De-Escalating Breast Cancer Surgery: Should We Apply Quality Indicators from Other Jurisdictions in Canada?
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Clinical Data
2.2. Outcome Measures
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, L.; Woods, R.; Brenner, D.; Bryan, S.; Louzado, C.; Shaw, A.; Turner, D.; Weir, H.K.; Demers, A.; Ellison, L. Canadian Cancer Statistics 2019. Available online: Cancer.ca/Canadian-Cancer-Statistics-2019-EN (accessed on 6 October 2021).
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.H.; Wolmark, N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 2002, 347, 1233–1241. [Google Scholar] [CrossRef] [Green Version]
- Jatoi, I.; Proschan, M.A. Randomized trials of breast-conserving therapy versus mastectomy for primary breast cancer: A pooled analysis of updated results. Am. J. Clin. Oncol. 2005, 28, 289–294. [Google Scholar] [CrossRef]
- Treatment of Early-Stage Breast Cancer. JAMA. 1991, 265, 391–395. [CrossRef]
- van Maaren, M.C.; de Munck, L.; de Bock, G.H.; Jobsen, J.J.; van Dalen, T.; Linn, S.C.; Poortmans, P.; Strobbe, L.J.A.; Siesling, S. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: A population-based study. Lancet Oncol. 2016, 17, 1158–1170. [Google Scholar] [CrossRef]
- Garcia-Etienne, C.A.; Tomatis, M.; Heil, J.; Friedrichs, K.; Kreienberg, R.; Denk, A.; Kiechle, M.; Lorenz-Salehi, F.; Kimmig, R.; Emons, G.; et al. Mastectomy trends for early-stage breast cancer: A report from the EUSOMA multi-institutional European database. Eur. J. Cancer. 2012, 48, 1947–1956. [Google Scholar] [CrossRef]
- Dragun, A.E.; Pan, J.; Riley, E.C.; Kruse, B.; Wilson, M.R.; Rai, S.; Jain, D. Increasing use of elective mastectomy and contralateral prophylactic surgery among breast conservation candidates: A 14-year report from a comprehensive cancer center. Am. J. Clin. Oncol. 2013, 36, 375–380. [Google Scholar] [CrossRef]
- Kiderlen, M.; Ponti, A.; Tomatis, M.; Boelens, P.G.; Bastiaannet, E.; Wilson, R.; van de Velde, C.J.H.; Audisio, R.A.; eusoma DB Working Group. Variations in compliance to quality indicators by age for 41,871 breast cancer patients across Europe: A European Society of Breast Cancer Specialists database analysis. Eur. J. Cancer. 2015, 51, 1221–1230. [Google Scholar] [CrossRef]
- Findlay-Shirras, L.; Lima, I.; Smith, G.; Clemons, M.; Arnaout, A. Canada follows the US in the rise of bilateral mastectomies for unilateral breast cancer: A 23-year population cohort study. Breast Cancer Res. Treat. 2021, 185, 517–525. [Google Scholar] [CrossRef]
- Lee, W.Q.; Tan, V.K.M.; Choo, H.M.C.; Ong, J.; Krishnapriya, R.; Khong, S.; Tan, M.; Sim, Y.R.; Tan, B.K.; Madhukumar, P.; et al. Factors influencing patient decision-making between simple mastectomy and surgical alternatives. BJS Open. 2019, 3, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Mac Bride, M.B.; Neal, L.; Dilaveri, C.A.; Sandhu, N.P.; Hieken, T.J.; Ghosh, K.; Wahner-Roedler, D.L. Factors Associated with Surgical Decision Making in Women with Early-Stage Breast Cancer: A Literature Review. J. Women’s Health. 2013, 22, 236–242. [Google Scholar] [CrossRef]
- Gu, J.; Groot, G.; Holtslander, L.; Engler-Stringer, R. Understanding Women’s Choice of Mastectomy Versus Breast Conserving Therapy in Early-Stage Breast Cancer. Clin. Med. Insights Oncol. 2017, 11, 1179554917691266. [Google Scholar] [CrossRef]
- Tollow, P.; Williams, V.S.; Harcourt, D.; Paraskeva, N. “It felt like unfinished business, it feels like that’s finished now”: Women’s experiences of decision making around contralateral prophylactic mastectomy (CPM). Psycho-Oncology 2019, 28, 1328–1334. [Google Scholar] [CrossRef]
- Fisher, C.S.; Martin-Dunlap, T.; Ruppel, M.B.; Gao, F.; Atkins, J.; Margenthaler, J.A. Fear of Recurrence and Perceived Survival Benefit are Primary Motivators for Choosing Mastectomy over Breast-Conservation Therapy Regardless of Age. Ann. Surg. Oncol. 2012, 19, 3246–3250. [Google Scholar] [CrossRef]
- Lee, M.C.; Rogers, K.; Griffith, K.; Diehl, K.A.; Breslin, T.M.; Cimmino, V.M.; Chang, A.E.; Newman, L.A.; Sabel, M.S. Determinants of Breast Conservation Rates: Reasons for Mastectomy at a Comprehensive Cancer Center. Breast J. 2009, 15, 34–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Turco, M.R.; Ponti, A.; Bick, U.; Biganzoli, L.; Cserni, G.; Cutuli, B.; Decker, T.; Dietel, M.; Gentilini, O.; Kuehn, T.; et al. Quality indicators in breast cancer care. Eur. J. Cancer. 2010, 46, 2344–2356. [Google Scholar] [CrossRef] [PubMed]
- Biganzoli, L.; Marotti, L.; Hart, C.D.; Cataliotti, L.; Cutuli, B.; Kühn, T.; Mansel, R.E.; Ponti, A.; Poortmans, P.; Regitnig, P.; et al. Quality indicators in breast cancer care: An update from the EUSOMA working group. Eur. J. Cancer. 2017, 86, 59–81. [Google Scholar] [CrossRef] [PubMed]
- American College of Surgeons. Available online: https://www.facs.org/quality-programs/napbc/standards (accessed on 1 October 2021).
- van Dam, P.A.; Tomatis, M.; Marotti, L.; Heil, J.; Wilson, R.; Rosselli del Turco, M.; Mayr, C.; Costa, A.; Danei, M.; Denk, A.; et al. The effect of EUSOMA certification on quality of breast cancer care. Eur. J. Surg. Oncol. 2015, 41, 1423–1429. [Google Scholar] [CrossRef]
- van Dam, P.A.; Tomatis, M.; Marotti, L.; Heil, J.; Mansel, R.E.; Rosselli del Turco, M.; van Dam, P.J.; Casella, D.; Bassani, L.G.; Danei, M.; et al. Time trends (2006–2015) of quality indicators in EUSOMA-certified breast centres. Eur. J. Cancer. 2017, 85, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Kapur, H.; Warburton, R.; Pao, J.S.; Dingee, C.; Chen, L.; McKevitt, E. Decreasing contralateral prophylactic mastectomy rates in average-risk women with unilateral breast cancer. Am. J. Surg. 2021, 221, 1172–1176. [Google Scholar] [CrossRef]
- Whelan, T.J.; Olivotto, I.A.; Parulekar, W.R.; Ackerman, I.; Chua, B.H.; Nabid, A.; Vallis, K.A.; White, J.R.; Rousseau, P.; Fortin, A.; et al. Regional Nodal Irradiation in Early-Stage Breast Cancer. N. Engl. J. Med. 2015, 373, 307–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poortmans, P.M.; Collette, S.; Kirkove, C.; Van Limbergen, E.; Budach, V.; Struikmans, H.; Collette, L.; Fourquet, A.; Maingon, P.; Valli, M.; et al. Internal Mammary and Medial Supraclavicular Irradiation in Breast Cancer. N. Engl. J. Med. 2015, 373, 317–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCahill, L.E.; Privette, A.R.; Hart, M.R.; James, T.A. Are mastectomy rates a reasonable quality measure of breast cancer surgery? Am. J. Surg. 2009, 197, 216–221. [Google Scholar] [CrossRef] [PubMed]
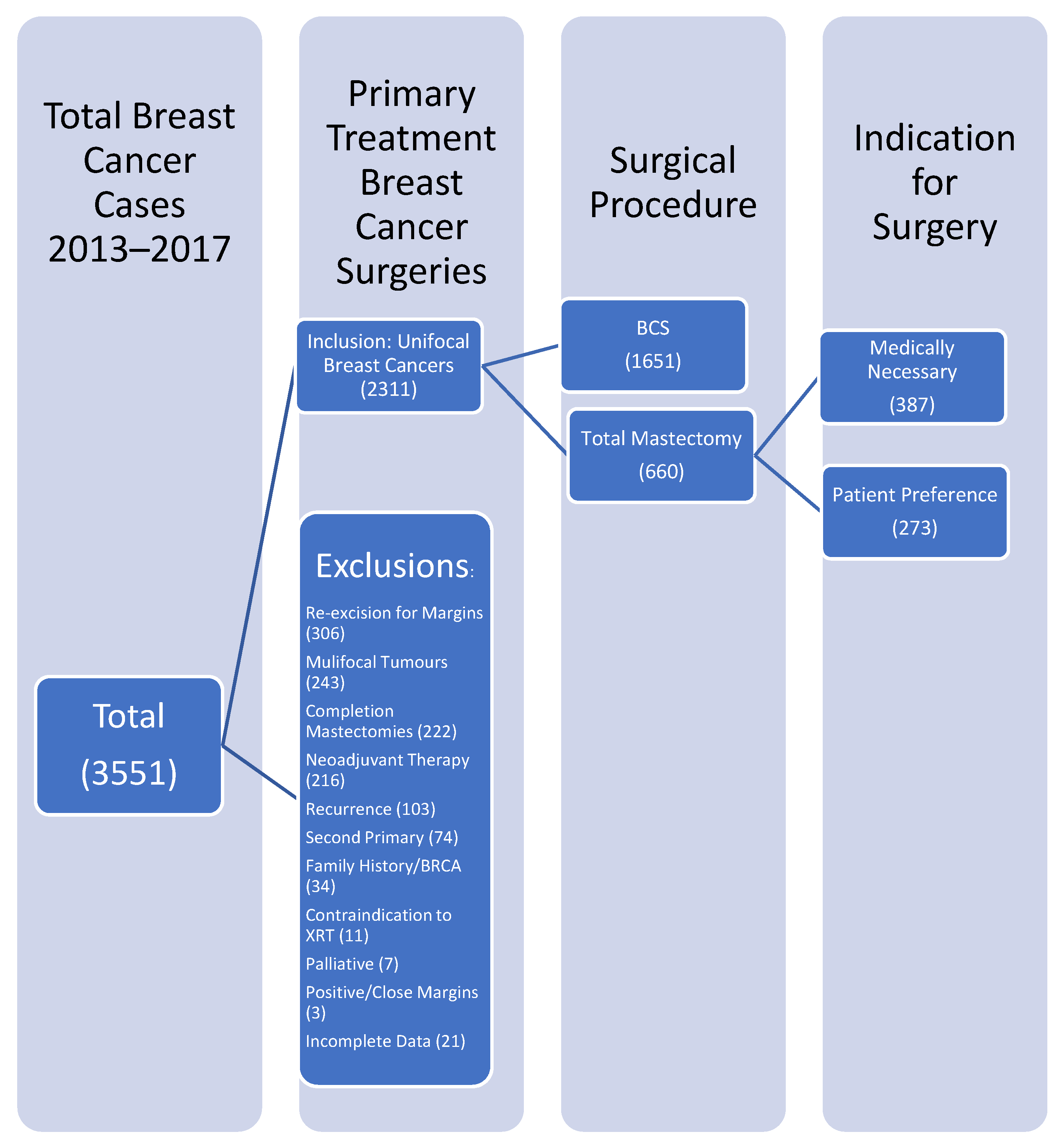
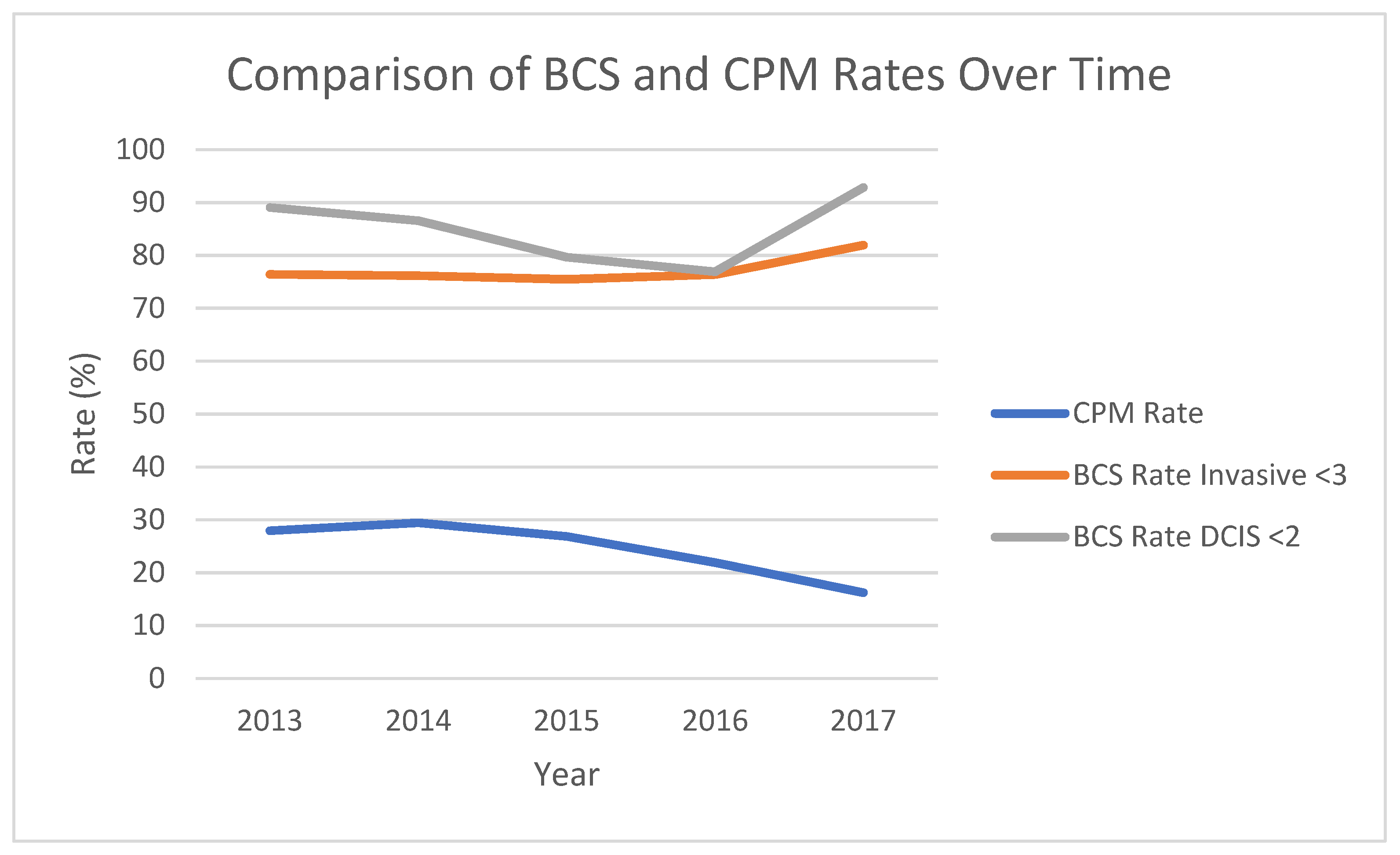
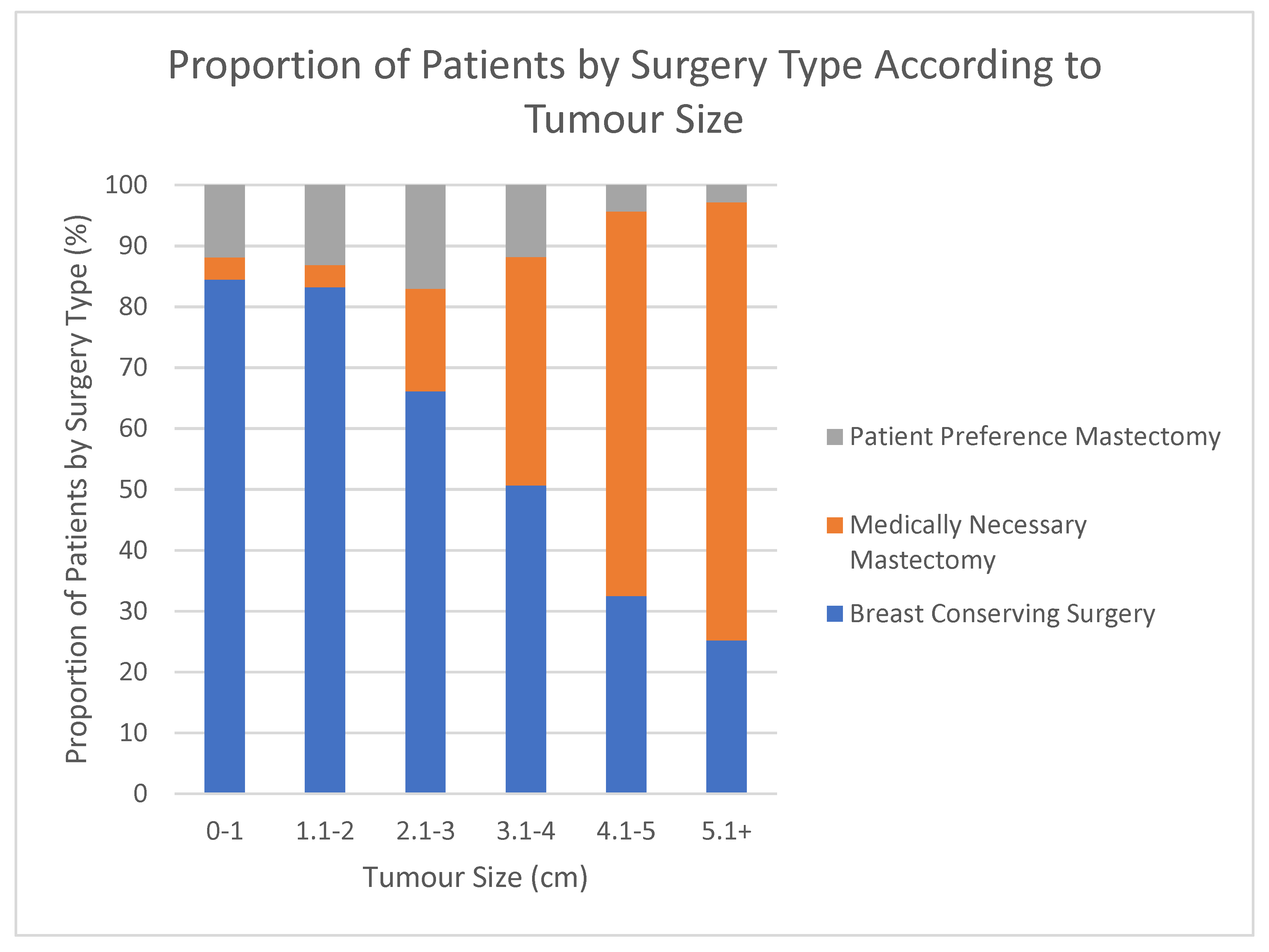
| Heading | BCS (N = 1651) | TMMN (N = 387) | TMPP (N = 273) | p-Value | |||
|---|---|---|---|---|---|---|---|
| TMMN vs. BCS | TMPP vs. BCS | TMMN vs. TMPP | |||||
| Patient Age (Continuous) | Mean | 60.2 | 58.1 | 61.5 | 0.007 | 0.107 | 0.001 |
| Median | 60 | 55 | 62 | <0.001 | 0.131 | <0.001 | |
| Range | 23–100 | 29–93 | 30–92 | n/a | n/a | n/a | |
| n | 1651 | 387 | 273 | n/a | n/a | n/a | |
| Patient Age (Categorical) | <40 | 47 (2.8%) | 29 (7.5%) | 8 (2.9%) | <0.001 | 0.001 | 0.042 |
| 40 to 75 | 1464 (88.7%) | 303 (78.3%) | 223 (81.7%) | ||||
| >75 | 140 (8.5%) | 55 (14.2%) | 42 (15.4%) | ||||
| n | 1651 | 387 | 273 | n/a | n/a | n/a | |
| CPM | Rate | n/a | 68 (17.6%) | 66 (24.2%) | n/a | n/a | 0.042 |
| n | 1651 | 387 | 273 | n/a | n/a | n/a | |
| Bilateral Cancer | Rate | 31 (1.9%) | 32 (8.3%) | 25 (9.2%) | <0.001 | <0.001 | 0.681 |
| n | 1651 | 387 | 272 | n/a | n/a | n/a | |
| Reconstruction | Rate | n/a | 211 (75.1%) | 106 (39.0%) | n/a | n/a | <0.001 |
| n | 1650 | 281 | 272 | n/a | n/a | n/a | |
| Presenting Problem | Mass | 588 (37.4%) | 249 (68.0%) | 131 (49.6%) | <0.001 | <0.001 | <0.001 |
| Imaging Abnormality | 958 (61.0%) | 101 (27.6%) | 121 (45.8%) | ||||
| Nipple Discharge | 10 (0.6%) | 10 (2.7%) | 7 (2.7%) | ||||
| Breast Pain | 6 (0.4%) | 1 (0.3%) | 1 (0.4%) | ||||
| n | 1571 | 366 | 264 | n/a | n/a | n/a | |
| Morphology | DCIS | 326 (20.2%) | 99 (26.4%) | 48 (17.7%) | 0.011 | 0.541 | 0.028 |
| IDC | 1204 (74.8%) | 252 (67.2%) | 207 (76.4%) | ||||
| Other (LCIS, Paget’s, ILC) | 80 (5.0%) | 24 (6.4%) | 16 (5.9%) | ||||
| n | 1610 | 375 | 271 | n/a | n/a | n/a | |
| Pre-Op Lymph Node Status | Positive | 62 (4.5%) | 63 (19.3%) | 17 (6.9%) | <0.001 | 0.164 | <0.001 |
| n | 1383 | 326 | 248 | n/a | n/a | n/a | |
| Post-Op Lymph Node Status | Positive | 354 (28.6%) | 149 (40.8%) | 63 (25.3%) | <0.001 | 0.552 | <0.001 |
| n | 1237 | 365 | 249 | n/a | n/a | n/a | |
| Pre-op Tumour Size (mm) | Mean | 17.5 | 42.2 | 20.7 | <0.001 | <0.001 | <0.001 |
| n | 1575 | 363 | 251 | n/a | n/a | n/a | |
| Post-op Tumour Size (mm) | Mean | 17.8 | 29.5 | 17.7 | <0.001 | 0.908 | <0.001 |
| n | 1527 | 358 | 243 | n/a | n/a | n/a | |
| ER | Positive | 1073 (90.8%) | 215 (85.0%) | 174 (85.3%) | 0.016 | 0.038 | 0.926 |
| n | 1182 | 253 | 204 | n/a | n/a | n/a | |
| PR | Positive | 971 (83.8%) | 196 (78.1%) | 146 (75.3%) | 0.045 | 0.010 | 0.486 |
| n | 1159 | 251 | 194 | n/a | n/a | n/a | |
| Her2 | Positive | 117 (10.6%) | 37 (15.3%) | 23 (12.0%) | 0.059 | 0.577 | 0.317 |
| n | 1107 | 242 | 192 | n/a | n/a | n/a | |
| LVI | Positive | 233 (21.3%) | 88 (38.6%) | 39 (20.1%) | <0.001 | 0.699 | <0.001 |
| n | 1093 | 228 | 194 | n/a | n/a | n/a | |
| Quality Indicator | Minimum Standard | Target | 5-Year Rate (2013–2017) | Remove Medically Necessary TM |
|---|---|---|---|---|
| NAPBC Standard 2.3: Breast-conserving surgery is offered to appropriate patients with breast cancer. A target rate of at least 50 percent of all eligible patients diagnosed with early-stage breast cancer (Stage 0, I, II) is treated with breast-conserving surgery | n/a | 50% | 71.44% | 81.25% |
| EUSOMA 9a: Proportion of patients (invasive cancers) who received a single (breast) operation for the primary tumour (excluding reconstruction) | 80% | 90% | 88.80% | 84.50% |
| EUSOMA 9b: Proportion of patients (DCIS only) who received just one operation (excluding reconstruction) | 70% | 90% | 80.30% | 69.64% |
| EUSOMA 9c: Proportion of patients receiving immediate reconstruction at the same time of mastectomy | 40% | none | 48.94% | 28.90% |
| EUSOMA 11c: Proportion of patients (BRCA1 and BRCA2 patients excluded) with invasive breast cancer not greater than 3 cm (total size, including DCIS component) who underwent BCT as primary treatment | 70% | 85% | 77.10% | 83.38% |
| EUSOMA 11d: Proportion of patients with non-invasive breast cancer not greater than 2 cm who underwent BCT | 80% | 90% | 84.90% | 90.14% |
| Predictor | Comparison vs. Reference | Tumour Type | Cut-Off | Odds Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Year of Operation | Each year later | DCIS | <2 cm | 1.040 | 0.858 | 1.262 | 0.691 |
| Each year later | Invasive | <2 cm | 1.066 | 0.964 | 1.178 | 0.213 | |
| Each year later | Invasive | <3 cm | 1.065 | 0.982 | 1.154 | 0.129 | |
| Age Category | 40 to <75 vs. <40 | DCIS | <2 cm | 3.689 | 0.888 | 14.385 | 0.058 |
| 75+ vs. <40 | DCIS | <2 cm | 2.933 | 0.476 | 20.358 | 0.250 | |
| 40 to <75 vs. <40 | Invasive | <2 cm | 3.145 | 1.571 | 6.221 | 0.001 | |
| 75+ vs. <40 | Invasive | <2 cm | 2.127 | 0.984 | 4.575 | 0.053 | |
| 40 to <75 vs. <40 | Invasive | <3 cm | 2.542 | 1.495 | 4.304 | 0.001 | |
| 75+ vs. <40 | Invasive | <3 cm | 1.806 | 0.996 | 3.272 | 0.050 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapur, H.; Chen, L.; Warburton, R.; Pao, J.-S.; Dingee, C.; Kuusk, U.; Bazzarelli, A.; McKevitt, E. De-Escalating Breast Cancer Surgery: Should We Apply Quality Indicators from Other Jurisdictions in Canada? Curr. Oncol. 2022, 29, 144-154. https://doi.org/10.3390/curroncol29010013
Kapur H, Chen L, Warburton R, Pao J-S, Dingee C, Kuusk U, Bazzarelli A, McKevitt E. De-Escalating Breast Cancer Surgery: Should We Apply Quality Indicators from Other Jurisdictions in Canada? Current Oncology. 2022; 29(1):144-154. https://doi.org/10.3390/curroncol29010013
Chicago/Turabian StyleKapur, Hannah, Leo Chen, Rebecca Warburton, Jin-Si Pao, Carol Dingee, Urve Kuusk, Amy Bazzarelli, and Elaine McKevitt. 2022. "De-Escalating Breast Cancer Surgery: Should We Apply Quality Indicators from Other Jurisdictions in Canada?" Current Oncology 29, no. 1: 144-154. https://doi.org/10.3390/curroncol29010013
APA StyleKapur, H., Chen, L., Warburton, R., Pao, J.-S., Dingee, C., Kuusk, U., Bazzarelli, A., & McKevitt, E. (2022). De-Escalating Breast Cancer Surgery: Should We Apply Quality Indicators from Other Jurisdictions in Canada? Current Oncology, 29(1), 144-154. https://doi.org/10.3390/curroncol29010013






