Immunotherapy in Extensive-Stage Small Cell Lung Cancer
Abstract
:1. Introduction
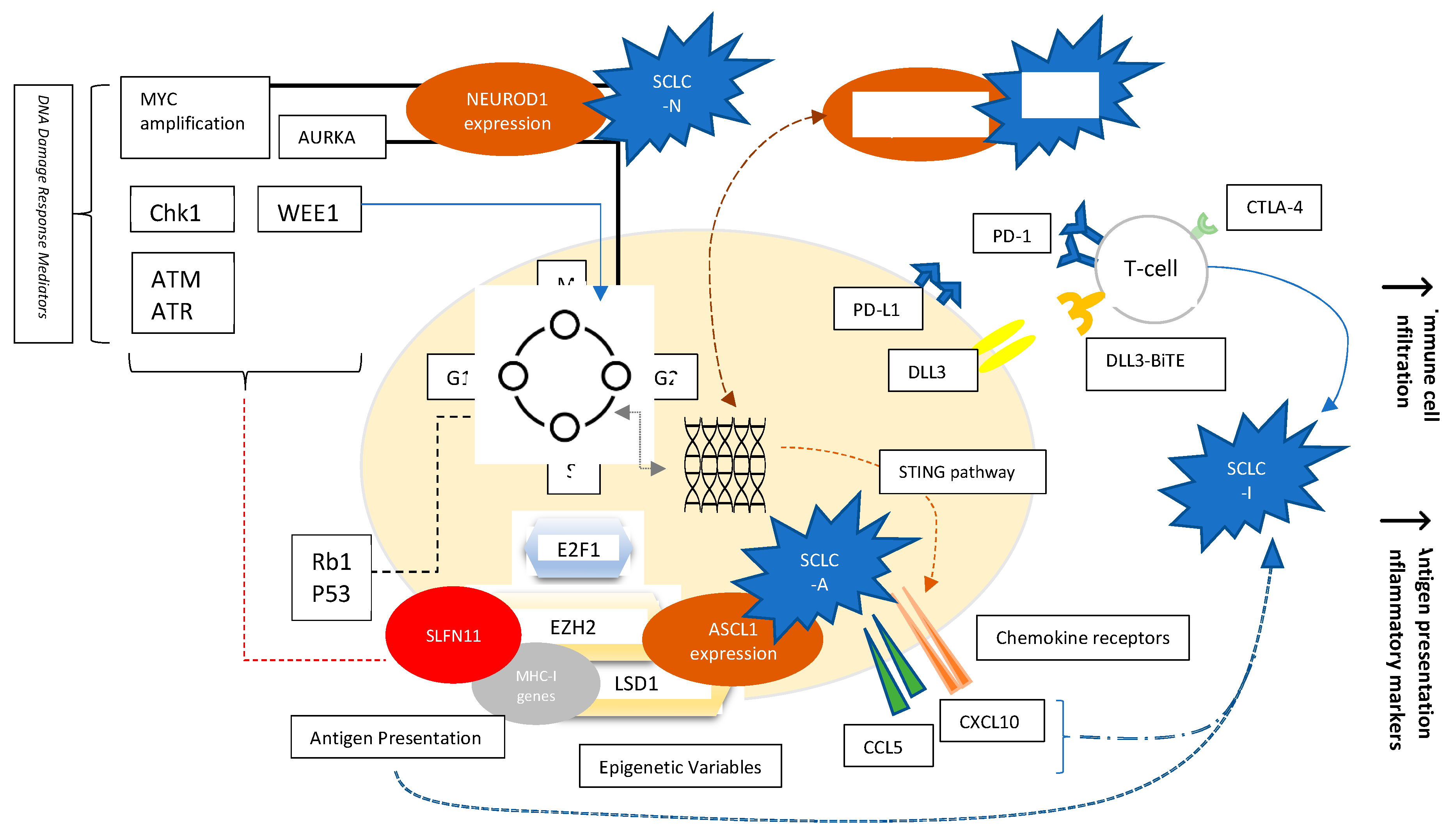
2. Subtypes of SCLC
3. Role of Immunotherapy in SCLC
4. Clinical Studies of Immunotherapy in ES-SCLC
4.1. First Line Studies
4.1.1. IMpower133
4.1.2. CASPIAN Trial
4.1.3. KEYNOTE-604
4.1.4. Arriola et al. (BMS/Cancer Research UK) and Reck et al. (BMS, Phase II)
4.1.5. Reck et al. (BMS, Phase III)
4.2. Second-Line and beyond Trials
4.2.1. KEYNOTE-028
4.2.2. KEYNOTE-158
4.2.3. CheckMate 032
4.2.4. CheckMate-331
4.3. Maintenance Trials
CheckMate-451
5. Biomarkers of Immunotherapy in SCLC
5.1. PD-L1 and PD-L1 Combined Score
5.2. Tumor Mutational Burden (TMB) and Micro-Satellite Instability (MSI)
5.3. SCLC Immunogenicity
5.4. SLFN11
6. Alternative Immune Activating Mechanisms
6.1. Cytokines/Interleukins/Interferons
6.2. Vaccines
6.2.1. Fucosyl GM1 and Polysialic Acid
6.2.2. Dendritic Cell Vaccines
6.3. Adoptive Cell Therapy
7. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SCLC | Small cell lung cancer |
| ES-SCLC | extensive-stage SCLC |
| PE | platinum-etoposide |
| TMB | Tumor Mutational Burden |
| Rb1 | retinoblastoma1 |
| DDR | DNA damage response |
| CHK1 | checkpoint kinase 1 |
| ATR | ataxia telangiectasia and RAD3-related protein |
| ATM | ataxia telangiectasia mutated |
| AURK | aurora kinase |
| EZH2 | enhancer of zeste homology 2 |
| LSD1 | lysine-specific demethylase 1A |
| DLL3 | delta-like ligand-3 |
| SSTR2 | somatostatin receptor 2 |
| TAZ | transcriptional coactivator with PDZ-binding motif |
| PD-L1 | Programmed death ligand-1 |
| BTK | Bruton Tyrosine Kinase |
| BTKi | Bruton Tyrosine Kinase inhibition |
| CPS | PD-L1 combined positive score |
| MSI | Micro-Satellite Instability |
| bTMB | blood-based tumor mutational burden |
| MMR | Mismatch repair |
| SLFN11 | protein Schlafen 11 |
| CPIs | check point inhibitors |
| PCI | prophylactic cranial irradiation |
| ORR | overall response rate |
| mPFS | median progression free survival |
| HR | hazard ration |
| CI | confidence interval |
| mOS | median overall survival |
| OS | overall survival |
| irBORR | best immune related ORR |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Huber, R.M.; Tufman, A. Update on small cell lung cancer management. Breathe 2012, 8, 314–330. [Google Scholar] [CrossRef]
- Qin, S.; Jiang, J.; Lu, Y.; Nice, E.C.; Huang, C.; Zhang, J.; He, W. Emerging role of tumor cell plasticity in modifying therapeutic response. Sig. Transduct. Target Ther. 2020, 5, 228. [Google Scholar] [CrossRef]
- Micke, P.; Faldum, A.; Metz, T.; Beeh, K.M.; Bittinger, F.; Hengstler, J.G.; Buhl, R. Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer-what limits limited disease? Lung Cancer 2002, 37, 271–276. [Google Scholar] [CrossRef]
- West, H.J. Moving Beyond Limited and Extensive Staging of Small Cell Lung Cancer. JAMA Oncol. 2019, 5, e185187. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Zhang, Z.; Wang, Q. Emerging therapies for small cell lung cancer. J. Hematol. Oncol. 2019, 12, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lara, P.N., Jr.; Moon, J.; Redman, M.W.; Semrad, T.J.; Kelly, K.; Allen, J.W.; Gitlitz, B.J.; Mack, P.C.; Gandara, D.R. Relevance of platinum-sensitivity status in relapsed/refractory extensive-stage small-cell lung cancer in the modern era: A patient-level analysis of southwest oncology group trials. J. Thorac. Oncol. 2015, 10, 110–115. [Google Scholar] [CrossRef] [Green Version]
- Khuder, S.A. Effect of cigarette smoking on major histological types of lung cancer: A meta-analysis. Lung Cancer 2001, 31, 139–148. [Google Scholar] [CrossRef]
- Wang, X.; Ricciuti, B.; Nguyen, T.; Li, X.; Rabin, M.S.; Awad, M.M.; Lin, X.; Johnson, B.E.; Christiani, D.C. Association between Smoking History and Tumor Mutation Burden in Advanced Non-Small Cell Lung Cancer. Cancer Res. 2021, 81, 2566–2573. [Google Scholar] [CrossRef]
- Kim, K.B.; Dunn, C.T.; Park, K.S. Recent progress in mapping the emerging landscape of the small-cell lung cancer genome. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Soomro, Z.; Youssef, M.; Yust-Katz, S.; Jalali, A.; Patel, A.J.; Mandel, J. Paraneoplastic syndromes in small cell lung cancer. J. Thorac. Dis. 2020, 12, 6253–6263. [Google Scholar] [CrossRef]
- Sen, T.; Rodriguez, B.L.; Chen, L.; Corte, C.M.D.; Morikawa, N.; Fujimoto, J.; Cristea, S.; Nguyen, T.; Diao, L.; Li, L.; et al. Targeting DNA Damage Response Promotes Antitumor Immunity through STING-Mediated T-Cell Activation in Small Cell Lung Cancer. Cancer Discov. 2019, 9, 646–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavan, A.; Attili, I.; Pasello, G.; Guarneri, V.; Conte, P.F.; Bonanno, L. Immunotherapy in small-cell lung cancer: From molecular promises to clinical challenges. J. Immunother. Cancer 2019, 7, 205. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, H.; Sen, T.; Rudin, C.M. Targeted Therapies and Biomarkers in Small Cell Lung Cancer. Front. Oncol. 2020, 10, 741. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Gay, C.M.; Byers, L.A. Targeting DNA damage repair in small cell lung cancer and the biomarker landscape. Transl Lung Cancer Res. 2018, 7, 50–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudin, C.M.; Poirier, J.T.; Byers, L.A.; Dive, C.; Dowlati, A.; George, J.; Heymach, J.V.; Johnson, J.E.; Lehman, J.M.; MacPherson, D.; et al. Molecular subtypes of small cell lung cancer: A synthesis of human and mouse model data. Nat. Rev. Cancer 2019, 19, 289–297. [Google Scholar] [CrossRef]
- Gay, C.M.; Stewart, C.A.; Park, E.M.; Diao, L.; Groves, S.M.; Heeke, S.; Nabet, B.Y.; Fujimoto, J.; Solis, L.M.; Lu, W.; et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell 2021, 39, 346–360.e7. [Google Scholar] [CrossRef]
- Frese, K.K.; Simpson, K.L.; Dive, C. Small cell lung cancer enters the era of precision medicine. Cancer Cell 2021, 39, 297–299. [Google Scholar] [CrossRef]
- Coleman, N.; Zhang, B.; Byers, L.A.; Yap, T.A. The role of Schlafen 11 (SLFN11) as a predictive biomarker for targeting the DNA damage response. Br. J. Cancer 2021, 124, 857–859. [Google Scholar] [CrossRef]
- Chan, J.M.; Quintanal-Villalonga, A.; Gao, V.; Allaj, V.; Masilionis, I.; Chaudhary, O.; Egger, J.V.; Chow, A.; Walle, T.; Mattar, M.; et al. Signatures of plasticity and immunosuppression in a single-cell atlas of human small cell lung cancer. J. Clin. Oncol. 2021, 39, 8509. [Google Scholar] [CrossRef]
- Baine, M.K.; Hsieh, M.S.; Lai, W.V.; Egger, J.V.; Jungbluth, A.A.; Daneshbod, Y.; Beras, A.; Spencer, R.; Lopardo, J.; Bodd, F.; et al. SCLC Subtypes Defined by ASCL1, NEUROD1, POU2F3, and YAP1: A Comprehensive Immunohistochemical and Histopathologic Characterization. J. Thorac. Oncol. 2020, 15, 1823–1835. [Google Scholar] [CrossRef] [PubMed]
- Owonikoko, T.K.; Dwivedi, B.; Chen, Z.; Zhang, C.; Barwick, B.; Ernani, V.; Zhang, G.; Gilbert-Ross, M.; Carlisle, J.; Khuri, F.R.; et al. YAP1 Expression in SCLC Defines a Distinct Subtype with T-Cell-Inflamed Phenotype. J. Thorac. Oncol. 2021, 16, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Raso, M.G.; Bota-Rabassedas, N.; Wistuba, I. Pathology and Classification of SCLC. Cancers 2021, 13, 820. [Google Scholar] [CrossRef] [PubMed]
- Schwendenwein, A.; Megyesfalvi, Z.; Barany, N.; Valko, Z.; Bugyik, E.; Lang, C.; Ferencz, B.; Paku, S.; Lantos, A.; Fillinger, J.; et al. Molecular profiles of small cell lung cancer subtypes: Therapeutic implications. Mol. Ther. Oncolytics 2021, 20, 470–483. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Okamoto, I.; Kameyama, H.; Kudoh, S.; Saito, H.; Sanada, M.; Kudo, N.; Wakimoto, J.; Fujino, K.; Ikematsu, Y.; et al. Integrated Immunohistochemical Study on Small-Cell Carcinoma of the Lung Focusing on Transcription and Co-Transcription Factors. Diagnostics 2020, 10, 949. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhao, Q.; Zhu, W.; Feng, Y.; Xiao, T.; Zhang, P.; Jiang, L.; Hou, Y.; Guo, C.; Huang, H.; et al. Identification of TAZ as the essential molecular switch in orchestrating SCLC phenotypic transition and metastasis. bioRxiv 2021, 454244. [Google Scholar] [CrossRef]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.W.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): Updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 51–65. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.; Ozguroglu, M.; Ji, J.; Voitko, O.; et al. Goldman. LBA61—Durvalumab ± tremelimumab + platinum-etoposide in first-line extensive-stage SCLC (ES-SCLC): 3-year overall survival update from the phase III CASPIAN study. Ann. Oncol. 2021, 32, S1283–S1346. [Google Scholar] [CrossRef]
- Rudin, C.M.; Awad, M.M.; Navarro, A.; Gottfried, M.; Peters, S.; Csőszi, T.; Cheema, P.K.; Rodriguez-Abreu, D.; Wollner, M.; Yang, J.C.-H.; et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J. Clin. Oncol. 2020, 38, 2369–2379. [Google Scholar] [CrossRef]
- Arriola, E.; Wheater, M.; Galea, I.; Cross, N.; Maishman, T.; Hamid, D.; Stanton, L.; Cave, J.; Geldart, T.; Mulatero, C.; et al. Outcome and Biomarker Analysis from a Multicenter Phase 2 Study of Ipilimumab in Combination with Carboplatin and Etoposide as First-Line Therapy for Extensive-Stage SCLC. J. Thorac. Oncol. 2016, 11, 1511–1521. [Google Scholar] [CrossRef] [Green Version]
- Reck, M.; Bondarenko, I.; Luft, A.; Serwatowski, P.; Barlesi, F.; Chacko, R.; Sebastian, M.; Lu, H.; Cuillerot, J.M.; Lynch, T.J. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: Results from a randomized, double-blind, multicenter phase 2 trial. Ann. Oncol. 2013, 24, 75–83. [Google Scholar] [CrossRef]
- Reck, M.; Luft, A.; Szczesna, A.; Havel, L.; Kim, S.W.; Akerley, W.; Pietanza, M.C.; Wu, Y.L.; Zielinski, C.; Thomas, M.; et al. Phase III Randomized Trial of Ipilimumab plus Etoposide and Platinum versus Placebo plus Etoposide and Platinum in Extensive-Stage Small-Cell Lung Cancer. J. Clin. Oncol. 2016, 34, 3740–3748. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Elez, E.; Hiret, S.; Kim, D.W.; Morosky, A.; Saraf, S.; Piperdi, B.; Mehnert, J.M. Pembrolizumab in Patients with Extensive-Stage Small-Cell Lung Cancer: Results from the Phase Ib KEYNOTE-028 Study. J. Clin. Oncol. 2017, 35, 3823–3829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, H.C.; Lopez-Martin, J.A.; Kao, S.C.-H.; Miller, W.H.; Ros, W.; Gao, B.; Marabelle, A.; Gottfried, M.; Zer, A.; Delord, J.-P.; et al. Phase 2 study of pembrolizumab in advanced small-cell lung cancer (SCLC): KEYNOTE-158. J. Clin. Oncol. 2018, 36, 8506. [Google Scholar] [CrossRef]
- Antonia, S.J.; López-Martin, J.A.; Bendell, J.; Ott, P.A.; Taylor, M.; Eder, J.P.; Jäger, D.; Pietanza, M.C.; Le, D.T.; de Braud, F.; et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): A multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016, 17, 883–895. [Google Scholar] [CrossRef] [Green Version]
- Spigel, D.R.; Vicente, D.; Ciuleanu, T.E.; Gettinger, S.; Peters, S.; Horn, L.; Audigier-Valette, C.; Pardo Aranda, N.; Juan-Vidal, O.; Cheng, Y.; et al. Second-line nivolumab in relapsed small-cell lung cancer: CheckMate 331. Ann. Oncol. 2021, 32, 631–641. [Google Scholar] [CrossRef]
- Owonikoko, T.K.; Park, K.; Govindan, R.; Ready, N.; Reck, M.; Peters, S.; Dakhil, S.R.; Navarro, A.; Rodríguez-Cid, J.; Schenker, M.; et al. Nivolumab and Ipilimumab as Maintenance Therapy in Extensive-Disease Small-Cell Lung Cancer: CheckMate 451. J. Clin. Oncol. 2021, 39, 1349–1359. [Google Scholar] [CrossRef]
- Tian, Y.; Zhai, X.; Han, A.; Zhu, H.; Yu, J. Potential immune escape mechanisms underlying the distinct clinical outcome of immune checkpoint blockades in small cell lung cancer. J. Hematol. Oncol. 2019, 12, 67. [Google Scholar] [CrossRef]
- Schmid, S.; Früh, M. Immune checkpoint inhibitors and small cell lung cancer: What’s new? J. Thorac. Dis. 2018, 10, S1503–S1508. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.C.; Piha-Paul, S.A.; Lopez-Martin, J.; Schellens, J.H.M.; Kao, S.; Miller, W.H., Jr.; Delord, J.-P.; Gao, B.; Planchard, D.; Gottfried, M.; et al. Pembrolizumab after Two or More Lines of Previous Therapy in Patients with Recurrent or Metastatic SCLC: Results from the KEYNOTE-028 and KEYNOTE-158 Studies. J. Thorac. Oncol. 2019, 15, 618–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.V.; Reck, M.; Mansfield, A.S.; Mok, T.; Scherpereel, A.; Reinmuth, N.; Garassino, M.C.; De Castro Carpeno, J.; Califano, R.; Nishio, M.; et al. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients with Extensive-Stage Small-Cell Lung Cancer Treated with Atezolizumab, Carboplatin, and Etoposide (IMpower133). J. Clin. Oncol. 2021, 39, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Goldman, J.W.; Garassino, M.C.; Dvorkin, M.; Trukhin, D.; Statsenko, G.; Hotta, K.; Ji, J.H.; Hochmair, M.J.; Voitko, O.; et al. LBA89—PD-L1 expression, patterns of progression and patient-reported outcomes (PROs) with durvalumab plus platinum-etoposide in ES-SCLC: Results from CASPIAN. Ann. Oncol. 2019, 30, v928–v929. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Callahan, M.K.; Awad, M.M.; Calvo, E.; Ascierto, P.A.; Atmaca, A.; Rizvi, N.A.; Hirsch, F.R.; Selvaggi, G.; Szustakowski, J.D.; et al. Tumor Mutational Burden and Efficacy of Nivolumab Monotherapy and in Combination with Ipilimumab in Small-Cell Lung Cancer. Cancer Cell 2018, 33, 853–861.e854. [Google Scholar] [CrossRef] [Green Version]
- Merlo, A.; Mabry, M.; Gabrielson, E.; Vollmer, R.; Baylin, S.B.; Sidransky, D. Frequent microsatellite instability in primary small cell lung cancer. Cancer Res. 1994, 54, 2098–2101. [Google Scholar]
- Chen, X.Q.; Stroun, M.; Magnenat, J.L.; Nicod, L.P.; Kurt, A.M.; Lyautey, J.; Lederrey, C.; Anker, P. Microsatellite alterations in plasma DNA of small cell lung cancer patients. Nat. Med. 1996, 2, 1033–1035. [Google Scholar] [CrossRef]
- Mahadevan, N.R.; Knelson, E.H.; Wolff, J.O.; Vajdi, A.; Saigí, M.; Campisi, M.; Hong, D.; Thai, T.C.; Piel, B.; Han, S.; et al. Intrinsic Immunogenicity of Small Cell Lung Carcinoma Revealed by Its Cellular Plasticity. Cancer Discov. 2021, 11, 1952–1969. [Google Scholar] [CrossRef]
- Goswami, S.; Apostolou, I.; Zhang, J.; Skepner, J.; Anandhan, S.; Zhang, X.; Xiong, L.; Trojer, P.; Aparicio, A.; Subudhi, S.K.; et al. Modulation of EZH2 expression in T cells improves efficacy of anti-CTLA-4 therapy. J. Clin. Investig. 2018, 128, 3813–3818. [Google Scholar] [CrossRef]
- Dunn, J.; Rao, S. Epigenetics and immunotherapy: The current state of play. Mol. Immunol. 2017, 87, 227–239. [Google Scholar] [CrossRef]
- Byers, L.A.; Wang, J.; Nilsson, M.B.; Fujimoto, J.; Saintigny, P.; Yordy, J.; Giri, U.; Peyton, M.; Fan, Y.H.; Diao, L.; et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov. 2012, 2, 798–811. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Ramkumar, K.; Cardnell, R.J.; Gay, C.M.; Stewart, C.A.; Wang, W.-L.; Fujimoto, J.; Wistuba, I.I.; Byers, L.A. A wake-up call for cancer DNA damage: The role of Schlafen 11 (SLFN11) across multiple cancers. Br. J. Cancer 2021. [Google Scholar] [CrossRef]
- Zhang, J.; Shih, D.J.H.; Lin, S.-Y. Role of DNA repair defects in predicting immunotherapy response. Biomark Res. 2020, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- NCT04334941. Available online: https://clinicaltrials.gov/ct2/show/NCT04334941 (accessed on 15 September 2021).
- Gladkov, O.; Biakhov, M.; Ramlau, R.; Serwatowski, P.; Milanowski, J.; Tomeczko, J.; Komarnitsky, P.B.; Bernard, L.; Kramer, D.; Krzakowski, M.J. Phase II trial of huKS-IL2 with cyclophosphamide (CTX) in patients with extensive disease small-cell lung cancer (ED-SCLC). J. Clin. Oncol. 2012, 30, 7090. [Google Scholar] [CrossRef]
- Ruotsalainen, T.M.; Mattson, K. Interferon trials in small cell lung cancer at one institution: A comparison of results obtained before and after initiation of systematic treatment trials using IFN-alpha in combination with other modalities. J. Interferon Cytokine Res. 2002, 22, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Dickler, M.N.; Ragupathi, G.; Liu, N.X.; Musselli, C.; Martino, D.J.; Miller, V.A.; Kris, M.G.; Brezicka, F.-T.; Livingston, P.O.; Grant, S.C. Immunogenicity of a Fucosyl-GM1-Keyhole Limpet Hemocyanin Conjugate Vaccine in Patients with Small Cell Lung Cancer. Clin. Cancer Res. 1999, 5, 2773–2779. [Google Scholar] [PubMed]
- Krug, L.M.; Ragupathi, G.; Ng, K.K.; Hood, C.; Jennings, H.J.; Guo, Z.; Kris, M.G.; Miller, V.; Pizzo, B.; Tyson, L.; et al. Vaccination of small cell lung cancer patients with polysialic acid or N-propionylated polysialic acid conjugated to keyhole limpet hemocyanin. Clin. Cancer Res. 2004, 10, 916–923. [Google Scholar] [CrossRef] [Green Version]
- Chiappori, A.A.; Soliman, H.; Janssen, W.E.; Antonia, S.J.; Gabrilovich, D.I. INGN-225: A dendritic cell-based p53 vaccine (Ad.p53-DC) in small cell lung cancer: Observed association between immune response and enhanced chemotherapy effect. Expert Opin. Biol. Ther. 2010, 10, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Giffin, M.J.; Cooke, K.; Lobenhofer, E.K.; Estrada, J.; Zhan, J.; Deegen, P.; Thomas, M.; Murawsky, C.M.; Werner, J.; Liu, S.; et al. AMG 757, a Half-Life Extended, DLL3-Targeted Bispecific T-Cell Engager, Shows High Potency and Sensitivity in Preclinical Models of Small-Cell Lung Cancer. Clin. Cancer Res. 2021, 27, 1526–1537. [Google Scholar] [CrossRef]
- Owen, D.H.; Giffin, M.J.; Bailis, J.M.; Smit, M.D.; Carbone, D.P.; He, K. DLL3: An emerging target in small cell lung cancer. J. Hematol. Oncol. 2019, 12, 61. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/ct2/show/NCT04885998 (accessed on 15 September 2021).
- Heinhuis, K.M.; Carlino, M.; Joerger, M.; Di Nicola, M.; Meniawy, T.; Rottey, S.; Moreno, V.; Gazzah, A.; Delord, J.-P.; Paz-Ares, L.; et al. Safety, Tolerability, and Potential Clinical Activity of a Glucocorticoid-Induced TNF Receptor-Related Protein Agonist Alone or in Combination with Nivolumab for Patients with Advanced Solid Tumors: A Phase 1/2a Dose-Escalation and Cohort-Expansion Clinical Trial. JAMA Oncol. 2020, 6, 100–107. [Google Scholar] [CrossRef] [Green Version]
- SKYSCRAPER-02. Available online: https://clinicaltrials.gov/ct2/show/NCT04256421 (accessed on 30 September 2021).
- Scherwitzl, I.; Opp, S.; Hurtado, A.M.; Pampeno, C.; Loomis, C.; Kannan, K.; Yu, M.; Meruelo, D. Sindbis Virus with Anti-OX40 Overcomes the Immunosuppressive Tumor Microenvironment of Low-Immunogenic Tumors. Mol. Ther. Oncolytics 2020, 17, 431–447. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Franco, A.; Ackermann, C.; Paz-Ares, L.; Califano, R. First-line immune checkpoint inhibitors for extensive stage small-cell lung cancer: Clinical developments and future directions. ESMO Open 2021, 6, 100003. [Google Scholar] [CrossRef] [PubMed]
- Singer, L.; Yom, S.S. Consolidative radiation therapy for extensive-stage small cell lung cancer. Transl. Lung Cancer Res. 2015, 4, 211–214. [Google Scholar] [CrossRef] [PubMed]
- SPACE. Available online: https://clinicaltrials.gov/ct2/show/NCT04221529 (accessed on 30 September 2021).
- MEDI4736. Available online: https://clinicaltrials.gov/ct2/show/NCT03963414 (accessed on 30 September 2021).
| SCLC Subtype | Transcription Factor Expression | Possible Targets |
|---|---|---|
| SCLC-A | High ASCL1, High DLL3 SLFN11 expression | DLL3 inhibition Platinum-based chemotherapy PARP inhibition BCL-2 inhibition |
| SCLC-N | High expression of NEUROD1 High expression of SSTR2 | Somatostatin analogs Aurora Kinase inhibitors |
| SCLC-P | High POU2F3 expression | PARP inhibition Anti-metabolites |
| SCLC-I | High expression of EMT+ BTK Increased Immune Infiltration (Higher antigen presentation + immune cell infiltration) | Ibrutinib Immune checkpoint inhibitors |
| Study | Phase | Setting | Agent | Patients | Primary Endpoint | Result |
|---|---|---|---|---|---|---|
| IMpower133 | III | First-line | Atezolizumab | 403 | PFS & OS | Improved PFS and OS in favor of atezolizumab |
| CASPIAN | III | First-line | Durvalumab +/− Tremelimumab | 805 | OS | Improved OS in favor of durvalumab. No benefit of addition of tremelimumab. |
| KEYNOTE-604 | III | First-line | Pembrolizumab | 453 | PFS & OS | Improved PFS in favor of pembrolizumab. No statistically significant difference in OS. |
| Arriola et al. | II | First-line | Ipilimumab | 42 | PFS at 1 year | 1-year PFS 15.8% |
| Reck et al. | II | First-line | Ipilimumab (+ carboplatin/paclitaxel in a concurrent/ phased manner) | 130 | irPFS | Improved irPFS in favor of Ipilimumab combination in a phased manner |
| Reck et al. | III | First-line | Ipilimumab (phased) | 1132 | OS | No difference in survival p = 0.38 |
| CheckMate-451 | III | Maintenance after first-line therapy | Nivolumab +/− Ipilimumab | 834 | OS | No difference in survival |
| KEYNOTE-028 | Ib | Recurrent/refractory | Pembrolizumab | 24 | Safety, Tolerability and ORR | Main AEs: Asthenia-fatigue-cough ORR 33% |
| KEYNOTE-158 | II | Recurrent/refractory | Pembrolizumab | 107 | ORR | ORR 18.7%, 35.7% PDL-1+ 6% PDL-1− |
| CheckMate-032 | I/II | Refractory/Recurrent | Nivolumab +/− Ipilimumab | 216 | ORR | N3 10%; N1I3 23%; N3I1 19% Promising results |
| CheckMate-331 | III | Recurrent/Refractory | Nivolumab | 284 receiving nivolumab vs. 285 on chemotherapy | OS | No improvement in OS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Sayed, R.; Blais, N. Immunotherapy in Extensive-Stage Small Cell Lung Cancer. Curr. Oncol. 2021, 28, 4093-4108. https://doi.org/10.3390/curroncol28050347
El Sayed R, Blais N. Immunotherapy in Extensive-Stage Small Cell Lung Cancer. Current Oncology. 2021; 28(5):4093-4108. https://doi.org/10.3390/curroncol28050347
Chicago/Turabian StyleEl Sayed, Rola, and Normand Blais. 2021. "Immunotherapy in Extensive-Stage Small Cell Lung Cancer" Current Oncology 28, no. 5: 4093-4108. https://doi.org/10.3390/curroncol28050347
APA StyleEl Sayed, R., & Blais, N. (2021). Immunotherapy in Extensive-Stage Small Cell Lung Cancer. Current Oncology, 28(5), 4093-4108. https://doi.org/10.3390/curroncol28050347





