Experiences and Perceptions of Older Adults with Lower-Risk Hormone Receptor-Positive Breast Cancer about Adjuvant Radiotherapy and Endocrine Therapy: A Patient Survey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Outcomes
2.3. Survey Development
2.4. Survey Implementation
2.5. Data Analysis
3. Results
3.1. Patient Characteristics
3.2. RT: Patient Experiences
3.3. ET: Patient Experiences
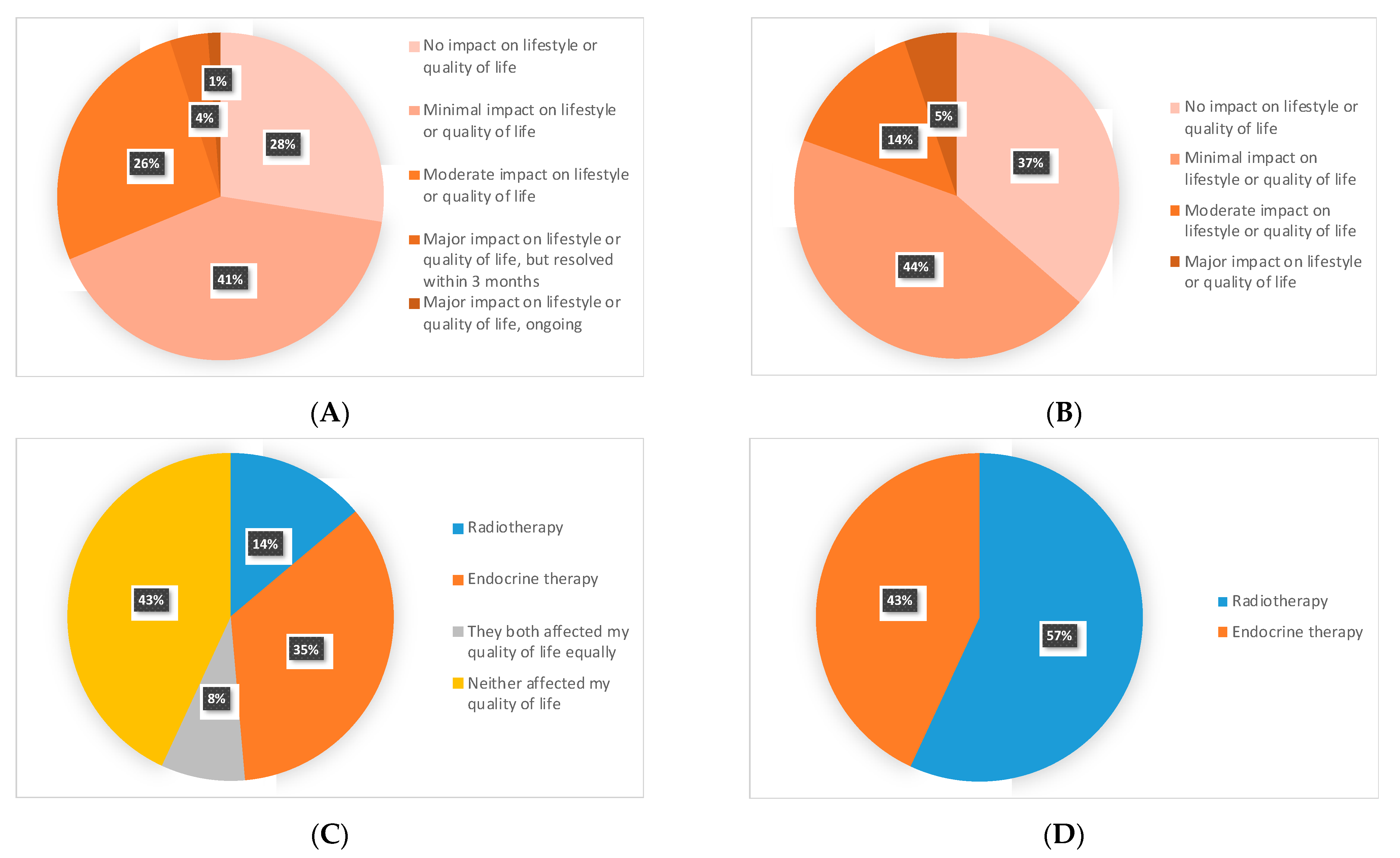
3.4. RT vs. ET: Impact on QoL and Patients’ Preferences
3.5. Patient’s Perceptions and Expectations of RT and ET Benefits
3.6. Clinical Scenario Evaluating Decision Making
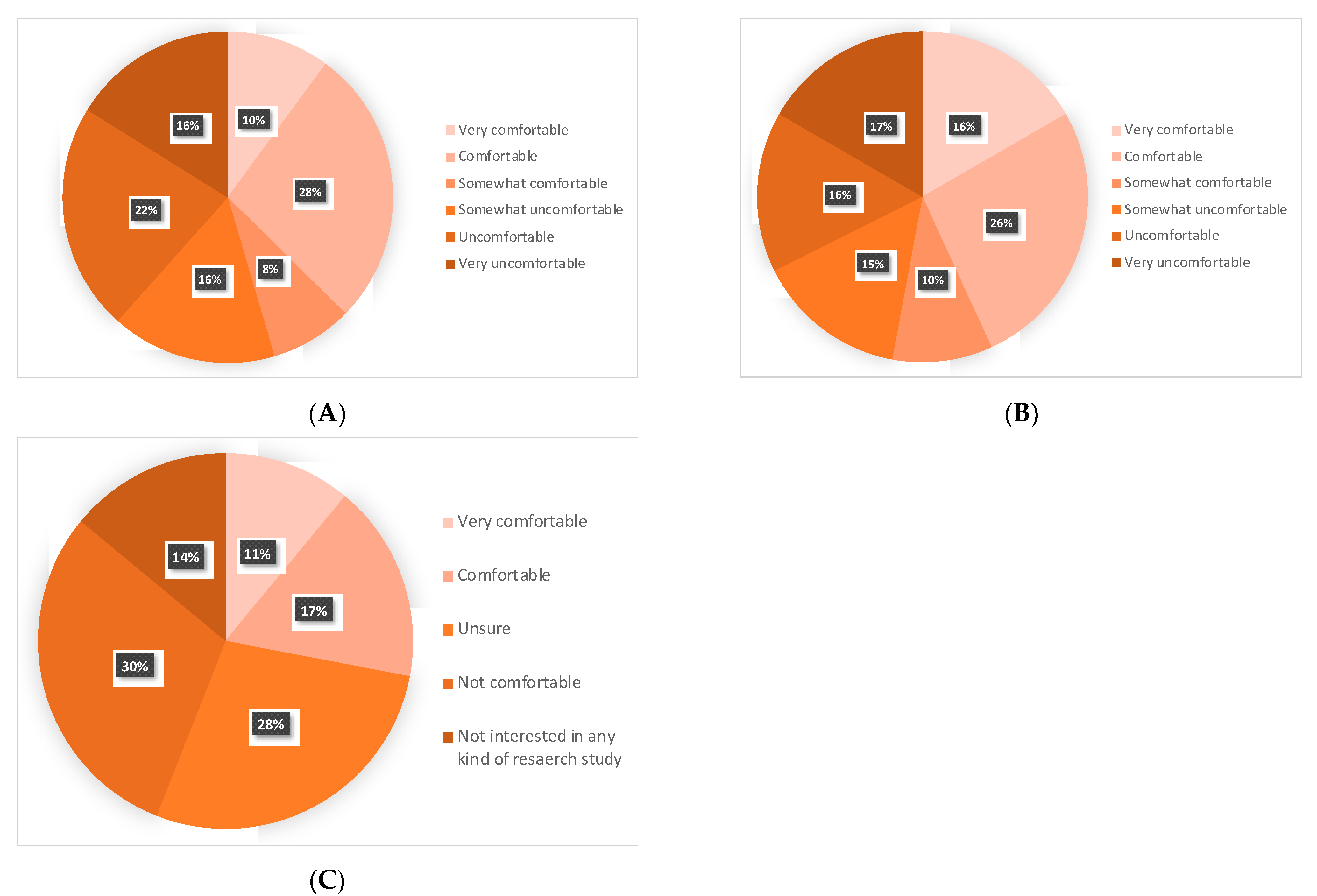
3.7. Patients’ Attitudes towards Omitting RT or ET, and a Future De-Escalation Clinical Trial
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- DeSantis, C.E.; Fedewa, S.A.; Sauer, A.G.; Kramer, J.L.; Smith, R.A.; Jemal, A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA A Cancer J. Clin. 2016, 66, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, M.; Sutradhar, R.; Paszat, L.; Whelan, T.J.; Gu, S.; Fong, C.; Rakovitch, E. Patterns of adjuvant care and outcomes of elderly women with stage I breast cancer after breast-conserving surgery: A population-based analysis. Breast Cancer Res. Treat. 2019, 176, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Lacchetti, C.; Anderson, H.; Buchholz, T.; Davidson, N.E.; Gelmon, K.A.; Giordano, S.H.; Hudis, C.A.; Solky, A.J.; Stearns, V.; et al. Adjuvant Endocrine Therapy for Women with Hormone Receptor–Positive Breast Cancer: ASCO Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2019, 37, 423–438. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology, Breast Cancer. Version 5. 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 30 June 2021).
- Smith, B.D.; Bellon, J.R.; Blitzblau, R.; Freedman, G.; Haffty, B.; Hahn, C.; Halberg, F.; Hoffman, K.; Horst, K.; Moran, J.; et al. Radiation therapy for the whole breast: Executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract. Radiat. Oncol. 2018, 8, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Biganzoli, L.; Wildiers, H.; Oakman, C.; Marotti, L.; Loibl, S.; Kunkler, I.; Reed, M.; Ciatto, S.; Voogd, A.; Brain, E.; et al. Management of elderly patients with breast cancer: Updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA). Lancet Oncol. 2012, 13, e148–e160. [Google Scholar] [CrossRef]
- Franco, P.; De Rose, F.; De Santis, M.C.; Pasinetti, N.; Lancellotta, V.; Meduri, B.; Meattini, I. Omission of postoperative radiation after breast conserving surgery: A progressive paradigm shift towards precision medicine. Clin. Transl. Radiat. Oncol. 2020, 21, 112–119. [Google Scholar] [CrossRef]
- Ethier, J.-L.; Anderson, G.M.; Austin, P.C.; Clemons, M.; Parulekar, W.; Shepherd, L.; Trasiewicz, L.S.; Tu, D.; Amir, E. Influence of the competing risk of death on estimates of disease recurrence in trials of adjuvant endocrine therapy for early-stage breast cancer: A secondary analysis of MA.27, MA.17 and MA.17R. Eur. J. Cancer 2021, 149, 117–127. [Google Scholar] [CrossRef]
- Templeton, A.J.; Booth, C.M.; Tannock, I.F. Informing Patients About Expected Outcomes: The Efficacy-Effectiveness Gap. J. Clin. Oncol. 2020, 38, 1651–1654. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Bryant, J.; Dignam, J.J.; Wickerham, D.L.; Mamounas, E.P.; Fisher, E.R.; Margolese, R.G.; Nesbitt, L.; Paik, S.; Pisansky, T.M.; et al. Tamoxifen, Radiation Therapy, or Both for Prevention of Ipsilateral Breast Tumor Recurrence After Lumpectomy in Women With Invasive Breast Cancers of One Centimeter or Less. J. Clin. Oncol. 2002, 20, 4141–4149. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.J.; Vicini, F.A.; Beitsch, P.; Goyal, S.; Kuerer, H.M.; Keisch, M.; Quiet, C.; Zannis, V.; Keleher, A.; Snyder, H.; et al. Local Control, Toxicity, and Cosmesis in Women >70 Years Enrolled in the American Society of Breast Surgeons Accelerated Partial Breast Irradiation Registry Trial. Int. J. Radiat. Oncol. 2012, 84, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Buszek, S.M.; Lin, H.Y.; Bedrosian, I.; Tamirisa, N.; Babiera, G.V.; Shen, Y.; Shaitelman, S.F. Lumpectomy Plus Hormone or Radiation Therapy Alone for Women Aged 70 Years or Older With Hormone Receptor–Positive Early Stage Breast Cancer in the Modern Era: An Analysis of the National Cancer Database. Int. J. Radiat. Oncol. 2019, 105, 795–802. [Google Scholar] [CrossRef]
- Joseph, K.; Zebak, S.; Alba, V.; Mah, K.; Au, C.; Vos, L.; Ghosh, S.; Abraham, A.; Chafe, S.; Wiebe, E.; et al. Adjuvant breast radiotherapy, endocrine therapy, or both after breast conserving surgery in older women with low-risk breast cancer: Results from a population-based study. Radiother. Oncol. 2021, 154, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Chesney, T.R.; Yin, J.X.; Rajaee, N.; Tricco, A.C.; Fyles, A.; Acuna, S.A.; Scheer, A.S. Tamoxifen with radiotherapy compared with Tamoxifen alone in elderly women with early-stage breast cancer treated with breast conserving surgery: A systematic review and meta-analysis. Radiother. Oncol. 2017, 123, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Matuschek, C.; Bölke, E.; Haussmann, J.; Mohrmann, S.; Nestle-Krämling, C.; Gerber, P.A.; Corradini, S.; Orth, K.; Kammers, K.; Budach, W. The benefit of adjuvant radiotherapy after breast conserving surgery in older patients with low risk breast cancer- a meta-analysis of randomized trials. Radiat. Oncol. 2017, 12, 60. [Google Scholar] [CrossRef] [Green Version]
- Savard, M.-F.; Clemons, M.; Hutton, B.; Alzahrani, M.J.; Caudrelier, J.-M.; Vandermeer, L.; Liu, M.; Saunders, D.; Sienkiewicz, M.; Stober, C.; et al. De-Escalating Adjuvant Therapies in Older Patients with Lower Risk Estrogen Receptor-Positive Breast Cancer Treated with Breast-Conserving Surgery: A Systematic Review and Meta-analysis. Cancer Treat. Rev. 2021, 99, 102254. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.C.; Vicini, F.; Chadha, M.; Pierce, L.; Recht, A.; Hayman, J.; Thaker, N.G.; Khan, A.; Keisch, M.; Shah, C. Radiation Therapy Without Hormone Therapy for Women Age 70 or Above with Low-Risk Early Breast Cancer: A Microsimulation. Int. J. Radiat. Oncol. 2019, 105, 296–306. [Google Scholar] [CrossRef]
- Rutter, C.E.; Lester-Coll, N.; Mancini, B.R.; Corso, C.D.; Park, H.; Yeboa, D.N.; Gross, C.P.; Evans, S. The evolving role of adjuvant radiotherapy for elderly women with early-stage breast cancer. Cancer 2015, 121, 2331–2340. [Google Scholar] [CrossRef]
- Nichol, A.M.; Chan, E.K.; Lucas, S.; Smith, S.L.; Gondara, L.; Speers, C.; Tyldesley, S. The Use of Hormone Therapy Alone Versus Hormone Therapy and Radiation Therapy for Breast Cancer in Elderly Women: A Population-Based Study. Int. J. Radiat. Oncol. 2017, 98, 829–839. [Google Scholar] [CrossRef]
- Chowdhary, M.; Chhabra, A.M.; Jhawar, S.R. Is It Time to Reevaluate Radiotherapy Omission in Older Patients With Favorable Early-Stage Breast Cancer? JAMA Oncol. 2021, 7, 965. [Google Scholar] [CrossRef] [PubMed]
- Hutton, B.; Morretto, P.; Emmenegger, U.; Mazzarello, S.; Kuchuk, I.; Addison, C.L.; Crawley, F.; Canil, C.; Malone, S.; Berry, S.; et al. Bone-targeted agent use for bone metastases from breast cancer and prostate cancer: A patient survey. J. Bone Oncol. 2013, 2, 105–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeVasseur, N.; Stober, C.; Daigle, K.; Robinson, A.; McDiarmid, S.; Mazzarello, S.; Hutton, B.; Joy, A.; Fergusson, D.; Hilton, J.; et al. Optimizing Vascular Access for Patients Receiving Intravenous Systemic Therapy for Early-Stage Breast Cancer—A Survey of Oncology Nurses and Physicians. Curr. Oncol. 2018, 25, 298–304. [Google Scholar] [CrossRef] [Green Version]
- Hughes, K.S.; Schnaper, L.A.; Bellon, J.R.; Cirrincione, C.T.; Berry, D.A.; Mc Cormick, B.; Muss, H.B.; Smith, B.L.; Hudis, C.A.; Winer, E.P.; et al. Lumpectomy Plus Tamoxifen With or Without Irradiation in Women Age 70 Years or Older With Early Breast Cancer: Long-Term Follow-Up of CALGB 9343. J. Clin. Oncol. 2013, 31, 2382–2387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunkler, I.H.; Williams, L.J.; Jack, W.J.L.; Cameron, D.A.; Dixon, J.M. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): A randomised controlled trial. Lancet Oncol. 2015, 16, 266–273. [Google Scholar] [CrossRef]
- Tinterri, C.; Gatzemeier, W.; Costa, A.; Gentilini, M.A.; Zanini, V.; Regolo, L.; Pedrazzoli, C.; Rondini, E.; Amanti, C.; Gentile, G.; et al. Breast-Conservative Surgery with and without Radiotherapy in Patients Aged 55–75 Years with Early-Stage Breast Cancer: A Prospective, Randomized, Multicenter Trial Analysis After 108 Months of Median Follow-up. Ann. Surg. Oncol. 2013, 21, 408–415. [Google Scholar] [CrossRef]
- Fyles, A.W.; McCready, D.R.; Manchul, L.A.; Trudeau, M.E.; Merante, P.; Pintilie, M.; Weir, L.M.; Olivotto, I.A. Tamoxifen with or without Breast Irradiation in Women 50 Years of Age or Older with Early Breast Cancer. N. Engl. J. Med. 2004, 351, 963–970. [Google Scholar] [CrossRef]
- Williams, L.; Kunkler, I.; King, C.; Jack, W.; Van Der Pol, M. A randomised controlled trial of post-operative radiotherapy following breast-conserving surgery in a minimum-risk population. Quality of life at 5 years in the PRIME trial. Health Technol. Assess. 2011, 15, i–xi, 1–57. [Google Scholar] [CrossRef]
- Rayan, G.; Dawson, L.; Bezjak, A.; Lau, A.; Fyles, A.W.; Yi, Q.-L.; Merante, P.; Vallis, K.A. Prospective comparison of breast pain in patients participating in a randomized trial of breast-conserving surgery and tamoxifen with or without radiotherapy. Int. J. Radiat. Oncol. 2003, 55, 154–161. [Google Scholar] [CrossRef]
- Martelli, G.; Boracchi, P.; Guzzetti, E.; Marano, G.; Lozza, L.; Agresti, R.; Ferraris, C.; Piromalli, D.; Greco, M. Omission of radiotherapy in elderly patients with early breast cancer: 15-Year results of a prospective nn-randomised trial. Eur. J. Cancer 2015, 51, 1358–1364. [Google Scholar] [CrossRef]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017, 17, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halli-Tierney, A.D.; Scarbrough, C.; Carroll, D. Polypharmacy: Evaluating Risks and Deprescribing. Am. Fam. Phys. 2019, 100, 32–38. [Google Scholar]
- Brunt, A.M.; Haviland, J.S.; Wheatley, D.A.; Sydenham, M.A.; Alhasso, A.; Bloomfield, D.J.; Chan, C.; Churn, M.; Cleator, S.; Coles, C.E.; et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet 2020, 395, 1613–1626. [Google Scholar] [CrossRef]
- Sedrak, M.S.; Freedman, R.A.; Cohen, H.J.; Muss, H.B.; Jatoi, A.; Klepin, H.D.; Wildes, T.M.; Le-Rademacher, J.G.; Kimmick, G.G.; Tew, W.P.; et al. Older adult participation in cancer clinical trials: A systematic review of barriers and interventions. CA A Cancer J. Clin. 2021, 71, 78–92. [Google Scholar] [CrossRef] [PubMed]
| N | N (%) | |
|---|---|---|
| Median Age (interquartile range) | 74 (71–76) | |
| Age Group | 102 | |
| 70–74 | 56 (55) | |
| 75–79 | 34 (33) | |
| 80+ | 12 (12) | |
| Health status patient’s perception | 100 | |
| Excellent | 20 (20) | |
| Good | 66 (66) | |
| Fair | 13 (13) | |
| Poor | 1 (1) | |
| Bad | 0 (0) | |
| Number of prescribed medications/days | 98 | |
| 0 | 10 (10) | |
| 1–3 | 52 (53) | |
| 4–5 | 23 (23) | |
| 6–9 | 10 (10) | |
| 10+ | 3 (3) | |
| Health problems (past or current) * | 102 | |
| Diabetes | 13 (13) | |
| Hypertension | 50 (49) | |
| Dyslipidemia | 39 (38) | |
| Heart disease | 7 (7) | |
| Stroke | 6 (6) | |
| Kidney disease | 4 (4) | |
| Liver disease | 2 (2) | |
| Lung disease | 15 (15) | |
| Stomach ulcers | 6 (6) | |
| Thromboembolic disease | 6 (6) | |
| Other cancers | 14 (14) | |
| Memory problems | 6 (6) | |
| Mobility problems | 18 (18) | |
| Others | 35 (34) | |
| Type of adjuvant therapy received | 102 | |
| Radiotherapy alone | 12 (12) | |
| Hormonal therapy alone | 9 (9) | |
| Both | 72 (71) | |
| Radiotherapy progress status | 100 | |
| Declined | 10 (10) | |
| Planned in the future | 7 (7) | |
| Ongoing | 1 (1) | |
| Completed <3 months ago | 7 (7) | |
| Completed 3–6 months ago | 11 (11) | |
| Completed 6–12 months ago | 27 (27) | |
| Completed >12 months | 37 (37) | |
| Endocrine therapy progress status | 101 | |
| Declined | 18 (18) | |
| Planned in future | 7 (7) | |
| Started <3 months ago | 8 (8) | |
| Started 3–6 months ago | 10 (10) | |
| Started 6–12 months ago | 17 (17) | |
| Started >12 months ago | 31 (31) | |
| Completed 5 years | 7 (7) | |
| Others (not recommended, stopped for side effects) | 3 (3) | |
| Types of Benefits and Concerns | Radiotherapy | Endocrine Therapy | ||
|---|---|---|---|---|
| N | N(%) | N | N (%) | |
| Benefits * | 99 | 99 | ||
| Reduce ipsilateral tumor recurrence | 90 (91) | 61 (62) | ||
| Reduce occurrence of a contralateral breast cancer | 25 (25) | 52 (53) | ||
| Reduce metastatic recurrence | 44 (44) | 49 (49) | ||
| Survival benefit | 55 (56) | 49 (49) | ||
| Improvement in quality of life | 35 (35) | 12 (12) | ||
| Cause side effects without benefit | 22 (22) | 29 (29) | ||
| Don’t know | 12 (12) | 18 (18) | ||
| Others | 1 (1) | 5 (5) | ||
| Concerns * | 95 | 99 | ||
| Possible side effects | 24 (25) | 50 (51) | ||
| Impact on quality of life | 14 (15) | 30 (30) | ||
| Impact on carrying daily activities | 11 (12) | 15 (15) | ||
| Lack of benefits | 17 (18) | 26 (26) | ||
| Treatment duration | 5 (5) | 11 (11) | ||
| Commuting for treatment | 8 (8) | |||
| No significant concerns | 58 (61) | 34 (34) | ||
| Others | 2 (2) | 1 (1) | ||
| Thresholds by Benefit Type | Radiotherapy | Endocrine Therapy | ||
|---|---|---|---|---|
| N | N(%) | N | N (%) | |
| Ipsilateral breast recurrence at 5 years | 98 | 100 | ||
| 1% | 0 (0) | 3 (3) | ||
| 5% | 5 (5) | 9 (9) | ||
| 10% | 6 (6) | 6 (6) | ||
| 15% | 4 (4) | 2 (2) | ||
| 20% | 6 (6) | 9 (9) | ||
| 30% | 3 (3) | 3 (3) | ||
| 50% | 8 (8) | 15 (15) | ||
| Any possible benefit | 65 (66) | 51 (51) | ||
| Not important to me | 1 (1) | 2 (2) | ||
| Metastatic recurrence at 5 years | 97 | |||
| 1% | 2 (2) | |||
| 5% | 10 (10) | |||
| 10% | 7 (7) | |||
| 15% | 2 (2) | |||
| 20% | 8 (8) | |||
| 30% | 2 (2) | |||
| 50% | 12 (12) | |||
| Any possible benefit | 52 (54) | |||
| Not important to me | 2 (2) | |||
| Survival at 5 years | 99 | |||
| 1% | 0 (0) | |||
| 2% | 2 (2) | |||
| 5% | 7 (7) | |||
| 10% | 5 (5) | |||
| 20% | 7 (7) | |||
| 30% | 1 (1) | |||
| 50% | 18 (18) | |||
| Any possible benefit | 56 (57) | |||
| Not important to me | 3 (3) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savard, M.-F.; Alzahrani, M.J.; Saunders, D.; Chang, L.; Arnaout, A.; Ng, T.L.; Brackstone, M.; Vandermeer, L.; Hsu, T.; Awan, A.A.; et al. Experiences and Perceptions of Older Adults with Lower-Risk Hormone Receptor-Positive Breast Cancer about Adjuvant Radiotherapy and Endocrine Therapy: A Patient Survey. Curr. Oncol. 2021, 28, 5215-5226. https://doi.org/10.3390/curroncol28060436
Savard M-F, Alzahrani MJ, Saunders D, Chang L, Arnaout A, Ng TL, Brackstone M, Vandermeer L, Hsu T, Awan AA, et al. Experiences and Perceptions of Older Adults with Lower-Risk Hormone Receptor-Positive Breast Cancer about Adjuvant Radiotherapy and Endocrine Therapy: A Patient Survey. Current Oncology. 2021; 28(6):5215-5226. https://doi.org/10.3390/curroncol28060436
Chicago/Turabian StyleSavard, Marie-France, Mashari Jemaan Alzahrani, Deanna Saunders, Lynn Chang, Angel Arnaout, Terry L. Ng, Muriel Brackstone, Lisa Vandermeer, Tina Hsu, Ari Ali Awan, and et al. 2021. "Experiences and Perceptions of Older Adults with Lower-Risk Hormone Receptor-Positive Breast Cancer about Adjuvant Radiotherapy and Endocrine Therapy: A Patient Survey" Current Oncology 28, no. 6: 5215-5226. https://doi.org/10.3390/curroncol28060436
APA StyleSavard, M.-F., Alzahrani, M. J., Saunders, D., Chang, L., Arnaout, A., Ng, T. L., Brackstone, M., Vandermeer, L., Hsu, T., Awan, A. A., Cole, K., Larocque, G., & Clemons, M. (2021). Experiences and Perceptions of Older Adults with Lower-Risk Hormone Receptor-Positive Breast Cancer about Adjuvant Radiotherapy and Endocrine Therapy: A Patient Survey. Current Oncology, 28(6), 5215-5226. https://doi.org/10.3390/curroncol28060436






