STRN-ALK Fusion in Lung Adenocarcinoma with Brain Metastasis Responded Well to Ensartinib: A Case Report
Abstract
1. Introduction
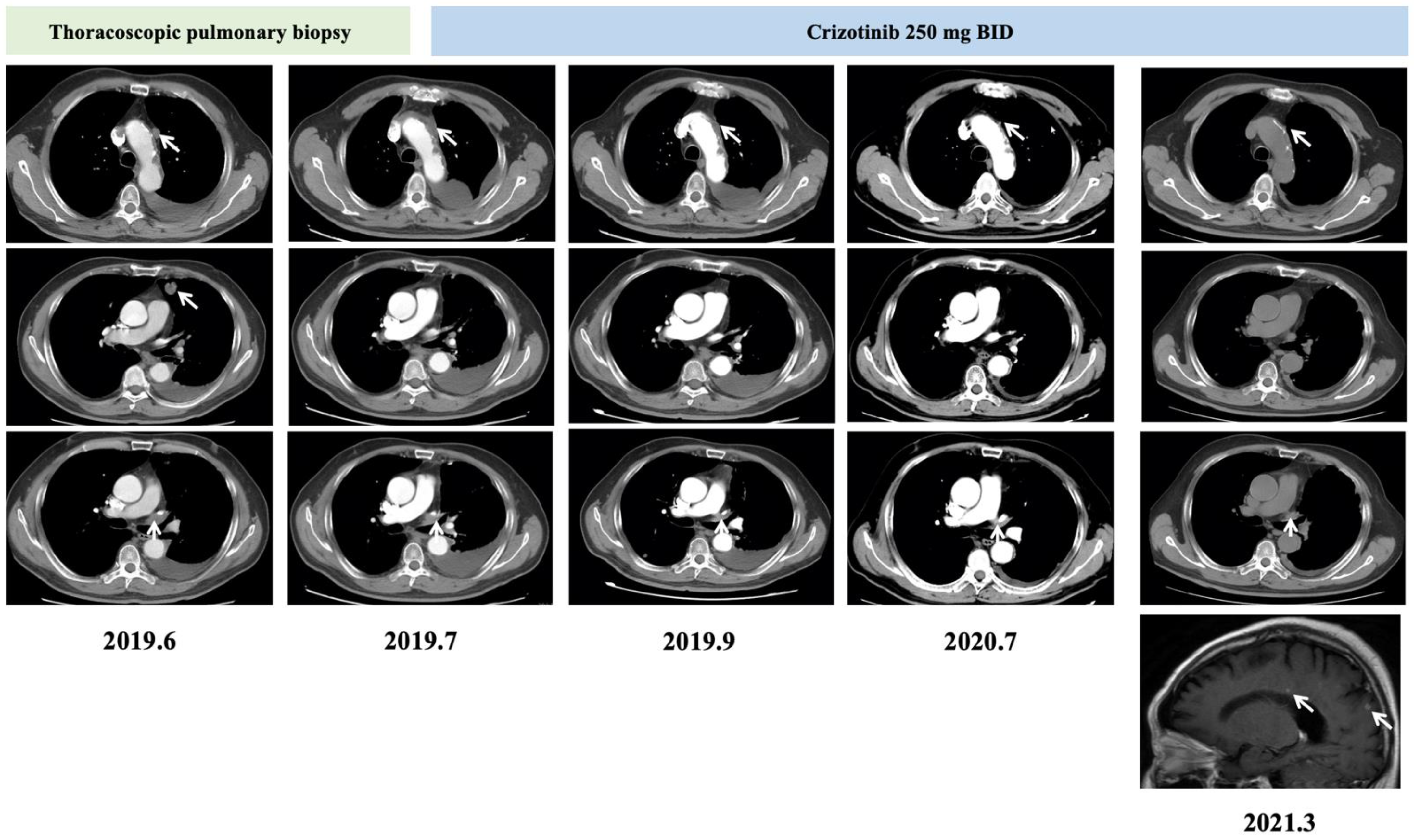
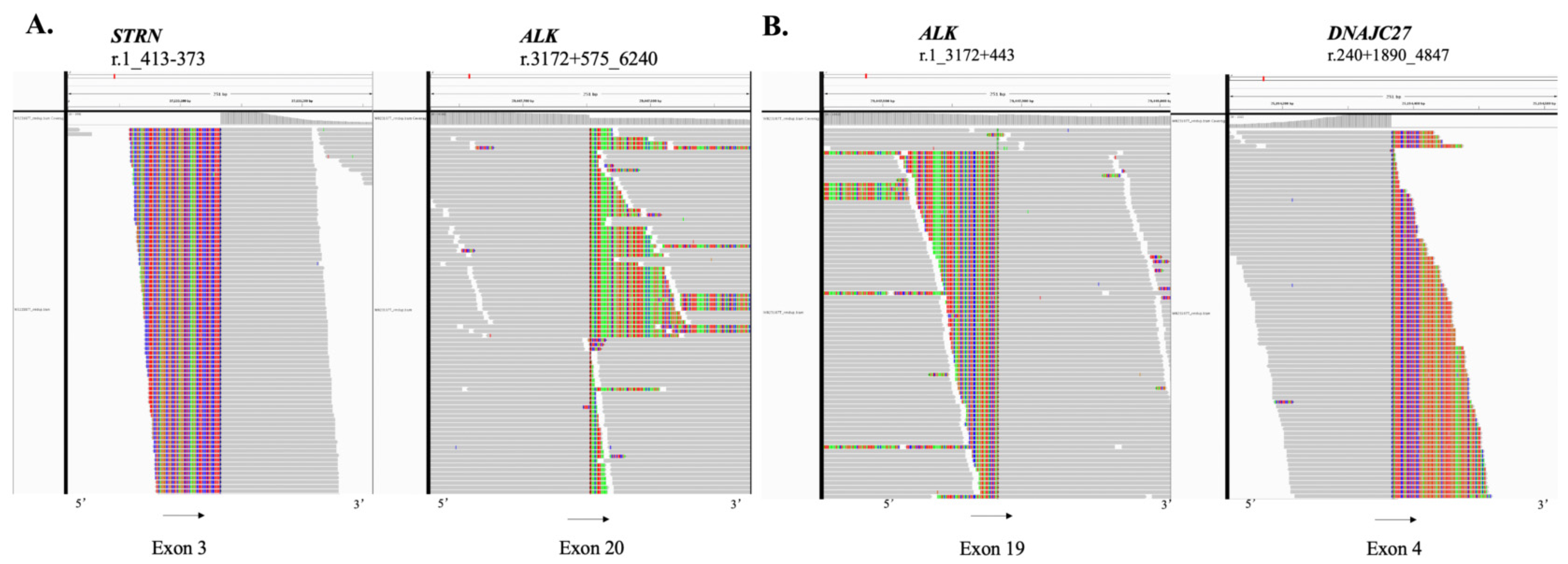
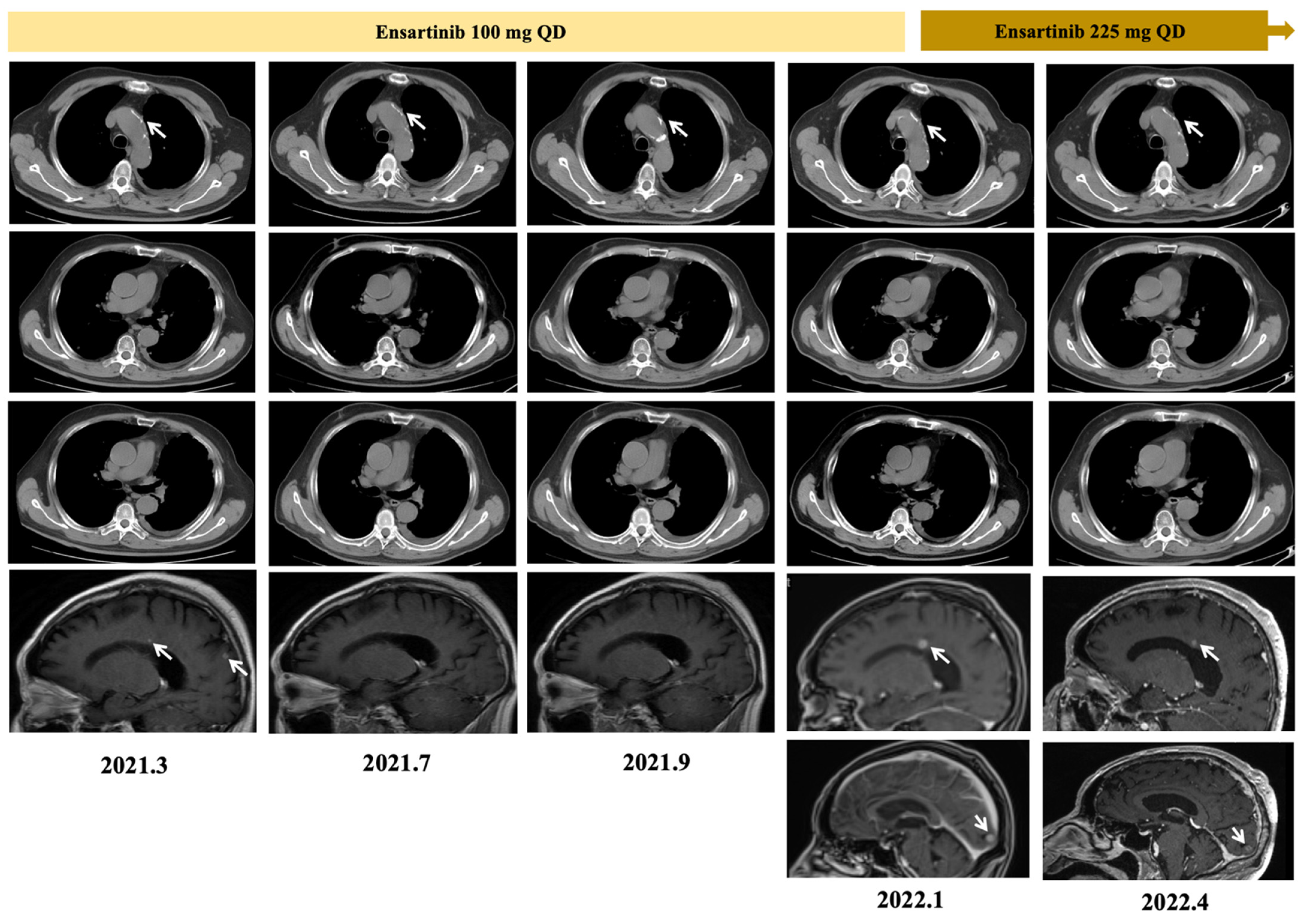
2. Case Report
3. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horn, L.; Wang, Z.; Wu, G.; Poddubskaya, E.; Mok, T.; Reck, M.; Wakelee, H.; Chiappori, A.A.; Lee, D.H.; Breder, V.; et al. Ensartinib vs Crizotinib for Patients with Anaplastic Lymphoma Kinase-Positive Non-Small Cell Lung Cancer: A Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Childress, M.A.; Himmelberg, S.M.; Chen, H.; Deng, W.; Davies, M.A.; Lovly, C.M. ALK Fusion Partners Impact Response to ALK Inhibition: Differential Effects on Sensitivity, Cellular Phenotypes, and Biochemical Properties. Mol. Cancer Res. 2018, 16, 1724–1736. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qin, S.K.; Zhu, J.; Wang, R.; Li, Y.M.; Xie, Z.Y.; Wu, Q. A Rare STRN-ALK Fusion in Lung Adenocarcinoma Identified Using Next-Generation Sequencing-Based Circulating Tumor DNA Profiling Exhibits Excellent Response to Crizotinib. Mayo Clin. Proc. Innov. Qual. Outcomes 2017, 1, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, Y.; Masuda, S.; Iida, Y.; Takahashi, N.; Hashimoto, S. Case Report of Non-Small Cell Lung Cancer with STRN-ALK Translocation: A Nonresponder to Alectinib. J. Thorac. Oncol. 2017, 12, e202–e204. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zeng, L.; Zhang, Y.; Yang, N. Responder of Gefitinib Plus Crizotinib in Osimertinib Failure EGFR-mutant NSCLC-Resistant with Newly Identified STRN-ALK by Next-Generation Sequencing. J. Thorac. Oncol. 2019, 14, e143–e144. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Jiang, Y.; Jiang, W.; Wang, H.; Liu, S.; Shao, Y.; Zhao, W.; Ning, R.; Yu, Q. STRN-ALK Fusion in Lung Adenocarcinoma with Excellent Response Upon Alectinib Treatment: A Case Report and Literature Review. Onco. Targets Ther. 2020, 13, 12515–12519. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, M.; Sarvadevabatla, N.; Iwata, S.; Ge, Y.; Sukari, A.; Klosowski, C.; Yanagihara, R. STRN-ALK, A Novel In-Frame Fusion with Response to Alectinib. JTO Clin. Res. Rep. 2020, 2, 100125. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Mixed responses to first-line alectinib in non-small cell lung cancer patients with rare ALK gene fusions: A case series and literature review. J. Cell. Mol. Med. 2021, 25, 9476–9481. [Google Scholar] [CrossRef]
- Zeng, H.; Li, Y.; Wang, Y.; Huang, M.; Zhang, Y.; Tian, P.; Li, W. Case Report: Identification of Two Rare Fusions, PDK1-ALK and STRN-ALK, That Coexist in a Lung Adenocarcinoma Patient and the Response to Alectinib. Front. Oncol. 2021, 13, 722843. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Nie, L.; Nong, L.; Cheng, Y. Primary resistance to alectinib in a patient with STRN-ALK-positive non-small cell lung cancer: A case report. Thorac. Cancer. 2021, 12, 1927–1930. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Gao, H.; Zhang, L.; Qin, S.; Gu, Y.; Chen, Q. Coexistence of a secondary STRN-ALK, EML4-ALK double-fusion variant in a lung adenocarcinoma patient with EGFR mutation: A case report. Anticancer Drugs 2021, 1, 890–893. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Infante, J.R.; Reckamp, K.L.; Blumenschein, G.R.; Leal, T.A.; Waqar, S.N.; Gitlitz, B.J.; Sanborn, R.E.; Whisenant, J.G.; Du, L.; et al. Ensartinib (X-396) in ALK-Positive Non-Small Cell Lung Cancer: Results from a First-in-Human Phase I/II, Multicenter Study. Clin. Cancer Res. 2018, 24, 2771–2779. [Google Scholar] [CrossRef] [PubMed]
| Gene Name | Nucleotide Change | Amino Acid Change | Mutation Type | Variant Allele Frequency (%) |
|---|---|---|---|---|
| ALK | c.2920G > C | p.E974Q | SNV | 42.7 |
| EPHA5 | c.188T > C | p.L63S | SNV | 30.4 |
| PRKDC | c.9587G > T | p.K3196N | SNV | 20.3 |
| PFWD2 | c.268A > T | p.S90C | SNV | 29.2 |
| RPL5 | c.262_264delinsTTTT | p.V88Ffs*2 | Deletion | 26.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Xiao, P.; Meng, F.; Zhong, D. STRN-ALK Fusion in Lung Adenocarcinoma with Brain Metastasis Responded Well to Ensartinib: A Case Report. Curr. Oncol. 2022, 29, 6749-6753. https://doi.org/10.3390/curroncol29100530
Zhang L, Xiao P, Meng F, Zhong D. STRN-ALK Fusion in Lung Adenocarcinoma with Brain Metastasis Responded Well to Ensartinib: A Case Report. Current Oncology. 2022; 29(10):6749-6753. https://doi.org/10.3390/curroncol29100530
Chicago/Turabian StyleZhang, Linlin, Ping Xiao, Fanlu Meng, and Diansheng Zhong. 2022. "STRN-ALK Fusion in Lung Adenocarcinoma with Brain Metastasis Responded Well to Ensartinib: A Case Report" Current Oncology 29, no. 10: 6749-6753. https://doi.org/10.3390/curroncol29100530
APA StyleZhang, L., Xiao, P., Meng, F., & Zhong, D. (2022). STRN-ALK Fusion in Lung Adenocarcinoma with Brain Metastasis Responded Well to Ensartinib: A Case Report. Current Oncology, 29(10), 6749-6753. https://doi.org/10.3390/curroncol29100530





