Abstract
Cognitive assessment is a cornerstone of geriatric care. Cognitive impairment has the potential to significantly impact multiple phases of a person’s cancer care experience. Accurately identifying this vulnerability is a challenge for many cancer care clinicians, thus the use of validated cognitive assessment tools are recommended. As international cancer guidelines for older adults recommend Geriatric Assessment (GA) which includes an evaluation of cognition, clinicians need to be familiar with the overall interpretation of the commonly used cognitive assessment tools. This rapid review investigated the cognitive assessment tools that were most frequently recommended by Geriatric Oncology guidelines: Blessed Orientation-Memory-Concentration test (BOMC), Clock Drawing Test (CDT), Mini-Cog, Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), and Short Portable Mental Status Questionnaire (SPMSQ). A detailed appraisal of the strengths and limitations of each tool was conducted, with a focus on practical aspects of implementing cognitive assessment tools into real-world clinical settings. Finally, recommendations on choosing an assessment tool and the additional considerations beyond screening are discussed.
1. Introduction
Cognitive impairment affects various aspects of care in older adults with cancer, such as the ability to participate in clinical decision-making, cope with treatment, and self-manage treatment regimens, as well as being associated with worse cancer and non-cancer outcomes [1,2,3,4,5]. Cancer and cognitive impairment are both associated with ageing [6]. Three out of every five new cancer diagnoses are in people aged 65 years or older, and 15–48% of patients 70 years and older with cancer have impairment detected on cognitive testing [6]. However, cognitive impairment is often subtle and easily overlooked by clinicians on a routine clinical assessment [7]. Therefore, a systematic approach to cognitive assessment in older adults with cancer is required [7].
At diagnosis, a patient is expected to participate in often complex decision-making that requires the ability to consider concepts which are multidimensional, as well as be able to weigh up potential trade-offs, such as quality of life and anticipated prognosis [1,2,5]. Moreover, patients need to consider decisions which have significant implications for future care, such as naming a power of attorney or substitute decision-maker, completing a will, and participating in advance care planning conversations on future care decisions [3,5,6,8]. During the treatment phase, cognitive limitations may increase the risk of medication administration errors, amplified in the context of self-management of oral chemotherapy regimens at home, as well as making the logistics of attending regular appointments more challenging [4]. Adapting to changes in daily routine due to treatment-associated side effects or reduction in functional ability may exceed the mental flexibility of those with executive impairment. This can have profound impacts such as increased hospitalizations during treatment, reduced ability to report complications, increased need for caregiver support potentially contributing to caregiver distress, and even compromised independence with potential increased relocation to supported accommodation [6,8]. It is not unexpected that cognitive impairment is associated with worse cancer and non-cancer outcomes including increased toxicity from the treatment, lower completion rate of treatment, worse overall survival, increased caregiver burden, cognitive decline with the treatment, and poorer patient-reported outcomes [1,2,3,4].
The term cognitive impairment encompasses a variety of neurocognitive conditions, including dementia, delirium, and Cancer-Related Cognitive Decline (CRCD). Cognitive impairment disorders range from Mild Cognitive Impairment (MCI) to dementia. MCI is a modest cognitive decline in one or more cognitive domains in history and formal testing, without change in functional independence (in the absence of other mental disorders) [9]. Dementia is a more substantial cognitive decline in one or more cognitive domains in history and formal testing, with an associated reduction in functional independence (in the absence of other mental disorders) [9]. Delirium is an acute confusional state characterized by deficits in attention and concentration, which tends to fluctuate during the course of the day and is associated with physiological stressors [10]. A pre-existing cognitive impairment increases the risk of delirium with physiological stressors such as chemotherapy and surgery [1,8]. Finally, CRCD refers to changes in cognition that occur during or after a cancer treatment and tend to impact domains of executive function, processing speed, memory, and attention [11,12]. This review focuses on assessing for pre-existing cognitive impairment disorders (e.g., MCI or dementia) in older adults with cancer, as distinct from delirium or CRCD [6].
Cognitive assessment is typically incorporated into the broader Geriatric Assessment (GA) of the older person with cancer. The GA evaluates various domains (cognition, comorbidities, function, mobility, nutrition, polypharmacy, psychological, and social support) with a view to guide interventions to improve the care of and outcomes for older adults with cancer [13,14]. GA has been demonstrated to alter the initial oncology treatment plan in at least a quarter of patients (median 28%; range 8–54%), with non-oncological interventions being recommended in almost three-quarters of patients (median 72%; range 26–100%) [15]. Recent randomized controlled trials demonstrated improvements in quality of life, unplanned hospital admissions, and reduced chemotherapy toxicity and early treatment discontinuation, confirming the importance of GA in older cancer patients [16,17,18,19]. In addition, several Oncological Societies internationally have published guidelines on GA, which include recommendations for cognitive assessment or screening [1,20,21,22]. If cancer care services do not screen for pre-existing cognitive impairment, this limits the information available to truly inform decision-making for older people and their families, as well as clinicians, and may lead to inadequate support for the upcoming cancer journey [2,3]. At the same time, many older people may not have heard of cognitive screening, nor seen themselves as at risk of impairments in thinking or memory, therefore assessing cognition needs to be approached with great sensitivity.
This rapid review seeks to evaluate cognitive assessment tools routinely used in older adults with cancer to provide clinical and practical information for cancer care clinicians. While the increased assessment of cognition in oncology is advocated, all cognitive assessment tools have inherent strengths and limitations. Awareness of these characteristics will aid the selection of a tool appropriate to the clinical setting and reduce the chances of misinterpretation of assessment results.
2. Materials and Methods
This narrative literature review consists of two components: (1) Identification of the recommended cognitive assessment tools; and (2) focused review of the evidence underpinning the identified tools.
2.1. Part 1: Identification of Cognitive Assessment Tools
A search was conducted to identify recent Oncology guidelines focused on geriatric assessment, both within the published literature and the web-based “grey literature”. Searches were limited to guidelines published in the last 5 years.
2.1.1. Published Resources
PubMed and Ovid Medline were searched using the terms in Table 1.

Table 1.
Search strategy published resources.
2.1.2. Web-Based Resources
To identify guidelines that may not be published in the peer-reviewed literature, a systematic search using predefined search terms was undertaken using the Google search engine to identify the most commonly viewed resources. The search strategy was refined after piloting to (cancer OR oncology) AND (geriatric OR elderly OR “older adult” OR “senior adult”) AND (guideline OR “position statement” OR “consensus statement” or recommendation). The first 100 websites featured in the results for the search were screened.
2.1.3. Inclusion Criteria
- Published between 1 July 2016 and 1 July 2021;
- Available in English;
- Guidelines, position statements, consensus statements or recommendations;
- Primary focus is the assessment of older adults with cancer;
- Authored or published by a national or international Medical or Oncological Society or Organization; and
- Refer to specific cognitive assessment tools.
2.1.4. Exclusion Criteria
- Opinion pieces, research articles, and review articles;
- Authored or published by a single institution;
- Related to a single cancer type or groups of cancers of a single body system or organ; and
- Tools designed specifically for screening or assessing acute confusional states or delirium.
Where there was uncertainty about whether an article/website met the inclusion/exclusion criteria, this was discussed by the research team, and a consensus decision was reached.
Any tools or instruments recommended for cognitive assessment or cognitive screening in these guidelines were identified. For this rapid review, the following operational definitions were adopted:
- Clinical practice guidelines: Evidence-based statements that include recommendations intended to optimize patient care and assist health care practitioners to make decisions on the appropriate health care for specific clinical circumstances [23].
- Cognitive assessment tool: Any instrument, tool or survey, developed or utilized to assess or screen cognitive function in adults.
2.2. Part 2: Focused Review of the Evidence Underpinning the Identified Tools
Characteristics of interest for the cognitive assessment tools were determined by an iterative consensus process among the authors. A focused search using PubMed and Medline was conducted for each cognitive assessment tool identified in Part 1 to establish their characteristics (Table 2). This was supplemented by the Google search engine if the relevant information about the cognitive assessment tool was not available in the peer-reviewed literature. Finally, a narrative synthesis was used to describe the findings and generate practice points to support clinicians in determining the best tools to use in clinical practice.

Table 2.
Characteristics of cognitive assessment tools.
3. Results
Eight guidelines on the assessment of older adults with cancer (Table 3) were identified from the search (Figure 1), from which six cognitive assessment tools were identified (Table 4 and Table 5). The guidelines recommended between one and five cognitive assessment tools. The Spanish Society for Medical Oncology (SEOM) 2018 [24] and National Comprehensive Cancer Network (NCCN) guidelines [22,25] endorsed a single brief cognitive screening tool, with recommendations for further cognitive assessment if screening was abnormal [22,24,25]. The International Geriatric Oncology Society (SIOG) COVID-19 guidelines recommended the Blessed Information Memory Concentration (BOMC) in the context of telehealth consultations [26]. Other guidelines suggested several cognitive assessment tools. The Mini-Mental Status Examination (MMSE) and Montreal Cognitive Assessment (MoCA) were most commonly recommended, closely followed by the Mini-Cog. Consistency was seen across most of the guidelines, with only the SEOM recommending a tool that was not recommended by any other guideline, the Short Portable Mental Status Questionnaire (SPMSQ) [24]. Only the American Society of Clinical Oncology (ASCO) [1] and Young International Society for Geriatric Oncology (SIOG) [21] guidelines commented specifically on the cut-off test scores indicating concern, and these are discussed in the relevant sections. The commonly utilized cognitive assessment tools encompassed variable combinations of cognitive domains, such as orientation (temporal and spatial), memory registration and recall, attention and concentration, language (written and verbal), visuo-spatial and visuo-constructional, and executive function.

Table 3.
Guidelines on the assessment of older adults with cancer.
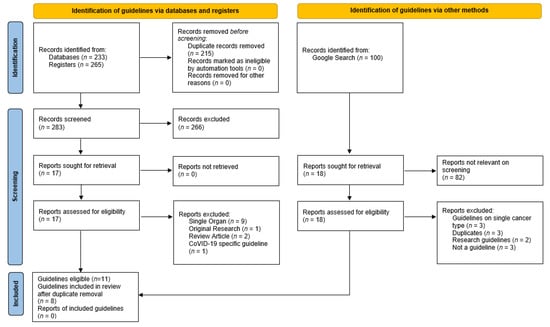
Figure 1.
Flow chart database search demonstrating the records identified, screened, and included with reasons for exclusion identified.

Table 4.
Psychometric considerations for clinical use.

Table 5.
Practical considerations for clinical use.
3.1. Blessed Information Memory Concentration
The Blessed Information Memory Concentration (BOMC) [28], also known as the six-item Cognitive Impairment Test (6-CIT) and the short Orientation-Memory-Concentration test, is a six-item cognitive assessment tool that can be completed in 2–3 min [68]. It is limited to assessing the three domains described in its title [28], thus not testing executive function. Typically, a score of 8 or more is considered abnormal [28]. However, the ASCO and Young SIOG guidelines recommend using lower cut-off scores of 6 and 4, respectively [1,21], which will increase the detection of milder cognitive problems. It has not been translated or validated as extensively as other tools. Although copyrighted, it is freely available to healthcare professionals and does not require specific training to be used. It has no visual elements, thus no adaptation is needed for telephone consultations or for those with visual impairment.
3.2. Clock Draw Test
The Clock Draw Test (CDT) [34] is a well-established and widely validated cognitive assessment tool. It is very brief, freely available, and does not require translation into other languages. It tests visuo-spatial skills and executive function, but not memory [83]. It is considered a cognitive screening tool for identifying those who should undergo further cognitive assessment rather than a diagnostic tool [84,85]. A variety of scoring methods are used, complicating the interpretation of the literature [36]. Adaptations using a digital clock have been studied [83,86].
3.3. Mini-Cog
The Mini-Cog incorporates the CDT and adds the three-item recall, thus adding a memory domain [42]. Moreover, it has a standardized scoring system. Although, a score of less than 3 is typically used to define an abnormal result [42], the Young SIOG guidelines recommend using a score of less than 4, to improve the detection of milder degrees of cognitive impairment [21]. It was developed as a screening tool for cognitive impairment in multi-lingual communities [42] and is available in several languages [73]. Minimal language interpretation is required, hence it can be easily used with an interpreter in additional languages [42]. It has been widely validated in different countries and populations [43,87,88,89]. Furthermore, it is copyrighted, but is freely available for use, reproduction, and distribution in clinical and educational settings without permission, and no formal training is required [73]. Its brevity and ease of use make it popular in the primary care and non-specialist secondary care setting.
3.4. Mini-Mental Status Examination
The Mini-Mental Status Examination (MMSE) is a well-established test [47]. It is widely studied with validation in multiple countries, languages, and clinical settings [48,49,50,51,52]. Many variations are available and have their own validation data. It is one of the longer tests and covers a range of cognitive domains, but not executive function [47]. Moreover, outcomes vary with education [90] and deficits may not be identified in those with high educational backgrounds [91]. It is copyrighted, thus cost and accessibility may limit its organizational use.
3.5. Montreal Cognitive Assessment
The Montreal Cognitive Assessment (MoCA) is one of the longer assessment tools in the guidelines, taking approximately 10 minutes to complete [57]. It covers a wide range of cognitive domains, including executive function [57]. It has been extensively validated in multiple languages and populations [58,59,60,61], as well as in varied neurological and neurocognitive disorders [92,93,94,95]. It is available in close to 100 languages, with adapted forms suitable for telephone administration (T-MoCA) [78]. It has high sensitivity and reasonable specificity in a population with dementia of Alzheimer’s type [57]. Moreover, unlike the other tools, it has good sensitivity for detecting mild cognitive impairment [57]. There is a training fee, and training requirement which, once completed, all versions of the tests become available for use, reproduction, and distribution without permission for clinical and teaching purposes [57].
3.6. Short Portable Mental Status Questionnaire
The Short Portable Mental Status Questionnaire (SPMSQ) [65] is a brief 10-item cognitive screening tool. It is less studied than the other identified tools. However, it is validated in its Spanish version [82] and in a number of populations [66,79]. Similar to the other brief tools, fewer cognitive domains are covered (memory, attention, and orientation) [65]. It is freely available, can be applied without formal training, and can be used via telephone while retaining comparable sensitivity for the detection of dementia [96].
4. Discussion
It can be challenging for cancer care clinicians to choose from the plethora of the available cognitive assessment tools. Of the six cognitive assessment tools that were recommended by geriatric oncology guidelines, the MOCA [57], MMSE [47], and Mini-Cog [42] were the most frequently endorsed tools. However, there is no ideal cognitive assessment tool, each has advantages and limitations. It is important for clinicians to be aware of the strengths and limitations of each tool and the neurocognitive domains they cover. This rapid review found that executive function is a notable gap in both the MMSE [47] and BOMC [28]. Moreover, the BOMC lacks visuo-constructional/visuo-spatial assessment. We hope this review will aid clinicians in considering which tool or tools might best meet their needs, taking into account the demographics of their patient population, access to other services, and the practicalities of their clinical setting. While we only identified validation studies of the CDT [34] and MMSE [47] in cancer populations [39,53], other tools have been validated in large populations that will include older people with cancer.
An initial consideration is whether a brief cognitive screen or a more detailed cognitive assessment is required and practical. The identified tools include brief screening tools (e.g., Mini-Cog or CDT) and short tools (e.g., BOMC or SPMSQ) that do not require specific training. In a busy oncology clinic, these may be most feasible. If a more detailed cognitive assessment is accessible through referral pathways, then a brief screen is likely to be adequate. However, in geriatric assessment clinics, clinicians may well prefer to utilize one of the longer recommended tools, the MoCA or MMSE, to evaluate a broader range of cognitive domains.
The choice of cognitive assessment tool should be influenced by the demographics of the patient population, as patient factors must be considered when undertaking any cognitive assessment. Patients from culturally and linguistically diverse backgrounds require tools validated in these populations and languages. The MoCA, MMSE, and Mini-Cog have been translated and validated in many languages [73,74,78]. The Mini-Cog has shown to be useful and feasible for cognitive screening across ethnically diverse groups and those with lower literacy and education levels [97,98]. Educational level does affect the cognitive assessment performance across all of these tools, except the BOMC, which is reportedly not sensitive to educational level [33]. For those with a higher educational background, a more sensitive tool such as the MoCA may be required to demonstrate the underlying cognitive impairment. Moreover, the MoCA has greater sensitivity and specificity for the detection of MCI in comparison to other tools [2,57,99,100,101,102]. Furthermore, visual and hearing deficits need to be factored into the tool choice. The BOMC has no visual elements, while the CDT is primarily visual. Therefore, it may be suitable when substantial hearing difficulties are present.
Pragmatic considerations will often determine the cognitive assessment tool chosen in a particular setting. An organization may have a preferred tool or may not have licensing for certain tools. In addition, clinicians may not have training for particular tools. For telephone consultations, the BOMC or SPMSQ can be used without adaptation, while the Mini-Cog or CDT cannot be utilized. If a tool has been utilized in an individual before, repeating the same tool allows for a longitudinal comparison. However, alternate versions should be used if the tool is repeated at time intervals below the recommended test-retest interval to counteract the practice effect [103,104]. There may be a geographical variation in the cognitive assessment tool preference. Observationally, the BOMC seems to be more widely utilized in North America than elsewhere, and is recommended in the ASCO guidelines [1], while the SPMQ is only endorsed by the SEOM [24], which may relate to local preferences for this tool that are available and validated in Spanish [82].
A brief screening tool such as the Mini-Cog could be implemented into routine oncology clinic appointments for older people. Abnormal screening results could be followed by a more detailed assessment or on-referral. Based on our own practices, the authors recommend the Mini-Cog for brief screening and the MMSE or MOCA for more detailed testing where time permits. Moreover, this selection corresponds with the most endorsed tools by the guidelines. Where screening via phone is required, the BOMC could be used rather than the Mini-Cog and the telephone version of the MoCA could be used if more detailed testing is required. Clinicians who are assessing cognition in older adults frequently may wish to familiarize themselves with a small number of tools to provide a cognitive screening “tool-kit”, which allows the clinician to select a tool to suit the individual patient and the clinical setting.
An abnormal result on cognitive screening requires further evaluation. Likewise, cognitive concerns in the context of a normal cognitive assessment tool result also warrant further assessment. To contextualize the abnormal cognitive screen, the clinician needs to obtain a broad understanding of a person’s level of function and the presence of longitudinal change in cognition [1,6]. A detailed history should be obtained from the patient ideally alongside an informant (usually the patient’s family or caregiver), with a focus on the patient’s ability to manage personal activities of daily living (e.g., dressing, bathing, and toileting) and instrumental activities of daily living (e.g., household tasks, medication management, shopping, finance management, and driving) [4]. In addition, validated informant tools can assist in obtaining relevant information [102,105,106,107,108]. Driving ability, financial, as well as medication self-management are considered tasks requiring higher cognitive abilities and may be some of the first functional areas where changes are observed in early dementia [109,110]. An assessment of this nature requires time and sensitive inquiry. Moreover, it may be most appropriate for the cancer care clinician to refer the person to a clinician with expertise in geriatric or dementia care.
The assessment can be complemented by an occupational therapist review to assess function-based cognition [4]. A physical examination followed by further pathological and radiological investigations are standard practice for cognitive work-up, to exclude alternate (and possibly reversible) causes of cognitive impairment. A work-up usually incorporates baseline blood tests (full blood count, renal function, electrolytes, liver function tests, thyroid function tests, vitamin B12 levels, calcium level, syphilis serology, HbA1C), urine microscopy culture, and may include cerebral radiological testing [3,111]. Similarly, although outside the scope of this review, the impact of mood disorders on cognitive performance should never be overlooked [112].
Depending on the available services, referral for more comprehensive neurocognitive testing either in a designated memory care clinic or referral to a Geriatrician for a Comprehensive Geriatric Assessment (CGA), whereby other geriatric domains can be reviewed in detail, is recommended [1,113]. Geriatrician input is not only for diagnostic purposes, but also to develop and implement an individualized plan to support the patient and their caregivers to optimize their cancer treatment, based upon the particular areas of deficits revealed [113]. The downstream implementation and integration of GA-guided interventions will differ across organizations. However, the timely communication of the CGA back to the primary treating oncology team to enable the incorporation of this information into the oncological plan is of the utmost importance [14,15].
A limitation of this review pertains to the narrow focus on pre-existing cognitive impairment at cancer diagnosis. It is important to demarcate this from other cognitive conditions associated with cancer, which were outside the scope of this review. Literature on the entity of CRCD is expanding, referring to the deterioration in cognitive domains (in particular memory, attention, concentration, and executive function) during and following the treatment for cancer [4,6]. Again, the screening tools discussed in this review may not be validated for detecting the subtle changes often seen in this pathophysiologically different entity.
There is a high prevalence of cognitive impairment in older cancer survivors, thus while we have considered screening for cognitive impairment at an initial assessment, a longitudinal review is also important [114]. This is not only a consideration in patients who received chemotherapy, but there is growing evidence that hormonal therapies and immunotherapies may also impact on cognition [114]. Similarly, while delirium is a separate entity of acute cognitive decline, it is important to be aware that those with baseline cognitive impairment are at a far higher risk of developing a delirium episode during their cancer treatment phase [1,8]. Education regarding delirium monitoring for these patients and their caregivers is required [10,115,116].
This study was a rapid review rather than a systematic review. We intended for our search to be broad and incorporate various international guidelines to make our study more generally applicable, but we acknowledge that our inclusion criteria required the availability of an English version of the guideline. Moreover, we note that our resulting guidelines originated in countries of similar global economic rankings and did not incorporate developing nations. Similarly, our practicalities and suggestions are more representative of the geriatric oncology practices described in the current geriatric oncology research (and within the geographical context of the Australian authors), which tend to focus on developed countries without incorporating the unique challenges of developing nations.
While we strongly advocate for routine cognitive screening, future research is needed to evaluate the optimal methods for routine cognitive screening and the associated interventions to support older adults with cancer and their caregivers. Moreover, it would be interesting to explore the varying models of care across geriatric oncology services and how cognitive assessment tools influence the service access and multidisciplinary team involvement in older adults with cancer with pre-existing cognitive impairment.
5. Conclusions
Cognitive impairment significantly impacts older cancer patients and their caregivers. International guidelines and position statements for older adults with cancer recommend the routine use of cognitive assessment tools, including the BOMC, CDT, Mini-Cog, MMSE, MoCA, and SPMSQ, to detect cognitive problems. Tool selection is influenced by the clinical environment and patient factors. Clinicians should be familiar with utilizing various tools and their limitations. Every effort should be made to ensure older adults with cancer receive the appropriate cognitive work-up when screening is abnormal.
Author Contributions
Conceptualization, H.L., W.K.S., M.A., G.T. and J.L.P.; methodology, H.L., W.K.S., M.A., G.T. and J.L.P.; validation, H.L., K.-Y.L., K.F. and E.L.O.; investigation, H.L., K.-Y.L., K.F. and E.L.O.; writing—original draft preparation, G.T. and H.L.; writing—review and editing, G.T., H.L., W.K.S., M.A. and J.L.P.; visualization, H.L., K.-Y.L., W.K.S. and G.T.; supervision, H.L. and W.K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Technical support, Joshua Dishon.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mohile, S.G.; Dale, W.; Somerfield, M.R.; Schonberg, M.A.; Boyd, C.M.; Burhenn, P.S.; Canin, B.; Cohen, H.J.; Holmes, H.M.; Hopkins, J.O. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J. Clin. Oncol. 2018, 36, 2326. [Google Scholar] [CrossRef]
- Magnuson, A.; Mohile, S.; Janelsins, M. Cognition and Cognitive Impairment in Older Adults with Cancer. Curr. Geriatr. Rep. 2016, 5, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Soo, W.K. Older adults with cancer and clinical decision-making: The importance of assessing cognition. Cancer Forum 2013, 37, 201–205. [Google Scholar]
- Pergolotti, M.; Battisti, N.M.L.; Padgett, L.; Sleight, A.G.; Abdallah, M.; Newman, R.; Van Dyk, K.; Covington, K.R.; Williams, G.R.; van den Bos, F.; et al. Embracing the complexity: Older adults with cancer-related cognitive decline-A Young International Society of Geriatric Oncology position paper. J. Geriatr. Oncol. 2020, 11, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Rostoft, S.; van den Bos, F.; Pedersen, R.; Hamaker, M.E. Shared decision-making in older patients with cancer—What does the patient want? J. Geriatr. Oncol. 2021, 12, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Magnuson, A.; Ahles, T.; Chen, B.T.; Mandelblatt, J.; Janelsins, M.C. Cognitive Function in Older Adults with Cancer: Assessment, Management, and Research Opportunities. J. Clin. Oncol. 2021, 39, 2138–2149. [Google Scholar] [CrossRef]
- Chodosh, J.; Petitti, D.B.; Elliott, M.; Hays, R.D.; Crooks, V.C.; Reuben, D.B.; Galen Buckwalter, J.; Wenger, N. Physician recognition of cognitive impairment: Evaluating the need for improvement. J. Am. Geriatr. Soc. 2004, 52, 1051–1059. [Google Scholar] [CrossRef]
- Magnuson, A.; Sattar, S.; Nightingale, G.; Saracino, R.; Skonecki, E.; Trevino, K.M. A practical guide to geriatric syndromes in older adults with cancer: A focus on falls, cognition, polypharmacy, and depression. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, e96–e109. [Google Scholar] [CrossRef] [PubMed]
- American Psychriatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Jung, P.; Puts, M.; Frankel, N.; Syed, A.T.; Alam, Z.; Yeung, L.; Malik, U.; Rosario, C.; Ayala, A.P.; Hudson, J.; et al. Delirium incidence, risk factors, and treatments in older adults receiving chemotherapy: A systematic review and meta-analysis. J. Geriatr. Oncol. 2021, 12, 352–360. [Google Scholar] [CrossRef]
- Ahles, T.A.; Root, J.C. Cognitive Effects of Cancer and Cancer Treatments. Ann. Rev. Clin. Psychol. 2018, 14, 425–451. [Google Scholar] [CrossRef]
- Wefel, J.S.; Vardy, J.; Ahles, T.; Schagen, S.B. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011, 12, 703–708. [Google Scholar] [CrossRef]
- Extermann, M.; Hurria, A. Comprehensive Geriatric Assessment for Older Patients with Cancer. J. Clin. Oncol. 2007, 25, 1824–1831. [Google Scholar] [CrossRef]
- Hamaker, M.E.; van Huis-Tanja, L.H.; Rostoft, S. Optimizing the geriatrician’s contribution to cancer care for older patients. J. Geriatr. Oncol. 2020, 11, 389–394. [Google Scholar] [CrossRef]
- Hamaker, M.E.; Te Molder, M.; Thielen, N.; van Munster, B.C.; Schiphorst, A.H.; van Huis, L.H. The effect of a geriatric evaluation on treatment decisions and outcome for older cancer patients—A systematic review. J. Geriatr. Oncol. 2018, 9, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Mohile, S.G.; Mohamed, M.R.; Culakova, E.; Xu, H.; Loh, K.P.; Magnuson, A.; Flannery, M.A.; Ramsdale, E.E.; Dunne, R.F.; Gilmore, N.; et al. A geriatric assessment (GA) intervention to reduce treatment toxicity in older patients with advanced cancer: A University of Rochester Cancer Center NCI community oncology research program cluster randomized clinical trial (CRCT). J. Clin. Oncol. 2020, 38, 12009. [Google Scholar] [CrossRef]
- Li, D.; Sun, C.-L.; Kim, H.; Chung, V.; Koczywas, M.; Fakih, M.; Chao, J.; Chien, L.; Charles, K.; Hughes, S.F.D.S.; et al. Geriatric assessment-driven intervention (GAIN) on chemotherapy toxicity in older adults with cancer: A randomized controlled trial. J. Clin. Oncol. 2020, 38, 12010. [Google Scholar] [CrossRef]
- Soo, W.K.; King, M.; Pope, A.; Parente, P.; Darzins, P.; Davis, I.D. Integrated geriatric assessment and treatment (INTEGERATE) in older people with cancer planned for systemic anticancer therapy. J. Clin. Oncol. 2020, 38, 12011. [Google Scholar] [CrossRef]
- Lund, C.M.; Vistisen, K.K.; Olsen, A.P.; Bardal, P.; Schultz, M.; Dolin, T.G.; Rønholt, F.; Johansen, J.S.; Nielsen, D.L. The effect of geriatric intervention in frail older patients receiving chemotherapy for colorectal cancer: A randomised trial (GERICO). Br. J. Cancer 2021, 124, 1949–1958. [Google Scholar] [CrossRef]
- Burhenn, P.S.; McCarthy, A.L.; Begue, A.; Nightingale, G.; Cheng, K.; Kenis, C. Geriatric assessment in daily oncology practice for nurses and allied health care professionals: Opinion paper of the Nursing and Allied Health Interest Group of the International Society of Geriatric Oncology (SIOG). J. Geriatr. Oncol. 2016, 7, 315–324. [Google Scholar] [CrossRef]
- Loh, K.P.; Soto-Perez-de-Celis, E.; Hsu, T.; de Glas, N.A.; Battisti, N.M.L.; Baldini, C.; Rodrigues, M.; Lichtman, S.M.; Wildiers, H. What Every Oncologist Should Know About Geriatric Assessment for Older Patients With Cancer: Young International Society of Geriatric Oncology Position Paper. J. Oncol. Pr. 2018, 14, 85–94. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Older Adult Oncology Version 1.2019. Available online: http://www.gaca.org.cn/uploadfolder/files/201903/ny27_219665.pdf (accessed on 4 August 2021).
- National Health and Medical Research Council. Clinical Practice Guidelines Portal. Available online: https://www.clinicalguidelines.gov.au/portal (accessed on 1 August 2021).
- Gironés Sarrió, R.; Antonio Rebollo, M.; Molina Garrido, M.J.; Guillén-Ponce, C.; Blanco, R.; Gonzalez Flores, E.; Saldaña, J. General recommendations paper on the management of older patients with cancer: The SEOM geriatric oncology task force’s position statement. Clin. Transl. Oncol. 2018, 20, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines: Older Adult Oncology Version 2.2017. Available online: https://oncolife.com.ua/doc/nccn/Older_Adult_Oncology.pdf (accessed on 4 August 2021).
- Battisti, N.M.L.; Mislang, A.R.; Cooper, L.; O’Donovan, A.; Audisio, R.A.; Cheung, K.-L.; Sarrió, R.G.; Stauder, R.; Soto-Perez-de-Celis, E.; Jaklitsch, M.; et al. Adapting care for older cancer patients during the COVID-19 pandemic: Recommendations from the International Society of Geriatric Oncology (SIOG) COVID-19 Working Group. J. Geriatr. Oncol. 2020, 11, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Fusco, D.; Ferrini, A.; Pasqualetti, G.; Giannotti, C.; Cesari, M.; Laudisio, A.; Ballestrero, A.; Scabini, S.; Odetti, P.R.; Colloca, G.F.; et al. Comprehensive geriatric assessment in older adults with cancer: Recommendations by the Italian Society of Geriatrics and Gerontology (SIGG). Eur. J. Clin. Investig. 2021, 51, e13347. [Google Scholar] [CrossRef] [PubMed]
- Katzman, R.; Brown, T.; Fuld, P.; Peck, A.; Schechter, R.; Schimmel, H. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am. J. Psychiatry 1983, 140, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, A.K.; Rajagopal, M.; Gale, T.M. The Six Item Cognitive Impairment Test (6-CIT) as a screening test for dementia: Comparison with Mini-Mental State Examination (MMSE). Curr. Aging Sci. 2010, 3, 138–142. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, D.; Brady, N.; Manning, E.; O’Shea, E.; O’Grady, S.; O’Regan, N.; Timmons, S. Validation of the 6-Item Cognitive Impairment Test and the 4AT test for combined delirium and dementia screening in older Emergency Department attendees. Age Ageing 2018, 47, 61–68. [Google Scholar] [CrossRef]
- Fillenbaum, G.G.; Heyman, A.; Wilkinson, W.E.; Haynes, C.S. Comparison of two screening tests in Alzheimer’s disease. The correlation and reliability of the Mini-Mental State Examination and the modified Blessed test. Arch. Neurol. 1987, 44, 924–927. [Google Scholar] [CrossRef] [PubMed]
- Yokomizo, J.E.; Simon, S.S.; Bottino, C.M. Cognitive screening for dementia in primary care: A systematic review. Int. Psychogeriatr 2014, 26, 1783–1804. [Google Scholar] [CrossRef]
- Tuijl, J.P.; Scholte, E.M.; de Craen, A.J.; van der Mast, R.C. Screening for cognitive impairment in older general hospital patients: Comparison of the Six-Item Cognitive Impairment Test with the Mini-Mental State Examination. Int. J. Geriatr. Psychiatry 2012, 27, 755–762. [Google Scholar] [CrossRef]
- Zama, I.N.; Maynard, W.K.; Davis, M.P. Clocking delirium: The value of the Clock Drawing Test with case illustrations. Am. J. Hosp. Palliat. Care 2008, 25, 385–388. [Google Scholar] [CrossRef]
- Shao, K.; Dong, F.M.; Guo, S.Z.; Wang, W.; Zhao, Z.M.; Yang, Y.M.; Wang, P.P.; Wang, J.H. Clock-drawing test: Normative data of three quantitative scoring methods for Chinese-speaking adults in Shijiazhuang City and clinical utility in patients with acute ischemic stroke. Brain Behav. 2020, 10, e01806. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, S.; Jin, X.; Lu, X.; Liu, L.; Lou, Y.; Wang, Y.; Li, Y.; Jin, Y. A comparison of six clock-drawing test scoring methods in a nursing home. Aging Clin. Exp. Res. 2018, 30, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Shao, K.; Guo, S.; Wang, W.; Yang, Y.; Zhao, Z.; Feng, R.; Wang, J. Clock-drawing test in vascular mild cognitive impairment: Validity of quantitative and qualitative analyses. J. Clin. Exp. Neuropsychol. 2020, 42, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Carnero-Pardo, C.; Rego-García, I.; Barrios-López, J.M.; Blanco-Madera, S.; Calle-Calle, R.; López-Alcalde, S.; Vílchez-Carrillo, R.M. Assessment of the diagnostic accuracy and discriminative validity of the Clock Drawing and Mini-Cog tests in detecting cognitive impairment. Neurología 2019. [Google Scholar] [CrossRef]
- Lycke, M.; Ketelaars, L.; Boterberg, T.; Pottel, L.; Pottel, H.; Vergauwe, P.; Goethals, L.; Van Eygen, K.; Werbrouck, P.; Debruyne, D.; et al. Validation of the Freund Clock Drawing Test as a screening tool to detect cognitive dysfunction in elderly cancer patients undergoing comprehensive geriatric assessment. Psycho-Oncology 2014, 23, 1172–1177. [Google Scholar] [CrossRef]
- Shulman, K.I. Clock-drawing: Is it the ideal cognitive screening test? Int. J. Geriatr. Psychiatry 2000, 15, 548–561. [Google Scholar] [CrossRef]
- Lourenço, R.A.; Ribeiro-Filho, S.T.; Moreira Ide, F.; Paradela, E.M.; Miranda, A.S. The Clock Drawing Test: Performance among elderly with low educational level. Braz. J. Psychiatry 2008, 30, 309–315. [Google Scholar] [CrossRef]
- Borson, S.; Scanlan, J.; Brush, M.; Vitaliano, P.; Dokmak, A. The mini-cog: A cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int. J. Geriatr. Psychiatry 2000, 15, 1021–1027. [Google Scholar] [CrossRef]
- Rezaei, M.; Rashedi, V.; Lotfi, G.; Shirinbayan, P.; Foroughan, M. Psychometric Properties of the Persian Adaptation of Mini-Cog Test in Iranian Older Adults. Int. J. Aging Hum. Dev. 2018, 86, 266–280. [Google Scholar] [CrossRef]
- Scanlan, J.; Borson, S. The Mini-Cog: Receiver operating characteristics with expert and naïve raters. Int. J. Geriatr. Psychiatry 2001, 16, 216–222. [Google Scholar] [CrossRef]
- Seitz, D.P.; Chan, C.C.; Newton, H.T.; Gill, S.S.; Herrmann, N.; Smailagic, N.; Nikolaou, V.; Fage, B.A. Mini-Cog for the diagnosis of Alzheimer’s disease dementia and other dementias within a primary care setting. Cochrane Database Syst. Rev. 2018, 2, Cd011415. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Filho, S.T.; Lourenço, R.A. The performance of the Mini-Cog in a sample of low educational level elderly. Dement. Neuropsychol. 2009, 3, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Boban, M.; Malojčić, B.; Mimica, N.; Vuković, S.; Zrilić, I.; Hof, P.R.; Simić, G. The reliability and validity of the mini-mental state examination in the elderly Croatian population. Dement. Geriatr. Cogn. Disord. 2012, 33, 385–392. [Google Scholar] [CrossRef]
- Elhan, A.H.; Kutlay, S.; Küçükdeveci, A.A.; Cotuk, C.; Oztürk, G.; Tesio, L.; Tennant, A. Psychometric properties of the Mini-Mental State Examination in patients with acquired brain injury in Turkey. J. Rehabil. Med. 2005, 37, 306–311. [Google Scholar] [CrossRef]
- El-Hayeck, R.; Baddoura, R.; Wehbé, A.; Bassil, N.; Koussa, S.; Abou Khaled, K.; Richa, S.; Khoury, R.; Alameddine, A.; Sellal, F. An Arabic Version of the Mini-Mental State Examination for the Lebanese Population: Reliability, Validity, and Normative Data. J. Alzheimers Dis. 2019, 71, 525–540. [Google Scholar] [CrossRef]
- Ong, H.L.; Subramaniam, M.; Abdin, E.; Wang, P.; Vaingankar, J.A.; Lee, S.P.; Shafie, S.; Seow, E.; Chong, S.A. Performance of Mini-Mental State Examination (MMSE) in long-stay patients with schizophrenia or schizoaffective disorders in a psychiatric institute. Psychiatry Res. 2016, 241, 256–262. [Google Scholar] [CrossRef]
- Ansari, N.N.; Naghdi, S.; Hasson, S.; Valizadeh, L.; Jalaie, S. Validation of a Mini-Mental State Examination (MMSE) for the Persian population: A pilot study. Appl. Neuropsychol. 2010, 17, 190–195. [Google Scholar] [CrossRef]
- Matuoka, J.Y.; Kurita, G.P.; Nordly, M.; Sjøgren, P.; de Mattos-Pimenta, C.A. Validation of a Battery of Neuropsychological Tests for Patients With Metastatic Cancer. Clin. Nurs. Res. 2019, 29, 607–615. [Google Scholar] [CrossRef]
- Marioni, R.E.; Chatfield, M.; Brayne, C.; Matthews, F.E.; Medical Research Council Cognitive, F.; Ageing, S. The reliability of assigning individuals to cognitive states using the Mini Mental-State Examination: A population-based prospective cohort study. BMC Med. Res. Methodol. 2011, 11, 127. [Google Scholar] [CrossRef]
- Bowie, P.; Branton, T.; Holmes, J. Should the Mini Mental State Examination be used to monitor dementia treatments? Lancet 1999, 354, 1527–1528. [Google Scholar] [CrossRef]
- Creavin, S.T.; Wisniewski, S.; Noel-Storr, A.H.; Trevelyan, C.M.; Hampton, T.; Rayment, D.; Thom, V.M.; Nash, K.J.; Elhamoui, H.; Milligan, R.; et al. Mini-Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Cochrane Database Syst. Rev. 2016, Cd011145. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Iiboshi, K.; Yoshida, K.; Yamaoka, Y.; Eguchi, Y.; Sato, D.; Kishimoto, M.; Funaki, K.; Mimura, M.; Kishimoto, T. A Validation Study of the Remotely Administered Montreal Cognitive Assessment Tool in the Elderly Japanese Population. Telemed. J. e-Health 2020, 26, 920–928. [Google Scholar] [CrossRef]
- Freud, T.; Vostrikov, A.; Dwolatzky, T.; Punchik, B.; Press, Y. Validation of the Russian Version of the MoCA Test as a Cognitive Screening Instrument in Cognitively Asymptomatic Older Individuals and Those With Mild Cognitive Impairment. Front. Med. 2020, 7, 447. [Google Scholar] [CrossRef] [PubMed]
- Gil, L.; Ruiz de Sánchez, C.; Gil, F.; Romero, S.J.; Pretelt Burgos, F. Validation of the Montreal Cognitive Assessment (MoCA) in Spanish as a screening tool for mild cognitive impairment and mild dementia in patients over 65 years old in Bogotá, Colombia. Int. J. Geriatr. Psychiatry 2015, 30, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Bartos, A.; Fayette, D. Validation of the Czech Montreal Cognitive Assessment for Mild Cognitive Impairment due to Alzheimer Disease and Czech Norms in 1,552 Elderly Persons. Dement. Geriatr. Cogn. Disord. 2018, 46, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Lifshitz, M.; Dwolatzky, T.; Press, Y. Validation of the Hebrew version of the MoCA test as a screening instrument for the early detection of mild cognitive impairment in elderly individuals. J. Geriatr. Psychiatry Neurol. 2012, 25, 155–161. [Google Scholar] [CrossRef]
- Tu, Q.Y.; Jin, H.; Ding, B.R.; Yang, X.; Lei, Z.H.; Bai, S.; Zhang, Y.D.; Tang, X.Q. Reliability, validity, and optimal cutoff score of the montreal cognitive assessment (changsha version) in ischemic cerebrovascular disease patients of hunan province, china. Dement. Geriatr. Cogn. Dis. Extra. 2013, 3, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, K.; Pichora-Fuller, M.K.; Chasteen, A.L.; Marchuk, V.; Singh, G.; Smith, S.L. Effects of hearing and vision impairments on the Montreal Cognitive Assessment. Aging Neuropsychol. Cogn. 2015, 22, 413–437. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J. Am. Geriatr. Soc. 1975, 23, 433–441. [Google Scholar] [CrossRef]
- Kojaie-Bidgoli, A.; Fadayevatan, R.; Sharifi, F.; Alizadeh-Khoei, M.; Vahabi, Z.; Aminalroaya, R. Applicability of SPMSQ in illiterate outpatients in clinics: The validity and reliability of the Short Portable Mental Status Questionnaire. Appl. Neuropsychol. Adult 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Foreman, M.D. Reliability and validity of mental status questionnaires in elderly hospitalized patients. Nurs. Res. 1987, 36, 216–220. [Google Scholar] [CrossRef] [PubMed]
- De Roeck, E.E.; De Deyn, P.P.; Dierckx, E.; Engelborghs, S. Brief cognitive screening instruments for early detection of Alzheimer’s disease: A systematic review. Alzheimers Res. 2019, 11, 21. [Google Scholar] [CrossRef]
- Apóstolo, J.L.A.; Paiva, D.D.S.; Silva, R.; Santos, E.; Schultz, T.J. Adaptation and validation into Portuguese language of the six-item cognitive impairment test (6CIT). Aging Ment. Health 2018, 22, 1184–1189. [Google Scholar] [CrossRef] [PubMed]
- Taussig, I.M.; Mack, W.J.; Henderson, V.W. Concurrent validity of Spanish-language versions of the Mini-Mental State Examination, Mental Status Questionnaire, Information-Memory-Concentration test, and Orientation-Memory-Concentration test: Alzheimer’s disease patients and nondemented elderly comparison subjects. J. Int. Neuropsychol. Soc. 1996, 2, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Borson, S.; Brush, M.; Gil, E.; Scanlan, J.; Vitaliano, P.; Chen, J.; Cashman, J.; Sta Maria, M.M.; Barnhart, R.; Roques, J. The Clock Drawing Test: Utility for dementia detection in multiethnic elders. J. Gerontol. A Biol. Sci. Med. Sci. 1999, 54, M534–M540. [Google Scholar] [CrossRef]
- Palsetia, D.; Rao, G.P.; Tiwari, S.C.; Lodha, P.; De Sousa, A. The Clock Drawing Test versus Mini-mental Status Examination as a Screening Tool for Dementia: A Clinical Comparison. Indian J. Psychol. Med. 2018, 40, 1–10. [Google Scholar] [CrossRef]
- Borson, S. Mini-Cog: Screening for Cognitive Impairment in Older Adults. Available online: https://mini-cog.com/ (accessed on 12 August 2021).
- PAR, Inc. MMSE Mini Mental State Examination. Available online: https://www.parinc.com/Products/Pkey/237 (accessed on 8 August 2021).
- Molloy, D.W.; Standish, T.I. A guide to the standardized Mini-Mental State Examination. Int. Psychogeriatr. 1997, 9 (Suppl. 1), 87–94; discussion 143–150. [Google Scholar] [CrossRef]
- Teng, E.L.; Chui, H.C. The Modified Mini-Mental State (3MS) examination. J. Clin. Psychiatry 1987, 48, 314–318. [Google Scholar]
- Kennedy, R.E.; Williams, C.P.; Sawyer, P.; Allman, R.M.; Crowe, M. Comparison of in-person and telephone administration of the Mini-Mental State Examination in the University of Alabama at Birmingham Study of Aging. J. Am. Geriatr. Soc. 2014, 62, 1928–1932. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z. MoCA Cognitive Assessment. Available online: https://www.mocatest.org/ (accessed on 8 August 2021).
- Erkinjuntti, T.; Sulkava, R.; Wikström, J.; Autio, L. Short Portable Mental Status Questionnaire as a screening test for dementia and delirium among the elderly. J. Am. Geriatr. Soc. 1987, 35, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Lorentz, W.J.; Scanlan, J.M.; Borson, S. Brief Screening Tests for Dementia. Can. J. Psychiatry 2002, 47, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.-C.J.; Liu, Y.-Y. Influence of social support on cognitive function in the elderly. BMC Health Serv. Res. 2003, 3, 9. [Google Scholar] [CrossRef]
- Martínez de la Iglesia, J.; Dueñas Herrero, R.; Onís Vilches, M.C.; Aguado Taberné, C.; Albert Colomer, C.; Luque Luque, R. Spanish language adaptation and validation of the Pfeiffer’s questionnaire (SPMSQ) to detect cognitive deterioration in people over 65 years of age. Med. Clin. 2001, 117, 129–134. [Google Scholar] [CrossRef]
- Dion, C.; Arias, F.; Amini, S.; Davis, R.; Penney, D.; Libon, D.J.; Price, C.C. Cognitive Correlates of Digital Clock Drawing Metrics in Older Adults with and without Mild Cognitive Impairment. J. Alzheimer’s Dis. JAD 2020, 75, 73–83. [Google Scholar] [CrossRef]
- Petrazzuoli, F.; Vestberg, S.; Midlöv, P.; Thulesius, H.; Stomrud, E.; Palmqvist, S. Brief Cognitive Tests Used in Primary Care Cannot Accurately Differentiate Mild Cognitive Impairment from Subjective Cognitive Decline. J. Alzheimer’s Dis. JAD 2020, 75, 1191–1201. [Google Scholar] [CrossRef]
- Rakusa, M.; Jensterle, J.; Mlakar, J. Clock Drawing Test: A Simple Scoring System for the Accurate Screening of Cognitive Impairment in Patients with Mild Cognitive Impairment and Dementia. Dement. Geriatr. Cogn. Disord. 2018, 45, 326–334. [Google Scholar] [CrossRef]
- Buckley, R.A.; Atkins, K.J.; Fortunato, E.; Silbert, B.; Scott, D.A.; Evered, L. A novel digital clock drawing test as a screening tool for perioperative neurocognitive disorders: A feasibility study. Acta Anaesthesiol. Scand. 2021, 65, 473–480. [Google Scholar] [CrossRef]
- Trongsakul, S.; Lambert, R.; Clark, A.; Wongpakaran, N.; Cross, J. Development of the Thai version of Mini-Cog, a brief cognitive screening test. Geriatr. Gerontol. Int. 2015, 15, 594–600. [Google Scholar] [CrossRef]
- Albanna, M.; Yehya, A.; Khairi, A.; Dafeeah, E.; Elhadi, A.; Rezgui, L.; Al Kahlout, S.; Yousif, A.; Uthman, B.; Al-Amin, H. Validation and cultural adaptation of the Arabic versions of the Mini-Mental Status Examination—2 and Mini-Cog test. Neuropsychiatr. Dis. Treat. 2017, 13, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Milian, M.; Leiherr, A.-M.; Straten, G.; Müller, S.; Leyhe, T.; Eschweiler, G.W. The Mini-Cog, Clock Drawing Test, and the Mini-Mental State Examination in a German Memory Clinic: Specificity of separation dementia from depression. Int. Psychogeriatr. 2013, 25, 96–104. [Google Scholar] [CrossRef] [PubMed]
- O’Bryant, S.E.; Humphreys, J.D.; Smith, G.E.; Ivnik, R.J.; Graff-Radford, N.R.; Petersen, R.C.; Lucas, J.A. Detecting dementia with the mini-mental state examination in highly educated individuals. Arch. Neurol. 2008, 65, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Franco-Marina, F.; García-González, J.J.; Wagner-Echeagaray, F.; Gallo, J.; Ugalde, O.; Sánchez-García, S.; Espinel-Bermúdez, C.; Juárez-Cedillo, T.; Rodríguez, M.A.; García-Peña, C. The Mini-mental State Examination revisited: Ceiling and floor effects after score adjustment for educational level in an aging Mexican population. Int. Psychogeriatr. 2010, 22, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Hoops, S.; Nazem, S.; Siderowf, A.D.; Duda, J.E.; Xie, S.X.; Stern, M.B.; Weintraub, D. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology 2009, 73, 1738–1745. [Google Scholar] [CrossRef] [PubMed]
- Burton, L.; Tyson, S.F. Screening for cognitive impairment after stroke: A systematic review of psychometric properties and clinical utility. J. Rehabil. Med. 2015, 47, 193–203. [Google Scholar] [CrossRef]
- Freitas, S.; Simões, M.R.; Alves, L.; Duro, D.; Santana, I. Montreal Cognitive Assessment (MoCA): Validation study for frontotemporal dementia. J. Geriatr. Psychiatry Neurol. 2012, 25, 146–154. [Google Scholar] [CrossRef]
- Freitas, S.; Simões, M.R.; Alves, L.; Vicente, M.; Santana, I. Montreal Cognitive Assessment (MoCA): Validation study for vascular dementia. J. Int. Neuropsychol. Soc. 2012, 18, 1031–1040. [Google Scholar] [CrossRef]
- Roccaforte, W.H.; Burke, W.J.; Bayer, B.L.; Wengel, S.P. Reliability and validity of the Short Portable Mental Status Questionnaire administered by telephone. J. Geriatr. Psychiatry Neurol. 1994, 7, 33–38. [Google Scholar] [CrossRef]
- Borson, S.; Scanlan, J.M.; Chen, P.; Ganguli, M. The Mini-Cog as a screen for dementia: Validation in a population-based sample. J. Am. Geriatr. Soc. 2003, 51, 1451–1454. [Google Scholar] [CrossRef]
- Borson, S.; Scanlan, J.M.; Watanabe, J.; Tu, S.P.; Lessig, M. Simplifying detection of cognitive impairment: Comparison of the Mini-Cog and Mini-Mental State Examination in a multiethnic sample. J. Am. Geriatr. Soc. 2005, 53, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Bravo, G.; Hébert, R. Age- and education-specific reference values for the Mini-Mental and modified Mini-Mental State Examinations derived from a non-demented elderly population. Int. J. Geriatr. Psychiatry 1997, 12, 1008–1018. [Google Scholar] [CrossRef]
- Scazufca, M.; Almeida, O.P.; Vallada, H.P.; Tasse, W.A.; Menezes, P.R. Limitations of the Mini-Mental State Examination for screening dementia in a community with low socioeconomic status: Results from the Sao Paulo Ageing & Health Study. Eur. Arch. Psychiatry Clin. Neurosci. 2009, 259, 8–15. [Google Scholar] [CrossRef]
- Lange, M.; Binarelli, G.; Joly, F. Geriatric phone follow-up including validated cognitive assessment in older patients treated for cancer. J. Geriatr. Oncol. 2021, 12, 838–839. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, K.K.; Chan, J.Y.; Hirai, H.W.; Wong, S.Y.; Kwok, T.C. Cognitive Tests to Detect Dementia: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2015, 175, 1450–1458. [Google Scholar] [CrossRef]
- Lezak, M.D.; Howieson, D.B.; Loring, D.W.; Hannay, H.J.; Fischer, J.S. Neuropsychological Assessment, 4th ed.; Oxford University Press: New York, NY, USA, 2004; pp. xiv, 1016–xiv, 1016. [Google Scholar]
- Calamia, M.; Markon, K.; Tranel, D. Scoring higher the second time around: Meta-analyses of practice effects in neuropsychological assessment. Clin. Neuropsychol. 2012, 26, 543–570. [Google Scholar] [CrossRef]
- Galvin, J.E.; Roe, C.M.; Xiong, C.; Morris, J.C. Validity and reliability of the AD8 informant interview in dementia. Neurology 2006, 67, 1942–1948. [Google Scholar] [CrossRef]
- Galvin, J.E.; Roe, C.M.; Powlishta, K.K.; Coats, M.A.; Muich, S.J.; Grant, E.; Miller, J.P.; Storandt, M.; Morris, J.C. The AD8: A brief informant interview to detect dementia. Neurology 2005, 65, 559–564. [Google Scholar] [CrossRef]
- Jorm, A.F. The Informant Questionnaire on cognitive decline in the elderly (IQCODE): A review. Int. Psychogeriatr. 2004, 16, 275–293. [Google Scholar] [CrossRef]
- Jorm, A.F.; Jacomb, P.A. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): Socio-demographic correlates, reliability, validity and some norms. Psychol. Med. 1989, 19, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, L.H.; Langa, K.M.; Bynum, J.P.W.; Hsu, J.W. Financial Presentation of Alzheimer Disease and Related Dementias. JAMA Intern. Med. 2021, 181, 220–227. [Google Scholar] [CrossRef]
- Ott, B.R.; Daiello, L.A. How does dementia affect driving in older patients? Aging Health 2010, 6, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Dementia Australia. Tests Used in Diagnosing Dementia. Available online: https://www.dementia.org.au/national/about-dementia/how-can-i-find-out-more/tests-used-in-diagnosing-dementia (accessed on 14 August 2021).
- Dementia Australia. Diagnosing Dementia. Available online: https://www.dementia.org.au/information/diagnosing-dementia (accessed on 14 August 2021).
- Hamaker, M.E.; Schiphorst, A.H.; ten Bokkel Huinink, D.; Schaar, C.; van Munster, B.C. The effect of a geriatric evaluation on treatment decisions for older cancer patients–a systematic review. Acta Oncol. 2014, 53, 289–296. [Google Scholar] [CrossRef]
- Blackwood, J.; Rybicki, K.; Huang, M. Cognitive measures in older cancer survivors: An examination of validity, reliability, and minimal detectable change. J. Geriatr. Oncol. 2021, 12, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Karuturi, M.; Wong, M.L.; Hsu, T.; Kimmick, G.G.; Lichtman, S.M.; Holmes, H.M.; Inouye, S.K.; Dale, W.; Loh, K.P.; Whitehead, M.I.; et al. Understanding cognition in older patients with cancer. J. Geriatr. Oncol. 2016, 7, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Malik, U.; Alam, Z.; Loucks, A.; Jin, R.; Yokom, D.; Watt, S.; Berger, A.; Romanovsky, L.; Puts, M.; Alibhai, S.M.H. Downstream consequences of abnormal cognitive screening in older adults seen pretreatment in a geriatric oncology clinic. J. Geriatr. Oncol. 2020, 11, 784–789. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).