELM)”, however, reveals a number of gaps and shortcomings. In most cases, PAN enlargement without other lateral metastasis is considered ELM. From an anatomical point of view, this definition includes the stations 16a2-b1 (lower end of celiac trunk and upper border of inferior mesenteric artery); in a preoperative radiological examination, a positive (metastatic) PAN found that the longest axis of the lymph node is at least 10 mm. As we consider the latest RECIST (Response Evaluation Criteria in Solid Tumors), a cut-off of ≥15 mm in the short axis should be analyzed [46]. Our experience based on analysis of CT scans found that ≥8 mm in the short axis is the value that we should take as suspected of PAN gastric cancer metastases [47]. The other definition of ELM was proposed by Japan Clinical Oncology Group (JCOG) studies about neoadjuvant treatment with ELM. They proposed to include not only PAN but also bulky nodes (bulky N2), defined as lymphatic tissue with a diameter of ≥3 cm or at least two adjacent nodes of ≥1.5 cm in diameter surrounding the coeliac artery and its branches [48,49,50]. (Figure 4) The reason for such a consideration is the inability to perform adequate resection, whereas even in the case of resection, the prognosis remains poor, similar to PAN metastases. In the JCOG0405 trial, survival outcomes were similar in PAN-only metastases as well as bulky N-only, while the worst outcome was for patients presenting metastases in both bulky N2 and PAN (results will be discussed further below) [48].
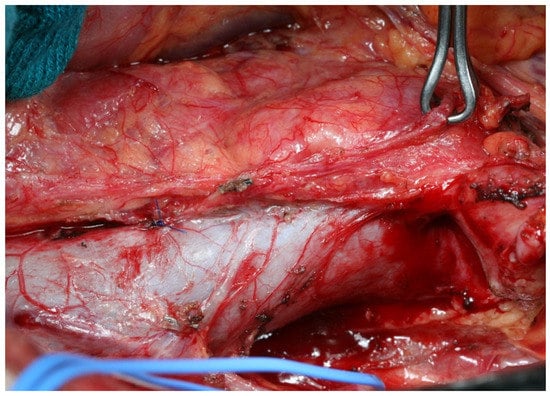
Figure 4.
Bulky nodes in 16a2-b1 stations.
In 2006, in fact, the phase III JCOG9501 randomized controlled trial investigated the role of “prophylactic” D2+ para-aortic lymph node dissection (PAND) compared to D2 in locally advanced gastric tumors without clinically evident metastases in the para-aortic area [51]. (Figure 5) Prophylactic D2+ PAND did not result in survival improvement, while it was associated with a higher morbidity rate. Therefore, the prophylactic removal of para-aortic lymph node 16a2 and 16b1 stations may not be recommended in all patients. Moreover, “curative” D2+ PAND, i.e., dissection of the para-aortic area in cases of clinically detected lymph nodes, was discouraged due to the rate of poor survival [52].
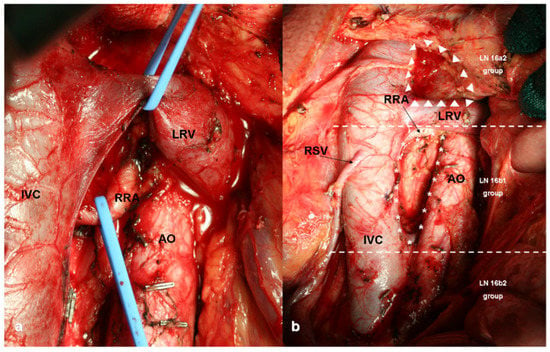
Figure 5.
Para-Aortic Lymph Node Dissection (PAND): (a) intraoperative view of 16a2 lymph node dissection; (b) 16a2 (upper third), 16b1 (medium third) and 16b2 (lower third) lymph nodes stations. IVC: Inferior Vena Cava, RRA: Right Renal Artery, LRV: Left Renal Vein, AO: Aorta, RSV: Right Spermatic Vein, LN: Lymph Nodes.
The stations 13 (retropancreatic) and 14v (superior mesenteric) are commonly considered the “gatekeepers” of the para-aortic nodes, and their dissection in middle- and lower-third advanced gastric cancer represents an independent prognostic factor when performed by experienced surgeons. [53,54,55].
The issue of the other “posterior” stations such as 8p and 12p has not been deeply discussed. Our group recently explored the benefit of “posterior” station removal in a large Western cohort undergoing D2plus dissection [56]. Posterior stations are “regional” nodes in distal tumor locations and expressions of an extensive nodal spread. Interestingly, middle- and lower-third gastric tumors showed a higher trend to metastasize to posterior nodes than the upper-third, whereas for PAN metastases it was the reverse. Only 6.3% of patients had metastases in posterior stations, showing a 5-year survival rate of 17% after adequate lymphadenectomy. Moreover, some retrospective data on D2plus lymphadenectomy showed that a higher probability of long-term survival was achieved in pT3 and pT4a patients with PAN metastasis [34,57].
Recent studies from the GIRCG, comparing D2 with D2plus lymphadenectomy in patients who underwent upfront surgery with both prophylactic and curative intents, showed that D2plus offers a better locoregional control in diffuse histotype [58]. Tumor histology largely affects the surgeon’s choice concerning the extent of lymphadenectomy. A European survey concluded that more extended lymphadenectomy was selected for tumors with Lauren diffuse histotype, even if no nodal metastases were detected on preoperative workup [59].
More extended lymphadenectomy should only be performed in dedicated high-volume hospitals, due to the high risk of post-operative complications and mortality [60].
2. Evolution in the Surgical Management of Gastric Cancer
It is not easy to offer a tailored lymphadenectomy (including sentinel node navigation surgery) for specific subgroups of patients, since the lymphatic network of the stomach is very complex. Specifically, the incidence of positive lymph node is related to the depth of invasion, and metastasis rates range from 2.3% for mucosal invasion to 86.6% for serosal pathological invasion. Nonetheless, most positive nodes are microscopic metastases, which often occur in small-sized lymph nodes, resulting in a difficult clinical and intraoperative diagnosis. Thus, gastrectomy with prophylactic lymphadenectomy is recommended as the standard surgical procedure for gastric cancer [61].
Widened lymphadenectomy involves mobilization of the duodenum, which is obtained performing the Kocher maneuver. This access provides exposure of the para-aortic node stations and improves the surgical exposure of lymph node stations 8p, 12p, and 13. Nodal stations 16a2 and 16b1 are included in PAND for gastric cancer. PAND is the lymphadenectomy performed between the upper margin of the origin of the celiac artery and the lower border of the left renal vein (station 16a2), and the lymph nodes between the lower border of the left renal vein and the upper border of the origin of the inferior mesenteric artery (station 16b1) [62].
Despite radical surgery still being the main treatment modality in gastric cancer, the validity of the complete mesogastric excision is still debated because of embryological restrictions of the gastric mesentery. Systematic mesogastric excision is a procedure consisting of three main steps: (i) the mesogastrium is dissociated from the adjacent mesenteries or the parietal wall following the embryological planes; (ii) the fat tissue containing lymph nodes is separated from the pancreas and its associated vessels; and (iii) the tumor-specific mesenteries are dissected. Therefore, D2 gastrectomy may be defined as the “en bloc” resection of the primary tumor and the tumor-specific mesenteries [63].
Interestingly, several studies have applied near-infrared (NIR) fluorescence imaging to gastric cancer for performing surgery in a real-time manner, including localizing cancers, assessing surgical margins, tracing lymph nodes, and mapping critical anatomical structures [64]. With the aid of lymphography, a significantly higher number of lymph nodes may be harvested [65]. However, some clinical situations, such as linitis plastica, post-chemotherapy fibrosis reaction, or cytotoxicity, and disturbed lymphatic flow following preoperative endoscopic submucosal dissection in early gastric cancer, could limit NIR application. From a pathological point of view, tumor cell metabolism is altered by chemotherapy drugs which lead to several histopathological changes, such as eosinophilic appearance of the cytoplasm, nuclear enlargement, condensation, cell necrosis, cell dissection, and destruction of ducts, neutrophils, and other inflammatory cells. Tumor cells are eventually replaced by the infiltration and aggregation of monocytes which cause interstitial fibrosis, which then leads to a shrinkage of the primary tumor and metastatic lymph nodes. This phenomenon does not allow lymphatic drainage and, therefore, may alter the visualization via indocyanine green tracer (ICG) [66]. At the same time, however, NIR has been proved to reduce intraoperative blood loss without increasing the operation time and overall complications, and to improve lymphadenectomy in terms of total number of lymph nodes harvested when performing minimally-invasive radical gastrectomy after neoadjuvant therapy (40.8 vs. 31.8), especially in 5–12a subgroups (19.1 vs. 13.2), and to reduce the lymph node non-compliance rate (35.1% vs. 51.1%). Clearly, the effect is marked in patients who underwent neoadjuvant therapy but with poor chemotherapy effects (23.3% vs. 55%) [66]. To our knowledge, there is no prospective research to confirm whether ICG technology can increase the number of lymph node dissections for patients who had undergone neoadjuvant chemotherapy. The study NCT04611997 explores the feasibility and the effect on 3-year disease-free survival using laparoscopic ICG tracing [67].
3. Contentious Issues in the Era of Multimodal Strategy
3.1. Preoperative Therapy
In 2012, De Manzoni and Roviello described D2 lymphadenectomy as “safe when conducted in specialized centers”, and D3 as “an emerging technical approach especially indicated for patients with upper-third gastric cancer” [68]. Is this still the case in the era of neoadjuvant therapy?
For many years, surgery has been the only treatment option in patients affected by gastric cancer. After the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial and the Fédération Nationale des Centres de Lutte contre le Cancer (FNCLCC) and the Fédération Francophone de Cancérologie Digestive (FFCD) trial in the Western world, the neoadjuvant approach has become a gold standard in treatment of locally advanced gastric cancer in Western countries and in parts of the Asia–Pacific region [38,69,70]. Neoadjuvant chemotherapy increases the chance for R0 resection, eliminates early micrometastases, downstages the lesions, and thus improves long-term outcomes [38], increasing the overall survival rate by 9% and R0 resection by 1.4-fold [71]. Another potential benefit may be the possibility for patients to receive systemic chemotherapy without having to wait for the surgery and the related postoperative recovery [72].
To date, FLOT schedule seems to provide the most successful results [73]; selected patients may also benefit from the use of fluoropyrimidine-platinum doublet or triplet chemotherapy [70]. The influence of human epidermal growth factor 2 (HER2)-targeted agents and vascular endothelial growth factor (VEGF) inhibition were recently analyzed in the metastatic setting. A new KEYNOTE-811 study suggested that adding pembrolizumab to standard therapy with trastuzumab and chemotherapy reduces tumor size and induces complete responses in unresectable or metastatic HER2-positive gastric cancers [74]. In the PETRARCA trial (NCT02581462), the combination of trastuzumab, pertuzumab, and perioperative FLOT showed improvement in the pathologic complete response and nodal negativity rates [75]. In the RAMSES/FLOT7 trial (NCT02661971), the addition of ramucirumab to FLOT has been showed to improve R0 resection rates but not the pathologic response rate [76,77].
The National Comprehensive Cancer Network (NCCN) [78] and the Italian Association of Medical Oncology (AIOM) [79] recommend neoadjuvant chemotherapy for resectable gastric cancer with clinical stage ≥ T2 or N+, while the European Society for Medical Oncology (ESMO) [80] indicate perioperative chemotherapy for patients with stage ≥ IB resectable gastric cancer. The GIRCG suggests considering it for clinical stage ≥ T3 and/or metastatic nodes, as the 5-year survival probability of T1 and T2 node-negative cases was greater than 80% in their series [81].
All studies conducted so far have demonstrated the superiority of preoperative chemotherapy compared to surgery alone, whether followed or not by adjuvant chemotherapy, while perioperative chemotherapy has rarely been investigated [82]. The GastroDOC trial investigated the compliance with neoadjuvant (four cycles of DOC regimen, i.e., docetaxel, oxaliplatin and capecitabine) and perioperative (two cycles pre- and two cycles post-surgery) therapy of stage II and III cancer patients, showing the neoadjuvant treatment to be more frequently completed [83].
Another critical open question is whether preoperative chemotherapy should be administered, taking into account the tumor’s (1) histopathological and (2) molecular characteristics.
3.1.1. Histopathological Characteristics
In diffuse-type tumors, only 14% of patients had a good pathological response, while a retrospective analysis from a French National registry suggested no benefit in signet-ring-cell type gastric cancer [84]. Similarly, in an early study aiming to evaluate the prognosis of patients who underwent neoadjuvant chemotherapy followed by surgery with PAND, patients with a diffuse-type histology tended to develop a worse prognosis (8-year survival halved when compared with the intestinal subtype) [85]. From a side-study of the multicenter LOGICA trial, lymph node metastases were more frequent in diffuse than intestinal tumors (66% vs. 52%, respectively), but not in cT3-4 than cT1-2-stage (59% vs. 51%, respectively) [86].
3.1.2. Molecular Characteristics
Following the introduction of molecular classifications [87,88,89], several studies have been conducted to clarify their potential impact in clinical decision-making and treatment of gastric cancer [47,90]. A different prognosis was attributed to the microsatellite instability (MSI) showing the best survival rate, and to the epithelial-mesenchymal transition (EMT) confirming the worst prognosis. The condition of MSI reflects approximately 10% of the cases of operable gastric cancer [91,92]. Although MSI was found to be a positive prognostic factor in the post hoc analysis of the MAGIC and the CLASSIC trials, the use of perioperative or adjuvant chemotherapy was not effective or even potentially detrimental. The same results were confirmed in the post hoc analysis of the CRITICS study and the ITACA-S trial [93,94]. Similarly, in 2019 a meta-analysis further demonstrated that MSS patients could benefit from adjuvant chemotherapy [95]. However, the limited number of MSI-high patients enrolled did not allow significant conclusions to be drawn. In our experience, MSI status was associated with a high rate of N0 stage (53.7% vs. 29.7%), a lower number of lymph node metastases (1 vs. 5), a less extensive spread to lymph node stations, and no skip metastases (0 vs. 6.1%) than microsatellite stable (MSS) tumors [91]. We therefore speculate that an approach consisting of preoperative therapy and more extended lymphadenectomy may not be indicated in all MSI tumors, due to the weak propensity to spread beyond the limit of the standard D2 dissection. Moreover, clinical trials enrolling advanced MSI-high gastric cancer patients showed prolonged survival following immunotherapy (pembrolizumab monotherapy), even in the first-line setting [76]. Similarly, Epstein–Barr virus (EBV)-associated gastric cancer, influencing tumors progression in about one patient in ten [96], has been described as being more susceptible to immune-checkpoint blockade, due to its increased PD-L1 and PD-L2 expression [76]).
Key points:
- Neoadjuvant chemotherapy is indicated in clinical stage > T2 or N+.
- Preoperative treatments increase R0 resection and improve overall survival.
- In cT3-T4 diffuse histotype, perioperative chemotherapy should be considered when staging laparoscopy confirms no peritoneal metastasis and negative peritoneal lavage cytology.
- MSI tumors may not benefit from chemotherapy and more extended lymphadenectomy.
3.2. Lymphadenectomy after Neoadjuvant Chemotherapy
After 10 years, the same authors raise the question of “whether a certain level of variability would reflect more likely the efforts by expert surgeons to choose the best treatment for their individual patients rather than technical issues” [97].
While the literature abounds with trials on the different choices of lymphadenectomy in patients undergoing upfront surgery, there is wide variation in practice patterns for lymph node dissection after neoadjuvant therapy. Studies have proven that patients who underwent neoadjuvant chemotherapy need more lymph node dissections to truly reflect the tumor stage and prognosis. The cut-off of 25 lymph nodes seemed to have had the best predictive power in terms of morbidity and mortality [98].
The collection of a high number of lymph nodes is associated with improved survival only for node-positive patients [28,99,100]. Although “extensive lymphadenectomy” is commonly practiced by surgeons in European high-volume centers, the extent of lymphadenectomy during gastrectomy for gastric cancer in the USA and in Asia is variable. Interestingly, an early report from the National Cancer Database proved no lymph nodes in the resection specimen of a quarter of gastrectomy performed [99,101].
Surgeons are forced to perform a more adequate lymphadenectomy in a condition of chemotherapy-driven tissue sclerosis and fibrosis. Since peripheral metastatic nodes may be difficult to palpate intraoperatively, the number of lymph nodes harvested greatly varies depending on the skills of the technician. Practically, these technical difficulties led to longer operative times, inadequate lymph node sampling, and increased blood loss [102]. In our experience, the fibrosis induced by neoadjuvant chemotherapy is particularly evident in the peri-pancreatic area and the retroperitoneal area (Figure 6).
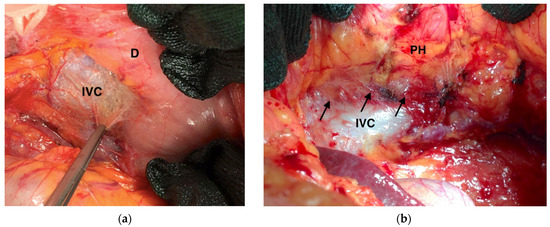
Figure 6.
Intraoperative frames highlighting fibrosis induced by neoadjuvant chemotherapy (black arrows): (a) Kocher’s maneuver; (b) Kocher’s maneuver showing adhesion between inferior cava vein, ovarian vein, and pancreatic head. IVC: Inferior Vena Cava; D: Duodenum; PH: Pancreatic Head.
As such, surgeons’ experience and hospital quality of care could have a strong impact on D2+ dissection after pre-operative chemotherapy. Gastrectomy with D2 is a complex surgical procedure that requires a significant learning curve [12,103,104]. A large South Korean study demonstrated that node retrieval and overall survival rate of patients was improved by institutional experience, and that surgeon subspecialty was an independent predictor of improved outcomes [105]. Most studies, including that of the Italian Gastric Cancer Collaborative, suggest a learning curve of approximately 15–25 cases or between 8 and 24 months in establishing a plateau [106,107].
In 2017, the Dutch Upper Gastrointestinal Cancer Audit (DUCA) group designed the Textbook Outcome, a multidimensional measure representing an ideal course after cancer surgery [108]. It consists of ten perioperative quality-of-care parameters, including adequate lymphadenectomy with “greater than 15 lymph nodes sampled” which, if met, are associated with better survival rates in gastric cancer. This suggests that a good patient selection, well-performed surgery, and optimal postoperative care are ways to allow fast discharge, optimize long-term outcome, and lower costs for the healthcare system [109,110]. In 2021, perioperative chemotherapy was added to the other features, resulting in a new Textbook Oncological Outcome. Parallel to it, GIRCG is conducting a consensus study aimed to modulate specific parameters (i.e., compliance/adherence to chemotherapy, or 25 lymph nodes sampled in neoadjuvant setting), defining a more actual and specific definition of Textbook Outcome in Gastric Surgery (TOGS).
It is intuitive, but also clearly demonstrated in all studies dealing with this issue, that the higher number of lymph nodes required to be excised and the sequelae of chemotherapy treatments further increase the learning curve. This implies that the extended lymph node dissection after neoadjuvant treatment could be associated with an increase in intra- and post-operative complications. Shrikhande described a 12% morbidity rate and no mortality in his series, mainly treated with EOX regimen [111]. Additionally, in the same study, the median blood loss and the number of lymph nodes harvested were similar to upfront surgery (500 vs. 400 mL, and 16 vs. 18 lymph nodes), but positive nodes were noted even in patients with a major pathological response of the primary tumor. In contrast, Wu observed greater intraoperative blood loss among neoadjuvant patients compared with those who underwent upfront surgery, probably due to the abovementioned chemotherapy-induced fibrosis surrounding the lymph nodes [112]. Thus, the administration of preoperative treatment does not obviate the need for a complete radical resection and D2 or more extended lymphadenectomy, which, although involving greater technical difficulties and longer operative time, may still be achieved with suitable oncological resectability [47].
Key points:
- The collection of more than 25 lymph nodes is associated with improved survival in N+ patients who underwent neoadjuvant chemotherapy.
- Good responders to neoadjuvant chemotherapy may have nodal micrometastases.
3.3. Closed Trials
The Stomach Cancer Study Group of the Japan Clinical Oncology Group (SCSG/JCOG) has conducted the following trials since 2000, in order to investigate how to improve the therapeutic effects of preoperative chemotherapy followed by gastrectomy with D2 plus PAND for gastric cancer patients with ELM:
JCOG0001: Concerning extended and super-extended lymphadenectomies, the phase II clinical trial enrolled patients who had received preoperative chemotherapy according to the schema irinotecan and cisplatin, and gastrectomy with D3 dissection for gastric cancer with ELM. Despite the study being stopped due to three treatment-related deaths, the available results showed a median survival of 14.6 months, and a 3-year survival rate of 27% [49].
JCOG0405: At present, PAND is considered safe after neoadjuvant chemotherapy in patients with bulky nodes in the D2 stations with or without lymphadenopathy in the para-aortic 16 a2–b1 area. Such a statement mainly derives from the results of a phase II trial [48]: patients with clinically detected ELM (i.e., the presence of bulky nodes in the D2 stations, with or without positive PAN (16 a2–b1)) could benefit from a multidisciplinary treatment including two courses of neoadjuvant chemotherapy with S-1 and cisplatin, followed by D2 plus PAND, with a 5-year survival rate of 53%. The presence of bulky nodes at the D2 stations or positive PANs alone demonstrated 3-year survival rates of 68% and 57%, while both conditions reached just 17% [48].
Extended surgery to PAN might be a promising and effective approach in prophylactic (bulky D2 nodes) intent to improve patients’ survival, due to closure of lymphatic pathways or presence of undetectable micrometastases.
JCOG1012A: In 2019, an integrated analysis of the two previous phase II trials (JCOG0001 and JCOG0405) regarding gastric cancer with ELM showed better survival for S-1 plus cisplatin scheme with respect to the irinotecan plus cisplatin scheme. Moreover, the 5-year overall survival rate was poor for patients with both positive bulky nodes and PAN (19.2%), compared with positive bulky nodes alone (50.7%) and positive PAN alone (43.5%) [113].
JCOG1002: On the basis of the significative results of a retrospective study demonstrating a 2-year overall survival rate of 93.8% [114], a phase II clinical trial using docetaxel, cisplatin and S-1 as neoadjuvant chemotherapy in advanced gastric cancer with ELM showed a response rate of 57.7%, according to the Response Evaluation Criteria in Solid Tumors (compared to 51% of the JCOG0405 study), with R0 resection achieved in 84.6% of patients (compared to 82% of the JCOG0405 study) [115].
Interestingly, Yoshida investigated the long-term survival rate of patients treated with chemotherapy alone for advanced gastric cancer. Overall, 2- and 5-year survival rates were respectively 14.3% and 10.4% for patients with metastases to abdominal nodes only [116]. Other studies obtained similar results, with a 3-year survival rate of 13.1% in the case of PAN-only metastases [117]. This evidence supports the pivotal role of surgery in the treatment of gastric cancer with curative intent.
Proper selection of patients currently remains the main issue as preoperative tumor staging is based only on radiological findings [118]. Indeed, preoperative nodal assessment is mainly obtained with the aid of computed tomography, but the specificity of this method is low because it is mainly based on the size of the lymph node [119]. In view of all the above considerations, the removal of para-aortic nodes is recommended with a “curative” intent; therefore, when para-aortic nodes register a good pathological response at restaging clinical examinations.
A Korean study analyzed a large cohort of patients who had undergone gastrectomy for gastric cancer: it very rarely extended to PAN (1.3% of cases) and described a 5-year locoregional recurrence rate of 8.5%, mostly in the stations 16a2 and 16b1 (46 and 60%, respectively) [120]. Regardless of the location of the primary tumor, indeed, lymphatic flow ultimately drains into the para-aortic region. It has been assumed that a subset of patients with advanced gastric cancer may have PAN micrometastases or metastases not detectable through the preoperative imaging examinations. A “prophylactic” PAND would prevent locoregional recurrence, especially in good responders to preoperative chemotherapy.
Another interesting aspect is that no clear benefit of neoadjuvant therapy has been found in older adults. This is because on the one hand, most of the studies conducted so far have excluded patients older than 70 years, and, on the other hand, elderly patients are more likely to die from non-cancer-related causes than younger patients [121].
Key points:
- PAND is safe and effective after neoadjuvant chemotherapy, in both therapeutic (positive PAN) and prophylactic (positive bulky nodes in D2 and negative PAN) surgery.
- Neoadjuvant chemotherapy followed by D2+ PAND improves long-term survival [122].
- Good responders to preoperative chemotherapy better benefit from PAND.
3.4. Benefits and Drawbacks of Minimally Invasive Approach
High referral centers for minimally invasive surgery are aiming to achieve a patient-targeted treatment [123,124,125]. It is supported that laparoscopic gastrectomy offers similar rates of survival when compared to open gastrectomy in both early and advanced gastric cancer [126]. Moreover, while laparoscopic D2 distal gastrectomy has been demonstrated as feasible and safe [123,127,128], laparoscopic total gastrectomy still represents a surgical challenge [123,124,129].
To overcome the intrinsic limitations of the laparoscopic approach, robotic surgery has been advocated to facilitate the lymph node dissection and complex reconstructions after gastrectomy, even without strong evidence [130]. Since the first robot-assisted gastrectomies described by Hashizume and Giulianotti in 2003 [131,132], robotic surgery has been claimed as safe and assures oncological safety in advanced gastric cancer patients [131,133,134]. When compared with laparoscopic subtotal and total gastrectomy, robotic approach results in decreased blood loss (176.6 vs. 212.5, respectively) and a higher number of retrieved lymph nodes (33 vs. 31.3, respectively) [135], especially supra-pancreatic [136]. These findings further support the hypothesis that the robotic wide range of motion allows us to overcome the abovementioned non-compliance lymphadenectomy. In our experience, the mean number of retrieved lymph nodes was 31.9 nodes in D2 dissection and a mean of 2.7 lymph nodes results positive [137]. Eastern retrospective studies highlighted no differences in stage-specific survival analysis between laparoscopic and robotic gastrectomy, in view of long operation time and high costs [136].
The latest published umbrella review on this topic, performed on more than 37,500 subjects, showed no difference in margins infiltration, conversion rate, postoperative complications, and hospital stay and mortality, between robotic and laparoscopic groups [138]. Interestingly, in 12 meta-analyses, the number of lymph nodes harvested in robotic gastrectomy was higher than in the laparoscopic approach and the distal margin of resection is higher.
A study was conducted on a combined cohort of patients from the United States and China which showed no statistically significant difference in the 5-year overall survival rate between minimally invasive and open approaches. A positive trend as TNM stage increased was noted [139]: long-term survival after minimally invasive gastrectomy tended to be higher in patients with stage III gastric cancer, probably due to lower stress responses, better preserved immune function, and earlier administration of adjuvant chemotherapy [139,140].
Although results appear consistent with previous evidence on the subject, it is more complex to define the role of minimally invasive surgery in advanced gastric cancer after neoadjuvant chemotherapy. Adhesions of tissues, destruction of anatomical dissection planes, perigastric edema and tissue fibrotic changes, occurring after chemotherapy, could increase the surgical difficulty. However, laparoscopy has several advantages, such as delicate manipulation, visual magnification, better exposure, faster recovery, and damage control which might reduce the surgical risk following preoperative chemotherapy.
Investigating the results of quality of life and 3-year overall survival, the European STOMACH trial proved that minimally invasive resection after neoadjuvant chemotherapy is as safe as open total gastrectomy, with a similar number of resected lymph nodes (41.8 vs. 43.4, respectively) and radicality (44 vs. 48 R0 resection, respectively) [141]. However, the inadequate dissection of lymph node station 10 determined a low rate of successful D2 lymphadenectomy. Thus, in the latest JGCA guidelines, standard dissection of station 10 is recommended only if the tumor invades the greater curvature [3].
Previous studies have confirmed that laparoscopic gastrectomy is a feasible method after neoadjuvant therapy, as it was associated with better short- and comparable long-term outcomes (3 years) compared to an open approach [142,143].
As far as we know, only a phase II trial [144] compared laparoscopic and open approach in a neoadjuvant setting [145]. Interestingly, the laparoscopic group had both more adherence to adjuvant chemotherapy (87% versus 70%) and a higher rate of completing therapy (60% versus 40%). Nevertheless, neither the safety and efficacy nor oncologic stability is yet widely established for laparoscopic gastric surgery in a neoadjuvant setting.
In Asian countries, many prospective trials are currently being conducted. The CLASS03a trial [146] and ClinicalTrials.gov NCT02902575 [147] aim to further evaluate safety, feasibility, and long-term outcomes of laparoscopic gastrectomy for advanced gastric cancer after neoadjuvant chemotherapy. In a similar setting, the REALIZATION trial [144] and the ClinicalTrials.gov NCT04658589 [148] will compare open and laparoscopic approach in distal gastrectomy and D2 lymph node dissection.
A further question is whether robotic gastrectomy could offer at least the same benefits as laparoscopic surgery compared to open surgery after neoadjuvant treatment. American data shows on one hand acceptable survival and recurrence rates, on the other hand no difference in R0 and successful lymphadenectomy, while showing an increased node harvest (18 vs. 17 vs. 16 in robotic, laparoscopic, and open gastrectomy, respectively) [99]. Neoadjuvant chemotherapy on the one hand has shown to affect lymph node harvest, on the other hand has proved to be the strongest predictor of open conversion rate, probably because of peritumoral connective tissue changes, liver enlargement, and margin blurring, which definitely led to the loss of visual definition of the proximal border of the tumor [149]. In contrast, neoadjuvant chemotherapy was not a risk factor for common early postoperative complications in Okabe analysis [150].
Because of the paucity of long-term follow-up results, Western reference centers for gastric cancer have implemented prospective data collection, as in the context of UGIRA (The Upper GI International Robotic Association).
In Italy, GIRCG recently investigated laparoscopic and open gastrectomy for advanced gastric cancer, a quarter of whom underwent pre-operative chemotherapy, between 2015 and 2018, showing similar 3-year survival outcomes and 30-day morbidity/mortality [126]. The same group designed a propensity score matching analysis aimed at comparing survival outcomes in patients undergoing curative-intent laparoscopic and robotic gastrectomy for advanced gastric cancer. Results will be presented shortly.
Definite evidence of the safety of minimally invasive surgery for the cure of gastric cancer in the era of neoadjuvant chemotherapy is not available yet; its broader use stressed a shift toward more tailored approach, with the intention of achieving better treatment efficacy for each patient, for each tumor [123,124].
Key points:
- No clear superiority of minimally invasive surgery has been proved.
- Minimally invasive surgery after neoadjuvant chemotherapy is safe and effective if performed in referral centers.
- Further trials are required to establish the viability and long-term outcomes of robotic gastrectomy.
4. Ongoing Trials and Future Perspectives
ClinicalTrials.gov NCT03961373: A phase III randomized controlled trial comparing D2 and D2plus lymphadenectomy after neoadjuvant treatment in patients with advanced gastric cancer, without clinically detectable PAN involvement and no evidence of distant metastases (stage IIA-IIIC), has just started in our center in Siena, Italy. In both groups, surgical treatment will be performed within 4–6 weeks after the end of the last cycle of neoadjuvant chemotherapy. The task of the trial “Neo-D2plus” is to assess if neoadjuvant chemotherapy together with more extended lymphadenectomy may improve the outcome of patients. Secondary endpoints are the patients’ response to chemotherapy, resection rate, quality of life, complications attributable to surgical intervention, side effects of chemotherapy, and the duration of hospitalization [151].
ClinicalTrials.gov NCT02139605: An Indian phase III randomized controlled trial aims to compare D2 and D3 lymphadenectomy after neoadjuvant treatment (after at least one complete cycle) for non-metastatic, locally advanced but resectable gastric cancer. Primary and secondary outcomes are a 5-year overall survival rate and a 2-year disease-free survival. Previously, just one multi-institutional, non-randomized study compared D2 and D3 lymphadenectomies, and reported that D3 had survival advantages in tumors of 50–100 mm in diameter, with or without metastatic nodes [152].
JCOG1704: The phase II “Bulky/PAN-GC DOS NAC” trial aims to evaluate the efficacy and safety of neoadjuvant chemotherapy with docetaxel, oxaliplatin and S-1 (DOS) followed by D2+ PAN dissection in advanced gastric cancer with ELM. The aim is to evaluate the pathological response rate (defined as patients achieving grade two or more according to the histological criteria of the Japanese Classification of Gastric Carcinoma [17]). Secondary endpoints are the overall survival, and the relapse-free survival, in patients with R0 resection, the proportion of R0 resection, the proportion of completion of surgery, the proportion of completion of protocol treatment, and the response rate of preoperative chemotherapy [153].
5. Conclusions
Gastric cancer remains a major global health problem, in terms of incidence and mortality. D2 dissection is the well-established standard treatment for resectable gastric cancer in most of the current guidelines. Despite this statement, the definition of the ”optimal” approach for lymphadenectomy in gastric cancer patients is still debated. Surgeons must be trained to perform more extended lymphadenectomy in advanced stages with high risk of metastases to distant nodes. In patients with a node-positive disease, the number of lymph nodes harvested is an independent predictor of survival, and relates to the number of lymph node metastases.
Current guidelines recommend preoperative chemotherapy for resectable gastric cancer with clinical stage ≥ T2 or N+. Patients with clinically positive para-aortic nodal metastases should undergo neoadjuvant chemotherapy followed by therapeutic PAND. In any case, prophylactic PAND and posterior nodes dissection after neoadjuvant treatment may be performed in patients at high risk of PAN metastases, such as positive bulky nodes in the second level perigastric nodal stations or diffuse histotype. GIRCG started a randomized controlled trial to explore the role of prophylactic D2+ compared to the standard D2 in locally advanced gastric cancer patients treated with perioperative chemotherapy in the Western countries.
To paraphrase Oscar Wilde, “nowadays people know the price of everything and the value of nothing”; modern surgeons should balance possible oncological value of D2plus lymphadenectomy with the price of post-operative complications and the risk of mortality. Research is developing fast and progress in health care delivery and new insights into tumor burden allow for more precise classifications, more efficacious therapies, more efficient tailored surgery, and better quality of care.
Author Contributions
Conceptualization, L.M., L.C. and D.M.; methodology, L.C. and G.E.P.; validation, L.V. and S.A.P.; investigation, L.M. and L.C.; data curation, L.C. and D.M.; writing—original draft preparation, L.C.; writing—review and editing, L.C., G.E.P. and V.R.; visualization, S.A.P. and V.R.; supervision, F.R.; project administration, F.R. and D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Deng, J.-Y. Clinical Significance of Lymph Node Metastasis in Gastric Cancer. World J. Gastroenterol. 2014, 20, 3967. [Google Scholar] [CrossRef]
- Biondi, A. R0 Resection in the Treatment of Gastric Cancer: Room for Improvement. World J. Gastroenterol. 2010, 16, 3358. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2018 (5th Edition). Gastric Cancer 2021, 24, 1–21. [Google Scholar] [CrossRef]
- de Manzoni, G.; Marrelli, D.; Baiocchi, G.L.; Morgagni, P.; Saragoni, L.; Degiuli, M.; Donini, A.; Fumagalli, U.; Mazzei, M.A.; Pacelli, F.; et al. The Italian Research Group for Gastric Cancer (GIRCG) Guidelines for Gastric Cancer Staging and Treatment: 2015. Gastric Cancer 2017, 20, 20–30. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2010 (Ver. 3). Gastric Cancer 2011, 14, 113–123. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2014 (Ver. 4). Gastric Cancer 2017, 20, 1–19. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-H.; Shen, L.; Li, J.; Zhou, Z.-W.; Liang, H.; Zhang, X.-T.; Tang, L.; Xin, Y.; Jin, J.; Zhang, Y.-J.; et al. The Chinese Society of Clinical Oncology (CSCO): Clinical Guidelines for the Diagnosis and Treatment of Gastric Cancer. Cancer Commun. 2019, 39, 10. [Google Scholar] [CrossRef]
- Marrelli, D.; de Franco, L.; Iudici, L.; Polom, K.; Roviello, F. Lymphadenectomy: State of the Art. Transl. Gastroenterol. Hepatol. 2017, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, A.-M.; Haas, O.; Piard, F.; Roignot, P.; Bonithon-Kopp, C.; Faivre, J. How Many Nodes Must Be Examined to Accurately Stage Gastric Carcinomas? Cancer 2002, 94, 2862–2866. [Google Scholar] [CrossRef]
- Smith, D.D.; Schwarz, R.R.; Schwarz, R.E. Impact of Total Lymph Node Count on Staging and Survival After Gastrectomy for Gastric Cancer: Data from a Large US-Population Database. J. Clin. Oncol. 2005, 23, 7114–7124. [Google Scholar] [CrossRef] [PubMed]
- Mogal, H.; Fields, R.; Maithel, S.K.; Votanopoulos, K. In Patients with Localized and Resectable Gastric Cancer, What Is the Optimal Extent of Lymph Node Dissection—D1 Versus D2 Versus D3? Ann. Surg. Oncol. 2019, 26, 2912–2932. [Google Scholar] [CrossRef]
- Cozzaglio, L.; Doci, R.; Celotti, S.; Roncalli, M.; Gennari, L. Gastric Cancer: Extent of Lymph Node Dissection and Requirements for a Correct Staging. Tumori 2004, 90, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.Y.; Kim, S.; Cheong, J.-H.; Kim, Y.I.; Hyung, W.J.; Choi, W.H.; Choi, S.H.; Wang, L.B.; Noh, S.H. The Impact of Total Retrieved Lymph Nodes on Staging and Survival of Patients with PT3 Gastric Cancer. Cancer 2007, 110, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lu, Y.; Jun, Z.; Zhang, R.; Yao, F.; Lu, P.; Jin, F.; Li, H.; Xu, H.; Wang, S.; et al. Impact of Total Retrieved Lymph Nodes on Staging and Survival of Patients with Gastric Cancer Invading the Subserosa. Surg. Oncol. 2009, 18, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Son, T.; Hyung, W.J.; Lee, J.H.; Kim, Y.M.; Kim, H.-I.; An, J.Y.; Cheong, J.-H.; Noh, S.H. Clinical Implication of an Insufficient Number of Examined Lymph Nodes after Curative Resection for Gastric Cancer. Cancer 2012, 118, 4687–4693. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma: 3rd English Edition. Gastric Cancer 2011, 14, 101–112. [Google Scholar] [CrossRef]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef]
- Coburn, N.; Seevaratnam, R.; Paszat, L.; Helyer, L.; Law, C.; Swallow, C.; Cardosa, R.; Mahar, A.; Lourenco, L.G.; Dixon, M.; et al. Optimal Management of Gastric Cancer. Ann. Surg. 2014, 259, 102–108. [Google Scholar] [CrossRef]
- Waddell, T.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D. Gastric Cancer: ESMO–ESSO–ESTRO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2013, 24, vi57–vi63. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.G.; Jung, H.-K.; Kim, J.H.; Jeong, W.K.; Jeon, T.J.; Kim, J.M.; Kim, Y.I.; Ryu, K.W.; Kong, S.-H.; et al. Clinical Practice Guidelines for Gastric Cancer in Korea: An Evidence-Based Approach. J. Gastric Cancer 2014, 14, 87. [Google Scholar] [CrossRef]
- American College of Surgeons. Cancer Programs Practice Profile Reports (CP3R). Available online: https://www.facs.org/~/media/files/quality%20programs/cancer/ncdb/measure%20specs%20gastric.ashx (accessed on 25 September 2022).
- Allum, W.H.; Blazeby, J.M.; Griffin, S.M.; Cunningham, D.; Jankowski, J.A.; Wong, R. Guidelines for the Management of Oesophageal and Gastric Cancer. Gut 2011, 60, 1449–1472. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Practice Guidelines in Oncology (NCCN Guidelines), Gastric Cancer, Version 1. 2019. Available online: www.nccn.org (accessed on 25 September 2022).
- Marrelli, D.; Pedrazzani, C.; Morgagni, P.; de Manzoni, G.; Pacelli, F.; Coniglio, A.; Marchet, A.; Saragoni, L.; Giacopuzzi, S.; Roviello, F. Changing Clinical and Pathological Features of Gastric Cancer over Time. Br. J. Surg. 2011, 98, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Wohnrath, D.R.; Araujo, R.L.C. D2 Lymphadenectomy for Gastric Cancer as an Independent Prognostic Factor of 10-Year Overall Survival. Eur. J. Surg. Oncol. 2019, 45, 446–453. [Google Scholar] [CrossRef]
- Naffouje, S.A.; Salti, G.I. Extensive Lymph Node Dissection Improves Survival among American Patients with Gastric Adenocarcinoma Treated Surgically: Analysis of the National Cancer Database. J. Gastric Cancer 2017, 17, 319. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Ye, Y.; Xie, Q.; Liang, B.; Jiang, K.; Wang, S. Effect of the Number of Lymph Nodes Harvested on the Long-Term Survival of Gastric Cancer Patients According to Tumor Stage and Location: A 12-Year Study of 1,637 Cases. Am. J. Surg. 2015, 210, 431–440.e3. [Google Scholar] [CrossRef] [PubMed]
- Macalindong, S.S.; Kim, K.H.; Nam, B.-H.; Ryu, K.W.; Kubo, N.; Kim, J.Y.; Eom, B.W.; Yoon, H.M.; Kook, M.-C.; Choi, I.J.; et al. Effect of Total Number of Harvested Lymph Nodes on Survival Outcomes after Curative Resection for Gastric Adenocarcinoma: Findings from an Eastern High-Volume Gastric Cancer Center. BMC Cancer 2018, 18, 73. [Google Scholar] [CrossRef]
- Siewert, J.R.; Kestlmeier, R.; Busch, R.; Böttcher, K.; Roder, J.D.; Müller, J.; Fellbaum, C.; Höfler, H. Benefits of D2 Lymph Node Dissection for Patients with Gastric Cancer and PN0 and PN1 Lymph Node Metastases. Br. J. Surg. 2005, 83, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Baiocchi, G.L.; Tiberio, G.A.; Minicozzi, A.M.; Morgagni, P.; Marrelli, D.; Bruno, L.; Rosa, F.; Marchet, A.; Coniglio, A.; Saragoni, L.; et al. A Multicentric Western Analysis of Prognostic Factors in Advanced, Node-Negative Gastric Cancer Patients. Ann. Surg. 2010, 252, 70–73. [Google Scholar] [CrossRef]
- di Leo, A.; Marrelli, D.; Roviello, F.; Bernini, M.; Minicozzi, A.; Giacopuzzi, S.; Pedrazzani, C.; Baiocchi, L.G.; de Manzoni, G. Lymph Node Involvement in Gastric Cancer for Different Tumor Sites and T Stage. J. Gastrointest. Surg. 2007, 11, 1146–1153. [Google Scholar] [CrossRef]
- Zhang, Y.-X.; Yang, K. Significance of Nodal Dissection and Nodal Positivity in Gastric Cancer. Transl. Gastroenterol. Hepatol. 2020, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Roviello, F.; Pedrazzani, C.; Marrelli, D.; di Leo, A.; Caruso, S.; Giacopuzzi, S.; Corso, G.; de Manzoni, G. Super-Extended (D3) Lymphadenectomy in Advanced Gastric Cancer. Eur. J. Surg. Oncol. EJSO 2010, 36, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Hartgrink, H.H.; van de Velde, C.J.H.; Putter, H.; Bonenkamp, J.J.; Klein Kranenbarg, E.; Songun, I.; Welvaart, K.; van Krieken, J.H.J.M.; Meijer, S.; Plukker, J.T.M.; et al. Extended Lymph Node Dissection for Gastric Cancer: Who May Benefit? Final Results of the Randomized Dutch Gastric Cancer Group Trial. J. Clin. Oncol. 2004, 22, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- Songun, I.; Putter, H.; Kranenbarg, E.M.-K.; Sasako, M.; van de Velde, C.J. Surgical Treatment of Gastric Cancer: 15-Year Follow-up Results of the Randomised Nationwide Dutch D1D2 Trial. Lancet Oncol. 2010, 11, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Degiuli, M.; Sasako, M.; Ponti, A.; Vendrame, A.; Tomatis, M.; Mazza, C.; Borasi, A.; Capussotti, L.; Fronda, G.; Morino, M.; et al. Randomized Clinical Trial Comparing Survival after D1 or D2 Gastrectomy for Gastric Cancer. Br. J. Surg. 2013, 101, 23–31. [Google Scholar] [CrossRef]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric Cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Degiuli, M.; Sasako, M.; Ponti, A. Morbidity and Mortality in the Italian Gastric Cancer Study Group Randomized Clinical Trial of D1 versus D2 Resection for Gastric Cancer. Br. J. Surg. 2010, 97, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Degiuli, M.; Reddavid, R.; Tomatis, M.; Ponti, A.; Morino, M.; Sasako, M.; Rebecchi, F.; Garino, M.; Vigano, L.; Scaglione, D.; et al. D2 Dissection Improves Disease-Specific Survival in Advanced Gastric Cancer Patients: 15-Year Follow-up Results of the Italian Gastric Cancer Study Group D1 versus D2 Randomised Controlled Trial. Eur. J. Cancer 2021, 150, 10–22. [Google Scholar] [CrossRef]
- Wu, C.-W.; Hsiung, C.A.; Lo, S.-S.; Hsieh, M.-C.; Chen, J.-H.; Li, A.F.-Y.; Lui, W.-Y.; Whang-Peng, J. Nodal Dissection for Patients with Gastric Cancer: A Randomised Controlled Trial. Lancet Oncol. 2006, 7, 309–315. [Google Scholar] [CrossRef] [PubMed]
- de Manzoni, G.; Roviello, F. Gastric Cancer: The 25-Year R-Evolution; Springer: Berlin/Heidelberg, Germany, 2022; pp. 111–118. [Google Scholar]
- Asoglu, O.; Matlim, T.; Kurt, A.; Onder, S.Y.; Kunduz, E.; Karanlik, H.; Sam, B.; Kapran, Y.; Bugra, D. Guidelines for Extended Lymphadenectomy in Gastric Cancer: A Prospective Comparative Study. Ann. Surg. Oncol. 2013, 20, 218–225. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma—2nd English Edition. Gastric Cancer 1998, 1, 10–24. [Google Scholar] [CrossRef]
- Yu, P.; Du, Y.; Xu, Z.; Huang, L.; Cheng, X. Comparison of D2 and D2 plus Radical Surgery for Advanced Distal Gastric Cancer: A Randomized Controlled Study. World J. Surg. Oncol. 2019, 17, 28. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Marrelli, D.; Mazzei, M.A.; Roviello, F. Gastric Cancer with Para-Aortic Lymph Node Metastases: Do Not Miss a Chance of Cure! Cancer Chemother. Pharm. 2014, 74, 433–434. [Google Scholar] [CrossRef] [PubMed]
- Tsuburaya, A.; Mizusawa, J.; Tanaka, Y.; Fukushima, N.; Nashimoto, A.; Sasako, M. Neoadjuvant Chemotherapy with S-1 and Cisplatin Followed by D2 Gastrectomy with Para-Aortic Lymph Node Dissection for Gastric Cancer with Extensive Lymph Node Metastasis. Br. J. Surg. 2014, 101, 653–660. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Sasako, M.; Yamamoto, S.; Sano, T.; Imamura, H.; Fujitani, K.; Oshita, H.; Ito, S.; Kawashima, Y.; Fukushima, N. Phase II Study of Neoadjuvant Chemotherapy and Extended Surgery for Locally Advanced Gastric Cancer. Br. J. Surg. 2009, 96, 1015–1022. [Google Scholar] [CrossRef]
- Xu, W.; Liu, W.; Wang, L.; He, C.; Lu, S.; Ni, Z.; Hua, Z.; Zhu, Z.; Sah, B.K.; Yang, Z.; et al. Is D2 Lymphadenectomy Alone Suitable for Gastric Cancer with Bulky N2 and/or Para-Aortic Lymph Node Metastases After Preoperative Chemotherapy? Front. Oncol. 2021, 11, 709617. [Google Scholar] [CrossRef]
- Sasako, M.; Sano, T.; Yamamoto, S.; Nashimoto, A.; Kurita, A.; Furukawa, H.; Tsujinaka, T.; Kinoshita, T.; Arai, K. Randomized Phase III Trial of Standard D2 versus D2 + Para-Aortic Lymph Node (PAN) Dissection (D) for Clinically M0 Advanced Gastric Cancer: JCOG9501. J. Clin. Oncol. 2006, 24, LBA4015. [Google Scholar] [CrossRef]
- Sasako, M.; Sano, T.; Yamamoto, S.; Kurokawa, Y.; Nashimoto, A.; Kurita, A.; Hiratsuka, M.; Tsujinaka, T.; Kinoshita, T.; Arai, K.; et al. D2 Lymphadenectomy Alone or with Para-Aortic Nodal Dissection for Gastric Cancer. N. Engl. J. Med. 2008, 359, 453–462. [Google Scholar] [CrossRef]
- Endo, S.; Ikenaga, M.; Yamada, T.; Tamura, S.; Sasaki, Y.O. Prognostic Factors for Para-aortic Lymph Node Dissection After Neoadjuvant Chemotherapy for Gastric Cancer. Anticancer Res. 2020, 40, 2351–2357. [Google Scholar] [CrossRef] [PubMed]
- Eom, B.W.; Joo, J.; Kim, Y.-W.; Park, B.; Park, J.Y.; Yoon, H.M.; Lee, J.H.; Ryu, K.W. Is There Any Role of Additional Retropancreatic Lymph Node Dissection on D2 Gastrectomy for Advanced Gastric Cancer? Ann. Surg. Oncol. 2013, 20, 2669–2675. [Google Scholar] [CrossRef]
- Eom, B.W.; Joo, J.; Kim, Y.-W.; Reim, D.; Park, J.Y.; Yoon, H.M.; Ryu, K.W.; Lee, J.Y.; Kook, M.-C. Improved Survival after Adding Dissection of the Superior Mesenteric Vein Lymph Node (14v) to Standard D2 Gastrectomy for Advanced Distal Gastric Cancer. Surgery 2014, 155, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, D.; Ferrara, F.; Giacopuzzi, S.; Morgagni, P.; di Leo, A.; de Franco, L.; Pedrazzani, C.; Saragoni, L.; de Manzoni, G.; Roviello, F. Incidence and Prognostic Value of Metastases to “Posterior” and Para-Aortic Lymph Nodes in Resectable Gastric Cancer. Ann. Surg. Oncol. 2017, 24, 2273–2280. [Google Scholar] [CrossRef]
- Bencivenga, M.; Verlato, G.; Mengardo, V.; Scorsone, L.; Sacco, M.; Torroni, L.; Giacopuzzi, S.; de Manzoni, G. Is There Any Role for Super-Extended Limphadenectomy in Advanced Gastric Cancer? Results of an Observational Study from a Western High Volume Center. J. Clin. Med. 2019, 8, 1799. [Google Scholar] [CrossRef]
- de Manzoni, G.; Verlato, G.; Bencivenga, M.; Marrelli, D.; di Leo, A.; Giacopuzzi, S.; Cipollari, C.; Roviello, F. Impact of Super-Extended Lymphadenectomy on Relapse in Advanced Gastric Cancer. Eur. J. Surg. Oncol. EJSO 2015, 41, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Bencivenga, M.; Torroni, L.; Verlato, G.; Mengardo, V.; Sacco, M.; Allum, W.H.; de Manzoni, G. Lymphadenectomy for Gastric Cancer at European Specialist Centres. Eur. J. Surg. Oncol. 2021, 47, 1048–1054. [Google Scholar] [CrossRef]
- Marano, L.; Carbone, L.; Poto, G.E.; Calomino, N.; Neri, A.; Piagnerelli, R.; Fontani, A.; Verre, L.; Savelli, V.; Roviello, F.; et al. Antimicrobial Prophylaxis Reduces the Rate of Surgical Site Infection in Upper Gastrointestinal Surgery: A Systematic Review. Antibiotics 2022, 11, 230. [Google Scholar] [CrossRef]
- Kinami, S.; Saito, H.; Takamura, H. Significance of Lymph Node Metastasis in the Treatment of Gastric Cancer and Current Challenges in Determining the Extent of Metastasis. Front. Oncol. 2022, 11, 806162. [Google Scholar] [CrossRef]
- de Manzoni, G.; Roviello, F. Gastric Cancer: The 25-Year R-Evolution; Springer: Berlin/Heidelberg, Germany, 2021; pp. 275–276. [Google Scholar]
- Kumamoto, T.; Kurahashi, Y.; Haruta, S.; Niwa, H.; Nakanishi, Y.; Ozawa, R.; Okumura, K.; Ishida, Y.; Shinohara, H. Laparoscopic Modified Lymphadenectomy in Gastric Cancer Surgery Using Systematic Mesogastric Excision: A Novel Technique Based on a Concept. Langenbecks Arch. Surg. 2019, 404, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Lou, K.; Wang, P.; Gao, Y.; Zhang, Y.; Chen, M.; Huang, W.; Zhang, G. Surgical Navigation for Malignancies Guided by Near-Infrared-II Fluorescence Imaging. Small Methods 2021, 5, 2001066. [Google Scholar] [CrossRef]
- Chen, Q.-Y.; Xie, J.-W.; Zhong, Q.; Wang, J.-B.; Lin, J.-X.; Lu, J.; Cao, L.-L.; Lin, M.; Tu, R.-H.; Huang, Z.-N.; et al. Safety and Efficacy of Indocyanine Green Tracer-Guided Lymph Node Dissection During Laparoscopic Radical Gastrectomy in Patients with Gastric Cancer. JAMA Surg. 2020, 155, 300. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-N.; Su-Yan; Qiu, W.-W.; Liu, C.-H.; Chen, Q.-Y.; Zheng, C.-H.; Li, P.; Wang, J.-B.; Lin, J.-X.; Lu, J.; et al. Assessment of Indocyanine Green Tracer-Guided Lymphadenectomy in Laparoscopic Gastrectomy after Neoadjuvant Chemotherapy for Locally Advanced Gastric Cancer: Results from a Multicenter Analysis Based on Propensity Matching. Gastric Cancer 2021, 24, 1355–1364. [Google Scholar] [CrossRef]
- IGG Using in Laparoscopic Gastrectomy for Locally Advanced Gastric Cancer After Neoadjuvant Chemotherapy. Available online: https://clinicaltrials.gov/ct2/show/NCT04611997 (accessed on 7 October 2022).
- De Manzoni, G.; Roviello, F.; Siquini, W. Surgery in the Multimodal Management of Gastric Cancer; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Ychou, M.; Boige, V.; Pignon, J.-P.; Conroy, T.; Bouché, O.; Lebreton, G.; Ducourtieux, M.; Bedenne, L.; Fabre, J.-M.; Saint-Aubert, B.; et al. Perioperative Chemotherapy Compared with Surgery Alone for Resectable Gastroesophageal Adenocarcinoma: An FNCLCC and FFCD Multicenter Phase III Trial. J. Clin. Oncol. 2011, 29, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; van de Velde, C.J.H.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef]
- Ronellenfitsch, U.; Schwarzbach, M.; Hofheinz, R.; Kienle, P.; Kieser, M.; Slanger, T.E.; Jensen, K. Perioperative Chemo(Radio)Therapy versus Primary Surgery for Resectable Adenocarcinoma of the Stomach, Gastroesophageal Junction, and Lower Esophagus. Cochrane Database Syst. Rev. 2013, 5, CD008107. [Google Scholar] [CrossRef] [PubMed]
- Kodera, Y. Neoadjuvant Chemotherapy for Gastric Adenocarcinoma in Japan. Surg. Today 2017, 47, 899–907. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.-G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative Chemotherapy with Fluorouracil plus Leucovorin, Oxaliplatin, and Docetaxel versus Fluorouracil or Capecitabine plus Cisplatin and Epirubicin for Locally Advanced, Resectable Gastric or Gastro-Oesophageal Junction Adenocarcinoma (FLOT4): A Randomised, Phase 2/3 Trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Kawazoe, A.; Yañez, P.; Li, N.; Lonardi, S.; Kolesnik, O.; Barajas, O.; Bai, Y.; Shen, L.; Tang, Y.; et al. The KEYNOTE-811 Trial of Dual PD-1 and HER2 Blockade in HER2-Positive Gastric Cancer. Nature 2021, 600, 727–730. [Google Scholar] [CrossRef]
- FLOT vs. FLOT/Herceptin/Pertuzumab for Perioperative Therapy of HER-2 Expressing Gastric or GEJ Cancer (PETRARCA). Available online: https://clinicaltrials.gov/ct2/show/NCT02581462 (accessed on 7 October 2022).
- Joshi, S.S.; Badgwell, B.D. Current Treatment and Recent Progress in Gastric Cancer. CA Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Hofheinz, R.D.; Schmalenberg, H.; Strumberg, D.; Goekkurt, E.; Angermeier, S.; Zander, T.; Potenberg, J.; Kopp, H.G.; Pink, D.; et al. Perioperative Ramucirumab in Combination with FLOT versus FLOT Alone for Resectable Esophagogastric Adenocarcinoma (RAMSES/FLOT7): Results of the Phase II-Portion—A Multicenter, Randomized Phase II/III Trial of the German AIO and Italian GOIM. J. Clin. Oncol. 2020, 38, 4501. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Practice Guidelines in Oncology (NCCN Guidelines), Gastric Cancer, Version 3. 2020. Available online: www.nccn.org/professional/physician_gls/pdf/gastric.pdf (accessed on 6 October 2022).
- Associazione Italiana Oncologia Medica (AIOM). Linee Guida AIOM: Neoplasie Dello Stomaco e Della Giunzione Esofago-Gastrica. Available online: https://www.aiom.it/linee-guida-aiom-2020-neoplasie-dello-stomaco-e-della-giunzione-esofago-gastrica/ (accessed on 6 October 2022).
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D. Gastric Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2016, 27, v38–v49. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, D.; Morgagni, P.; de Manzoni, G.; Coniglio, A.; Marchet, A.; Saragoni, L.; Tiberio, G.; Roviello, F. Prognostic Value of the 7th AJCC/UICC TNM Classification of Noncardia Gastric Cancer. Ann. Surg. 2012, 255, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Coccolini, F.; Nardi, M.; Montori, G.; Ceresoli, M.; Celotti, A.; Cascinu, S.; Fugazzola, P.; Tomasoni, M.; Glehen, O.; Catena, F.; et al. Neoadjuvant Chemotherapy in Advanced Gastric and Esophago-Gastric Cancer. Meta-Analysis of Randomized Trials. Int. J. Surg. 2018, 51, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.; Morgagni, P.; Nanni, O.; Framarini, M.; Saragoni, L.; Marrelli, D.; Roviello, F.; Petrioli, R.; Fumagalli Romario, U.; Rimassa, L.; et al. Preoperative or Perioperative Docetaxel, Oxaliplatin, and Capecitabine (GASTRODOC Regimen) in Patients with Locally-Advanced Resectable Gastric Cancer: A Randomized Phase-II Trial. Cancers 2020, 12, 2790. [Google Scholar] [CrossRef]
- Messager, M.; Lefevre, J.H.; Pichot-Delahaye, V.; Souadka, A.; Piessen, G.; Mariette, C.; Arnaud, J.P.; Balon, J.M.; Bonnetain, F.; Borie, F.; et al. The Impact of Perioperative Chemotherapy on Survival in Patients with Gastric Signet Ring Cell Adenocarcinoma. Ann. Surg. 2011, 254, 684–693. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Omori, T.; Demura, K.; Miyata, H.; Sugimura, K.; Ohue, M.; Kobayashi, S.; Takahashi, H.; Doki, Y.; Yano, M. A Multidisciplinary Approach for Advanced Gastric Cancer with Paraaortic Lymph Node Metastasis. Anticancer Res. 2015, 35, 6739–6745. [Google Scholar]
- de Jongh, C.; Triemstra, L.; van der Veen, A.; Brosens, L.A.A.; Luyer, M.D.P.; Stoot, J.H.M.B.; Ruurda, J.P.; van Hillegersberg, R.; Brenkman, H.J.F.; Seesing, M.F.J.; et al. Pattern of Lymph Node Metastases in Gastric Cancer: A Side-Study of the Multicenter LOGICA-Trial. Gastric Cancer 2022, 25, 1060–1072. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Comprehensive Molecular Characterization of Gastric Adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, G.; Hu, C. Molecular Classification of Gastric Adenocarcinoma. Gastroenterol. Res. 2019, 12, 275–282. [Google Scholar] [CrossRef]
- Marrelli, D.; Marano, L.; Ambrosio, M.R.; Carbone, L.; Spagnoli, L.; Petrioli, R.; Ongaro, A.; Piccioni, S.; Fusario, D.; Roviello, F. Immunohistochemical Markers of the Epithelial-to-Mesenchymal Transition (EMT) Are Related to Extensive Lymph Nodal Spread, Peritoneal Dissemination, and Poor Prognosis in the Microsatellite-Stable Diffuse Histotype of Gastric Cancer. Cancers 2022, 14, 6023. [Google Scholar] [CrossRef]
- Tirino, G.; Pompella, L.; Petrillo, A.; Laterza, M.; Pappalardo, A.; Caterino, M.; Orditura, M.; Ciardiello, F.; Galizia, G.; de Vita, F. What’s New in Gastric Cancer: The Therapeutic Implications of Molecular Classifications and Future Perspectives. Int. J. Mol. Sci. 2018, 19, 2659. [Google Scholar] [CrossRef] [PubMed]
- Polom, K.; Marrelli, D.; Pascale, V.; Ferrara, F.; Voglino, C.; Marini, M.; Roviello, F. The Pattern of Lymph Node Metastases in Microsatellite Unstable Gastric Cancer. Eur. J. Surg. Oncol. 2017, 43, 2341–2348. [Google Scholar] [CrossRef] [PubMed]
- Polom, K.; Marano, L.; Marrelli, D.; de Luca, R.; Roviello, G.; Savelli, V.; Tan, P.; Roviello, F. Meta-Analysis of Microsatellite Instability in Relation to Clinicopathological Characteristics and Overall Survival in Gastric Cancer. Br. J. Surg. 2018, 105, 159–167. [Google Scholar] [CrossRef] [PubMed]
- di Bartolomeo, M.; Morano, F.; Raimondi, A.; Miceli, R.; Corallo, S.; Tamborini, E.; Perrone, F.; Antista, M.; Niger, M.; Pellegrinelli, A.; et al. Prognostic and Predictive Value of Microsatellite Instability, Inflammatory Reaction and PD-L1 in Gastric Cancer Patients Treated with Either Adjuvant 5-FU/LV or Sequential FOLFIRI Followed by Cisplatin and Docetaxel: A Translational Analysis from the ITACA-S Trial. Oncologist 2020, 25, e460–e468. [Google Scholar] [CrossRef] [PubMed]
- Biesma, H.D.; Soeratram, T.T.D.; Sikorska, K.; Caspers, I.A.; van Essen, H.F.; Egthuijsen, J.M.P.; Mookhoek, A.; van Laarhoven, H.W.M.; van Berge Henegouwen, M.I.; Nordsmark, M.; et al. Response to Neoadjuvant Chemotherapy and Survival in Molecular Subtypes of Resectable Gastric Cancer: A Post Hoc Analysis of the D1/D2 and CRITICS Trials. Gastric Cancer 2022, 25, 640–651. [Google Scholar] [CrossRef] [PubMed]
- Pietrantonio, F.; Miceli, R.; Raimondi, A.; Kim, Y.W.; Kang, W.K.; Langley, R.E.; Choi, Y.Y.; Kim, K.-M.; Nankivell, M.G.; Morano, F.; et al. Individual Patient Data Meta-Analysis of the Value of Microsatellite Instability as a Biomarker in Gastric Cancer. J. Clin. Oncol. 2019, 37, 3392–3400. [Google Scholar] [CrossRef] [PubMed]
- Sexton, R.E.; al Hallak, M.N.; Diab, M.; Azmi, A.S. Gastric Cancer: A Comprehensive Review of Current and Future Treatment Strategies. Cancer Metastasis Rev. 2020, 39, 1179–1203. [Google Scholar] [CrossRef]
- de Manzoni, G.; Roviello, F. Correction to: Gastric Cancer: The 25-Year R-Evolution. In Gastric Cancer: The 25-Year R-Evolution; de Manzoni, G., Roviello, F., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 105–110. ISBN 978-3-03073-158-8. [Google Scholar]
- Liu, Y.-Y.; Fang, W.-L.; Wang, F.; Hsu, J.-T.; Tsai, C.-Y.; Liu, K.-H.; Yeh, C.-N.; Chen, T.-C.; Wu, R.-C.; Chiu, C.-T.; et al. Does a Higher Cutoff Value of Lymph Node Retrieval Substantially Improve Survival in Patients With Advanced Gastric Cancer?—Time to Embrace a New Digit. Oncologist 2017, 22, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.D.; Trufan, S.; Benbow, J.H.; Gower, N.L.; Hill, J.S.; Salo, J.C. Effect of Surgical Approach on Node Harvest in Gastrectomy: Analysis of the National Cancer Database. World J. Surg. 2020, 44, 3061–3069. [Google Scholar] [CrossRef]
- Kim, Y.I. Is Retrieval of at Least 15 Lymph Nodes Sufficient Recommendation in Early Gastric Cancer? Ann. Surg. Treat. Res. 2014, 87, 180. [Google Scholar] [CrossRef]
- Wanebo, H.J.; Kennedy, B.J.; Winchester, D.P.; Fremgen, A.; Stewart, A.K. Gastric Carcinoma: Does Lymph Node Dissection Alter Survival? J. Am. Coll. Surg. 1996, 183, 616–624. [Google Scholar]
- Sachs, T.E.; Tseng, J.F. Laparoscopic Resection After Neoadjuvant Chemotherapy for Distal Gastric Tumors. JAMA Surg. 2019, 154, 1101. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ryu, K.W.; Lee, J.-H.; Park, S.R.; Kim, C.G.; Kook, M.C.; Nam, B.-H.; Kim, Y.-W.; Bae, J.-M. Learning Curve for Total Gastrectomy with D2 Lymph Node Dissection: Cumulative Sum Analysis for Qualified Surgery. Ann. Surg. Oncol. 2006, 13, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Woodall, C.E.; Scoggins, C.R.; McMasters, K.M.; Martin, R.C.G. Adequate Lymphadenectomy Results in Accurate Nodal Staging without an Increase in Morbidity in Patients with Gastric Adenocarcinoma. Am. J. Surg. 2008, 196, 413–417. [Google Scholar] [CrossRef]
- Jang, Y.-J. Surgeon Subspecialty as a Factor in Improving Long-Term Outcomes for Gastric Cancer. Arch. Surg. 2010, 145, 1091. [Google Scholar] [CrossRef]
- Parikh, D.; Johnson, M.; Chagla, L.; Lowe, D.; McCulloch, P. D2 Gastrectomy: Lessons from a Prospective Audit of the Learning Curve. Br. J. Surg. 2005, 83, 1595–1599. [Google Scholar] [CrossRef]
- Degiuli, M.; Sasako, M.; Calgaro, M.; Garino, M.; Rebecchi, F.; Mineccia, M.; Scaglione, D.; Andreone, D.; Ponti, A.; Calvo, F. Morbidity and Mortality after D1 and D2 Gastrectomy for Cancer: Interim Analysis of the Italian Gastric Cancer Study Group (IGCSG) Randomised Surgical Trial. Eur. J. Surg. Oncol. EJSO 2004, 30, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Busweiler, L.A.D.; Schouwenburg, M.G.; van Berge Henegouwen, M.I.; Kolfschoten, N.E.; de Jong, P.C.; Rozema, T.; Wijnhoven, B.P.L.; van Hillegersberg, R.; Wouters, M.W.J.M.; van Sandick, J.W.; et al. Textbook Outcome as a Composite Measure in Oesophagogastric Cancer Surgery. Br. J. Surg. 2017, 104, 742–750. [Google Scholar] [CrossRef]
- van der Werf, L.R.; Wijnhoven, B.P.L.; Fransen, L.F.C.; van Sandick, J.W.; Nieuwenhuijzen, G.A.P.; Busweiler, L.A.D.; van Hillegersberg, R.; Wouters, M.W.J.M.; Luyer, M.D.P.; van Berge Henegouwen, M.I. A National Cohort Study Evaluating the Association Between Short-Term Outcomes and Long-Term Survival After Esophageal and Gastric Cancer Surgery. Ann. Surg. 2019, 270, 868–876. [Google Scholar] [CrossRef]
- van der Kaaij, R.T.; de Rooij, M.V.; van Coevorden, F.; Voncken, F.E.M.; Snaebjornsson, P.; Boot, H.; van Sandick, J.W. Using Textbook Outcome as a Measure of Quality of Care in Oesophagogastric Cancer Surgery. Br. J. Surg. 2018, 105, 561–569. [Google Scholar] [CrossRef]
- Shrikhande, S.V.; Barreto, S.G.; Talole, S.D.; Vinchurkar, K.; Annaiah, S.; Suradkar, K.; Mehta, S.; Goel, M. D2 Lymphadenectomy Is Not Only Safe but Necessary in the Era of Neoadjuvant Chemotherapy. World J. Surg. Oncol. 2013, 11, 31. [Google Scholar] [CrossRef]
- Wu, L.; Ge, L.; Qin, Y.; Huang, M.; Chen, J.; Yang, Y.; Zhong, J. Postoperative Morbidity and Mortality after Neoadjuvant Chemotherapy versus Upfront Surgery for Locally Advanced Gastric Cancer: A Propensity Score Matching Analysis. Cancer Manag. Res. 2019, 11, 6011–6018. [Google Scholar] [CrossRef] [PubMed]
- Katayama, H.; Tsuburaya, A.; Mizusawa, J.; Nakamura, K.; Katai, H.; Imamura, H.; Nashimoto, A.; Fukushima, N.; Sano, T.; Sasako, M. An Integrated Analysis of Two Phase II Trials (JCOG0001 and JCOG0405) of Preoperative Chemotherapy Followed by D3 Gastrectomy for Gastric Cancer with Extensive Lymph Node Metastasis. Gastric Cancer 2019, 22, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Oyama, K.; Fushida, S.; Kinoshita, J.; Makino, I.; Nakamura, K.; Hayashi, H.; Nakagawara, H.; Tajima, H.; Fujita, H.; Takamura, H.; et al. Efficacy of Pre-Operative Chemotherapy with Docetaxel, Cisplatin, and S-1 (DCS Therapy) and Curative Resection for Gastric Cancer with Pathologically Positive Para-Aortic Lymph Nodes. J. Surg. Oncol. 2012, 105, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Sano, T.; Mizusawa, J.; Takahari, D.; Katayama, H.; Katai, H.; Kawashima, Y.; Kinoshita, T.; Terashima, M.; Nashimoto, A.; et al. A Phase II Study of Preoperative Chemotherapy with Docetaxel, Cisplatin, and S-1 Followed by Gastrectomy with D2 plus Para-Aortic Lymph Node Dissection for Gastric Cancer with Extensive Lymph Node Metastasis: JCOG1002. Gastric Cancer 2017, 20, 322–331. [Google Scholar] [CrossRef]
- Yoshida, M. Long-Term Survival and Prognostic Factors in Patients with Metastatic Gastric Cancers Treated with Chemotherapy in the Japan Clinical Oncology Group (JCOG) Study. Jpn. J. Clin. Oncol. 2004, 34, 654–659. [Google Scholar] [CrossRef]
- Park, I.H.; Kim, S.Y.; Kim, Y.-W.; Ryu, K.W.; Lee, J.H.; Lee, J.S.; Park, Y.-I.; Kim, N.K.; Park, S.R. Clinical Characteristics and Treatment Outcomes of Gastric Cancer Patients with Isolated Para-Aortic Lymph Node Involvement. Cancer Chemother. Pharm. 2011, 67, 127–136. [Google Scholar] [CrossRef]
- Marrelli, D.; Mazzei, M.A.; Pedrazzani, C.; di Martino, M.; Vindigni, C.; Corso, G.; Morelli, E.; Volterrani, L.; Roviello, F. High Accuracy of Multislices Computed Tomography (MSCT) for Para-Aortic Lymph Node Metastases from Gastric Cancer: A Prospective Single-Center Study. Ann. Surg. Oncol. 2011, 18, 2265–2272. [Google Scholar] [CrossRef]
- Berlth, F.; Chon, S.-H.; Chevallay, M.; Jung, M.K.; Mönig, S.P. Preoperative Staging of Nodal Status in Gastric Cancer. Transl. Gastroenterol. Hepatol. 2017, 2, 8. [Google Scholar] [CrossRef]
- Chang, J.S.; Kim, K.H.; Yoon, H.I.; Hyung, W.J.; Rha, S.Y.; Kim, H.S.; Lee, Y.C.; Lim, J.S.; Noh, S.H.; Koom, W.S. Locoregional Relapse after Gastrectomy with D2 Lymphadenectomy for Gastric Cancer. Br. J. Surg. 2017, 104, 877–884. [Google Scholar] [CrossRef]
- Motoori, M.; Ito, Y.; Miyashiro, I.; Sugimura, K.; Miyata, H.; Omori, T.; Fujiwara, Y.; Yano, M. Impact of Age on Long-Term Survival in Patients with Esophageal Cancer Who Underwent Transthoracic Esophagectomy. Oncology 2019, 97, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Mengardo, V.; Bencivenga, M.; Weindelmayer, J.; Pavarana, M.; Giacopuzzi, S.; de Manzoni, G. Para-Aortic Lymphadenectomy in Surgery for Gastric Cancer: Current Indications and Future Perspectives. Updates Surg. 2018, 70, 207–211. [Google Scholar] [CrossRef]
- Rausei, S.; Lianos, G.D. Treatment of Gastric Cancer. Cancers 2020, 12, 2627. [Google Scholar] [CrossRef] [PubMed]
- Lianos, G.D.; Hasemaki, N.; Glantzounis, G.K.; Mitsis, M.; Rausei, S. Assessing Safety and Feasibility of ‘Pure’ Laparoscopic Total Gastrectomy for Advanced Gastric Cancer in the West. Review Article. Int. J. Surg. 2018, 53, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Lianos, G.D.; Rausei, S.; Ruspi, L.; Galli, F.; Mangano, A.; Roukos, D.H.; Dionigi, G.; Boni, L. Laparoscopic Gastrectomy for Gastric Cancer: Current Evidences. Int. J. Surg. 2014, 12, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, P.M.; Bernasconi, D.; Baiocchi, G.L.; Berselli, M.; Biondi, A.; Castoro, C.; Catarci, M.; Degiuli, M.; Fumagalli Romario, U.; Giacopuzzi, S.; et al. Open versus Laparoscopic Gastrectomy for Advanced Gastric Cancer: A Propensity Score Matching Analysis of Survival in a Western Population—On Behalf of the Italian Research Group for Gastric Cancer. Gastric Cancer 2022, 25, 1105–1116. [Google Scholar] [CrossRef]
- Kim, H.-H.; Han, S.-U.; Kim, M.-C.; Kim, W.; Lee, H.-J.; Ryu, S.W.; Cho, G.S.; Kim, C.Y.; Yang, H.-K.; Park, D.J.; et al. Effect of Laparoscopic Distal Gastrectomy vs Open Distal Gastrectomy on Long-Term Survival Among Patients with Stage I Gastric Cancer. JAMA Oncol. 2019, 5, 506. [Google Scholar] [CrossRef]
- Hyung, W.J.; Yang, H.-K.; Park, Y.-K.; Lee, H.-J.; An, J.Y.; Kim, W.; Kim, H.-I.; Kim, H.-H.; Ryu, S.W.; Hur, H.; et al. Long-Term Outcomes of Laparoscopic Distal Gastrectomy for Locally Advanced Gastric Cancer: The KLASS-02-RCT Randomized Clinical Trial. J. Clin. Oncol. 2020, 38, 3304–3313. [Google Scholar] [CrossRef] [PubMed]
- Trapani, R.; Rausei, S.; Reddavid, R.; Degiuli, M.; Bencivenga, M.; Dal Cero, M.; Rosa, F.; Alfieri, S.; Tiberio, G.A.; Alfano, M.S.; et al. Risk Factors for Esophago-Jejunal Anastomosis Leakage after Total Gastrectomy for Cancer. A Multicenter Retrospective Study of the Italian Research Group for Gastric Cancer. Eur. J. Surg. Oncol. 2020, 46, 2243–2247. [Google Scholar] [CrossRef]
- Degiuli, M.; de Manzoni, G.; di Leo, A.; D’Ugo, D.; Galasso, E.; Marrelli, D.; Petrioli, R.; Polom, K.; Roviello, F.; Santullo, F.; et al. Gastric Cancer: Current Status of Lymph Node Dissection. World J. Gastroenterol. 2016, 22, 2875. [Google Scholar] [CrossRef]
- Hashizume, M.; Shimada, M.; Tomikawa, M.; Ikeda, Y.; Takahashi, I.; Abe, R.; Koga, F.; Gotoh, N.; Konishi, K.; Maehara, S.; et al. Early Experiences of Endoscopic Procedures in General Surgery Assisted by a Computer-Enhanced Surgical System. Surg. Endosc. Other Interv. Tech. 2002, 16, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Giulianotti, P.C. Robotics in General Surgery. Arch. Surg. 2003, 138, 777. [Google Scholar] [CrossRef] [PubMed]
- Suda, K.; Man-i, M.; Ishida, Y.; Kawamura, Y.; Satoh, S.; Uyama, I. Potential Advantages of Robotic Radical Gastrectomy for Gastric Adenocarcinoma in Comparison with Conventional Laparoscopic Approach: A Single Institutional Retrospective Comparative Cohort Study. Surg. Endosc. 2015, 29, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Son, T.; Lee, J.H.; Kim, Y.M.; Kim, H.-I.; Noh, S.H.; Hyung, W.J. Robotic Spleen-Preserving Total Gastrectomy for Gastric Cancer: Comparison with Conventional Laparoscopic Procedure. Surg. Endosc. 2014, 28, 2606–2615. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Xi, H.; Wei, B.; Cui, J.; Bian, S.; Zhang, K.; Wang, N.; Huang, X.; Chen, L. Robotic versus Laparoscopic Gastrectomy for Gastric Cancer: Comparison of Short-Term Surgical Outcomes. Surg. Endosc. 2016, 30, 574–580. [Google Scholar] [CrossRef]
- Tian, Y.; Cao, S.; Kong, Y.; Shen, S.; Niu, Z.; Zhang, J.; Chen, D.; Jiang, H.; Lv, L.; Liu, X.; et al. Short- and Long-Term Comparison of Robotic and Laparoscopic Gastrectomy for Gastric Cancer by the Same Surgical Team: A Propensity Score Matching Analysis. Surg. Endosc. 2022, 36, 185–195. [Google Scholar] [CrossRef]
- Marano, L.; D’Ignazio, A.; Resca, L.; Marrelli, D.; Roviello, F. Robotic-Assisted Gastrectomy for Gastric Cancer: Single Western Center Results. Updat. Surg. 2021, 73, 865–872. [Google Scholar] [CrossRef]
- Marano, L.; Fusario, D.; Savelli, V.; Marrelli, D.; Roviello, F. Robotic versus Laparoscopic Gastrectomy for Gastric Cancer: An Umbrella Review of Systematic Reviews and Meta-Analyses. Updates Surg. 2021, 73, 1673–1689. [Google Scholar] [CrossRef]
- Lu, J.; Yoon, C.; Xu, B.; Xie, J.; Li, P.; Zheng, C.; Huang, C.; Yoon, S.S. Long-Term Survival after Minimally Invasive Versus Open Gastrectomy for Gastric Adenocarcinoma: A Propensity Score-Matched Analysis of Patients in the United States and China. Ann. Surg. Oncol. 2020, 27, 802–811. [Google Scholar] [CrossRef]
- Veenhof, A.A.F.A.; Vlug, M.S.; van der Pas, M.H.G.M.; Sietses, C.; van der Peet, D.L.; de Lange-de Klerk, E.S.M.; Bonjer, H.J.; Bemelman, W.A.; Cuesta, M.A. Surgical Stress Response and Postoperative Immune Function After Laparoscopy or Open Surgery with Fast Track or Standard Perioperative Care. Ann. Surg. 2012, 255, 216–221. [Google Scholar] [CrossRef]
- van der Wielen, N.; Straatman, J.; Daams, F.; Rosati, R.; Parise, P.; Weitz, J.; Reissfelder, C.; Diez del Val, I.; Loureiro, C.; Parada-González, P.; et al. Open versus Minimally Invasive Total Gastrectomy after Neoadjuvant Chemotherapy: Results of a European Randomized Trial. Gastric Cancer 2021, 24, 258–271. [Google Scholar] [CrossRef]
- Hu, H.-T.; Ma, F.-H.; Xiong, J.-P.; Li, Y.; Jin, P.; Liu, H.; Ma, S.; Kang, W.-Z.; Tian, Y.-T. Laparoscopic vs Open Total Gastrectomy for Advanced Gastric Cancer Following Neoadjuvant Therapy: A Propensity Score Matching Analysis. World J. Gastrointest. Surg. 2022, 14, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Khaled, I.; Priego, P.; Soliman, H.; Faisal, M.; Saad Ahmed, I. Oncological Outcomes of Laparoscopic versus Open Gastrectomy after Neoadjuvant Chemotherapy for Locally Advanced Gastric Cancer: A Retrospective Multicenter Study. World J. Surg. Oncol. 2021, 19, 206. [Google Scholar] [CrossRef] [PubMed]
- A Comparison of Laparoscopic with Open Distal Gastrectomy in Advanced Gastric Cancer After Neoadjuvant Chemotherapy (REALIZATION). Available online: https://clinicaltrials.gov/ct2/show/NCT02404753 (accessed on 7 October 2022).
- Li, Z.; Shan, F.; Ying, X.; Zhang, Y.; Yu, J.; Wang, Y.; Ren, H.; Su, X.; Ji, J. Assessment of Laparoscopic Distal Gastrectomy After Neoadjuvant Chemotherapy for Locally Advanced Gastric Cancer. JAMA Surg. 2019, 154, 1093. [Google Scholar] [CrossRef]
- Laparoscopic D2 Distal Gastrectomy Following Neoadjuvant Chemotherapy for Locally Advanced Gastric Cancers (CLASS-03a). Available online: https://clinicaltrials.gov/ct2/show/NCT03468712 (accessed on 7 October 2022).
- The Safety and Feasibility of Laparoscopic-Assisted Gastrectomy for Advanced Gastric Cancer After Neoadjuvant Chemotherapy. Available online: https://clinicaltrials.gov/ct2/show/NCT02902575 (accessed on 7 October 2022).
- Laparoscopic vs. Open Distal Gastrectomy After Neoadjuvant Chemotherapy. Available online: https://clinicaltrials.gov/ct2/show/NCT04658589 (accessed on 7 October 2022).
- Strong, V.E.; Russo, A.E.; Nakauchi, M.; Schattner, M.; Selby, L.V.; Herrera, G.; Tang, L.; Gonen, M. Robotic Gastrectomy for Gastric Adenocarcinoma in the USA: Insights and Oncologic Outcomes in 220 Patients. Ann. Surg. Oncol. 2021, 28, 742–750. [Google Scholar] [CrossRef]
- Okabe, H.; Sunagawa, H.; Saji, M.; Hirai, K.; Hisamori, S.; Tsunoda, S.; Obama, K. Comparison of Short-Term Outcomes between Robotic and Laparoscopic Gastrectomy for Gastric Cancer: A Propensity Score-Matching Analysis. J. Robot Surg. 2021, 15, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Standard Versus Super-Extended Lymphadenectomy After Neo-Adjuvant Chemotherapy for Gastric Cancer (Neo-D2plus). Available online: https://clinicaltrials.gov/ct2/show/NCT03961373 (accessed on 7 October 2022).
- Phase III Randomized Trial Comparing D2 vs D3 Lymphadenectomy with Gastric Cancer Following Neoadjuvant Chemotherapy. Available online: https://clinicaltrials.gov/ct2/show/NCT02139605 (accessed on 7 October 2022).
- Sato, Y.; Kurokawa, Y.; Doki, Y.; Mizusawa, J.; Tanaka, K.; Katayama, H.; Boku, N.; Yoshikawa, T.; Terashima, M. A Phase II Study of Preoperative Chemotherapy with Docetaxel, Oxaliplatin and S-1 in Gastric Cancer with Extensive Lymph Node Metastasis (JCOG1704). Future Oncol. 2020, 16, 31–38. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).