ERCC1 19007 Polymorphism in Greek Patients with Advanced Urothelial Cancer Treated with Platinum-Based Chemotherapy: Effect of the Changing Treatment Paradigm: A Cohort Study by the Hellenic GU Cancer Group
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. PCR Amplification
2.3. RFLP
2.4. Statistics
3. Results
3.1. Patients
3.2. ERCC1 19007 C>T Polymorphism
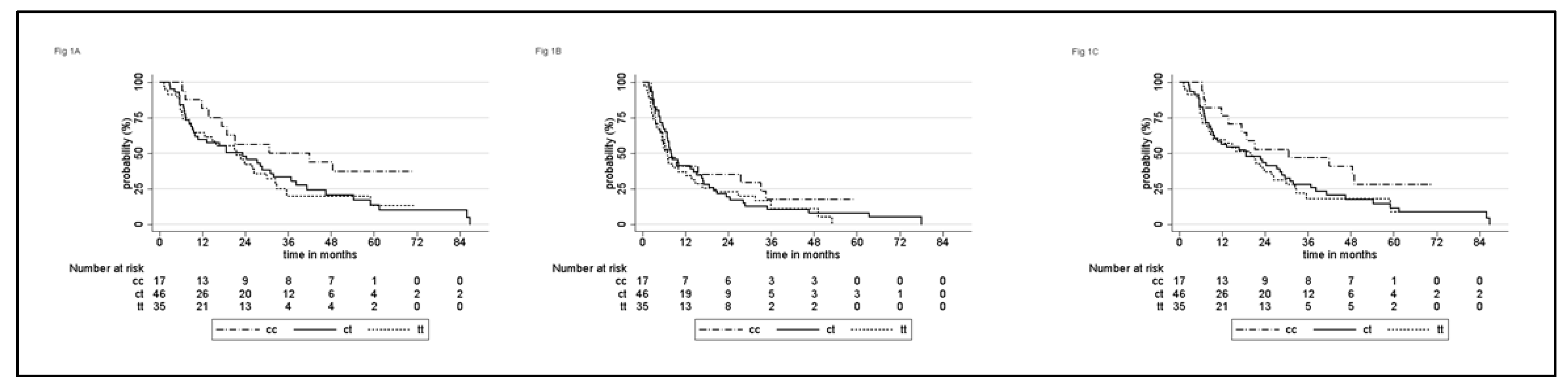
3.3. Correlation of SNPs with CSS, OS, PFS, and Tumor Response
3.3.1. Whole Population
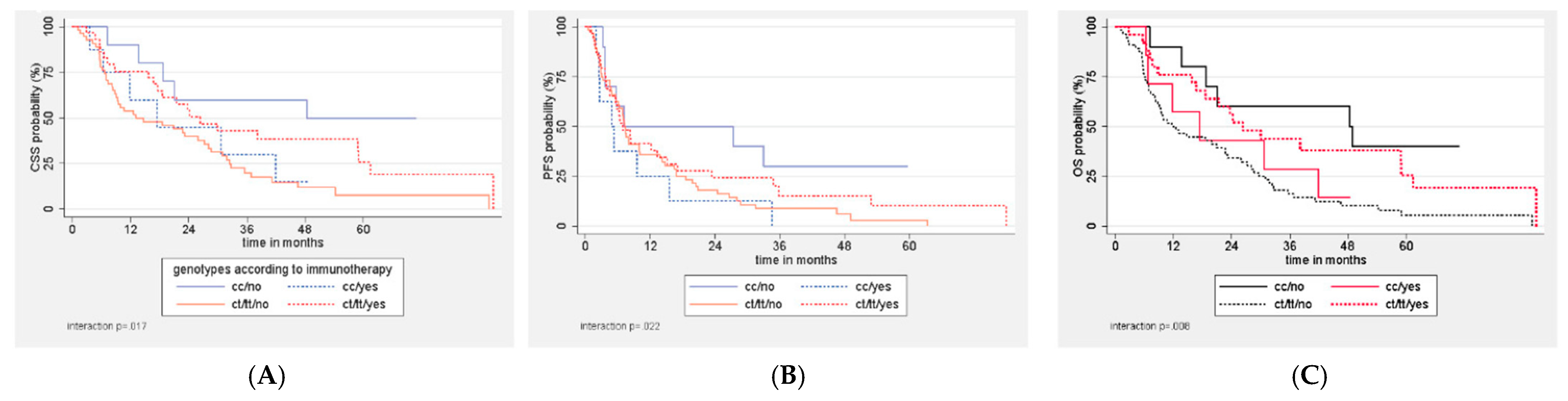
3.3.2. Subgroup Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bladder Cancer Statistics. Available online: www.wcrf.org (accessed on 21 June 2021).
- Sternberg, C.N.; Yagoda, A.; Scher, H.I.; Watson, R.C.; Herr, H.W.; Morse, M.J.; Sogani, P.C.; Vaughan, E.D., Jr.; Bander, N.; Weiselberg, L.R.; et al. M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for advanced transitional cell carcinoma of the urothelium. J. Urol. 1988, 139, 461–469. [Google Scholar] [CrossRef]
- Von der Maase, H.; Hansen, S.W.; Roberts, J.T.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Bodrogi, I.; Albers, P.; Knuth, A.; Lippert, C.M.; et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J. Clin. Oncol. 2000, 18, 3068–3077. [Google Scholar] [CrossRef]
- Bamias, A.; Tzannis, K.; Harshman, L.C.; Crabb, S.J.; Wong, Y.N.; Kumar Pal, S.; De Giorgi, U.; Ladoire, S.; Agarwal, N.; Yu, E.Y.; et al. RISC Investigators. Impact of contemporary patterns of chemotherapy utilization on survival in patients with advanced cancer of the urinary tract: A Retrospective International Study of Invasive/Advanced Cancer of the Urothelium (RISC). Ann. Oncol. 2019, 30, 1841. [Google Scholar] [CrossRef] [Green Version]
- Sternberg, C.N.; De Mulder, P.H.; Schornagel, J.H.; Theodore, C.; Fossa, S.D.; Van Oosterom, A.T.; Witjes, F.; Spina, M.; Van Groeningen, C.J.; De Balincourt, C.; et al. European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J. Clin. Oncol. 2001, 19, 2638–2646. [Google Scholar]
- Bamias, A.; Dafni, U.; Karadimou, A.; Timotheadou, E.; Aravantinos, G.; Psyrri, A.; Xanthakis, I.; Tsiatas, M.; Koutoulidis, V.; Constantinidis, C.; et al. Prospective, open-label, randomized, phase III study of two dose-dense regimens MVAC versus gemcitabine/cisplatin in patients with inoperable, metastatic or relapsed urothelial cancer: A Hellenic Cooperative Oncology Group study (HE 16/03). Ann. Oncol. 2013, 24, 1011–1017. [Google Scholar] [CrossRef]
- Bajorin, D.F.; Dodd, P.M.; Mazumdar, M.; Fazzari, M.; McCaffrey, J.A.; Scher, H.I.; Herr, H.; Higgins, G.; Boyle, M.G. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J. Clin. Oncol. 1999, 17, 3173–3181. [Google Scholar] [CrossRef]
- Bamias, A.; Tzannis, K.; Bamia, C.; Harshman, L.C.; Crabb, S.; Plimack, E.R.; Pal, S.; De Giorgi, U.; Ladoire, S.; Theodore, C.; et al. The Impact of Cisplatin- or Non-Cisplatin-Containing Chemotherapy on Long-Term and Conditional Survival of Patients with Advanced Urinary Tract Cancer. Oncologist 2019, 24, 1348–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellmunt, J.; De Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. KEYNOTE-045 Investigators. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. IMvigor Study Group. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Retz, M.; Siefker-Radtke, A.; Baron, A.; Necchi, A.; Bedke, J.; Plimack, E.R.; Vaena, D.; Grimm, M.O.; Bracarda, S.; et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017, 18, 312–322. [Google Scholar] [CrossRef]
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Duran, I.; Lee, J.L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. EnfortumabVedotin in Previously Treated Advanced Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 1125–1135. [Google Scholar] [CrossRef]
- Loriot, Y.; Necchi, A.; Park, S.H.; Garcia-Donas, J.; Huddart, R.; Burgess, E.; Fleming, M.; Rezazadeh, A.; Mellado, B.; Varlamov, S.; et al. 348 BLC2001 Study Group. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2019, 381, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Reardon, J.T.; Sancar, A. Nucleotide excision repair. Prog. Nucleic Acid. Res. Mol. Biol. 2005, 79, 183–235. [Google Scholar]
- Hanawalt, P.C. Subpathways of nucleotide excision repair and their regulation. Oncogene 2002, 21, 8949–8956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabik, C.A.; Dolan, M.E. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat. Rev. 2007, 33, 9–23. [Google Scholar] [CrossRef] [Green Version]
- Altaha, R.; Liang, X.; Yu, J.J.; Reed, E. Excision repair cross complementing-group 1: Gene expression and platinum resistance. Int. J. Mol. Med. 2004, 14, 959–970. [Google Scholar] [PubMed]
- Arriagada, R.; Bergman, B.; Dunant, A.; Le Chevalier, T.; Pignon, J.P.; Vansteenkiste, J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N. Engl. J. Med. 2004, 350, 351–360. [Google Scholar]
- Olaussen, K.A.; Dunant, A.; Fouret, P.; Brambilla, E.; Andre, F.; Haddad, V.; Taranchon, E.; Filipits, M.; Pirker, R.; Popper, H.H.; et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N. Engl. J. Med. 2006, 355, 983–991. [Google Scholar] [CrossRef]
- Kim, K.H.; Do, I.G.; Kim, H.S.; Chang, M.H.; Kim, H.S.; Jun, H.J.; Uhm, J.; Yi, S.Y.; Lim, D.H.; Ji, S.H.; et al. Excision repair cross-complementation group 1 (ERCC1) expression in advanced urothelial carcinoma patients receiving cisplatin-based chemotherapy. APMIS 2010, 118, 941–948. [Google Scholar] [CrossRef]
- Hoffmann, A.C.; Wild, P.; Leicht, C.; Bertz, S.; Danenberg, K.D.; Danenberg, P.V.; Stohr, R.; Stockle, M.; Lehmann, J.; Schuler, M.; et al. MDR1 and ERCC1 expression predict outcome of patients with locally advanced bladder cancer receiving adjuvant chemotherapy. Neoplasia 2010, 12, 628–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellmunt, J.; Paz-Ares, L.; Cuello, M.; Cecere, F.L.; Albiol, S.; Guillem, V.; Gallardo, E.; Carles, J.; Mendez, P.; De la Cruz, J.J.; et al. Gene expression of ERCC1 as a novel prognostic marker in advanced bladder cancer patients receiving cisplatin-based chemotherapy. Ann. Oncol. 2007, 18, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Nikitas, N.; Karadimou, A.; Tsitoura, E.; Soupos, N.; Tsiatas, M.; Karavasilis, V.; Pectasides, D.; Pavlidis, N.; Chrisofos, M.; Adamakis, I.; et al. Association of ERCC1 SNPs with outcome in platinum-treated patients with advanced urothelial cancer: A Hellenic Cooperative Oncology Group study. Pharmacogenomics 2012, 13, 1595–1607. [Google Scholar] [CrossRef]
- Stoehlmacher, J.; Park, D.J.; Zhang, W.; Yang, D.; Groshen, S.; Zahedy, S.; Lenz, H.J. A multivariate analysis of genomic polymorphisms: Prediction of clinical outcome to 5-FU/oxaliplatin combination chemotherapy in refractory colorectal cancer. Br. J. Cancer 2004, 91, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Matuszczak, M.; Salagierski, M. Diagnostic and Prognostic Potential of Biomarkers CYFRA 21.1, ERCC1, p53, FGFR3 and TATI in Bladder Cancers. Int. J. Mol. Sci. 2020, 21, 3360. [Google Scholar] [CrossRef]
- Horwich, A.; Babjuk, M.; Bellmunt, J.; Bruins, H.M.; De Reijke, T.M.; De Santis, M.; Gillessen, S.; James, N.; Maclennan, S.; Palou, J.; et al. EAU-ESMO consensus statements on the management of advanced and variant bladder cancer-an international collaborative multi-stakeholder effort: Under the auspices of the EAU and ESMO Guidelines Committees. Ann. Oncol. 2019, 30, 1697–1727. [Google Scholar] [CrossRef] [Green Version]
- Bamias, A.; Merseburger, A.S.; Loriot, Y.; James, N.; Choy, E.; Castellano, D.; Lopez-Rios, F.; Calabro, F.; Kramer, M.; De Velasco, G.; et al. SAUL, a single-arm study of atezolizumab for chemotherapy-pretreated locally advanced or metastatic carcinoma of the urinary tract: Outcomes by key baseline factors, PD-L1 expression and prior platinum therapy. ESMO Open 2021, 6, 100152. [Google Scholar] [CrossRef]
- Aiello, M.M.; Solinas, C.; Santoni, M.; Battelli, N.; Restuccia, N.; Latteri, F.; Paratore, S.; Verderame, F.; Albanese, G.V.; Bruzzi, P.; et al. Excision Repair Cross Complementation Group 1 Single Nucleotide Polymorphisms and Nivolumab in Advanced Non-Small Cell Lung Cancer. Front. Oncol. 2020, 10, 1167. [Google Scholar] [CrossRef]
- Nikanjam, M.; Arguello, D.; Gatalica, Z.; Swensen, J.; Barkauskas, D.A.; Kurzrock, R. Relationship between protein biomarkers of chemotherapy response and microsatellite status, tumor mutational burden and PD-L1 expression in cancer patients. Int. J. Cancer 2020, 146, 3087–3097. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.C.; Cai, H.Z.; Li, X.; Xu, W.Z.; Xu, T.; Yu, B.; Zou, Q.; Xu, L. ERCC1 C118T polymorphism has predictive value for platinum-based chemotherapy in patients with late-stage bladder cancer. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Galsky, M.D.; Saci, A.; Szabo, P.M.; Han, G.C.; Grossfeld, G.; Collette, S.; Siefker-Radtke, A.; Necchi, A.; Sharma, P. Nivolumab in Patients with Advanced Platinum-resistant Urothelial Carcinoma: Efficacy, Safety, and Biomarker Analyses with Extended Follow-up from CheckMate 275. Clin. Cancer Res. 2020, 26, 5120–5128. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulovic, S.; Demey, W.; Ullen, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Total | ERCC1 19007 Genotypes | |||
|---|---|---|---|---|---|
| C/C | C/T | T/T | p | ||
| Median (25th–75th percentile) | |||||
| Age | 68.8 (63–73.7) | 68.7 (59.1–75.4) | 69.1 (64.6–73.7) | 68.7 (58.8–73.4) | 0.94 └ |
| BMI | 25.9 (23.1–29) | 24.3 (23.4–29.4) | 25.9 (23.5–29.1) | 27 (22.8–28) | 0.85 └ |
| BSA | 1.9 (1.8–2) | 1.9 (1.8–2) | 1.9 (1.8–2) | 1.9 (1.8–2) | 0.78 ┘ |
| n (%) | |||||
| Gender | 0.33 ⅟ | ||||
| Female | 13 (13.3) | 2 (11.8) | 4 (8.7) | 7 (20) | |
| Male | 85 (86.7) | 15 (88.2) | 42 (91.3) | 28 (80) | |
| Primary site | 0.26 ⅟ | ||||
| Bladder | 82 (83.7) | 12 (70.6) | 40 (87) | 30 (85.7) | |
| Pelvis | 10 (10.1) | 3 (17.6) | 4 (8.7) | 3 (8.6) | |
| Ureter | 3 (3.1) | 0 (0) | 1 (2.2) | 2 (5.7) | |
| Urethra | 3 (3.1) | 2 (11.8) | 1 (2.2) | 0 (0) | |
| ECOG PS | 0.103 ⅟ | ||||
| 0 | 56 (57.1) | 9 (52.9) | 24 (52.2) | 23 (65.7) | |
| 1 | 33 (33.7) | 8 (47.1) | 19 (41.3) | 6 (17.1) | |
| 2 | 6 (6.2) | 0 (0) | 3 (6.5) | 3 (8.6) | |
| 3 | 2 (2) | 0 (0) | 0 (0) | 2 (5.7) | |
| missing | 1 (1) | 0 (0) | 0 (0) | 1 (2.9) | |
| Disease status | 0.043 | ||||
| Distant metastases | 46 (46.9) | 8 (47.1) | 16 (34.8) | 22 (62.9) | |
| Locally advanced | 52 (53.1) | 9 (52.9) | 30 (65.2) | 13 (37.1) | |
| MSKCC risk * | 0.079 ⅟ | ||||
| Low | 48 (49) | 9 (52.9) | 28 (60.9) | 11 (31.4) | |
| Intermediate | 45 (45.9) | 8 (47.1) | 17 (37) | 20 (57.1) | |
| High | 4 (4.1) | 0 (0) | 1 (2.2) | 3 (8.6) | |
| missing | 1 (1) | 0 (0) | 0 (0) | 1 (2.9) | |
| Treatment | |||||
| Carbo based | 41 (41.8) | 9 (52.9) | 17 (37) | 15 (42.9) | 0.52 |
| Cis based | 57 (58.2) | 8 (47.1) | 29 (63) | 20 (57.1) | |
| Adjuvant | 14 (14.3) | 4 (23.5) | 5 (10.9) | 5 (14.3) | 0.39⅟ |
| Neoadjuvant | 11 (11.2) | 1 (5.9) | 7 (15.2) | 3 (8.3) | |
| Lines of treatment for aUC | 0.78 | ||||
| 1 | 39 (39.8) | 6 (35.3) | 20 (43.5) | 13 (37.1) | |
| ≥2 | 59 (60.2) | 11 (64.7) | 26 (56.5) | 22 (62.9) | |
| Immunotherapy | 0.6 | ||||
| Yes | 32 (32.7) | 7 (41.2) | 13 (28.3) | 12 (34.3) | |
| No | 66 (67.3) | 10 (58.8) | 33 (71.7) | 23 (65.7) | |
| Vinflunine | 0.19 | ||||
| Yes | 19 (19.4) | 6 (35.3) | 7 (15.2) | 6 (17.1) | |
| No | 79 (80.6) | 11 (64.7) | 39 (84.8) | 29 (82.9) | |
| Characteristic | Total | p | ERCC1 19007 Genotypes | p1 | ||
|---|---|---|---|---|---|---|
| C/C | C/T | T/T | ||||
| Median CSS (95% CI) | ||||||
| Total | 22.7 (15.8–30) | 41.9 (13.7–NR) | 23.2 (9.4–31.8) | 21.4 (8.8–30) | 0.19 | |
| Age at chemo start | 0.085 | 0.046 | ||||
| ≤68 | 30.7 (21–46.5) | 30.7 (6.4–NR) | 36.9 (9.9–61.4) | 22.7 (6.5–NR) | ||
| >68 | 17.5 (9.5–24.2) | 18.8 (7.1–NR) | 13.1 (7.2–24.2) | 21.4 (5.7–30) | ||
| BMI | 0.12 | 0.052 | ||||
| ≤26 | 17.5 (9.1–22.7) | 30.7 (13.7–NR) | 10.7 (7.2–24.2) | 14.7 (5.6–22.7) | ||
| >26 | 30 (18.6–38.2) | NR | 31 (18.6–54.3) | 26.3 (9.5–35.6) | ||
| BSA | 0.29 | 0.33 | ||||
| ≤1.9 | 21 (9.5–28.7) | 30.7 (6.4–48.4) | 9.9 (6.7–28.7) | 22.7 (8.3–35.6) | ||
| >1.9 | 28.1 (13.7–36.9) | NR | 31 (13.1–54.3) | 15.8 (5.8–32.7) | ||
| Gender | 0.32 | 0.27 | ||||
| Female | 30 (6.5–NR) | 11.8 (11.8–NR) | 6.7 (5.5–NR) | 30 (4.9–NR) | ||
| Male | 21.4 (14.7–28.7) | 41.9 (13.7–NR) | 23.2 (9.4–31) | 21 (8.3–26.3) | ||
| Primary site | −0.58 | 0.64 | ||||
| Bladder | 21 (13.1–28.1) | 18.8 (7.1–NR) | 24.2 (9.4–36.9) | 21 (8.3–30) | ||
| Pelvis | 30.7 (6.7–41.9) | 41.9 (30.7–NR) | 7.2 (6.7–NR) | 21.4 (21.4–NR) | ||
| Ureter | 25.9 (5.8–NR) | - | NR | 5.8 (5.8–NR) | ||
| Urethra | NR | NR | NR | - | ||
| ECOG PS | <0.001 | 0.002 | ||||
| 0 | 28.1 (21.4–38.2) | 41.9 (6.4–NR) | 38.2 (16.7–54.3) | 22.7 (8.8–32.3) | ||
| 1 | 18.6 (9.4–24.2) | 21 (7.1–NR) | 10.7 (7–28.7) | 9.5 (1.2–NR) | ||
| 2 | 5.6 (4.2–NR) | - | 5.6 (4.2–NR) | 30 (4.9–NR) | ||
| 3 | 1.6 (1.6–NR) | - | - | 1.6 (1.6–NR) | ||
| Disease status | 0.23 | 0.41 | ||||
| Distant | 21 (9.4–25.9) | 18.8 (7.1–NR) | 23.2 (7–59) | 21 (6.3–25.9) | ||
| Locally advanced/other | 28.7 (13.7–36.9) | 48.4 (6.4–NR) | 18.6 (8.8–31) | 32.4 (9.5–NR) | ||
| MSKCC risk * | 0.017 | 0.076 | ||||
| Low | 28.7 (16.7–36.9) | 48.4 (6.4–NR) | 27.2 (9.1–31.8) | 32.4 (9.5–NR) | ||
| Intermediate | 21 (8.8–25.9) | 18.8 (7.1–NR) | 23.2 (7–41.1) | 21 (6.3–26.3) | ||
| High | 5.6 (1.6–NR) | - | NR | 5.7 (1.6–NR) | ||
| Treatment | ||||||
| Carbo based | 21 (11.8–31.8) | 0.21 | 17.5 (7.1–NR) | 8.8 (5.6–36.9) | 23.7 (9.5–32.4) | 0.19 |
| Cis based | 25.9 (12.6–35.6) | 48.4 (6.4–NR) | 27.2 (10.7–38.2) | 12.6 (5.6–35.6) | ||
| Adjuvant | 32.7 (8.3–NR) | 0.65 | 48.4 (30.7–NR) | 27.2 (8.3–NR) | 32.7 (5.6–NR) | 0.86 |
| Neoadjuvant | 35.6 (18.6–61.4) | NR | 31 (18.6–38.2) | 35.6 (12.6–NR) | ||
| Lines of therapy for advanced disease | 0.11 | 0.085 | ||||
| 1 | 9.5 (6.9–28.1) | NR | 9.9 (5.6–28.7) | 8.3 (2.3–25.9) | ||
| ≥2 lines | 27.2 (18.8–35.6) | 30.7 (13.7–NR) | 27.2 (10.7–41.1) | 23.7 (15.8–32.7) | ||
| Immunotherapy | 0.12 | 0.08 | ||||
| Yes | 30 (17.5–58.9) | 30.7 (6.4–NR) | 38.2 (8.8–NR) | 26.3 (6.5–58.9) | ||
| No | 18.8 (9.5–28.1) | 48.4 (7.1–NR) | 13.1 (7.3–28.7) | 14.7 (6.3–32.4) | ||
| Vinflunine | 0.3 | 0.37 | ||||
| Yes | 31.8 (21–41.1) | 21 (11.8–NR) | 31.8 (18.6–NR) | 32.4 (12.6–NR) | ||
| No | 18.6 (9.5–26.3) | NR | 13.1 (8.3–31) | 15.8 (6.5–26.3) | ||
| No Immunotherapy (n = 66) | |||||
|---|---|---|---|---|---|
| Total | ERCC1 19007 Genotypes | ||||
| C/C | C/T | T/T | p | ||
| Median CSS | 18.8 (9.5–28.1) | 48.4 (7.1–NR) | 13.1 (7.3–28.7) | 14.7 (6.3–32.4) | 0.035 |
| Median PFS | 7.2 (5.7–10) | 7.1 (3.2–NR) | 7.5 (5.5–14.2) | 6.9 (2.6–13.8) | 0.12 |
| Median OS | 14.7 (9.4–23.2) | 48.4 (7.1–NR) | 11.7 (7.3–27.2) | 12.6 (5.9–22.7) | 0.028 |
| Immunotherapy (n = 32) | |||||
| Total | ERCC1 19007 genotypes | ||||
| C/C | C/T | T/T | p | ||
| Median CSS | 30 (17.5–58.9) | 30.7 (6.4–NR) | 38.2 (8.8–NR) | 26.3 (6.5–NR) | 0.67 |
| Median PFS | 8.3 (5.3–15.5) | 5.3 (2.5–15.5) | 13.3 (3.8–23.4) | 6.3 (3.9–35.8) | 0.32 |
| Median OS | 24.2 (15.8–41.9) | 17.5 (6.4–41.9) | 38.2 (16.6–NR) | 23.7 (6.5–58.9) | 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bamias, A.; Koutsoukos, K.; Gavalas, N.; Zakopoulou, R.; Tzannis, K.; Dedes, N.; Boulouta, A.; Fragkoulis, C.; Kostouros, E.; Dellis, A.; et al. ERCC1 19007 Polymorphism in Greek Patients with Advanced Urothelial Cancer Treated with Platinum-Based Chemotherapy: Effect of the Changing Treatment Paradigm: A Cohort Study by the Hellenic GU Cancer Group. Curr. Oncol. 2021, 28, 4474-4484. https://doi.org/10.3390/curroncol28060380
Bamias A, Koutsoukos K, Gavalas N, Zakopoulou R, Tzannis K, Dedes N, Boulouta A, Fragkoulis C, Kostouros E, Dellis A, et al. ERCC1 19007 Polymorphism in Greek Patients with Advanced Urothelial Cancer Treated with Platinum-Based Chemotherapy: Effect of the Changing Treatment Paradigm: A Cohort Study by the Hellenic GU Cancer Group. Current Oncology. 2021; 28(6):4474-4484. https://doi.org/10.3390/curroncol28060380
Chicago/Turabian StyleBamias, Aristotelis, Konstantinos Koutsoukos, Nikos Gavalas, Roubini Zakopoulou, Kimon Tzannis, Nikos Dedes, Anna Boulouta, Charalampos Fragkoulis, Eythymios Kostouros, Athanasios Dellis, and et al. 2021. "ERCC1 19007 Polymorphism in Greek Patients with Advanced Urothelial Cancer Treated with Platinum-Based Chemotherapy: Effect of the Changing Treatment Paradigm: A Cohort Study by the Hellenic GU Cancer Group" Current Oncology 28, no. 6: 4474-4484. https://doi.org/10.3390/curroncol28060380
APA StyleBamias, A., Koutsoukos, K., Gavalas, N., Zakopoulou, R., Tzannis, K., Dedes, N., Boulouta, A., Fragkoulis, C., Kostouros, E., Dellis, A., Mitsogiannis, I., Adamakis, I., Anastasiou, I., Skolarikos, A., Papatsoris, A., Stravodimos, K., Ferakis, N., Pagoni, S., Ntoumas, K., ... Dimopoulos, M. A. (2021). ERCC1 19007 Polymorphism in Greek Patients with Advanced Urothelial Cancer Treated with Platinum-Based Chemotherapy: Effect of the Changing Treatment Paradigm: A Cohort Study by the Hellenic GU Cancer Group. Current Oncology, 28(6), 4474-4484. https://doi.org/10.3390/curroncol28060380







