Real World Outcomes in Patients with Advanced Melanoma Treated in Alberta, Canada: A Time-Era Based Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Statistical and Survival Analyses
3. Results
3.1. Patient Characteristics
3.2. Outcomes Based on era of Treatment
3.3. Outcomes by First Treatment Received
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2014, 372, 320–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larkin, J.; Ascierto, P.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Maio, M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N. Engl. J. Med. 2014, 371, 1867–1876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flaherty, K.T.; Robert, C.; Hersey, P.; Nathan, P.; Garbe, C.; Milhem, M.; Demidov, L.V.; Hassel, J.C.; Rutkowski, P.; Mohr, P.; et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N. Engl. J. Med. 2012, 367, 107–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert, C.; Grob, J.J.; Stroyakovskiy, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion Sileni, V.; Schachter, J.; Garbe, C.; Bondarenko, I.; et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N. Engl. J. Med. 2019, 381, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Ascierto, P.A.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1315–1327. [Google Scholar] [CrossRef]
- Schadendorf, D.; Hodi, F.S.; Robert, C.; Weber, J.S.; Margolin, K.; Hamid, O.; Patt, D.; Chen, T.T.; Berman, D.M.; Wolchok, J.D. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J. Clin. Oncol. 2015, 33, 1889–18894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topalian, S.L.; Sznol, M.; McDermott, D.F.; Kluger, H.M.; Carvajal, R.D.; Sharfman, W.H.; Brahmer, J.R.; Lawrence, D.P.; Atkins, M.B.; Powderly, J.D.; et al. Durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 2014, 32, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Long, G.V.; Robert, C.; Brady, B.; Dutriaux, C.; Di Giacomo, A.M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; et al. Survival outcomes in patients with previously untreated BRAF wild-type advanced melanoma treated with nivolumab therapy. JAMA Oncol. 2019, 5, 187–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schachter, J.; Ribas, A.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017, 390, 1853–1862. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauschild, A.; Grob, J.J.; Demidov, L.V.; Jouary, T.; Gutzmer, R.; Millward, M.; Rutkowski, P.; Blank, C.U.; Miller, W.H., Jr.; Kaempgen, E.; et al. Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012, 380, 358–365. [Google Scholar] [CrossRef]
- Long, G.V.; Grob, J.J.; Nathan, P.; Ribas, A.; Robert, C.; Schadendorf, D.; Lane, S.R.; Mak, C.; Legenne, P.; Flaherty, K.T.; et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: A pooled analysis of individual patient data from randomised trials. Lancet Oncol 2016, 17, 1743–1754. [Google Scholar] [CrossRef]
- Donia, M.; Ellebaek, E.; Øllegaard, T.H.; Duval, L.; Aaby, J.B.; Hoejberg, L.; Køhler, U.H.; Schmidt, H.; Bastholt, L.; Svane, I.M. The real-world impact of modern treatments on the survival of patients with metastatic melanoma. Eur. J. Cancer 2019, 108, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Pavlick, A.C.; Zhao, R.; Lee, C.-H.; Ritchings, C.; Rao, S. First-line immunotherapy versus targeted therapy in patients with BRAF -mutant advanced melanoma: A real-world analysis. Future Oncol. 2021, 17, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Keilholz, U.; Ascierto, P.A.; Dummer, R.; Robert, C.; Lorigan, P.; Van Akkooi, A.; Arance, A.; Blank, C.U.; Chiarion Sileni, V.; Donia, M.; et al. ESMO consensus conference recommendations on the management of metastatic melanoma: Under the auspices of the ESMO Guidelines Committee. Ann. Oncol. 2020, 31, 1435–1448. [Google Scholar] [CrossRef] [PubMed]
- Ugurel, S.; Röhmel, J.; Ascierto, P.A.; Becker, J.C.; Flaherty, K.T.; Grob, J.J.; Hauschild, A.; Larkin, J.; Livingstone, E.; Long, G.V.; et al. Survival of patients with advanced metastatic melanoma: The impact of MAP kinase pathway inhibition and immune checkpoint inhibition—Update 2019. Eur. J. Cancer 2020, 130, 126–138. [Google Scholar] [CrossRef] [PubMed]
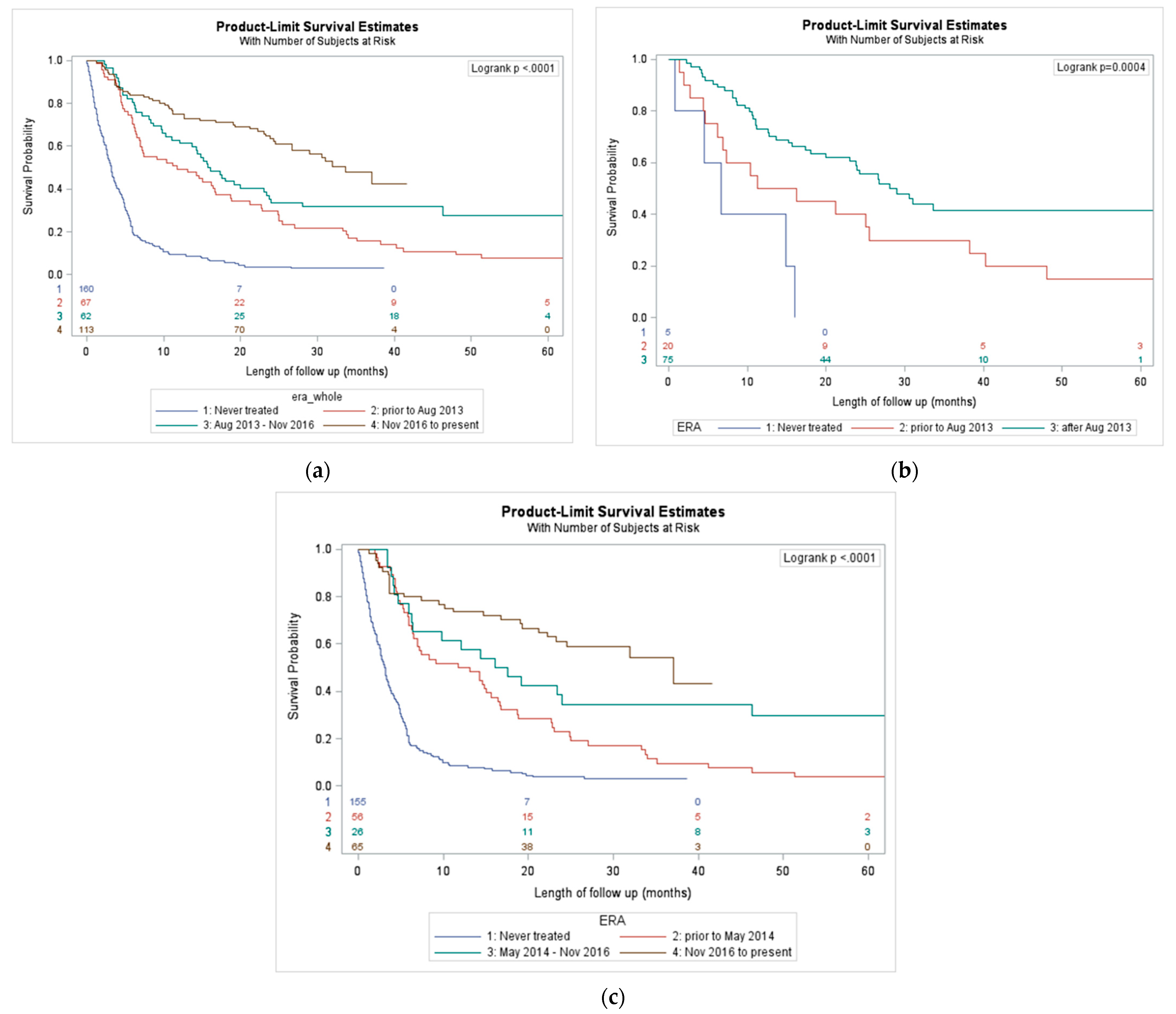
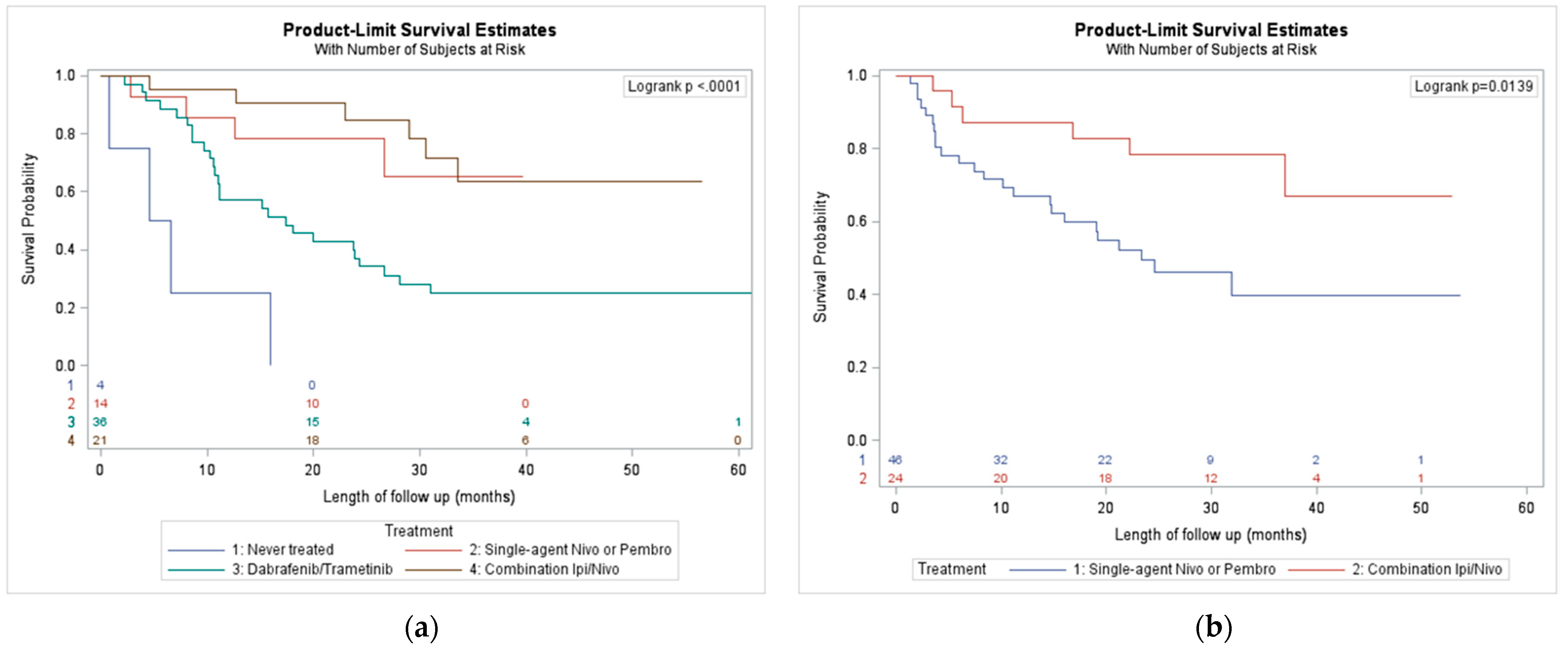
| Variables | Category | Total | 1st Era (Mar 2007–July 2013) | 2nd Era (August 2013–November 2016) | 3rd Era (November 2016–July 2020) | Never Treated | p-Value |
|---|---|---|---|---|---|---|---|
| Number of patients | 402 | 67 | 62 | 113 | 160 | ||
| Treatment | DTIC Ipilimumab Vemurafenib | Pembrolizumab Nivolumab Dabrafenib/ Trametinib | Ipilimumab/ Nivolumab | ||||
| Age | Mean (STD) | 63 (15) | 68 (15) | 55 (14) | 59 (14) | 65 (13) | <0.0001 |
| Sex | Female | 123 (30.6%) | 52 (32.5%) | 19 (28.4%) | 17 (27.4%) | 35 (31.0%) | <0.0001 |
| Male | 279 (69.4%) | 108 (67.5%) | 48 (71.6%) | 45 (72.6%) | 78 (69.0%) | ||
| BRAF mutation status | Mutant | 100 (24.9%) | 5 (3.1%) | 20 (29.9%) | 27 (43.5%) | 48 (42.5%) | <0.0001 |
| Wild | 165 (41.0%) | 53 (33.1%) | 29 (43.3%) | 35 (56.5%) | 48 (42.5%) | ||
| Unknown | 137 (34.1%) | 102 (63.7%) | 18 (26.9%) | 0 (0.0%) | 17 (15.0%) |
| Variables | Category | Total (n = 402) | Alive (n = 94) | Death (n = 308) | p-Value |
|---|---|---|---|---|---|
| Age | Mean (STD) | 63 (15.3) | 63.1 (13.4) | 63 (15.9) | |
| Sex | Female | 123 (30.6%) | 33 (35.1%) | 90 (29.2%) | |
| Male | 279 (69.4%) | 61 (64.9%) | 218 (70.8%) | ||
| ECOG | 0–1 | 255 (63.4%) | 86 (91.5%) | 169 (54.9%) | <0.0001 |
| 2+ | 147 (36.6%) | 8 (8.5%) | 139 (45.1%) | ||
| Melanoma stage | Stage III | 80 (19.9%) | 44 (46.8%) | 36 (11.7%) | <0.0001 |
| Stage IV | 322 (80.1%) | 50 (53.2%) | 272 (88.3%) | ||
| BRAF mutation | Mutant | 100 (24.9%) | 39 (41.5%) | 61 (19.8%) | <0.0001 |
| Wild | 165 (41%) | 41 (43.6%) | 124 (40.3%) | ||
| Unknown | 137 (34.1%) | 14 (14.9%) | 123 (39.9%) | ||
| LDH | LDH high | 140 (34.8%) | 6 (6.4%) | 134 (43.5%) | <0.0001 |
| LDH not high | 166 (41.3%) | 66 (70.2%) | 100 (32.5%) | ||
| Unknown | 96 (23.9%) | 22 (23.4%) | 74 (24%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigo, R.; Doherty, J.; Koczka, K.; Kong, S.; Ding, P.Q.; Cheng, T.; Cheung, W.Y.; Monzon, J.G. Real World Outcomes in Patients with Advanced Melanoma Treated in Alberta, Canada: A Time-Era Based Analysis. Curr. Oncol. 2021, 28, 3978-3986. https://doi.org/10.3390/curroncol28050338
Rigo R, Doherty J, Koczka K, Kong S, Ding PQ, Cheng T, Cheung WY, Monzon JG. Real World Outcomes in Patients with Advanced Melanoma Treated in Alberta, Canada: A Time-Era Based Analysis. Current Oncology. 2021; 28(5):3978-3986. https://doi.org/10.3390/curroncol28050338
Chicago/Turabian StyleRigo, Rodrigo, Jordan Doherty, Kim Koczka, Shiying Kong, Philip Q. Ding, Tina Cheng, Winson Y. Cheung, and Jose G. Monzon. 2021. "Real World Outcomes in Patients with Advanced Melanoma Treated in Alberta, Canada: A Time-Era Based Analysis" Current Oncology 28, no. 5: 3978-3986. https://doi.org/10.3390/curroncol28050338
APA StyleRigo, R., Doherty, J., Koczka, K., Kong, S., Ding, P. Q., Cheng, T., Cheung, W. Y., & Monzon, J. G. (2021). Real World Outcomes in Patients with Advanced Melanoma Treated in Alberta, Canada: A Time-Era Based Analysis. Current Oncology, 28(5), 3978-3986. https://doi.org/10.3390/curroncol28050338





