Abstract
Cancer immunotherapy has the goal of enhancing a patient’s intrinsic immune processes in order to mount a successful immune response against tumor cells. Cancer cells actively employ tactics to evade, delay, alter, or attenuate the anti-tumor immune response. Immune checkpoint inhibitors (ICIs) modulate endogenous regulatory immune mechanisms to enhance immune system activation, and have become the mainstay of therapy in many cancer types. This activation occurs broadly and as a result, activation is supraphysiologic and relatively non-specific, which can lead to immune-related adverse events (irAEs), the frequency of which depends on the patient, the cancer type, and the specific ICI antibody. Careful assessment of patients for irAEs through history taking, physical exam, and routine laboratory assessments are key to identifying irAEs at early stages, when they can potentially be managed more easily and before progressing to higher grades or more serious effects. Generally, most patients with low grade irAEs are eligible for re-challenge with ICIs, and the use of corticosteroids to address an irAE is not associated with poorer patient outcomes. This paper reviews immune checkpoint inhibitors (ICIs) including their mechanisms of action, usage, associated irAEs, and their management.
1. Introduction
Cancer immunotherapy has the goal of enhancing a patient’s intrinsic immune processes in order to mount a successful immune response against tumor cells. Generally, these approaches fall under a “passive” immunotherapy approach, which uses therapies (e.g., antibody-drug conjugates) to recruit effector cells/molecules of the immune system to directly attack tumor cells, and “active” immune approaches (e.g., CAR-T, type I interferons, anti-CTLA4/PD-1/PD-L1 antibodies), which modulate endogenous regulatory immune mechanisms to enhance immune system activation [1]. Cancer cells actively employ tactics to evade, delay, alter, or attenuate the anti-tumor immune response. Often, strategies that modulate endogenous regulatory immune mechanisms broadly enhance or activate the immune system. As a result, activation is supraphysiologic, which can lead to immune-related adverse events (irAEs). This paper reviews immune checkpoint inhibitors (ICIs) including their mechanisms of action, usage, associated irAEs, and their management.
2. Overview of Mechanisms of Action
ICIs induce anti-tumor immune responses by blocking immune checkpoints. These immune checkpoints play an important role in normal physiology to downregulate T cell responses, such as in autoimmune disease regulation. Two important immune checkpoint signaling pathways include the cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed death 1 (PD-1) pathways.
To be activated, T cells require major histocompatibility complex (MHC) class II molecules on an antigen presenting cell (APC) to present an antigen (Ag) that is recognized by the T cell receptor, as well as engagement with other co-stimulatory molecules (Figure 1) [2,3]. The engagement of CTLA-4 and PD-1 receptors on T cells downregulates T cell activation. Cancer cells exploit these important physiologic immune checkpoints by engaging these receptors and attenuating a T cell-mediated anti-tumor immune response [2,3,4]. ICIs such as anti-CTLA-4, anti-PD-1, and anti-PD-Ligand 1 (PD-L1) antibodies have been developed to restore the immune system’s ability to mount an anti-tumor immune response.
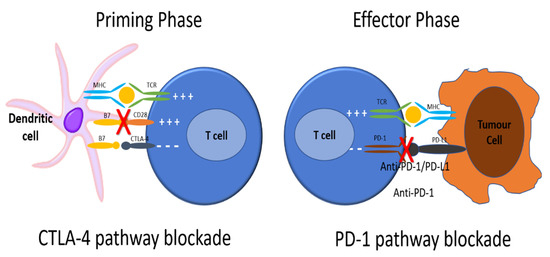
Figure 1.
To be activated, T cells require major histocompatibility complex (MHC) class II molecules on an antigen presenting cell (APC) to present an antigen (Ag) that is recognized by the T cell receptor. Next, the CD28 receptor on the T cell is bound by CD80/86 on the APC, signaling the T cell to be activated. CTLA4 is found on T cell surfaces and competes with CD28 for binding to CD80/86 on the APC. When this interaction predominates, T cell activation signaling is attenuated. As well, PD-1 receptors are expressed on the surface of T cells. When PD-1 receptors are engaged by programmed death ligand 1 (PD-L1) on an APC, the T cell that recognizes the Ag being presented by the APC activates signaling pathways that downregulate activation and promote apoptosis. There is also reduced apoptosis of T-regulatory cells (Treg), facilitating downregulation of the immune response to that antigen. Cancer cells exploit these important physiologic mechanisms by upregulating PD-L1 expression on their cell surface, thereby attenuating any anti-tumor immune response through inducing the quiescence of tumor-reactive T cells. ICIs such as anti-CTLA-4, anti-PD-1, and anti-PD-L1 antibodies have been developed to restore the immune system’s ability to mount an anti-tumor immune response. Anti-CTLA-4 antibodies block the interaction between CTLA-4 and CD80/86 on the APCs, allowing for increased T cell activation. Anti-PD-1 and anti-PD-L1 antibodies block the interaction between PD-1 and PD-L1, allowing for increased T cell activation to Ag being presented by the tumor cell. In this way, the tumor cell is no longer recognized as “self”, tumor-reactive T cells are no longer shunted towards quiescence, and an anti-tumor immune response can be mounted.
3. Immune-Related Adverse Events
ICIs are used in a variety of disease sites as they universally upregulate the immune response, independent of the tumor-antigen being presented. In lung cancer, anti-PD-1 antibodies cemiplimab, nivolumab, and pembrolizumab, and anti-PD-L1 antibodies atezolizumab and durvalumab are used in the curative or metastatic settings to produce an anti-tumor immune response. However, the challenge with ICIs are the effects of a highly activated immune system on normal/non-tumor tissues via an autoimmune process, also called irAEs. irAEs are graded by severity (mild, moderate, severe, life-threatening, or death) on a scale from 1–5, respectively. In a meta-analysis of 36 phase II/III trials, the estimated incidence of any grade irAEs ranged from 54% to 76%, while the incidence of grades 3 and 4 adverse events ranged from 14.1% to 28.6% [5]. Interestingly, patients treated with ICIs and who develop an irAE have been shown to have a statistically significant reduced risk of death (~51%) and progression (~49%), suggesting that this may be proportional to the robustness of the T cell activation [6].
irAEs can occur in any tissue/organ system. Median onset ranges from 2–16 weeks from the start of treatment and varies depending on the organ system involved [2]. However, irAEs have been reported as early as one week after treatment commencement or as late as one year after discontinuation, presumably due to the presence of autoreactive T cell clones that remain in the body after treatment has stopped [7,8]. The most common irAEs are pruritis, rash, diarrhea, colitis, hypo- or hyper-thyroidism, and pneumonitis.
Combining one ICI with another ICI or conventional chemotherapy increases both the toxicity profile and severity in patients. Moreover, patients receiving the same ICI do not present with the same irAEs, even if being treated for the same type of cancer, suggesting there are patient/organ-specific microenvironments that can drive irAE [9,10]. While there are no known genetic risk factors for experiencing an irAE, personal risk factors such as a family or personal history of previous autoimmune disorder, high BMI, and elevated creatinine can increase the likelihood of ICI toxicity [11,12,13]. Interestingly, CTLA4 and PDCD1 polymorphisms are associated with autoimmune disorders [14,15].
4. How Do ICIs Cause Toxicity?
ICIs upregulate immune-activation pathways in a non-specific manner. Because of the differences in the types and frequencies of irAEs depending on the ICI used, there must be differences in the mechanisms by which they cause toxicity. As stated, normally CTLA4 maintains self-tolerance. Animal models and congenital genetic diseases in humans that cause functional abnormalities in CTLA-4 pathways demonstrate that abrogation of this regulation leads to T cell lymphoproliferation and lymphocytic infiltration, Treg defects, and auto-antibody (Ab) production [16,17,18]. In both mice and patients treated with CTLA-4 inhibitor, there are decreased circulating Treg and increased T helper (Th) 17 cells, whose enhancement is known to be involved in the pathogenesis of autoimmune diseases [19,20,21]. With respect to PD-1/PD-L1 inhibition, there are fewer circulating Treg cells in melanoma patients treated with ICI, and mice deficient for PD-1/PD-L1 develop auto-antibodies to various normal endogenous murine proteins [22,23,24,25,26].
T cell activation can also increase cross-talk between T and B cells, leading to increased auto-antibody production. In one study, patients treated with ICIs were shown to have changes in proportions of B cell populations, including reduced circulating B cells and increased CD21low B cells and plasmablasts, which were highly indicative of subsequent irAEs [27].
Finally, ICIs may cause toxicity through cross-reactivity. When the immune system recognizes an Ag presented by a tumor cell and mounts a response, there may be cross-reactivity with similar Ag on normal/non-cancerous cells [28]. This is evidenced by the high number of melanoma patients with vitiligo after being treated with ICIs, and has also been demonstrated in fatal cases of irAE myocarditis at autopsy [7,29,30,31].
5. Organ-Specific ICIs
For patients on ICI, careful self-assessment by the patient and history gathering by the medical team is integral to identifying complications early. In this way, an attempt can be made to institute management strategies to both minimize the need to interrupt or discontinue ICI treatment, and prevent an irAE from progressing to higher grades with increased toxicity and morbidity for the patient. Using Cancer Care Ontario (CCO) and the American Society of Clinical Oncology (ASCO) guidelines, this section will briefly describe some of the more common organ-specific irAEs and their management [32,33]. A high yield summary of these sections can be found in Table 1, including definitions for how these irAEs are graded (Table 2). For any reference to steroid use, please see Table 1 for dosage recommendations.

Table 1.
High yield management guidelines, including steroid doses, for common irAEs. This table is an abbreviated high yield summary of the CCO Immune Checkpoint Inhibitor Management Clinical Practice Guidelines and the ASCO Management of Immune-Related Adverse Events Clinical Practice Guidelines [32,33].

Table 2.
Definition of grades of severity for various common irAEs. These definitions have been taken from the CCO Immune Checkpoint Inhibitor Management Clinical Practice Guidelines [32].
5.1. Dermatologic
Cutaneous irAEs are most common, occurring in >30% of patients on ICIs, and tend to occur earlier than other organ-specific irAEs [34,35]. Generally, these occur irrespective of cancer type or number of treatments received, and more frequently with CTLA-4 inhibitors (~45%) [35,36,37]. Most are grade 1 or 2 toxicities; however, 1–3% of cases are grade 3 or higher, irrespective of the ICI [35]. Cutaneous reactions include lichenoid, psoriaform, granulomatous, eczematous and immunobullous reactions, vitiligo, drug rash with eosinophila and systemic symptoms (DRESS), toxic epidermal necrolysis (TEN), Stevens–Johnson syndrome (SJS), and Sweet syndrome [34,35]. More severe reactions include mucosal and palmoplantar surfaces. Interestingly, improved progression free survival (PFS) and overall survival (OS) have been reported in patients who develop cutaneous irAEs [38,39,40].
Per CCO guidelines, cutaneous irAEs are graded as 1–4 based on the percent of body surface area (BSA) covered, and type of reaction (Table 2) [32]. Any signs of desquamation should be considered a medical emergency and classified as grade 4. For grades 1 and 2 dermatitis, supportive therapy such as thick emollients is often all that is required. Topical steroids can be considered and are generally prescribed for grade 2 dermatitis, with anti-histamines to address pruritus as needed. These patients can be monitored while continuing on ICI. If the dermatitis persists, oral steroids and referral to a dermatologist can be considered.
If the patient has grade 3 or 4 dermatitis, a dermatologist consult is required. For grade 3 dermatitis, oral prednisone with a taper, and possible antibiotics are required. ICI should be held until resolution to grade 0 or 1, with the potential for re-challenge. If there is no improvement, or if the patient has grade 4 dermatitis (<5% of patients), the ICI should be permanently discontinued. Grade 4 dermatitis requires longer term IV steroids with a slower taper, as well as hospital admission for supportive care.
5.2. Endocrine
The incidence of endocrine irAEs ranges in the literature; however, one meta-analysis reported an overall incidence of clinically significant endocrinopathies of approximately 10% [41]. The most common endocrinopathies include acute hypophysitis and thyroid disease, though others such as development of type one diabetes mellitus (DM1), primary adrenal insufficiency (AI), hypercalcemia, and hypoparathyroidism occur more rarely [41,42]. Hypophysitis occurs most frequently with dual ICI or anti-CTLA4 Ab, whereas hypo- or hyperthyroidism occurs more frequently with PD-1 inhibitors [42,43]. Diagnosis of endocrine irAEs can be difficult due to their presentation with vague symptoms that can mimic a patient’s cancer such as fatigue, anorexia, and nausea. With the exception of labs assessing thyroid function and electrolytes including calcium, there are no routine laboratory tests that are monitored to detect endocrine dysfunction. However, some physicians using CTLA-4 inhibitors will assess other endocrine hormone levels (e.g., ACTH, testosterone, LH/FSH) at the onset of treatment to record the patient’s baseline for future comparison.
Endocrine irAEs are rarely grade 3–4, and though steroids can be given, efficacy is not well-established in improving the pituitary–thyroid–adrenal axis [44]. Generally, hormone replacement is sufficient, though in rare severe cases admission to hospital for supportive care and/or other investigations may be required. For both hypo- and hyperthyroidism, grade 1 adverse events (AEs) occur in an asymptomatic patient with altered thyroid stimulating hormone (TSH) levels. These patients can be followed and their thyroid chemistry monitored without stopping the ICI. In the case of grade 2 AEs, the patient may have moderate symptoms that necessitate treatment. This includes levothyroxine in the case of hypothyroidism. For hyperthyroidism, beta blockers are the mainstay of symptomatic treatment as well as hydration and anti-diarrheals. Agents such as methimazole or propylthiouracil in the case of hyperthyroidism may be less effective in ICI induced hyperthyroidism unless true Graves’ disease is present, and consultation with an endocrinologist is warranted in grade 3 or 4 cases [45]. Immune-related thyrotoxicosis generally transitions to hypothyroidism and ultimately requires thyroid hormone supplementation [46,47,48]. For any grade 3 or 4 hypo-/hyperthyroidism, hospitalization may be indicated for supportive management. Steroids have not been shown to decrease the duration of toxicity [49]. If grade 2 or 3, ICI should be withheld until the patient is stable on hormone therapy and prednisone has been tapered to <7.5 mg of prednisone equivalents per day, with the possibility of re-challenge. If grade 4, re-challenge can be reasonable once the patient is stable on hormone replacement, although individual patient situations need to be considered [33].
Hypophysitis typically presents as low TSH and low free T4. It occurs more frequently in males, and after approximately 2–6 months of treatment with an ICI [50]. Symptoms can be vague, or mimic the patient’s cancer, and so diagnosis can be delayed [51]. Once suspected however, endocrinology should be involved. Chemistry to assess morning cortisol, adrenocorticotropic hormone (ACTH), luteinizing hormone (LH), follicle stimulating hormone (FSH), and growth hormone (GH) will confirm the diagnosis. Imaging to rule out a cause that would require intervention should be completed. If a patient has grade 1 hypophysitis, monitor closely and continue the ICI. If the patient has grade 2 hypophysitis, withhold the ICI until grade 0–1, and re-challenge if the patient is stable on hormone replacement and asymptomatic. As with other endocrinopathies, if grade 3 or 4 hypophysitis is identified, the ICI may be resumed with appropriate hormone supplementation and monitoring once the initial clinical symptoms resolve.
Adrenal insufficiency (AI) as an irAE is rare though can be life-threatening. It can manifest as primary (0.7% and 4.2% of cases with single or double ICI, respectively [52,53]) or secondary AI (via hypophysitis), depending on the target of autoimmune antibodies. If primary AI, it is imperative to rule out inciting causes such as sepsis, which can present similarly, and complete a CT of the adrenals to assess for hemorrhage or metastases [54]. In general, once the patient reaches at least grade 2 AI, corticosteroids should be initiated, with likely long-term replacement required. In grades 2 and higher, ICI should be withheld until the irAE reaches grade 0 or 1, though re-challenge with ICI can resume once the patient is stable on hormone replacement and asymptomatic.
5.3. Gastrointestinal
Diarrhea is a common irAE occurring in ~35%, 20%, and >40% of patients on CTLA-4, PD-1 inhibitors, or combination therapy, respectively, though colitis is found in only 12%, 1%, and 14% of patients, respectively [55,56]. Similar symptoms occur with enteritis; however, constipation due to inflammation and abdominal pain are also possible presenting features. Typically, the work-up consists of a history and physical exam, as well as stool samples to rule out infection. Depending on the severity and type of symptoms elicited, computed tomography (CT) to evaluate for perforation or the extent of inflammation can be helpful. The median time to onset of diarrhea/colitis is 6–8 weeks for ipilimumab and nivolumab, and 3–4 months for pembrolizumab [57]. Therapy can range from supportive therapy with loperamide, in the case of grade 1 irAEs, the addition of IV hydration, electrolyte replacement, and oral steroids in the case of grade 2 irAE, to IV steroids and possible antibiotics with hospital admission for supportive care in the case of grade 3 or 4 irAEs. For grade 1 AEs, ICI can continue as long as patient symptoms are controlled with supportive therapy. For grade 2 AEs, ICI should be withheld until grade 0 and the patient is on <7.5 mg/day prednisone equivalents if on a CTLA-4 inhibitor, or <10 mg/day prednisone equivalents if on a PD-1 inhibitor. For grades 3 and 4 diarrhea, ICI should be discontinued. In some cases, the addition of infliximab is necessary, though caution is advised with grade 4 AE due to the risk of bowl perforation.
Liver toxicity can occur in 1–17% of patients on ICI [51,58,59]. The incidence varies by regimen with those on CTLA-4 inhibitors occurring slightly more commonly. Most events are grade 1 or 2. Liver chemistry should be reviewed with each ICI cycle as asymptomatic elevations in transaminases are the most common initial presentation [51]. There are many reasons a patient with cancer on ICI can present with hepatitis; therefore, alternative diagnoses should be carefully considered and can include disease progression, thrombosis, drug-induced liver injury, acute infections, alcohol-induced hepatitis, effects of other concomitant systemic therapies, as well as irAEs. If grade 1, the patient can be monitored while continuing on ICI. If grade 2, prednisone should be initiated with a slow taper provided liver transaminases normalize with treatment. Once normalized, and provided the patient is on ≤10 mg prednisone equivalents per day, re-challenge with ICI can be considered. If grade 3 or 4, hepatology/gastroenterology should be consulted, and biopsy considered. In such cases, high dose IV steroids are required with a long taper. If there is no downward trend to suggest the resolution of transaminitis by 3 days, mycofenolate mofetil (MMF) should be added. Again, if there is no improvement by 7 days, a switch to another immunosuppressant such as tacrolimus should be made. As a last resort, infliximab, after consultation with expert opinion and the patient, can be considered, as it may also cause transaminitis. If the hepatitis is grade 3 or 4, the ICI should be permanently discontinued.
5.4. Lung
Though pneumonitis as an irAE is rare (<5% of cases and <1% meeting criteria for grade 3 or 4 toxicity, or <10% of cases for mono- and combination therapy, respectively), it can be life-threatening and warrants careful consideration [60,61]. Patients can present with a dry, unproductive cough, tachypneic, dyspneic, tachycardic, cyanosed, and/or fatigue [61]. They can have exertional hypoxia, or hypoxia as lung inflammation and interstitial/alveolar infiltrates increase [61]. Grade 1 disease is asymptomatic and requires no intervention; however, prednisone can be considered and oxygen saturation, chest x-ray (CXR), or possibly CT should be completed with subsequent cycles. If the patient is placed on steroids for any reason, consider withholding the ICI until resolution. Patients are symptomatic with grade 2 irAEs and therefore require medical intervention. Respirology and infectious disease consults are recommended, as well as starting prednisone with a long taper once resolution begins. If there is no improvement by 48–72 h, the patient should be approached as grade 3 or 4 pneumonitis and the ICI should be withheld until symptom resolution and the patient is on <10 mg/day prednisone equivalents. Re-challenge can occur, but if toxicity recurs, the ICI should be discontinued.
Grade 3 and 4 pneumonitis are treated the same. Respirology and infectious disease consults are recommended, as well as consideration for bronchoscopy/biopsy to aid with the diagnosis. High dose steroids should be initiated with a long taper once symptoms improve. If there is no improvement at 48 h, additional immunosuppression with infliximab should be added to the treatment. Supportive care such as oxygen and prophylactic antibiotics should be included; the ICI should be permanently discontinued.
5.5. Renal
Nephritis is typically asymptomatic at onset and is found with rising serum creatinine on routine labs [62]. On progression, symptoms can include edema, oliguria, and other electrolyte abnormalities. Renal toxicity occurs in <5% of patients [63,64]. The management of grade 1 disease includes hydration, cessation of nephrotoxic medications, and correcting electrolyte imbalances. ICI can be continued provided creatinine stabilizes or decreases. Grade 2, 3, and 4 nephritis are treated similarly. As with other irAEs, other causes of elevated creatinine should be ruled out with urine microscopy and ultrasound +/− biopsy; nephrologist consultation should be considered. For grade 2 AE, prednisone should be started and tapered once creatinine reaches grade 0 or 1 levels. The ICI should be withheld until creatinine decreases to grade 1 criteria, and the patient is on <10 mg/day of prednisone equivalents. For grades 3 and 4 AE, methylprednisolone should be initiated with a slow taper once resolution occurs. Addition of MMF could be considered in refractory cases, and hemodialysis may be necessary. If grade 3 or 4 irAEs, discontinue the ICI.
5.6. Neurologic
There are a wide variety of neurotoxicities at various degrees of severity that can occur due to ICI therapy, but occur in <5% of patients [65,66]. These include potential antibody-mediated toxicities such as paresthesias, Guillian–Barre syndrome, and myasthenia gravis, or other sensory, motor, and CNS toxicities like enteric neuropathy, inflammatory myopathy, lymphocytic meningitis, cerebral vasculitis, and optic neuritis [65,66]. Neurologic irAEs usually occur within one to six weeks of starting ICI treatment [65,66]. If grade 1, the ICI can be continued, and the patient monitored closely for progression. If grade 2 or greater, a neurology consult is recommended as well as magnetic resonance imaging, lumbar puncture, nerve conduction studies, or electromyography based on the patient’s symptoms to rule out other non-ICI-related causes. For grade 2, oral steroids should be initiated while ICI is withheld. Re-challenge can be considered once symptoms are grade 0 or 1, and a review with a multidisciplinary team to weigh the risks and benefits occurs. If grades 3 or 4, the ICI should be permanently discontinued and higher doses of prednisone to control the AE are recommended. For any grade 2–4 irAEs, consider a separate immunosuppressive agent if there is no improvement on prednisone (e.g., infliximab or MMF). Some patients may require IV immunoglobulin (IVIG), plasmapheresis, or other supportive approaches.
5.7. Cardiac
The incidence of cardiac toxicities is <1% for both single and double agent ICI, and presents as a wide variety of toxicities including myocarditis, pericarditis, arrhythmias, cardiomyopathy, and impaired ventricular function [33,60]. Though infrequent, cardiotoxicity can be life-threatening. If suspected, a consultation with a cardiologist is recommended, as well as holding the ICI and instituting high dose corticosteroids. If required, escalation to other immunosuppressive agents can be performed. It is unclear whether re-challenge should be conducted.
6. Efficacy of Immunotherapy after Treatment with Corticosteroids for Any Reason
Corticosteroids affect and attenuate numerous points along a pro-inflammatory pathway. As such, it was thought that perhaps glucocorticoids may reduce the efficacy of ICI, and ultimately patient outcomes. Because of this, patients on systemic glucocorticoids have generally been excluded from clinical trials of ICIs, leaving it to retrospective analyses to address the use of steroids in patients on ICI and their outcomes, and whether there is a causal relationship, or merely an association if outcomes differ.
One such retrospective study included 640 patients treated for NSCLC with single agent ICI, 14% of which were on corticosteroids ≥ 10 mg prednisone-equivalents per day, and 75% of whom had steroids prescribed for cancer-related dyspnea, fatigue, or control of symptoms from brain metastases [67]. Upon taking anti-PD-1/PD-L1 ICIs, patients experienced significantly lower objective response rates (ORR) and reduced PFS and OS, even when multivariate analyses were completed to take into account other potentially confounding variables [67]. As well, the use of corticosteroids or other immune-modulating medications (e.g., infliximab) to treat irAEs were not subsequently associated with decreased efficacy [67]. The authors hypothesized that commencing corticosteroids before initiating ICIs could reduce efficacy, though not once the patient had already responded to ICI. Though interesting, these conclusions should be interpreted with caution as patients on steroids for cancer-related symptom management may, at baseline, have a lower performance status than the comparator group, which would also confer a poorer PFS and OS, confounding the final analysis.
A more recent systematic review and meta-analysis assessing OS and PFS outcomes in patients with NSCLC treated with ICI +/− corticosteroids for any reason included 5461 patients from 14 studies [68]. The authors found that despite the retrospective nature, low quality studies, and significant heterogeneity and publication bias, the use of corticosteroids for any reason while on ICI significantly reduced OS and PFS versus patients who did not take corticosteroids [68]. A subgroup analysis stratifying patients by reason for corticosteroid use showed worse OS if being used for supportive therapy versus those in which they were used to manage brain metastases, though PFS was no different [68].
Another meta-analysis included 15 studies with over 14,000 patients of any cancer type who had corticosteroid administration before and/or after initiation of ICI treatment [69]. Corticosteroid use significantly reduced PFS and OS in cancer patients treated with ICI. In a planned subgroup analysis, the reason for corticosteroid use impacted efficacy: those for whom steroids had been prescribed for cancer-related symptoms had a shorter PFS and OS versus those patients for whom corticosteroids were prescribed to address an irAE where there was no detrimental impact on OS [69].
Finally, one study prospectively followed 341 patients with hepatocellular carcinoma (HCC) treated with ICI therapy alone to assess the differences between PFS, OS, and ORR between patients receiving and not receiving corticosteroid therapy [70]. Overall, corticosteroid use did not predict for worse OS, PFS, or ORR in uni-/multivariate analyses. However, corticosteroids prescribed for cancer-related indications were predictive for significantly shorter PFS and were associated with refractoriness to ICI. The authors proposed that this was due to the fact that patients with symptomatic HCC have a poorer prognosis, rather than a causal relationship between corticosteroid use and outcomes [70].
Generally, these studies use a threshold of 10 mg of prednisone equivalents per day, which is slightly above physiologic levels. In practice, if corticosteroids are required, patients should be on the lowest possible dose while on ICI, though they are not excluded from ICI therapy if they exceed the studied threshold of 10 mg of prednisone equivalents per day. It is reassuring that studies have demonstrated no adverse effect on the efficacy of ICI in patients prescribed corticosteroids to manage irAEs.
7. Re-Challenge with ICI after Interruption Due to irAE
When an ICI is held due to an irAE, a decision needs to be made with respect to re-initiating/re-challenging a patient with the drug due to concerns that re-challenge will be associated with recurrence of the irAE, which is especially worrisome if the event was of a higher grade or serious. Unfortunately, understanding which factors to take into account when weighing the risks and benefits of ICI re-challenge are unknown. One study that attempted to address this question found that patients who responded to an ICI prior to holding due to an irAE may not benefit from re-challenge, while for those without an OR at the time ICI was held, re-challenge was associated with improved PFS and OS versus those who were not re-challenged [71]. Though interesting, this study was of a small sample size, and there are reasons other than an irAE that can lead to discontinuation of the ICI that appear not to have been accounted for (e.g., worse performance status).
One observational study aimed to identify the rate of recurrence of the same irAE that prompted ICI interruption and management upon ICI re-challenge, in an attempt to identify clinical features associated with recurrence [72]. In a population of 452 cases of irAEs where re-challenge occurred, 28.8% of patients experienced recurrence of the initial irAE, with those on CTLA-4 or combination therapy experiencing recurrence most often. Colitis, hepatitis, and pneumonitis were associated with higher recurrence rates compared to adrenal events, which were found to have lower recurrence rates in a multivariate analysis [72]. Recurrence of a different irAE after re-challenge was reported in only 4.4% of cases, with colitis being the most frequent [72]. Similar recurrence rates have been shown in other studies [71,73,74,75].
Several approaches to re-challenge post-holding an ICI due to an irAE exist, including: (i) no re-challenge, (ii) switching to another class of ICI, (iii) re-challenge with the same ICI regimen, and (iv) restarting ICI with prophylactic immunosuppressive therapy. As above, depending on which organ system was affected and provided the irAE was lower grade, re-challenge with the same ICI is reasonable, and does not necessarily require prophylactic immunosuppressive therapy. There is insufficient evidence to support re-challenge with another class of ICI as traditionally ICI have not been studied in sequence in clinical trials; however, there are data to support ceasing doublet ICI treatment and continuing on with one of the single agent ICIs from the doublet regimen. If the irAE was severe enough, re-challenge is not recommended.
8. Hyper-Progression
A relatively new and controversial side effect of ICI use is the concept of hyperprogressive disease (HPD). There appear to be emerging data that a subset of patients treated with ICI experience rapid progression of disease and receive no benefit from IO. A full discussion on HPD is beyond the scope of this review; however, please see the Canadian consensus guideline by Dr. S. Laurie and colleagues for a review of the topic [76].
9. Conclusions
ICIs have become the mainstay of therapy in many cancer types, including lung cancers. Through their relatively non-specific upregulation of the immune system, a variety of irAEs can occur, the frequency of which depends on the patient, the cancer type, and the specific ICI antibody. Careful assessment of patients for irAEs through history taking, physical exam, and routine laboratory assessments are key to identifying irAEs at early stages, when they can potentially be managed more easily and before progressing to higher grades or more serious effects. Generally, most patients are eligible for re-challenge, and the use of corticosteroids to address an irAE is not associated with more poor patient outcomes.
Author Contributions
Writing—original draft preparation, C.H.C.; writing—review and editing, R.A.J.; supervision, R.A.J. All authors have read and agreed to the published version of the manuscript.
Funding
This article, as well as several others in the Special Issue, was supported by grants from Amgen Canada, AstraZeneca Canada Inc., Eisai Canada Limited, Hoffman La Roche Canada (journal publication fees only), Jazz Pharmaceuticals Canada Inc., Novartis Canada, Sanofi Canada, Pfizer Canada Inc. Funds were used to pay journal publication fees, provide administrative support, and honorariums for some authors. These entities did not influence the content of the articles, nor did they review the article prior to publication.
Conflicts of Interest
C.H.C.: none. R.A.J.: has participated on advisory boards for AbbVie, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, EMD Serono, Fusion Pharmaceuticals, Glaxo Smith Klein, Jazz Pharmaceuticals, Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Roche Canada, Sanofi/Regeneron, and Takeda; has received honoraria from Amgen, AstraZeneca, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis Pharmaceuticals Canada, and Roche Canada; and has received research funding from Astellas, AstraZeneca/MedImmune, Bristol-Myers Squibb, Debiopharm Group, Merck Sharp & Dohme, Novartis and Turnstone Bio.
References
- Sanmamed, M.F.; Chen, L. A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell 2018, 175, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chávez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suárez-Almazor, M.E. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Prim. 2020, 6, 1–21. [Google Scholar] [CrossRef]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.; Labarriere, N. PD-1 expression on tumor-specific T cells: Friend or foe for immunotherapy? OncoImmunology 2018, 7, e1364828. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Chen, Y.; Du, X.-J.; Liu, J.-Q.; Huang, C.-L.; Chen, L.; Zhou, G.-Q.; Li, W.-F.; Mao, Y.-P.; Hsu, C.; et al. Comparative safety of immune checkpoint inhibitors in cancer: Systematic review and network meta-analysis. BMJ 2018, 363, k4226. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Grizzi, G.; Ghidini, M.; Ghidini, A.; Ratti, M.; Panni, S.; Cabiddu, M.; Ghilardi, M.; Borgonovo, K.; Parati, M.C.; et al. Immune-related Adverse Events and Survival in Solid Tumors Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. J. Immunother. 2020, 43, 1–7. [Google Scholar] [CrossRef]
- Yoest, J.M. Clinical features, predictive correlates, and pathophysiology of immune-related adverse events in immune checkpoint inhibitor treatments in cancer: A short review. ImmunoTargets Ther. 2017, 6, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Parakh, S.; Cebon, J.; Klein, O. Delayed Autoimmune Toxicity Occurring Several Months After Cessation of Anti-PD-1 Therapy. Oncologist 2018, 23, 849–851. [Google Scholar] [CrossRef]
- Pauken, K.E.; Dougan, M.; Rose, N.R.; Lichtman, A.H.; Sharpe, A.H. Adverse Events Following Cancer Immunotherapy: Obstacles and Opportunities. Trends Immunol. 2019, 40, 511–523. [Google Scholar] [CrossRef]
- Khoja, L.; Day, D.; Chen, T.W.-W.; Siu, L.L.; Hansen, A.R. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: A systematic review. Ann. Oncol. 2017, 28, 2377–2385. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Saito, Y.; Okamoto, K.; Narumi, K.; Furugen, A.; Takekuma, Y.; Sugawara, M.; Kobayashi, M. Preexisting autoimmune disease is a risk factor for immune-related adverse events: A meta-analysis. Support. Care Cancer 2021, 29, 7747–7753. [Google Scholar] [CrossRef]
- Kartolo, A.; Sattar, J.; Sahai, V.; Baetz, T.; Lakoff, J.M. Predictors of Immunotherapy-Induced Immune-Related Adverse Events. Curr. Oncol. 2018, 25, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Eun, Y.; Kim, I.Y.; Sun, J.-M.; Lee, J.; Cha, H.-S.; Koh, E.-M.; Kim, H.; Lee, J. Risk factors for immune-related adverse events associated with anti-PD-1 pembrolizumab. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, C.; Garcia-Diaz, D.; Codner, E.; Salas-Pérez, F.; Carrasco, E.; Pérez-Bravo, F. PD-L1 gene polymorphisms and low serum level of PD-L1 protein are associated to type 1 diabetes in Chile. Diabetes/Metab. Res. Rev. 2014, 30, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, B.; Pearce, S.H.S.; Charlton, S.; Marshall, N.; Rowan, A.D.; Griffiths, I.D.; Kendall-Taylor, P.; Cawston, T.E.; Young-Min, S. An association between the CTLA4 exon 1 polymorphism and early rheumatoid arthritis with autoimmune endocrinopathies. Rheumatology 2002, 41, 180–183. [Google Scholar] [CrossRef]
- Klocke, K.; Sakaguchi, S.; Holmdahl, R.; Wing, K. Induction of autoimmune disease by deletion of CTLA-4 in mice in adulthood. Proc. Natl. Acad. Sci. USA 2016, 113, E2383–E2392. [Google Scholar] [CrossRef] [PubMed]
- Wing, K.; Onishi, Y.; Prieto-Martin, P.; Yamaguchi, T.; Miyara, M.; Fehervari, Z.; Nomura, T.; Sakaguchi, S. CTLA-4 Control over Foxp3+ Regulatory T Cell Function. Science 2008, 322, 271–275. [Google Scholar] [CrossRef]
- Lo, B.; Fritz, J.M.; Su, H.C.; Uzel, G.; Jordan, M.B.; Lenardo, M.J. CHAI and LATAIE: New genetic diseases of CTLA-4 checkpoint insufficiency. Blood 2016, 128, 1037–1042. [Google Scholar] [CrossRef]
- Selby, M.J.; Engelhardt, J.J.; Quigley, M.; Henning, K.A.; Chen, T.; Srinivasan, M.; Korman, A.J. Anti-CTLA-4 Antibodies of IgG2a Isotype Enhance Antitumor Activity through Reduction of Intratumoral Regulatory T Cells. Cancer Immunol. Res. 2013, 1, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Knochelmann, H.M.; Dwyer, C.; Bailey, S.; Amaya, S.M.; Elston, D.M.; Mazza-McCrann, J.M.; Paulos, C.M. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell. Mol. Immunol. 2018, 15, 458–469. [Google Scholar] [CrossRef] [PubMed]
- von Euw, E.; Chodon, T.; Attar, N.; Jalil, J.; Koya, R.C.; Comin-Anduix, B.; Ribas, A. CTLA4 blockade increases Th17 cells in patients with metastatic melanoma. J. Transl. Med. 2009, 7, 35. [Google Scholar] [CrossRef]
- Latchman, Y.E.; Liang, S.C.; Wu, Y.; Chernova, T.; Sobel, R.A.; Klemm, M.; Kuchroo, V.K.; Freeman, G.J.; Sharpe, A.H. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc. Natl. Acad. Sci. USA 2004, 101, 10691–10696. [Google Scholar] [CrossRef]
- Gianchecchi, E.; Fierabracci, A. Inhibitory Receptors and Pathways of Lymphocytes: The Role of PD-1 in Treg Development and Their Involvement in Autoimmunity Onset and Cancer Progression. Front. Immunol. 2018, 9, 2374. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Tanaka, Y.; Nishio, R.; Mitsuiye, T.; Mizoguchi, A.; Wang, J.; Ishida, M.; Hiai, H.; Matsumori, A.; Minato, N.; et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat. Med. 2003, 9, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Nose, M.; Hiai, H.; Minato, N.; Honjo, T. Development of Lupus-like Autoimmune Diseases by Disruption of the PD-1 Gene Encoding an ITIM Motif-Carrying Immunoreceptor. Immunity 1999, 11, 141–151. [Google Scholar] [CrossRef]
- Okazaki, T.; Otaka, Y.; Wang, J.; Hiai, H.; Takai, T.; Ravetch, J.V.; Honjo, T. Hydronephrosis associated with antiurothelial and antinuclear autoantibodies in BALB/c-Fcgr2b−/−Pdcd1−/− mice. J. Exp. Med. 2005, 202, 1643–1648. [Google Scholar] [CrossRef]
- Das, R.; Bar, N.; Ferreira, M.; Newman, A.; Zhang, L.; Bailur, J.K.; Bacchiocchi, A.; Kluger, H.; Wei, W.; Halaban, R.; et al. Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J. Clin. Investig. 2018, 128, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Michot, J.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Msc, E.H.B.; Fisher, D.E. Immune and molecular correlates in melanoma treated with immune checkpoint blockade. Cancer 2017, 123, 2143–2153. [Google Scholar] [CrossRef]
- Sandigursky, S.; Mor, A. Immune-Related Adverse Events in Cancer Patients Treated with Immune Checkpoint Inhibitors. Curr. Rheumatol. Rep. 2018, 20, 65. [Google Scholar] [CrossRef]
- Cheng, F.; Loscalzo, J. Autoimmune Cardiotoxicity of Cancer Immunotherapy. Trends Immunol. 2017, 38, 77–78. [Google Scholar] [CrossRef]
- Cancer Care Ontario Clinical Practice Guideline—Immune Checkpoint Inhibitor Toxicity Management 2018. Available online: https://www.cancercareontario.ca/sites/ccocancercare/files/guidelines/full/ImmuneCheckpointInhibitor.pdf (accessed on 22 September 2021).
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. Am. Soc. Clin. Oncol. J. 2018, 36, 1714–1768. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Chaudhary, N.; Garg, M.; Floudas, C.S.; Soni, P.; Chandra, A.B. Current Diagnosis and Management of Immune Related Adverse Events (irAEs) Induced by Immune Checkpoint Inhibitor Therapy. Front. Pharmacol. 2017, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Muntyanu, A.; Netchiporouk, E.; Gerstein, W.; Gniadecki, R.; Litvinov, I.V. Cutaneous Immune-Related Adverse Events (irAEs) to Immune Checkpoint Inhibitors: A Dermatology Perspective on Management. J. Cutan. Med. Surg. 2021, 25, 59–76. [Google Scholar] [CrossRef]
- Coleman, E.; Ko, C.; Dai, F.; Tomayko, M.M.; Kluger, H.; Leventhal, J.S. Inflammatory eruptions associated with immune checkpoint inhibitor therapy: A single-institution retrospective analysis with stratification of reactions by toxicity and implications for management. J. Am. Acad. Dermatol. 2019, 80, 990–997. [Google Scholar] [CrossRef]
- Sibaud, V. Dermatologic Reactions to Immune Checkpoint Inhibitors. Am. J. Clin. Dermatol. 2018, 19, 345–361. [Google Scholar] [CrossRef]
- Freeman-Keller, M.; Kim, Y.; Cronin, H.; Richards, A.; Gibney, G.T.; Weber, J. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin. Cancer Res. 2016, 22, 886–894. [Google Scholar] [CrossRef]
- Lee, C.K.M.; Li, S.; Tran, D.C.; Zhu, G.; Kim, J.; Kwong, B.Y.; Chang, A.L.S. Characterization of dermatitis after PD-1/PD-L1 inhibitor therapy and association with multiple oncologic outcomes: A retrospective case-control study. J. Am. Acad. Dermatol. 2018, 79, 1047–1052. [Google Scholar] [CrossRef]
- Sanlorenzo, M.; Vujic, I.; Daud, A.; Algazi, A.; Gubens, M.A.; Luna, S.A.; Lin, K.; Quaglino, P.; Rappersberger, K.; Ortiz-Urda, S. Pembrolizumab Cutaneous Adverse Events and Their Association with Disease Progression. JAMA Dermatol. 2015, 151, 1206–1212. [Google Scholar] [CrossRef]
- Barroso-Sousa, R.; Barry, W.T.; Garrido-Castro, A.C.; Hodi, F.S.; Min, L.; Krop, I.E.; Tolaney, S.M. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens. JAMA Oncol. 2018, 4, 173–182. [Google Scholar] [CrossRef]
- Byun, D.J.; Wolchok, J.D.; Rosenberg, L.M.; Girotra, M. Cancer immunotherapy—immune checkpoint blockade and associated endocrinopathies. Nat. Rev. Endocrinol. 2017, 13, 195–207. [Google Scholar] [CrossRef]
- Chang, L.-S.; Barroso-Sousa, R.; Tolaney, S.M.; Hodi, F.S.; Kaiser, U.B.; Min, L. Endocrine Toxicity of Cancer Immunotherapy Targeting Immune Checkpoints. Endocr. Rev. 2019, 40, 17–65. [Google Scholar] [CrossRef]
- Del Rivero, J.; Cordes, L.M.; Klubo-Gwiezdzinska, J.; Madan, R.A.; Nieman, L.K.; Gulley, J.L. Endocrine-Related Adverse Events Related to Immune Checkpoint Inhibitors: Proposed Algorithms for Management. Oncologist 2019, 25, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Brancatella, A.; Lupi, I.; Montanelli, L.; Ricci, D.; Viola, N.; Sgro, D.; Antonangeli, L.; Sardella, C.; Aragona, M.; Antonuzzo, A.; et al. Management of thyrotoxicosis induced by PD1 and PD-L1 blockade. J. Endocr. Soc. 2021, 5, bvab093. [Google Scholar] [CrossRef] [PubMed]
- de Filette, J.; Jansen, Y.; Schreuer, M.; Everaert, H.; Velkeniers, B.; Neyns, B.; Bravenboer, B. Incidence of Thyroid-Related Adverse Events in Melanoma Patients Treated with Pembrolizumab. J. Clin. Endocrinol. Metab. 2016, 101, 4431–4439. [Google Scholar] [CrossRef] [PubMed]
- Delivanis, D.A.; Gustafson, M.; Bornschlegl, S.; Merten, A.M.M.; Kottschade, A.L.; Withers, S.; Dietz, P.A.B.; Ryder, M. Pembrolizumab-Induced Thyroiditis: Comprehensive Clinical Review and Insights into Underlying Involved Mechanisms. J. Clin. Endocrinol. Metab. 2017, 102, 2770–2780. [Google Scholar] [CrossRef]
- Castinetti, F.; Albarel, F.; Archambeaud, F.; Bertherat, J.; Bouillet, B.; Buffier, P.; Briet, C.; Cariou, B.; Caron, P.; Chabre, O.; et al. French Endocrine Society Guidance on endocrine side effects of immunotherapy. Endocr.-Relat. Cancer 2018, 26, G1–G18. [Google Scholar] [CrossRef]
- El Sabbagh, R.; Azar, N.S.; Eid, A.A.; Azar, S.T. Thyroid Dysfunctions Due to Immune Checkpoint Inhibitors: A Review. Int. J. Gen. Med. 2020, 13, 1003–1009. [Google Scholar] [CrossRef]
- Marrone, K.A.; Ying, W.; Naidoo, J. Immune-Related Adverse Events from Immune Checkpoint Inhibitors. Clin. Pharmacol. Ther. 2016, 100, 242–251. [Google Scholar] [CrossRef]
- Fay, A.P.; Moreira, R.B.; Filho, P.R.S.N.; Albuquerque, C.; Barrios, C.H. The management of immune-related adverse events associated with immune checkpoint blockade. Expert Rev. Qual. Life Cancer Care 2016, 1, 89–97. [Google Scholar] [CrossRef][Green Version]
- Grouthier, V.; Lebrun-Vignes, B.; Moey, M.; Johnson, D.B.; Moslehi, J.J.; Salem, J.-E.; Bachelot, A. Immune Checkpoint Inhibitor-Associated Primary Adrenal Insufficiency: WHO VigiBase Report Analysis. Oncologist 2020, 25, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Lacchetti, C.; Thompson, J.A. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline Summary. J. Oncol. Pr. 2018, 14, 247–249. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, E.; Rodríguez-Abreu, D.; on behalf of the Spanish Group for Cancer. Immuno-Biotherapy (GETICA) Immune Checkpoint Inhibitors: Review and Management of Endocrine Adverse Events. Oncologist 2016, 21, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Foppen, M.H.G.; Rozeman, E.A.; van Wilpe, S.; Postma, C.; Snaebjornsson, P.; van Thienen, J.V.; van Leerdam, M.E.; Heuvel, M.V.D.; Blank, C.U.; van Dieren, J.; et al. Immune checkpoint inhibition-related colitis: Symptoms, endoscopic features, histology and response to management. ESMO Open 2018, 3, e000278. [Google Scholar] [CrossRef] [PubMed]
- Hassel, J.C.; Heinzerling, L.; Aberle, J.; Bähr, O.; Eigentler, T.K.; Grimm, M.-O.; Grünwald, V.; Leipe, J.; Reinmuth, N.; Tietze, J.K.; et al. Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): Evaluation and management of adverse drug reactions. Cancer Treat. Rev. 2017, 57, 36–49. [Google Scholar] [CrossRef]
- Friedman, C.; Proverbs-Singh, T.A.; Postow, M.A. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors. JAMA Oncol. 2016, 2, 1346–1353. [Google Scholar] [CrossRef]
- Larkin, J.; Sileni, V.C.; Gonzalez, R.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Schachter, J.; Long, G.; Arance, A.; Grob, J.-J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Haanen, J.; Carbonnel, F.; Robert, C.; Kerr, K.; Peters, S.; Larkin, J.; Jordan, K. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv119–iv142. [Google Scholar] [CrossRef]
- Tabchi, S.; Messier, C.; Blais, N. Immune-mediated respiratory adverse events of checkpoint inhibitors. Curr. Opin. Oncol. 2016, 28, 269–277. [Google Scholar] [CrossRef]
- Sise, M.E.; Seethapathy, H.; Reynolds, K.L. Diagnosis and Management of Immune Checkpoint Inhibitor-Associated Renal Toxicity: Illustrative Case and Review. Oncologist 2019, 24, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Espi, M.; Teuma, C.; Novel-Catin, E.; Maillet, D.; Souquet, P.; Dalle, S.; Koppe, L.; Fouque, D. Renal adverse effects of immune checkpoints inhibitors in clinical practice: ImmuNoTox study. Eur. J. Cancer 2021, 147, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, F.B.; Marrone, K.A.; Troxell, M.L.; Ralto, K.M.; Hoenig, M.P.; Brahmer, J.R.; Le, D.T.; Lipson, E.J.; Glezerman, I.G.; Wolchok, J.; et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016, 90, 638–647. [Google Scholar] [CrossRef]
- Cuzzubbo, S.; Javeri, F.; Tissier, M.; Roumi, A.; Barlog, C.; Doridam, J.; Lebbe, C.; Belin, C.; Ursu, R.; Carpentier, A. Neurological adverse events associated with immune checkpoint inhibitors: Review of the literature. Eur. J. Cancer 2017, 73, 1–8. [Google Scholar] [CrossRef]
- Marini, A.; Bernardini, A.; Gigli, G.L.; Valente, M.; Muniz-Castrillo, S.; Honnorat, J.; Vogrig, A. Neurologic adverse events of immune checkpoint inhibitors: A systematic review. Neurology 2021, 96, 754–766. [Google Scholar] [CrossRef]
- Arbour, K.C.; Mezquita, L.; Long, N.; Rizvi, H.; Auclin, E.; Ni, A.; Martínez-Bernal, G.; Ferrara, R.; Lai, W.V.; Hendriks, L.E.L.; et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients with Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 2872–2878. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Huang, X.; Li, J.; Ma, H.; Zeng, R. Impact of corticosteroid use on outcomes of non–small-cell lung cancer patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. J. Clin. Pharm. Ther. 2021, 46, 927–935. [Google Scholar] [CrossRef]
- Li, J.; Yang, K.; Zhao, L.; Bai, C.; Sun, Z. Impact of corticosteroids use on efficacy of immune checkpoint inhibitors in cancer patients: A meta-analysis. J. Clin. Oncol. 2020, 38, e15234. [Google Scholar] [CrossRef]
- Pinato, D.J.; Kaseb, A.; Wang, Y.; Saeed, A.; Szafron, D.; Jun, T.; Dharmapuri, S.; Naqash, A.R.; Muzaffar, M.; Navaid, M.; et al. Impact of corticosteroid therapy on the outcomes of hepatocellular carcinoma treated with immune checkpoint inhibitor therapy. J. Immunother. Cancer 2020, 8, e000726. [Google Scholar] [CrossRef] [PubMed]
- Santini, F.C.; Rizvi, H.; Plodkowski, A.J.; Ni, A.; Lacouture, M.E.; Gambarin-Gelwan, M.; Wilkins, O.; Panora, E.; Halpenny, D.F.; Long, N.M.; et al. Safety and Efficacy of Re-treating with Immunotherapy after Immune-Related Adverse Events in Patients with NSCLC. Cancer Immunol. Res. 2018, 6, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Dolladille, C.; Ederhy, S.; Sassier, M.; Cautela, J.; Thuny, F.; Cohen, A.A.; Fedrizzi, S.; Chrétien, B.; DA Silva, A.; Plane, A.-F.; et al. Immune Checkpoint Inhibitor Rechallenge After Immune-Related Adverse Events in Patients with Cancer. JAMA Oncol. 2020, 6, 865–871. [Google Scholar] [CrossRef]
- Abu-Sbeih, H.; Ali, F.S.; Naqash, A.R.; Owen, D.; Patel, S.; Otterson, G.A.; Kendra, K.; Ricciuti, B.; Chiari, R.; De Giglio, A.; et al. Resumption of Immune Checkpoint Inhibitor Therapy After Immune-Mediated Colitis. J. Clin. Oncol. 2019, 37, 2738–2745. [Google Scholar] [CrossRef] [PubMed]
- Pollack, M.; Betof, A.; Dearden, H.; Rapazzo, K.; Valentine, I.; Brohl, A.; Ancell, K.; Long, G.; Menzies, A.; Eroglu, Z.; et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann. Oncol. 2018, 29, 250–255. [Google Scholar] [CrossRef]
- Simonaggio, A.; Michot, J.M.; Voisin, A.L.; Le Pavec, J.; Collins, M.; Lallart, A.; Cengizalp, G.; Vozy, A.; Laparra, A.; Varga, A.; et al. Evaluation of Readministration of Immune Checkpoint Inhibitors After Immune-Related Adverse Events in Patients with Cancer. JAMA Oncol. 2019, 5, 1310–1317. [Google Scholar] [CrossRef]
- Laurie, S.A.; Banerji, S.; Blais, N.; Brule, S.; Cheema, P.K.; Cheung, P.; Daaboul, N.; Hao, D.; Hirsh, V.; Juergens, R.; et al. Canadian Consensus: Oligoprogressive, Pseudoprogressive, and Oligometastatic Non-Small-Cell Lung Cancer. Curr. Oncol. 2019, 26, 81–93. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).