The Role of a Navigational Radiofrequency Ablation Device and Concurrent Vertebral Augmentation for Treatment of Difficult-to-Reach Spinal Metastases
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population and Inclusion Criteria
2.2. Group Definition and Preoperative Assessment
- (1)
- Group A: patients with 1 or 2 vertebral lesions.
- (2)
- Group B: patients having more than 2 metastatic spine lesions.
2.3. Target Radiofrequency Ablation (tRFA) and Vertebral Augmentation (VA) Procedure
2.4. Postoperative Follow Up
2.5. Statistical Analysis
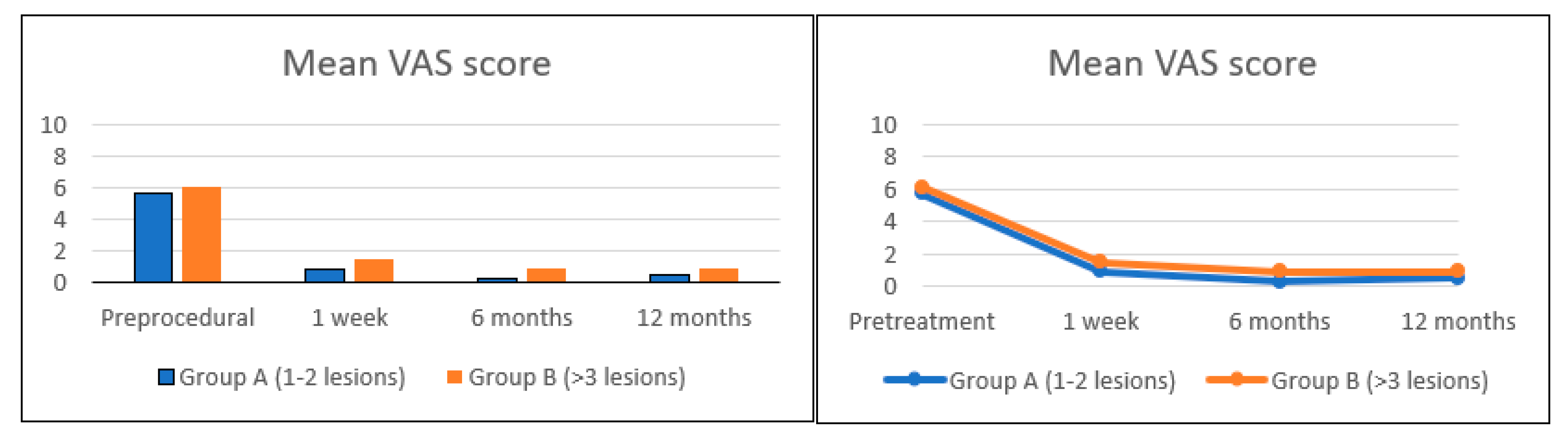
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coleman, R. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001, 27, 165–176. [Google Scholar] [CrossRef]
- Yang, P.-L.; He, X.-J.; Li, H.-P.; Zang, Q.-J.; Wang, G.-Y. Image-guided minimally invasive percutaneous treatment of spinal metastasis. Exp. Ther. Med. 2017, 13, 705–709. [Google Scholar] [CrossRef]
- Shah, L.M.; Salzman, K.L. Imaging of Spinal Metastatic Disease. Int. J. Surg. Oncol. 2011, 2011, 769753. [Google Scholar] [CrossRef] [PubMed]
- Reyes, M.; Georgy, M.; Brook, L.; Ortiz, O.; Brook, A.; Agarwal, V.; Muto, M.; Manfre, L.; Marcia, S.; Georgy, B.A. Multicenter clinical and imaging evaluation of targeted radiofrequency ablation (t-RFA) and cement augmentation of neoplastic vertebral lesions. J. Neurointerv. Surg. 2018, 10, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wallace, A.N.; Madaelil, T.P.; Jennings, J.W. Treatment of osseous metastases using the Spinal Tumor Ablation with Radiofrequency (STAR) system. Expert Rev. Med. Devices 2016, 13, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Dosani, M.; Lucas, S.; Wong, J.; Weir, L.; Lomas, S.; Cumayas, C.; Fisher, C.; Tyldesley, S. Impact of the Spinal Instability Neoplastic Score on Surgical Referral Patterns and Outcomes. Curr. Oncol. 2018, 25, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Bollen, L.; Van Der Linden, Y.M.; Pondaag, W.; Fiocco, M.; Pattynama, B.P.M.; Marijnen, C.; Nelissen, R.; Peul, W.C.; Dijkstra, P.S. Prognostic factors associated with survival in patients with symptomatic spinal bone metastases: A retrospective cohort study of 1 043 patients. Neuro-Oncology 2014, 16, 991–998. [Google Scholar] [CrossRef]
- Pusceddu, C.; Sotgia, B.; Fele, R.M.; Ballicu, N.; Melis, L. Combined Microwave Ablation and Cementoplasty in Patients with Painful Bone Metastases at High Risk of Fracture. Cardiovasc. Interv. Radiol. 2016, 39, 74–80. [Google Scholar] [CrossRef]
- Tomasian, A.; Jennings, J.W. Percutaneous minimally invasive thermal ablation for management of osseous metastases: Recent advances. Int. J. Hyperth. 2019, 36, 3–12. [Google Scholar] [CrossRef]
- Pusceddu, C.; Dessì, G.; Melis, L.; Fancellu, A.; Ruggiu, G.; Sailis, P.; Congia, S.; Derudas, D.; Cau, R.; Senis, I.; et al. Combined Microwave Ablation and Osteosynthesis for Long Bone Metastases. Medicina 2021, 57, 825. [Google Scholar] [CrossRef]
- Delank, K.S.; Wendtner, C.; Eich, H.T.; Eysel, P. The treatment of spinal metastases. Dtsch. Arztebl. Int. 2011, 108, 71–79; quiz 80. [Google Scholar] [CrossRef]
- NCCN Guidelines Version 2.2021. Metastatic Spine Tumors. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf (accessed on 15 June 2021).
- Hillen, T.J.; Anchala, P.; Friedman, M.V.; Jennings, J.W. Treatment of metastatic posterior vertebral body osseous tumors by using a targeted bipolar radiofrequency ablation device: Technical note. Radiology 2014, 273, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Anchala, P.R.; Irving, W.D.; Hillen, T.J.; Friedman, M.V.; Georgy, B.A.; Coldwell, D.M.; Tran, N.D.; Vrionis, F.D.; Brook, A.; Jennings, J.W. Treatment of metastatic spinal lesions with a navigational bipolar radiofrequency ablation device: A multicenter retrospective study. Pain Physician 2014, 17, 317–327. [Google Scholar] [PubMed]
- Tomasian, A.; Jennings, J.W. Spinal Osteoid Osteoma: Percutaneous Radiofrequency Ablation Using a Navigational Bipolar Electrode System. AJR Am. J. Roentgenol. 2018, 211, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Delgado, D.A.; Lambert, B.S.; Boutris, N.; Mcculloch, P.; Robbins, A.B.; Moreno, M.; Harris, J.D. Validation of Digital Visual Analog Scale Pain Scoring with a Traditional Paper-based Visual Analog Scale in Adults. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2018, 2, e088. [Google Scholar] [CrossRef]
- Deschamps, F.; Farouil, G.; Ternes, N.; Gaudin, A.; Hakime, A.; Tselikas, L.; Teriitehau, C.; Baudin, E.; Auperin, A.; De Baere, T. Thermal ablation techniques: A curative treatment of bone metastases in selected patients? Eur. Radiol. 2014, 24, 1971–1980. [Google Scholar] [CrossRef]
- Khan, L.; Mitera, G.; Probyn, L.; Ford, M.; Christakis, M.; Finkelstein, J.; Donovan, A.; Zhang, L.; Zeng, L.; Rubenstein, J.; et al. Inter-Rater Reliability between Musculoskeletal Radiologists and Orthopedic Surgeons on Computed Tomography Imaging Features of Spinal Metastases. Curr. Oncol. 2011, 18, e282–e287. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Clemons, M.; Gelmon, K.A.; Pritchard, K.I.; Paterson, A.H. Bone-targeted agents and skeletal-related events in breast cancer patients with bone metastases: The state of the art. Curr. Oncol. 2012, 19, 259–268. [Google Scholar] [CrossRef]
- Muto, M.; Guarnieri, G.; Giurazza, F.; Manfrè, L. What’s new in vertebral cementoplasty? Br. J. Radiol. 2016, 89, 20150337. [Google Scholar] [CrossRef]
- Robson, P. Metastatic spinal cord compression: A rare but important complication of cancer. Clin. Med. 2014, 14, 542–554. [Google Scholar] [CrossRef]
- Boussios, S.; Cooke, D.; Hayward, C.; Kanellos, F.S.; Tsiouris, A.K.; Chatziantoniou, A.A.; Zakynthinakis-Kyriakou, N.; Karathanasi, A. Metastatic Spinal Cord Compression: Unraveling the Diagnostic and Therapeutic Challenges. Anticancer. Res. 2018, 38, 4987–4997. [Google Scholar] [CrossRef]
- Coleman, R.E.; Croucher, P.I.; Padhani, A.R. Bone metastases. Nat. Rev. Dis. Primers 2020, 15, 83. [Google Scholar] [CrossRef] [PubMed]
- Sahgal, A.; Whyne, C.M.; Ma, L.; Larson, D.A.; Fehlings, M.G. Vertebral compression fracture after stereotactic body radiotherapy for spinal metastases. Lancet Oncol. 2013, 14, e310–e320. [Google Scholar] [CrossRef]
- Sahgal, A.; Atenafu, E.G.; Chao, S.; Al-Omair, A.; Boehling, N.; Balagamwala, E.H.; Cunha, M.; Thibault, I.; Angelov, L.; Brown, P.; et al. Vertebral compression fracture after spine stereotactic body radiotherapy: A multi-institutional analysis with a focus on radiation dose and the spinal instability neoplastic score. J. Clin. Oncol. 2013, 31, 3426–3431. [Google Scholar] [CrossRef] [PubMed]
- Tomasian, A.; Gangi, A.; Wallace, A.N.; Jennings, J.W. Percutaneous Thermal Ablation of Spinal Metastases: Recent Advances and Review. AJR Am. J. Roentgenol. 2018, 210, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Georgy, B.A. Metastatic Spinal Lesions: State-of-theArt, treatment options and future trends. AJNR Am. J. Neuroradiol. 2008, 29, 1605–1611. [Google Scholar] [CrossRef]
- Deschamps, F.; de Baere, T. Cementoplasty of bone metastases. Diagn. Interv. Imaging 2012, 93, 685–689. [Google Scholar] [CrossRef]
- Anselmetti, G.C.; Manca, A.; Kanika, K.; Murphy, K.; Eminefendic, H.; Masala, S.A.; Regge, D. Temperature Measurement During Polymerization of Bone Cement in Percutaneous Vertebroplasty: An In Vivo Study in Humans. Cardiovasc. Interv. Radiol. 2009, 32, 491–498. [Google Scholar] [CrossRef]
- Mohme, M.; Riethdorf, S.; Dreimann, M.; Werner, S.; Maire, C.L.; Joosse, S.; Bludau, F.; Mueller, V.; Neves, R.; Stoecklein, N.; et al. Circulating Tumour Cell Release after Cement Augmentation of Vertebral Metastases. Sci. Rep. 2017, 7, 7196. [Google Scholar] [CrossRef]
- Greenwood, T.J.; Wallace, A.; Friedman, M.V.; Hillen, T.J.; Robinson, C.G.; Jennings, J.W. Combined Ablation and Radiation Therapy of Spinal Metastases: A Novel Multimodality Treatment Approach. Pain Physician 2015, 18, 573–581. [Google Scholar] [CrossRef]
- Wallace, A.N.; Tomasian, A.; Vaswani, D.; Vyhmeister, R.; Chang, R.O.; Jennings, J.W. Radiographic Local Control of Spinal Metastases with Percutaneous Radiofrequency Ablation and Vertebral Augmentation. AJNR Am. J. Neuroradiol. 2016, 37, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; DeGroot, H., III. Evaluation of the risk of pathologic fractures secondary to metastatic bone disease. Orthopedics 2001, 24, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Willeumier, J.J.; van der Linden, Y.M.; Dijkstra, P.D. Lack of clinical evidence for postoperative radiotherapy after surgical fixation of impending or actual pathologic fractures in the long bones in patients with cancer; a systematic review. Radiother. Oncol. 2016, 121, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Di Staso, M.; Zugaro, L.; Gravina, G.L.; Bonfili, P.; Marampon, F.; di Nicola, L.; Conchiglia, A.; Ventura, L.; Franzese, P.; Gallucci, M.; et al. A feasibility study of percutaneous Radiofrequency Ablation followed by Radiotherapy in the management of painful osteolytic bone metastases. Eur. Radiol. 2011, 21, 2004–2010. [Google Scholar] [CrossRef]
- Cappuccio, M.; Bandiera, S.; Babbi, L. Management of bone metastases. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 407–414. [Google Scholar] [PubMed]
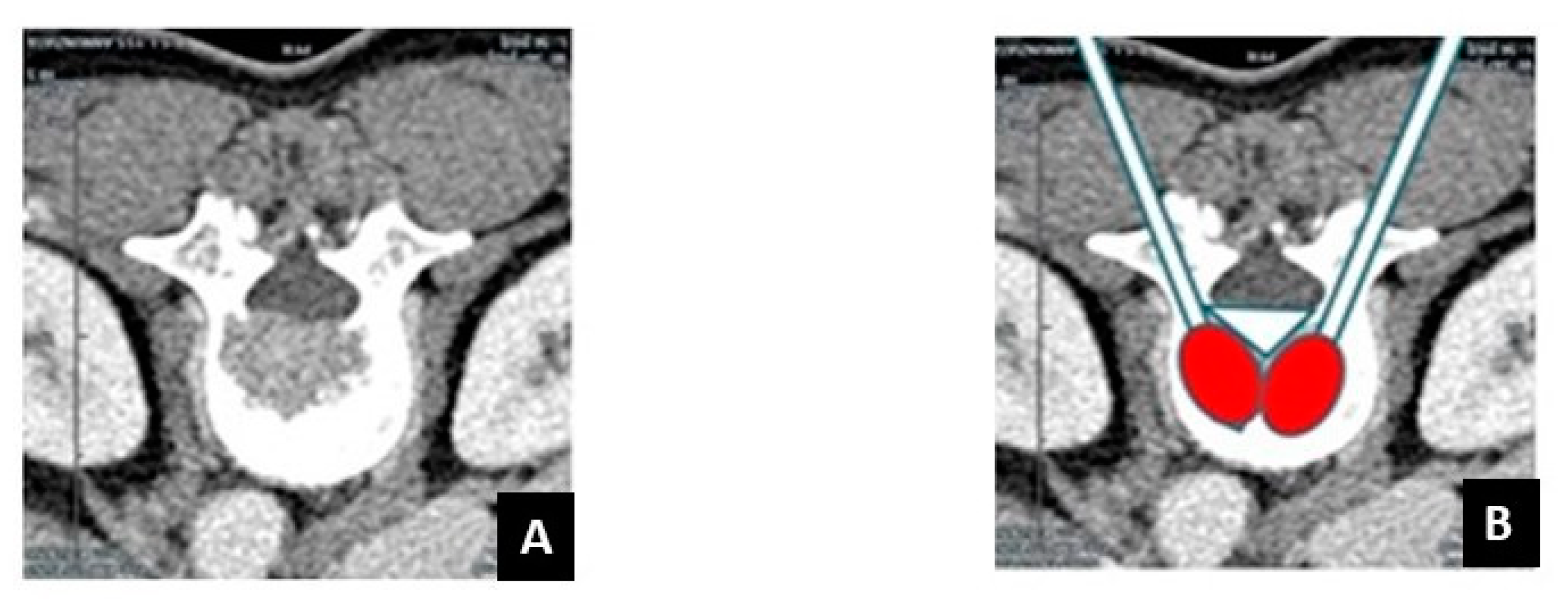
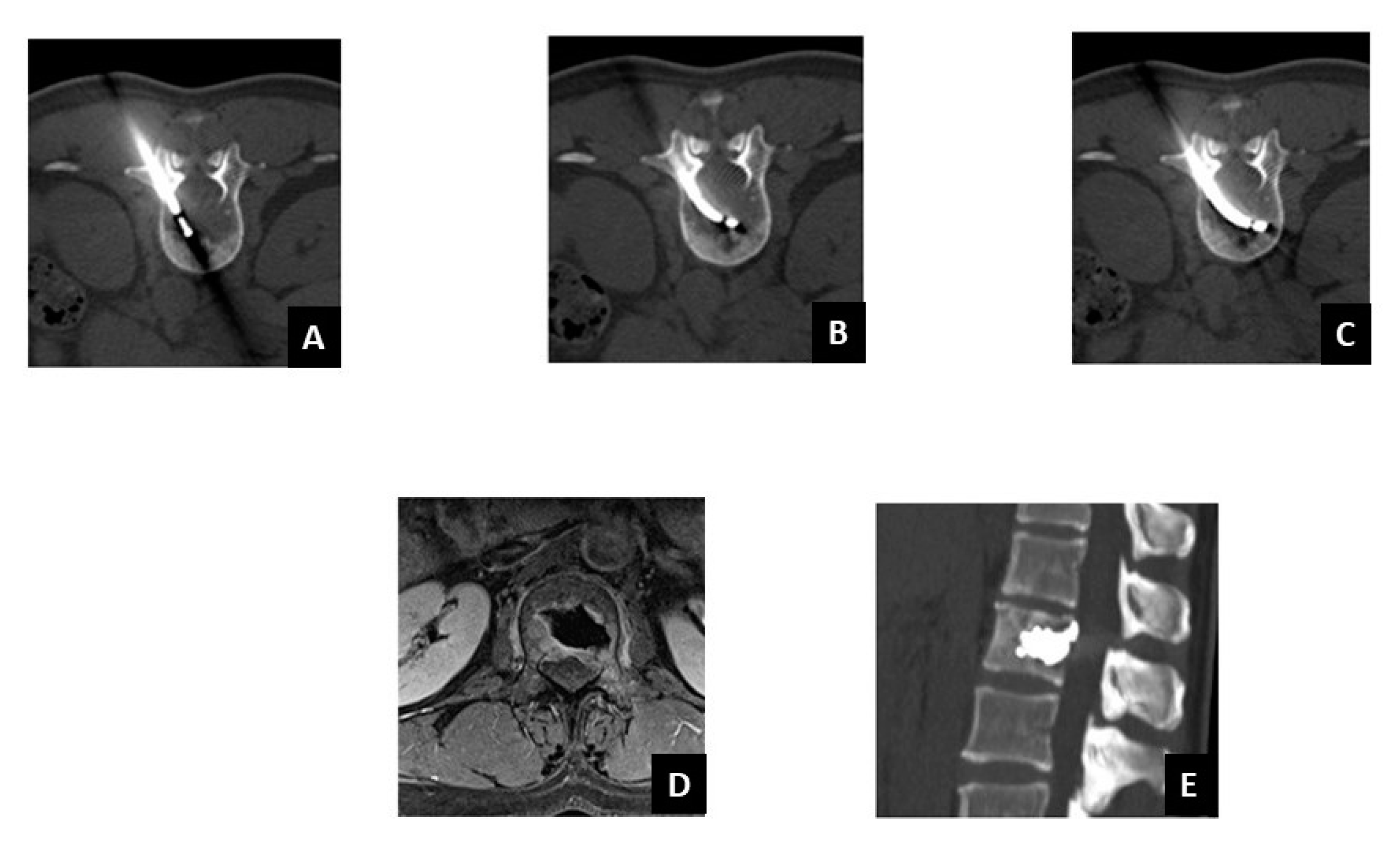
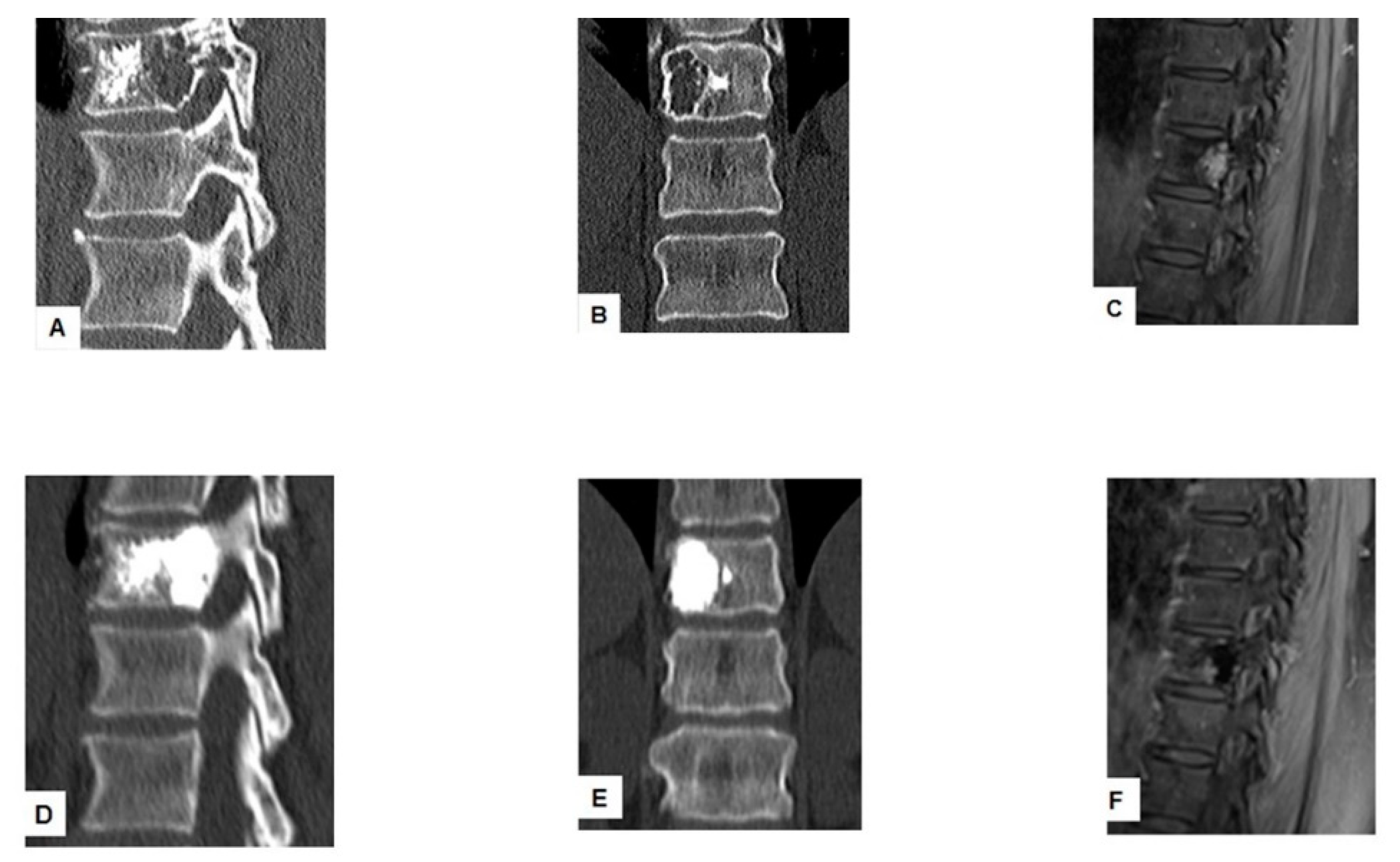
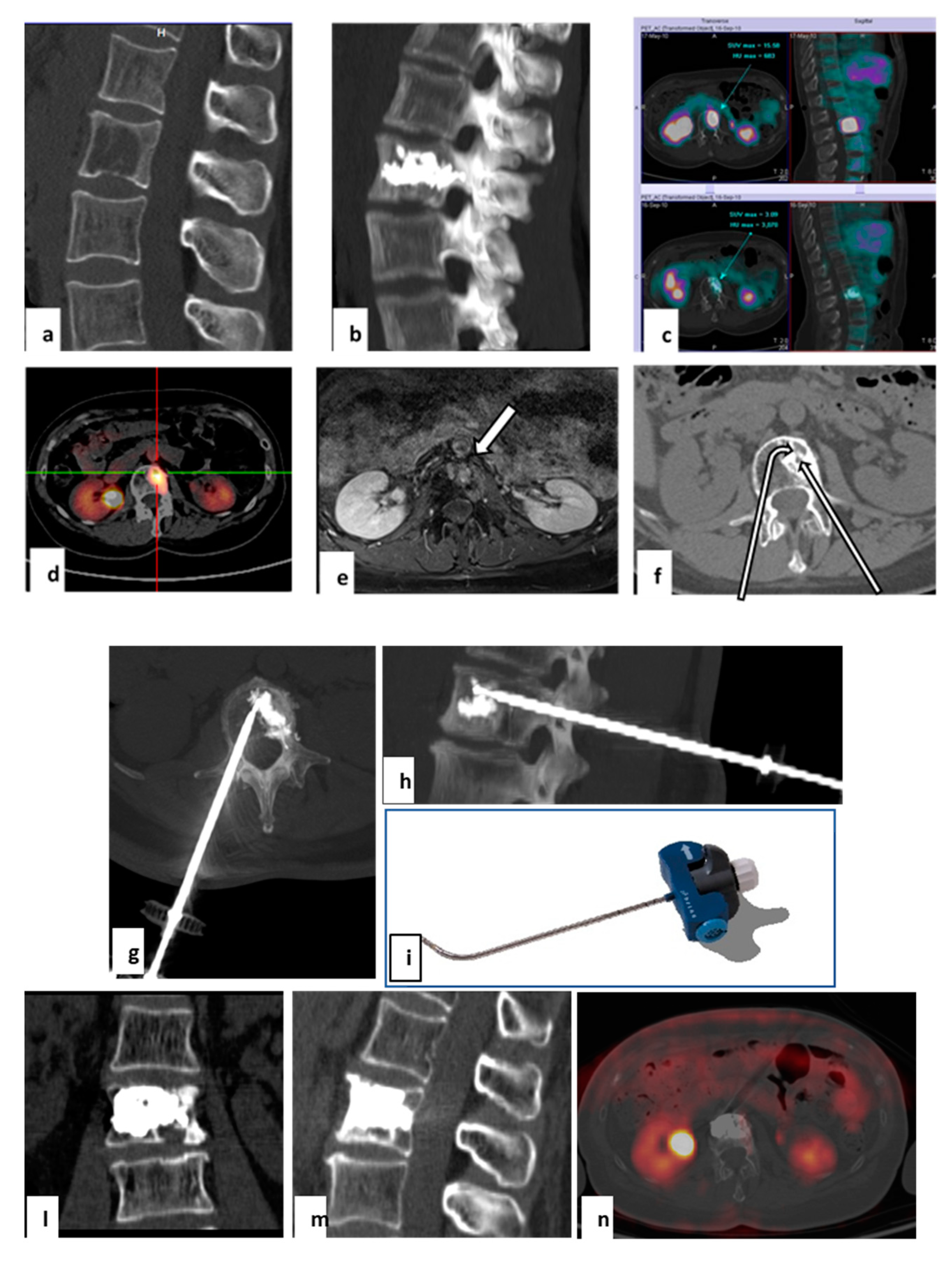
| Author | Country | Year | No of Patients/No of Treated Lesions | Inclusion Criteria | Treatment of Spinal Lesions | Main Conclusion(s) |
|---|---|---|---|---|---|---|
| Anchala [14] * | US | 2014 | 92/128 | Spine metastatic lesions | tRFA ± VA | The STAR System is an RFA device that was safely and effectively used in the treatment of spine metastatic osseous lesions. |
| Hillen [13] | US | 2014 | 26/47 | Posterior vertebral body tumors | tRFA + VA | Targeted RFA with a newly developed articulating device is both feasible and safe for the treatment of painful posterior vertebral body metastatic tumors. |
| Reyes [4] * | US/Italy | 2017 | 49/72 | Vertebral metastases | tRFA + VA | tRFA followed by vertebral augmentation in malignant vertebral lesions resulted in significant pain reduction and functional status improvement. |
| Tomasian [15] | US | 2018 | 7/7 | Spinal osteoid osteomas | tRFA | Safe and effective percutaneous CT-guided radiofrequency ablation of spinal osteoid osteomas can be performed using a targeted navigational bipolar electrode system. |
| 3 | Primary Tumor | Baseline Imaging | Site of Treated Vertebrae | Baseline LXD (cm × cm) | RT before/after tRFA | Complication | VAS before/after tRFA | VAS 6 Months | VAS 12 Months | Imaging Post tRFA | Status-Follow Up (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 49-F | Melanoma | CT-PET | T10 | 1.5 × 1.2 | No | no | 3/0 | 0 | 0 | CT-Scintigraphy | alive—46 |
| 51-F | Breast | CT- PET | L1 | 3.2 × 2 | No | no | 3/0 | 0 | 0 | MRI | alive—45 |
| 39-F | Breast | Scintigraphy-MRI | T8 | 1.8 × 1.6 | After | no | 4/0 | 0 | 0 | MRI | alive—40 |
| 58-F | Breast | PET-CT-MRI | L2, L4 | 2.1 × 1.8–2 × 1.4 | After | no | 4/0 | 0 | 0 | MRI | alive—40 |
| 47-F | Breast | MRI-CT-PECT | T9 | 2.6 × 2 | Before | no | 5/0 | 0 | 0 | CT-Scintigraphy | alive—34 |
| 57-F | Breast | CT-MRI-PET | L2 | 2 × 1 | Before | no | 2/0 | 0 | 0 | MRI-PET | alive—28 |
| 78-M | Prostate | CT | T3 | 1.8 × 1.5 | No | no | 5/0 | 0 | 0 | CT-MRI | death—7 |
| 66-F | Breast | CT-MRI-PET | L3 | 1.2 × 1.3 | No | no | 5/0 | 0 | 0 | MRI-PET | alive—22 |
| 72-F | Colon | CT-PET | L3 | 2.4 × 2 | Before | no | 6/0 | 0 | 0 | CT | death—10 |
| 65-F | Breast | CT-PET | L3 | 2.3 × 2 | Before | no | 7/2 | 0 | 0 | MRI | alive—21 |
| 70-M | Prostate | CT-PET | T10 | 2 × 2.2 | No | no | 6/0 | 0 | 0 | CT-MRI | alive—19 |
| 70-M | Colon | CT | T12 | 2.9 × 2.4 | Before | no | 4/0 | 0 | 0 | MRI | alive—19 |
| 56-F | Breast | CT | L3 | 3 × 2.3 | No | no | 7/2 | 0 | 0 | CT-MRI | alive—19 |
| 62-F | Lung | CT-MRI | L1 | 2.4 × 1.6 | No | no | 7/0 | 0 | 0 | CT | alive—15 |
| 76-F | Breast | CT | T7, T10 | 2.1 × 1.8–2 × 1 | No | no | 8/2 | 0 | 0 | CT | alive—13 |
| 56-F | Breast | CT-MRI | T9 | 1.3 × 1.1 | No | no | 4/0 | 0 | 0 | CT-PET | alive—12 |
| 57-F | Breast | CT-MRI-PET | L2 | 1 × 1 | No | no | 4/0 | 0 | 0 | MRI-PET | alive—8 |
| 71-F | Multiple myeloma | CT-MRI | S1 | 5 × 4 | No | no | 7/2 | 0 | N/A | CT-MRI | alive—10 |
| 54-M | Lung | CT | T8 | 3.2 × 1.2 | No | no | 8/0 | N/A | N/A | CT | alive—4 |
| 57-F | Breast | CT | T6, T8 | 2 × 2–2 × 2.05 | No | no | 8/0 | N/A | N/A | CT | alive—4 |
| 55-M | Prostate | CT | T11 | 3 × 3 | No | no | 6/2 | N/A | N/A | CT | alive—4 |
| Age-Gender | Primary Tumor | Baseline Imaging | Site of Treated Vertebrae | Baseline LXD (cm × cm) | RT before/after tRFA | Complication | VAS before/after tRFA | VAS 6 Months | VAS 12 Months | Imaging Post tRFA | Status-Follow Up (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 67-F | Breast | CT-PET-MRI | L2 | 1.9 × 2 | before | No | 4/0 | 0 | 0 | PET | alive—33 |
| 57-F | Breast | CT-MRI | T8 | 1.06 × 1 | No | No | 5/0 | 0 | 0 | MRI-CT | alive—37 |
| 59-M | Lung | CT | L1 | 3 × 3.5 | before | No | 6/0 | 0 | 0 | CT-Scintigraphy | death—4 |
| 57-M | Colon | CT | L5 | 4.5 × 3.8 | after | No | 8/3 | 3 | N/A | MRI | death—12 |
| 70-F | Thyroid | CT-Scintigraphy | T10 | 2.3 × 0.7 | No | No | 3/0 | 0 | 0 | MRI-Scintigraphy | alive—24 |
| 68-M | Kidney | CT | T11, L2 | 2.4 × 2.2–2.4 × 1.5 | before | posterior leakage | 8/2 | 3 | 5 | MRI | death—19 |
| 41 -F | Sarcoma | CT | L3, L4 | 2.2 × 1.7–2 × 1.4 | No | No | 8/3 | 2 | 0 | CT | death—12 |
| 79-M | Lung | CT | T4 | 3.1 × 2.5 | No | No | 8/5 | N/A | N/A | CT | death—4 |
| 40-M | Adrenal gland | CT | L1 | 2.9 × 3.1 | No | No | 7/1 | 0 | 0 | CT | death—4 |
| 71-M | Lung | CT-PET | L4 | 4.6 × 3.7 | After | posterior leakage | 8.5/3 | N/A | N/A | CT | death—5 |
| 66-M | Kidney | CT | T12 | 5 × 3 | After | lateral leakage | 9/3 | 0 | 0 | CT | alive—11 |
| 65-M | Colon | CT-MRI | L1 | 1.8 × 1.3 | before | No | 6/0 | 0 | 0 | CT | death—6 |
| 71-F | Breast | CT-MRI | L2, L3 | 3 × 2.7–2.9 × 2.8 | before | No | 6/0 | 3 | 3 | CT | alive—10 |
| 58-F | Breast | CT | T12 | 1.7 × 1.5 | No | No | 6/0 | N/A | N/A | CT | alive—5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pusceddu, C.; De Francesco, D.; Melis, L.; Ballicu, N.; Fancellu, A. The Role of a Navigational Radiofrequency Ablation Device and Concurrent Vertebral Augmentation for Treatment of Difficult-to-Reach Spinal Metastases. Curr. Oncol. 2021, 28, 4004-4015. https://doi.org/10.3390/curroncol28050340
Pusceddu C, De Francesco D, Melis L, Ballicu N, Fancellu A. The Role of a Navigational Radiofrequency Ablation Device and Concurrent Vertebral Augmentation for Treatment of Difficult-to-Reach Spinal Metastases. Current Oncology. 2021; 28(5):4004-4015. https://doi.org/10.3390/curroncol28050340
Chicago/Turabian StylePusceddu, Claudio, Davide De Francesco, Luca Melis, Nicola Ballicu, and Alessandro Fancellu. 2021. "The Role of a Navigational Radiofrequency Ablation Device and Concurrent Vertebral Augmentation for Treatment of Difficult-to-Reach Spinal Metastases" Current Oncology 28, no. 5: 4004-4015. https://doi.org/10.3390/curroncol28050340
APA StylePusceddu, C., De Francesco, D., Melis, L., Ballicu, N., & Fancellu, A. (2021). The Role of a Navigational Radiofrequency Ablation Device and Concurrent Vertebral Augmentation for Treatment of Difficult-to-Reach Spinal Metastases. Current Oncology, 28(5), 4004-4015. https://doi.org/10.3390/curroncol28050340






