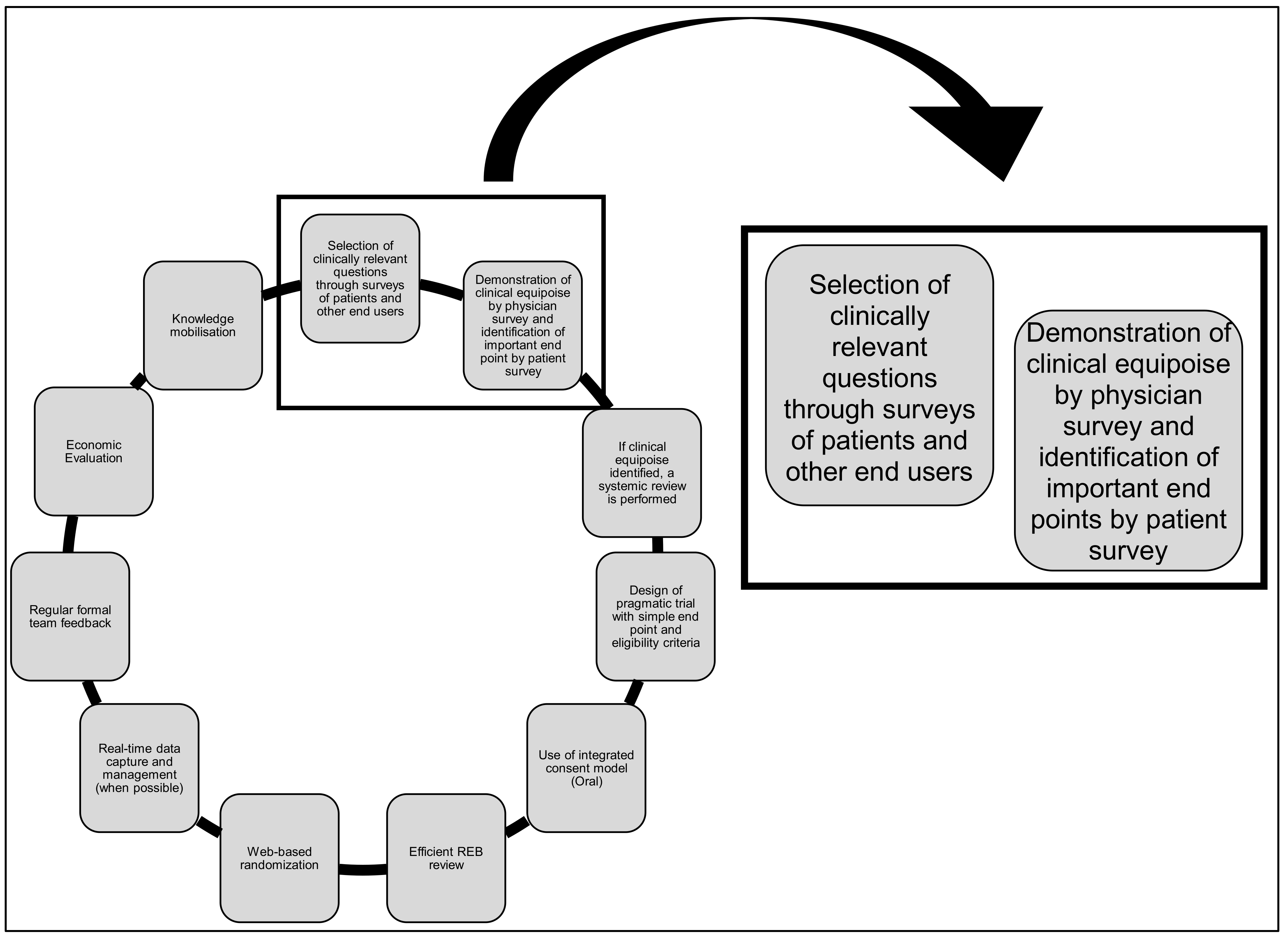
The Rethinking Clinical Trials (REaCT) Program. A Canadian-Led Pragmatic Trials Program: Strategies for Integrating Knowledge Users into Trial Design
Abstract
1. Introduction
2. Materials and Methods
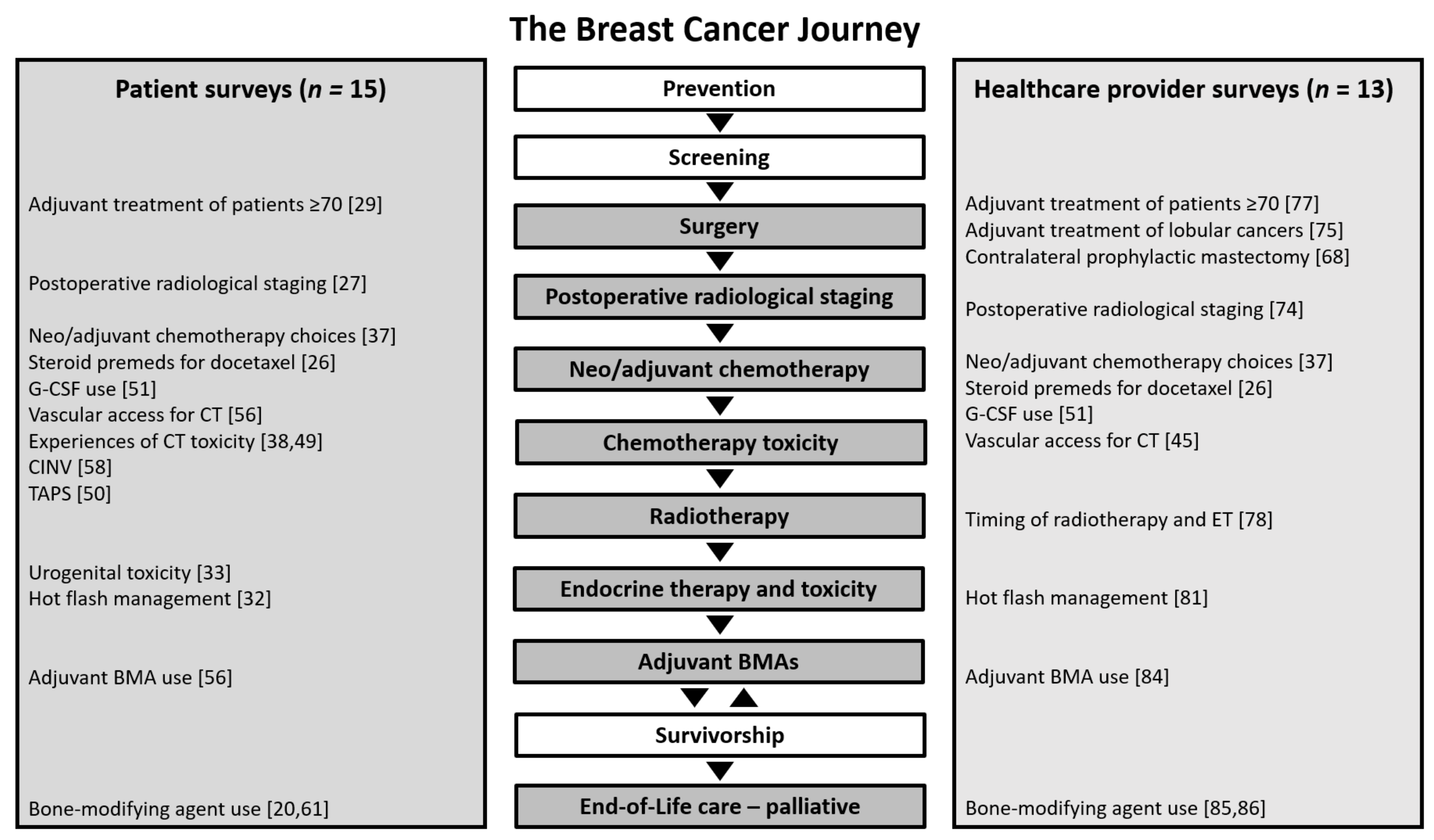
2.1. Patient Survey Outcomes
2.2. Health Care Provider Survey Outcomes
3. Results
3.1. Process for Designing Surveys
3.2. Choice of Research Ethics Board (REB)
3.3. Use of Incentives
3.4. Patient Surveys
3.5. Health Care Provider Surveys
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nipp, R.D.; Hong, K.; Paskett, E.D. Overcoming barriers to clinical trial enrollment. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Hilton, J.; Mazzarello, S.; Fergusson, D.; Joy, A.A.; Robinson, A.; Arnaout, A.; Hutton, B.; Vandermeer, L.; Clemons, M. Novel Methodology for Comparing Standard-of-Care Interventions in Patients With Cancer. J. Oncol. Pract. 2016, 12, e1016–e1024. [Google Scholar] [CrossRef] [PubMed]
- Basulaiman, B.; Awan, A.A.; Fergusson, D.; Vandermeer, L.; Arnaout, A.; Hilton, J.; Hutton, B.; Joy, A.A.; Robinson, A.; Califaretti, N.; et al. Creating a pragmatic trials program for breast cancer patients: Rethinking Clinical Trials (REaCT). Breast Cancer Res. Treat. 2019, 177, 93–101. [Google Scholar] [CrossRef]
- Ibrahim, M.F.K.; Hilton, J.; Mazzarello, S.; Fergusson, D.; Hutton, B.; Robinson, A.; Califaretti, N.; Hsu, T.; Gertler, S.; Mates, M.; et al. A multi-center pragmatic, randomized, feasibility trial comparing standard of care schedules of filgrastim administration for primary febrile neutropenia prophylaxis in early-stage breast cancer. Breast Cancer Res. Treat. 2018, 168, 371–379. [Google Scholar] [CrossRef]
- Kim, S.Y.; Miller, F.G. Informed consent for pragmatic trials--the integrated consent model. N. Engl. J. Med. 2014, 370, 769–772. [Google Scholar] [CrossRef] [PubMed]
- Sugarman, J.; Califf, R.M. Ethics and regulatory complexities for pragmatic clinical trials. JAMA 2014, 311, 2381–2382. [Google Scholar] [CrossRef]
- Arnaout, A.; Zhang, J.; Frank, S.; Momtazi, M.; Cordeiro, E.; Roberts, A.; Ghumman, A.; Fergusson, D.; Stober, C.; Pond, G. A Randomized Controlled Trial Comparing Alloderm-RTU with DermACELL in Immediate Subpectoral Implant-Based Breast Reconstruction. Curr. Oncol. 2021, 28, 20. [Google Scholar] [CrossRef]
- Robertson, S.J.; Pond, G.R.; Hilton, J.; Petkiewicz, S.L.; Ayroud, Y.; Kos, Z.; Gravel, D.H.; Stober, C.; Vandermeer, L.; Arnaout, A. Selecting patients for Oncotype DX testing using standard clinicopathologic information. Clin. Breast Cancer 2020, 20, 61–67. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. An Integrated Consent Model Study to Compare Two Standard of Care Schedules for Monitoring Cardiac Function in Patients Receiving Trastuzumab for Early Stage Breast Cancer. Available online: https://www.clinicaltrials.gov/ct2/show/NCT02696707 (accessed on 9 June 2021).
- Clemons, M.; Stober, C.; Kehoe, A.; Bedard, D.; MacDonald, F.; Brunet, M.-C.; Saunders, D.; Vandermeer, L.; Mazzarello, S.; Awan, A. A randomized trial comparing vascular access strategies for patients receiving chemotherapy with trastuzumab for early-stage breast cancer. Support. Care Cancer 2020, 28, 4891–4899. [Google Scholar] [CrossRef]
- Robinson, A.; Stober, C.; Fergusson, D.; Kehoe, A.; Bedard, D.; MacDonald, F.; Brunet, M.-C.; Saunders, D.; Mazzarello, S.; Vandermeer, L. A multicentre, randomized pilot trial comparing vascular access strategies for early stage breast cancer patients receiving non-trastuzumab containing chemotherapy. Breast Cancer Res. Treat. 2019, 178, 337–345. [Google Scholar] [CrossRef]
- Clemons, M.; Bouganim, N.; Smith, S.; Mazzarello, S.; Vandermeer, L.; Segal, R.; Dent, S.; Gertler, S.; Song, X.; Wheatley-Price, P. Risk model–guided antiemetic prophylaxis vs physician’s choice in patients receiving chemotherapy for early-stage breast cancer: A randomized clinical trial. JAMA Oncol. 2016, 2, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Clemons, M.; Dranitsaris, G.; Sienkiewicz, M.; Sehdev, S.; Ng, T.; Robinson, A.; Mates, M.; Hsu, T.; McGee, S.; Freedman, O. A randomized trial of individualized versus standard of care antiemetic therapy for breast cancer patients at high risk for chemotherapy-induced nausea and vomiting. Breast 2020, 54, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Hilton, J.; Stober, C.; Mazzarello, S.; Vandermeer, L.; Fergusson, D.; Hutton, B.; Clemons, M. Randomised feasibility trial to compare three standard of care chemotherapy regimens for early stage triple-negative breast cancer (REaCT-TNBC trial). PLoS ONE 2018, 13, e0199297. [Google Scholar] [CrossRef] [PubMed]
- Clemons, M.; Fergusson, D.; Simos, D.; Mates, M.; Robinson, A.; Califaretti, N.; Zibdawi, L.; Bahl, M.; Raphael, J.; Ibrahim, M. A multicentre, randomised trial comparing schedules of G-CSF (filgrastim) administration for primary prophylaxis of chemotherapy-induced febrile neutropenia in early stage breast cancer. Ann. Oncol. 2020, 31, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Clemons, M.; Ong, M.; Stober, C.; Ernst, S.; Booth, C.; Canil, C.; Mates, M.; Robinson, A.; Blanchette, P.; Joy, A.A. A randomised trial of 4-versus 12-weekly administration of bone-targeted agents in patients with bone metastases from breast or castration-resistant prostate cancer. Eur. J. Cancer 2021, 142, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Clemons, M.; Mazzarello, S.; Hilton, J.; Joy, A.; Price-Hiller, J.; Zhu, X.; Verma, S.; Kehoe, A.; Ibrahim, M.F.; Sienkiewicz, M.; et al. Feasibility of using a pragmatic trials model to compare two primary febrile neutropenia prophylaxis regimens (ciprofloxacin versus G-CSF) in patients receiving docetaxel-cyclophosphamide chemotherapy for breast cancer (REaCT-TC). Support. Care Cancer 2019, 27, 1345–1354. [Google Scholar] [CrossRef]
- Awan, A.; Ng, T.; Conter, H.; Raskin, W.; Stober, C.; Simos, D.; Pond, G.; Dhesy-Thind, S.; Mates, M.; Kumar, V.; et al. Feasibility outcomes of a randomised, multicentre, pilot trial comparing standard 6-monthly dosing of adjuvant zoledronate with a single one-time dose in patients with early stage breast cancer. J. Bone Oncol. 2021, 26, 100343. [Google Scholar] [CrossRef]
- Parry, D.; Salsberg, J.; Macaulay, A.C.; FCPC, C. A Guide to Researcher and Knowledge-User Collaboration in Health Research. Available online: https://cihr-irsc.gc.ca/e/44954 (accessed on 27 July 2021).
- Schroter, S.; Price, A.; Flemyng, E.; Demaine, A.; Elliot, J.; Harmston, R.R.; Richards, T.; Staniszewska, S.; Stephens, R. Perspectives on involvement in the peer-review process: Surveys of patient and public reviewers at two journals. BMJ Open 2018, 8, e023357. [Google Scholar] [CrossRef]
- Surujballi, J.; Shah, H.; Hutton, B.; Alzahrani, M.; Beltran-Bless, A.A.; Shorr, R.; Larocque, G.; McGee, S.; Cole, K.; Ibrahim, M.F.K.; et al. The COVID-19 pandemic: An opportunity to rethink and harmonise the frequency of follow-up visits for patients with early stage breast cancer. Cancer Treat. Rev. 2021, 97, 102188. [Google Scholar] [CrossRef]
- Dillman, D.A. The design and administration of mail surveys. Annu. Rev. Sociol. 1991, 17, 225–249. [Google Scholar] [CrossRef]
- Mazzarello, S.; Clemons, M.; Graham, I.D.; Jacobs, C. Surviving Surveys. J. Oncol. Pract. 2015, 11, 44–46. [Google Scholar] [CrossRef]
- Wiebe, E.R.; Kaczorowski, J.; MacKay, J. Why are response rates in clinician surveys declining? Can. Fam. Physician 2012, 58, e225–e228. [Google Scholar] [PubMed]
- Mazzarello, S.; Clemons, M.; Graham, I.; Joy, A.; Smith, S.; Jacobs, C. Third-party online surveys—Science, selling, or sugging? Curr. Oncol. 2015, 22, 182. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.; Hutton, B.; Mazzarello, S.; Smith, S.; Joy, A.; Amir, E.; Ibrahim, M.F.; Gregario, N.; Daigle, K.; Eggert, L. Optimisation of steroid prophylaxis schedules in breast cancer patients receiving docetaxel chemotherapy—A survey of health care providers and patients. Support. Care Cancer 2015, 23, 3269–3275. [Google Scholar] [CrossRef]
- Simos, D.; Hutton, B.; Graham, I.D.; Arnaout, A.; Caudrelier, J.-M.; Mazzarello, S.; Clemons, M. Patient perceptions and expectations regarding imaging for metastatic disease in early stage breast cancer. Springerplus 2014, 3, 176. [Google Scholar] [CrossRef] [PubMed]
- Simos, D.; Catley, C.; van Walraven, C.; Arnaout, A.; Booth, C.M.; McInnes, M.; Fergusson, D.; Dent, S.; Clemons, M. Imaging for distant metastases in women with early-stage breast cancer: A population-based cohort study. CMAJ 2015, 187, E387–E397. [Google Scholar] [CrossRef] [PubMed]
- Savard, M.F.; AlZahrani, M.J.; Saunders, D.; Chang, L.; Ng, T.L.; Brackstone, M.; Vandermeer, L.; Hsu, T.; Awan, A.A.; Cole, K.; et al. Experiences and perceptions of older adults with lower-risk hormone receptor-positive breast cancer about adjuvant radiotherapy and endocrine therapy: A patient survey. Ottawa Hospital, Ottawa, ON, Canada. Unpublished work. 2021. [Google Scholar]
- Savard, M.F.; Clemons, M.; Hutton, B. De-escalating adjuvant therapies in older patients with lower risk estrogen receptor-positive breast cancer treated with breast-conserving surgery: A systematic review and meta-analysis. Cancer Treat. Rev. 2021, 99, 102254. [Google Scholar] [CrossRef] [PubMed]
- Clinicaltrials.gov. Evaluating Harms and Benefits of Endocrine Therapy in Patients ≥70 Years of Age with Lower Risk Breast Cancer. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04921137 (accessed on 9 June 2021).
- Cole, K.; McGee, S.; Liu, M. Developing patient-centered strategies to optimize the management of vasomotor symptoms in breast cancer patients: A patient survey. Breast Cancer In press. 2021. [Google Scholar]
- Chin, S.N.; Trinkaus, M.; Simmons, C.; Flynn, C.; Dranitsaris, G.; Bolivar, R.; Clemons, M. Prevalence and severity of urogenital symptoms in postmenopausal women receiving endocrine therapy for breast cancer. Clin. Breast Cancer 2009, 9, 108–117. [Google Scholar] [CrossRef]
- Trinkaus, M.; Chin, S.; Wolfman, W.; Simmons, C.; Clemons, M. Should urogenital atrophy in breast cancer survivors be treated with topical estrogens? Oncol. 2008, 13, 222–231. [Google Scholar] [CrossRef]
- Mazzarello, S.; Hutton, B.; Ibrahim, M.F.; Jacobs, C.; Shorr, R.; Smith, S.; Ng, T.; Clemons, M. Management of urogenital atrophy in breast cancer patients: A systematic review of available evidence from randomized trials. Breast Cancer Res. Treat. 2015, 152, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Simmons, C.; Kuchuk, I.; Freedman, O.; Colgan, T.; Dodd, A.; Kulhanek, K.; Sheiner, J.; Dranitsaris, G.; Dowsett, M.; Folkerd, E. Are Estring® and Vagifem® equally effective and safe for the treatment of urogenital atrophy in breast cancer patients on aromatase inhibitor therapy? Clin. Oncol. 2012, 24, e128–e129. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.; Clemons, M.; Mazzarello, S.; Hutton, B.; Joy, A.A.; Brackstone, M.; Freedman, O.; Vandermeer, L.; Ibrahim, M.; Fergusson, D.; et al. Enhancing accrual to chemotherapy trials for patients with early stage triple-negative breast cancer: A survey of physicians and patients. Support. Care Cancer 2017, 25, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Beusterien, K.; Grinspan, J.; Kuchuk, I.; Mazzarello, S.; Dent, S.; Gertler, S.; Bouganim, N.; Vandermeer, L.; Clemons, M. Use of conjoint analysis to assess breast cancer patient preferences for chemotherapy side effects. Oncologist 2014, 19, 127. [Google Scholar] [CrossRef]
- Fernandes, R.; Mazzarello, S.; Hutton, B.; Shorr, R.; Ibrahim, M.F.; Jacobs, C.; Ong, M.; Clemons, M. A systematic review of the incidence and risk factors for taxane acute pain syndrome in patients receiving taxane-based chemotherapy for prostate cancer. Clin. Genitourin. Cancer 2017, 15, 1–6. [Google Scholar] [CrossRef]
- Fernandes, R.; Mazzarello, S.; Hutton, B.; Shorr, R.; Majeed, H.; Ibrahim, M.F.; Jacobs, C.; Ong, M.; Clemons, M. Taxane acute pain syndrome (TAPS) in patients receiving taxane-based chemotherapy for breast cancer—A systematic review. Support. Care Cancer 2016, 24, 3633–3650. [Google Scholar] [CrossRef]
- Fernandes, R.; Mazzarello, S.; Majeed, H.; Smith, S.; Shorr, R.; Hutton, B.; Ibrahim, M.F.; Jacobs, C.; Ong, M.; Clemons, M. Treatment of taxane acute pain syndrome (TAPS) in cancer patients receiving taxane-based chemotherapy—A systematic review. Support. Care Cancer 2016, 24, 1583–1594. [Google Scholar] [CrossRef]
- Hutton, B.; Clemons, M.; Mazzarello, S.; Kuchuk, I.; Skidmore, B.; Ng, T. Identifying an optimal antiemetic regimen for patients receiving anthracycline and cyclophosphamide-based chemotherapy for breast cancer–an inspection of the evidence base informing clinical decision-making. Cancer Treat. Rev. 2015, 41, 951–959. [Google Scholar] [CrossRef]
- Dranitsaris, G.; Molassiotis, A.; Clemons, M.; Roeland, E.; Schwartzberg, L.; Dielenseger, P.; Jordan, K.; Young, A.; Aapro, M. The development of a prediction tool to identify cancer patients at high risk for chemotherapy-induced nausea and vomiting. Ann. Oncol. 2017, 28, 1260–1267. [Google Scholar] [CrossRef]
- Ng, T.; Mazzarello, S.; Wang, Z.; Hutton, B.; Dranitsaris, G.; Vandermeer, L.; Smith, S.; Clemons, M. Choice of study endpoint significantly impacts the results of breast cancer trials evaluating chemotherapy-induced nausea and vomiting. Breast Cancer Res. Treat. 2016, 155, 337–344. [Google Scholar] [CrossRef]
- Clemons, M.; Simos, D.; Sienkiewicz, M.; Ng, T.; Zibdawi, L.; Basulaiman, B.; Awan, A.; Fergusson, D.; Vandermeer, L.; Saunders, D. A prospective multi-centre, randomized study comparing the addition of tapering dexamethasone to other standard of care therapies for taxane-associated pain syndrome (TAPS) in breast cancer patients. Support. Care Cancer 2021, 29, 5787–5795. [Google Scholar] [CrossRef]
- Thavorn, K.; Coyle, D.; Hoch, J.S.; Vandermeer, L.; Mazzarello, S.; Wang, Z.; Dranitsaris, G.; Fergusson, D.; Clemons, M. A cost-utility analysis of risk model-guided versus physician’s choice antiemetic prophylaxis in patients receiving chemotherapy for early-stage breast cancer: A net benefit regression approach. Support. Care Cancer 2017, 25, 2505–2513. [Google Scholar] [CrossRef]
- Fernandes, R.; Mazzarello, S.; Joy, A.; Pond, G.; Hilton, J.; Ibrahim, M.; Canil, C.; Ong, M.; Stober, C.; Vandermeer, L. Taxane acute pain syndrome (TAPS) in patients receiving chemotherapy for breast or prostate cancer: A prospective multi-center study. Support. Care Cancer 2018, 26, 3073–3081. [Google Scholar] [CrossRef] [PubMed]
- AlZahrani, M.J.; Dranitsaris, G.; Sienkiewicz, M.; Vandermeer, L.; Clemons, M. Clinical utility of a prediction tool to differentiate between breast cancer patients at high or low risk of chemotherapy induced nausea and vomiting. Support. Care Cancer 2021. [Google Scholar] [CrossRef] [PubMed]
- Kuchuk, I.; Bouganim, N.; Beusterien, K.; Grinspan, J.; Vandermeer, L.; Gertler, S.; Dent, S.; Song, X.; Segal, R.; Mazzarello, S. Preference weights for chemotherapy side effects from the perspective of women with breast cancer. Breast Cancer Res. Treat. 2013, 142, 101–107. [Google Scholar] [CrossRef]
- Saibil, S.; Fitzgerald, B.; Freedman, O.C.; Amir, E.; Napolskikh, J.; Salvo, N.; Dranitsaris, G.; Clemons, M. Incidence of taxane-induced pain and distress in patients receiving chemotherapy for early-stage breast cancer: A retrospective, outcomes-based survey. Curr. Oncol. 2010, 17, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Hilton, J.; Vandermeer, L.; Sienkiewicz, M.; Mazzarello, S.; Hutton, B.; Stober, C.; Fergusson, D.; Blanchette, P.; Joy, A.A.; Bota, A.B. Filgrastim use in patients receiving chemotherapy for early-stage breast cancer—A survey of physicians and patients. Support. Care Cancer 2018, 26, 2323–2331. [Google Scholar] [CrossRef]
- Fernandes, R.; Mazzarello, S.; Stober, C.; Ibrahim, M.F.; Dudani, S.; Perdrizet, K.; Majeed, H.; Vandermeer, L.; Shorr, R.; Hutton, B. Primary febrile neutropenia prophylaxis for patients who receive FEC-D chemotherapy for breast cancer: A systematic review. J. Glob. Oncol. 2017, 4, 1–8. [Google Scholar] [CrossRef]
- Fernandes, R.; Mazzarello, S.; Stober, C.; Vandermeer, L.; Dudani, S.; Ibrahim, M.F.; Majeed, H.; Perdrizet, K.; Shorr, R.; Hutton, B. Optimal primary febrile neutropenia prophylaxis for patients receiving docetaxel–cyclophosphamide chemotherapy for breast cancer: A systematic review. Breast Cancer Res. Treat. 2017, 161, 1–10. [Google Scholar] [CrossRef]
- Clemons, M.; Fergusson, D.; Joy, A.A.; Thavorn, K.; Meza-Junco, J.; Hiller, J.P.; Mackey, J.; Ng, T.; Zhu, X.; Ibrahim, M.F. A multi-centre study comparing granulocyte-colony stimulating factors to antibiotics for primary prophylaxis of docetaxel-cyclophosphamide induced febrile neutropenia. Breast 2021, 58, 42–49. [Google Scholar] [CrossRef]
- Hsu, T.; Fergusson, D.; Stober, C.; Daigle, K.; Moledina, N.; Vandermeer, L.; Pond, G.; Hilton, J.; Hutton, B.; Clemons, M. A randomized clinical trial comparing physician-directed or fixed-dose steroid replacement strategies for incomplete dexamethasone dosing prior to docetaxel chemotherapy. Support. Care Cancer 2021, 29, 3113–3120. [Google Scholar] [CrossRef] [PubMed]
- LeVasseur, N.; Stober, C.; Ibrahim, M.; Gertler, S.; Hilton, J.; Robinson, A.; McDiarmid, S.; Fergusson, D.; Mazzarello, S.; Hutton, B. Perceptions of vascular access for intravenous systemic therapy and risk factors for lymphedema in early-stage breast cancer—A patient survey. Curr. Oncol. 2018, 25, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.; Souied, O.; Bota, A.B.; Levasseur, N.; Stober, C.; Hilton, J.; Kamel, D.; Hutton, B.; Vandermeer, L.; Mazzarello, S. Optimal vascular access strategies for patients receiving chemotherapy for early-stage breast cancer: A systematic review. Breast Cancer Res. Treat. 2018, 171, 607–620. [Google Scholar] [CrossRef]
- Torres, C.H.; Mazzarello, S.; Ng, T.; Dranitsaris, G.; Hutton, B.; Smith, S.; Munro, A.; Jacobs, C.; Clemons, M. Defining optimal control of chemotherapy-induced nausea and vomiting—Based on patients’ experience. Support. Care Cancer 2015, 23, 3341–3359. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.; AlZahrani, M.; Stober, C.; Ng, T.L.; Cole, K.; Larocque, G.; Awan, A.; Sehdev, S.; Hilton, J.; Vandermeer, L.; et al. Adjuvant bisphosphonate use in patients with early stage breast cancer: Patient perspectives on treatment acceptability and potential de-escalation. J. Bone Oncol. 2021, 27, 100351. [Google Scholar] [CrossRef]
- Dhesy-Thind, S.; Fletcher, G.G.; Blanchette, P.S.; Clemons, M.J.; Dillmon, M.S.; Frank, E.S.; Gandhi, S.; Gupta, R.; Mates, M.; Moy, B.; et al. Use of Adjuvant Bisphosphonates and Other Bone-Modifying Agents in Breast Cancer: A Cancer Care Ontario and American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2017, 35, 2062–2081. [Google Scholar] [CrossRef] [PubMed]
- Hutton, B.; Morretto, P.; Emmenegger, U.; Mazzarello, S.; Kuchuk, I.; Addison, C.L.; Crawley, F.; Canil, C.; Malone, S.; Berry, S.; et al. Bone-targeted agent use for bone metastases from breast cancer and prostate cancer: A patient survey. J. Bone Oncol. 2013, 2, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Awan, A.A.; Hutton, B.; Hilton, J.; Mazzarello, S.; Van Poznak, C.; Vandermeer, L.; Bota, B.; Stober, C.; Sienkiewicz, M.; Fergusson, D.; et al. De-escalation of bone-modifying agents in patients with bone metastases from breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2019, 176, 507–517. [Google Scholar] [CrossRef]
- Ibrahim, M.F.; Mazzarello, S.; Shorr, R.; Vandermeer, L.; Jacobs, C.; Hilton, J.; Hutton, B.; Clemons, M. Should de-escalation of bone-targeting agents be standard of care for patients with bone metastases from breast cancer? A systematic review and meta-analysis. Ann. Oncol. 2015, 26, 2205–2213. [Google Scholar] [CrossRef] [PubMed]
- Southcott, D.; Awan, A.; Ghate, K.; Clemons, M.; Fernandes, R. Practical Update for the Use of Bone-Targeted Agents in Patients with Bone Metastases from Metastatic Breast Cancer or Castration-Resistant Prostate Cancer. Curr. Oncol. 2020, 27, 220–224. [Google Scholar] [CrossRef]
- Hong, B.Y.; Ibrahim, M.F.; Fernandes, R.; Mazzarello, S.; Hutton, B.; Shorr, R.; Clemons, M. De-escalation of bone-targeted agents for metastatic prostate cancer. Curr. Oncol. 2016, 23, 77–78. [Google Scholar] [CrossRef][Green Version]
- Hilton, J.; Clemons, M.; Pond, G.; Zhao, H.; Mazzarello, S.; Vandermeer, L.; Addison, C. Effects on bone resorption markers of continuing pamidronate or switching to zoledronic acid in patients with high risk bone metastases from breast cancer. J. Bone Oncol. 2018, 10, 6–13. [Google Scholar] [CrossRef]
- Addison, C.L.; Pond, G.R.; Zhao, H.; Mazzarello, S.; Vandermeer, L.; Goldstein, R.; Amir, E.; Clemons, M. Effects of de-escalated bisphosphonate therapy on bone turnover biomarkers in breast cancer patients with bone metastases. Springerplus 2014, 3, 577. [Google Scholar] [CrossRef][Green Version]
- Alzahrani, M.; Clemons, M.; Sienkiewicz, M.; Shrem, N.S.; McGee, S.F.; Vandermeer, L.; Sehdev, S.; Savard, M.F.; Awan, A.; Canil, C.; et al. Perceptions around bone-modifying agent use in patients with bone metastases from breast and castration resistant prostate cancer: A patient survey. Support. Care Cancer 2021, 29, 6903–6912. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.; Ng, T.; Ong, M.; Clemons, M. Long-term benefits versus side-effects from bone-targeted therapies for cancer patients: Minimizing risk while maximizing benefits. Curr. Opin. Support. Palliat. Care 2014, 8, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.L.; Tu, M.M.; Ibrahim, M.F.K.; Basulaiman, B.; McGee, S.F.; Srikanthan, A.; Fernandes, R.; Vandermeer, L.; Stober, C.; Sienkiewicz, M.; et al. Long-term impact of bone-modifying agents for the treatment of bone metastases: A systematic review. Support. Care Cancer 2020, 29, 925–943. [Google Scholar] [CrossRef]
- Jacobs, C.; Kuchuk, I.; Bouganim, N.; Smith, S.; Mazzarello, S.; Vandermeer, L.; Dranitsaris, G.; Dent, S.; Gertler, S.; Verma, S. A randomized, double-blind, phase II, exploratory trial evaluating the palliative benefit of either continuing pamidronate or switching to zoledronic acid in patients with high-risk bone metastases from breast cancer. Breast Cancer Res. Treat. 2016, 155, 77–84. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. A Randomised Trial Comparing Continuation or De-escalation of Bone Modifying Agents (BMA) in Patients Treated for Over 2 Years for Bone Metastases From Either Breast or Castration-Resistant Prostate Cancer (REaCT-Hold BMA). Available online: https://www.clinicaltrials.gov/ct2/show/NCT04549207 (accessed on 9 June 2021).
- Squires, J.; Stacey, D.; Coughlin, M.; Greenough, M.; Roberts, A.; Dorrance, K.; Clemons, M.; Caudrelier, J.; Graham, I.; Zhang, J. Patient decision aid for contralateral prophylactic mastectomy for use in the consultation: A feasibility study. Curr. Oncol. 2019, 26, 137. [Google Scholar] [CrossRef]
- Simos, D.; Hutton, B.; Graham, I.D.; Arnaout, A.; Caudrelier, J.M.; Clemons, M. Imaging for metastatic disease in patients with newly diagnosed breast cancer: Are doctor’s perceptions in keeping with the guidelines? J. Eval. Clin. Pract. 2015, 21, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.; Ibrahim, M.F.; Clemons, M.; Hutton, B.; Simos, D.; Caudrelier, J.M.; Graham, I.D.; Smith, S.; Addison, C.; Arnaout, A. Treatment choices for patients with invasive lobular breast cancer: A doctor survey. J. Eval. Clin. Pract. 2015, 21, 740–748. [Google Scholar] [CrossRef]
- Al-Baimani, K.; Bazzarelli, A.; Clemons, M.; Robertson, S.J.; Addison, C.; Arnaout, A. Invasive pleomorphic lobular carcinoma of the breast: Pathologic, clinical, and therapeutic considerations. Clin. Breast Cancer 2015, 15, 421–425. [Google Scholar] [CrossRef] [PubMed]
- AlZahrani, M.J.; Clemons, M.; Chang, L.; Vandermeer, L.; Arnaout, A.; Larocque, G.; Cole, K.; Hsu, T.; Saunders, D.; Savard, M.F. Management strategies for older patients with low-risk early stage breast cancer: A physician survey. Ottawa Hospital, Ottawa, Ontario, Canada. Unpublished work. 2021. [Google Scholar]
- McGee, S.F.; Vandermeer, L.; Mazzarello, S.; Sienkiewicz, M.; Stober, C.; Hutton, B.; Fergusson, D.; Hilton, J.; Caudrelier, J.-M.; Blanchette, P. Physician Survey of Timing of Adjuvant Endocrine Therapy Relative to Radiotherapy in Early Stage Breast Cancer Patients. Clin. Breast Cancer 2019, 19, e40–e47. [Google Scholar] [CrossRef]
- McGee, S.; Mazzarello, S.; Caudrelier, J.; Lima, M.; Hutton, B.; Sienkiewicz, M.; Stober, C.; Fernandes, R.; Ibrahim, M.; Vandermeer, L. Optimal sequence of adjuvant endocrine and radiation therapy in early-stage breast cancer—A systematic review. Cancer Treat. Rev. 2018, 69, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Clinicaltrials.gov. Evaluating Optimal Timing of Endocrine Therapy and Radiation Therapy in Early-Stage Breast Cancer (REaCT-RETT). Available online: https://www.clinicaltrials.gov/ct2/show/NCT03948568 (accessed on 12 May 2021).
- Cole, K.M.; Clemons, M.; AlZahrani, M.J.; Larocque, G.; MacDonald, F.; Vandermeer, L.; Hutton, B.; Piper, A.; Pond, G.; McGee, S. Developing patient-centered strategies to optimize the management of vasomotor symptoms in breast cancer patients: A survey of health care providers. Breast Cancer Res. Treat. 2021, 188, 343–350. [Google Scholar] [CrossRef]
- Hutton, B.; Hersi, M.; Cheng, W.; Pratt, M.; Barbeau, P.; Mazzarello, S.; Ahmadzai, N.; Skidmore, B.; Morgan, S.C.; Bordeleau, L.; et al. Comparing Interventions for Management of Hot Flashes in Patients With Breast and Prostate Cancer: A Systematic Review With Meta-Analyses. Oncol. Nurs. Forum 2020, 47, E86–E106. [Google Scholar] [CrossRef] [PubMed]
- LeVasseur, N.; Stober, C.; Daigle, K.; Robinson, A.; McDiarmid, S.; Mazzarello, S.; Hutton, B.; Joy, A.; Fergusson, D.; Hilton, J. Optimizing vascular access for patients receiving intravenous systemic therapy for early-stage breast cancer—A survey of oncology nurses and physicians. Curr. Oncol. 2018, 25, 298–304. [Google Scholar] [CrossRef]
- McGee, S.; Alzahrani, M.; Vandermeer, L.; Cole, K.; Larocque, G.; Awan, A.; Hutton, B.; Pond, G.; Saunders, D.; Clemons, M. Adjuvant bisphosphonate use in patients with early stage breast cancer: A physician survey. Breast Cancer Res Treat 2021, 187, 477–486. [Google Scholar] [CrossRef]
- Hutton, B.; Addison, C.; Mazzarello, S.; Joy, A.A.; Bouganim, N.; Fergusson, D.; Clemons, M. De-escalated administration of bone-targeted agents in patients with breast and prostate cancer-A survey of Canadian oncologists. J. Bone Oncol. 2013, 2, 77–83. [Google Scholar] [CrossRef]
- AlZahrani, M.; Clemons, M.; Vandermeer, L.; Sienkiewicz, M.; Awan, A.A.; Hutton, B.; Pond, G.R.; Ng, T.L. Real-world practice patterns and attitudes towards de-escalation of bone-modifying agents in patients with bone metastases from breast and prostate cancer: A physician survey. J. Bone Oncol. 2021, 26, 100339. [Google Scholar] [CrossRef]
- Clemons, M.; Liu, M.; Stober, C.; Pond, G.; AlZahrani, M.J.; Ong, M.; Ernst, S.; Booth, C.; Mates, M.; Joy, A.A.; et al. Two-year results of a randomised trial comparing 4- versus 12-weekly bone-targeted agent use in patients with bone metastases from breast or castration-resistant prostate cancer. J. Bone Oncol. 2021. [CrossRef]
- Awan, A.; Basulaiman, B.; Stober, C.; Clemons, M.; Fergusson, D.; Hilton, J.; Al Ghareeb, W.; Goodwin, R.; Ibrahim, M.; Hutton, B.; et al. Oral magnesium supplements for cancer treatment-induced hypomagnesmia: Results from a pilot randomized trial. Health Sci. Rep. In Press.
| Reference and Year of Publication | Survey Topic | Population Surveyed | Sample Size (Response Rate) | Consent | Duration | How were Participants Identified | Methods of Approach | Methods of Completion | Pertinent Findings | Other Studies the Survey Led To |
|---|---|---|---|---|---|---|---|---|---|---|
| Postoperative radiological staging | ||||||||||
| Simos et al., 2014 [27] | Patient perceptions regarding postoperative imaging for metastatic disease | Patients with EBC who had completed their definitive breast surgery | 245/282 (87%) | Written | 3 months | Eligible participants identified by their physician | Approached by their physician during a regularly scheduled visit | Paper in clinic | >80% recalled having imaging tests for distant metastases. Over half indicated they would want imaging even if the chance of detecting metastases was </=10%, | Led to a population-based cohort study [28] |
| Adjuvant surgical, systemic, and radiotherapy choices in patients >70 years of age | ||||||||||
| Savard et al. 2021 [29] | Patient experience of the harms and benefits of radiotherapy and endocrine therapy | Patients with low risk EBC, 70 years of age or older and had been offered radiation and hormonal therapy | 102/130 (78.5%) | Oral | 7 months | Eligible participants identified either in outpatient clinic by their HCP or CRA if participating in other studies | Approached by their HCP in clinic or if previously transferred to Wellness program and had consented to research contact, telephoned by physicians or CRA | Paper in clinic/Mail/ Emailed web-based survey/Telephone | Most patient received radiation and endocrine therapy and that have minimal or no impact on their quality of life. Most respondents preferred radiation over endocrine therapy if they had to choose between the two treatment modalities. | Led to systematic review [30] and pilot clinical trial [31] |
| Supportive care—endocrine therapy | ||||||||||
| Cole et al., 2021 [32] | Patient experience of hot flashes and efficacy of prior treatments | Patients with EBC who were experiencing hot flashes | 373/448 (83%) | Oral | 9 months | Eligible participants identified either in outpatient clinic by HPC, or by CRAs if participating in other studies | Approached by their HCP in clinic or if previously trasferred to Wellness program and had consented to research contact, telephoned by physicians or CRA | Paper in clinic/Mail/ Emailed web-based survey /Telephone | Most patients with VMS did not feel the issue was adequately acknowledged or addressed. Patients wanted better and more personalized approaches to VMS management. | Led to grant application |
| Chin et al., 2009 [33] * | Prevalence of urogenital symptoms in postmenopausal patients with BC receiving endocrine therapy | Postmenopausal women receiving endocrine therapy for EBC or metastatic BC | 251 (response rate N/A) | Written | 3 months | Eligible participants were identified by their physician | Eligible participants were approached by their physician during a regularly scheduled visit | Paper in clinic | Urogenital side effects reported by 63% of patients. Less than one third of patients had used some form of treatment for these symptoms. | Led to review article [34], systematic review [35] and clinical trial [36] |
| Adjuvant chemotherapy choices for EBC and metastatic breast cancer | ||||||||||
| Jacobs et al., 2017 [37] | Adjuvant CT choices for EBC. Willlingness to participate in trials. Thoughts on the ICM | Patients with EBC and all receptor types treated with neo/adjuvant CT | 74 (response rate N/A) | Oral | 4 months | Eligible participants identified by their physician | Participants approached by their physician during a regularly scheduled visit | Paper in clinic/Take home | Most respondents willing to participate in trials to determine optimal CT regimens. Respondents interested in studies to minimize side effects, even if this means longer duration of treatment. Most respondents willing to enter clinical trials if administrative processes around trial entry were streamlined. | Led to a clinical trial [14] |
| Beusterien et al., 2014 [38] | Conjoint analysis to assess BC patient preferences for CT side effects | Female patients with BC receiving CT for any stage of breast cancer | 102 (response rate N/A) | Written | 7 months | Eligible participants identified by their physician | Participants approached by their physician during a regularly scheduled visit | Web-based (laptop in the clinic or at home) | Identified relative preferences for side effects from the patient perspective. Patients willing to make trade-offs between side effects and different routes and schedules of treatment. | Led to systematic review [39,40,41,42], reviews [43,44] and clinical studies [12,13,45,46,47,48] |
| Kuchuk et al., 2013 [49] | To obtain utility weights from patients with BC for common side effects of CT | Female patients with BC receiving CT for any stage of breast cancer | 69 (response rate N/A) | Written | 7 months | Eligible participants identified by their physician | Participants approached by their physician during a regularly scheduled visit | Web-based (laptop in the clinc or at home) | The least preferred side effects of CT were: nausea/vomiting, diarrhea, neuropathy. Survival was more important than slowing cancer growth and maintaining quality of life. | Led to systematic review [39,40,41,42], reviews [43,44] and clinical studies [12,13,45,46,47,48] |
| Saibil et al., 2010 [50] * | Incidence of taxane-induced pain and distress | Patients with EBC treated with anthracycline-taxane CT | 82 (response rate N/A) | Written | N/A | Eligible participants identified through pharmacy and hospital records | Participants approached by their physician during a regularly scheduled visit | Interview | Distressing taxane-induced pain was common. Myalgias and arthralgias were major component of distress experienced. Pain required narcot ics in 43% of patients. | Led to systematic reviews [39,40,41], guidelines , clinical study [47,45] |
| Supportive care—adjuvant chemotherapy | ||||||||||
| Hilton et al., 2018 [51] | Filgrastim use in patients receiving CT | Patients with EBC treated with CT | 95/97 (98%) | Oral | 3 months | Eligible participants identified by their physician | Participants approached by their physician during a regularly scheduled visit | Paper in clinic/ Emailed web-based survey | Patients willing to participate in clinical trials to evaluate optimal duration of G-CSF. Respondent preference was for prophylaxis with antibiotics over G-CSF, if there is no difference between the two. | Led to systemaitic reviews [52,53], clinicl trials [4,15,17,54] |
| Jacobs et al., 2015 [26] | Optimisation of steroid prophylaxis schedules for patients with BC receiving docetaxel CT | Patients with EBC treated with docetaxel CT | 72/87 (82.3%) | N/A | N/A | Eligible participants identified by their physician | Participants approached by their physician during a regularly scheduled visit | Paper in clinic | A single steroid protocol for pre- and post-medication prophylaxis is required. A single protocol for post-medications required when pre-medication not taken as prescribed. | Led to a clinical trial [55] |
| LeVasseur et al., 2018 [56] | Determine patient experience of vascular access (peripheral access, PICC and PORT) for administering CT | Patients with EBC who had received anthracycline-cyclophosphamide-based CT | 187/200 (93.5%) | Oral | 3 months | Eligible participants identified by their physician | Participants approached by their physician during a regularly scheduled visit | Paper in clinic | Respondents report being satisfied with the vascular access used for their treatment. Perceived risk factors for lymphedema were variable and are not evidence-based. | Led to systematic review [57] and clinical trials [10,11] |
| Hernandez Torres et al., 2015 [58] | Patient experiences of CINV and perceptions of different CINV assessment tools | Patients with EBC who had received anthracycline-cyclophosphamide-based CT | 168/201 (83.6%) | Oral | 7 months | Eligible participants identified by their physician | Participants approached by their physician during a regularly scheduled visit | Paper in clinic/Mail/ Telephone | Respondents strongly favor a CINV endpoint that includes the absence of both nausea and vomiting. Respondents experience with CINV is underestimated when nausea is not included in composite end points. | Led to systematic review [42], review [43,44], 2 grant applications and clinical trials [12,13,48] |
| Adjuvant bisphosphonate therapy | ||||||||||
| McGee et al., 2021 [59] | Patient experiences adjuvant BP use and future trial designs for adjuvant BPs | Patients with EBC who had either completed or were currently receiving adjuvant BPs | 164/255 (64.3%) | Oral | 2 months | Eligible participants identified by their physician | Participants approached by their physician during a regularly scheduled visit | Paper in clinic/Mail/ Emailed web-based survey /Telephone | More than 50% of respondents were interested in a BP de-escalation trial | Led to guidelines [60], pilot study of different dosing durations [18] |
| Palliative/Supportive Care: bone-modifying agents (BMAs) | ||||||||||
| Hutton et al., 2013 [61] | Patient experiences of palliative BMA use and future trials of treatment de-escalation | Patients receiving BMAs for metastatic prostate or BC | 141 patients, 76 (53.9%) with prostate cancer and 65 (46.1%) with BC | N/A | 3 months | Eligible participants identified by their physician | Participants approached by their physician during a regularly scheduled visit | Paper in clinic/Take home/Web-based in clinic | Different BMAs used in prostate and BC. Perceptions of the goals of therapy similar. Patients were interested in participating in trials of de-escalated therapy. | Led to systematic review [62,63] guidelines [64,65] and clinical trials [16,66,67] |
| AlZahrani, 2021 [68] | Patient experiences of palliative BMA use and future trials de-escalation after 2 years of treatment | Patients receiving BMAs for metastatic prostate or BC | 172/220 (78.2%) | Oral | 2 months | Eligible participants identified by their physician and from pharmacy lists | Participants approached by their physician during a regularly scheduled visit or cold calling by CRA | Paper in clinic/Mail/ Emailed web-based survey /Telephone | Respondents interested in trials of de-escalated therapy. Quality of life is an important clinical endpoint. | Led to review paper [69], systematic reviews [70] and clinical trials [16,71,72] |
| Reference and Year of Publication | Survey Topic | Population Surveyed | Sample Size (Response Rate) | Duration | How were Participants Identified | Methods of Approach | Methods of Completion | Summary of Pertinent Findings | Other Studies the Survey Led To |
|---|---|---|---|---|---|---|---|---|---|
| Contralateral prophylactic mastectomy | |||||||||
| Squires et al., 2019 [73] | Development of a patient decision aid for contralateral prophylactic mastectomy (cpm) | Medical/ surgical/ radiation oncologists, plastic surgeons, general surgeons, oncology nurses, geneticists | 39 (response rate N/A) | N/A | Master lists were compiled using publicly available information in databases | Invited by email | Emailed web-based survey | The cpm patient decision aid can be used by clinicians in consultation with women who have unilateral BC to enhance evidence-informed and shared decision-making with respect to undergoing cpm | N/A |
| Postoperative radiological staging | |||||||||
| Simos et al., 2015 [74] | Physician perceptions around radiological imaging of patients with newly diagnosed BC | Canadian breast cancer surgical, radiation, and medical oncologists | 173/665 (26%) | 4 months | Email lists from Canadian Society of Surgical Oncology, Canadian Association of General Surgeons, Canadian Association of Radiation Oncologists and Canadian Association of Medical Oncologists | Invited by email | Emailed web-based survey | The majority of physicians treating BC patients are aware of and generally agree that guidelines pertaining to staging imaging for EBC are reflective of evidence. Despite this, adherence is variable. | Led to a population-based cohort study [28] |
| Adjuvant surgical, systemic, and radiotherapy choices for breast cancer patients | |||||||||
| Jacobs et al., 2015 [75] | Management approaches, evidence supporting practice, and future research needs for management of invasive lobular carcinoma | Canadian breast cancer surgical, radiation, and medical oncologists | 88/428 (20.6%) | N/A | Canadian Society of Surgical Oncology, Canadian Association of General Surgeons, Canadian Association of Radiation Oncologists and Canadian Association of Medical Oncologists | Invited by email | Emailed web-based survey | Variation exists in physicians’ beliefs around the quality of evidence for the management of invasive lobular carcinoma | Led to a review [76] |
| AlZahrani et al. 2021 [77] | Adjuvant management strategies for older patients with low risk HR positive early stage breast cancer | Canadian breast cancer surgical, radiation, and medical oncologists | 50/242 (21%) | 3 months | Collection of publicly available email addresses used by the research team in previous surveys | Invited by email | Emailed web-based survey | There is interest in trials of different adjuvant strategies in regard of radiation and endocrine therapy | Led to systematic review [30] and pilot clinical trial [31] |
| McGee et al., 2019 [78] | Physician recommendations for the timing of starting endocrine therapy either before, concurrent with, or sequential to radiotherapy for patients with EBC | Canadian breast cancer radiation and medical oncologists | 65/220 (30%) | 3 months | Collection of publicly available email addresses used by the research team in previous surveys | Invited by email | Emailed web-based survey /Paper | Decisions around the timing of endocrine therapy and radiotherapy are largely made based on physicians’ personal choices. | Led to a systematic review [79] and a clinical trial [80] |
| Jacobs et al., 2017 [37] | Physician preferred CT for early stage TNBC and clinical trial strategies. | Medical oncologists | 41/84(48.8%) | 3 months | Medical oncologists who had responded to previous practice-based surveys | Invited by email | Emailed web-based survey | Optimization of chemotherapy for TNBC is an important and unmet clinical need. The majority of medical oncologists are interested in entering trials to optimise CT choices | Led to a clinical trial [14] |
| Supportive care—endocrine therapy | |||||||||
| Cole et al., 2021 [81] | HCP recommendations for management of hot flashes in patients with EBC | Canadian surgical, radiation, and medical oncologists, general practitioners in oncology, nurse practitioners, oncology nurses specializing in BC | Physicians: 36/212 (17%) Nurses: 29 (response rate N/A) | 4 months | Collection of publicly available email addresses used by the research team in previous surveys. Canadian Association of Nurses in Oncology (CANO) membership email pool | Invited by email | Emailed web-based survey | 54% of HCPs reported being confident in managing these symptoms. The most commonly recommended intervention was antidepressants. HCPs desire optimal treatment strategies. HCPs lack comfort and experience in prescribing complementary/ alternative medicine therapies. | Led to systematic review [82], grant application |
| Supportive care—adjuvant chemotherapy | |||||||||
| LeVasseur et al., 2018 [83] | Determine current access practices, perceptions of complications with vascular access (peripheral access, PICC and PORT) for administering CT. Evaluated perceived risk factors for lymphedema | Canadian oncologists and oncology nurses responsible for the care of breast cancer patients | Physicians: 25/27 (93%) Nurses: 57 (response rate N/A) | 4 months | Collection of publicly available email addresses used by the research team in previous surveys. Nurses were approached by their respective nurse managers. | Invited by email/ Approached by manager | Emailed web-based survey /Paper | Type of venous access used for administering CT treatment varies significantly, as do perceptions about the risks of vascular device use. Many ”urban legends” about risk factors for lymphedema persist amongst HCPs | Led to systematic review [57] and clinical trials [10,11] |
| Hilton et al., 2018 [51] | Determine current practices for granulocyte colony-stimulating factor (G-CSF) use for CT in EBC. | Canadian oncologists involved in the treatment of breast cancer patients | 38/50 (76%) | 3 months | Collection of publicly available email addresses used by the research team in previous surveys | Invited by email | Emailed web-based survey | Significant variability in practice exists. Definitive studies are required to standardize and improve care. | Led to systematic reviews [52,53], clinical trials [4,6,15,17] |
| Jacobs et al., 2015 [26] | Optimisation of steroid prophylaxis schedules for patients with BC receiving docetaxel CT | Oncology nurses, oncology pharmacists and medical oncologists | 184/698 (26.4%) | N/A | Members of Canadian oncology societies, and oncology nurses working at cancer centres. | Invited by email/ Nurses approached at cancer centres | Emailed web-based survey/Paper | A single steroid protocol for pre- and post-medication prophylaxis is required. A single protocol for post-medications is required when pre-medication not taken as prescribed. | Led to a clinic trial [55] |
| Adjuvant bisphosphonate therapy | |||||||||
| McGee et al., 2021 [84] | Determine real world practice patterns of adjuvant BMA use in treatment of patients with EBC and to determine interest in clinical trials of alternative strategies for BMA administration. | Canadian oncologists treating patients with EBC | 53/127 (41.7%) | 1 month | Collection of publicly available email addresses used by the research team in previous surveys | Invited by email | Emailed web-based survey | Questions around optimal use of adjuvant BMAs still exist. There is interest in performing trials of de-escalation of these agents. | Led to pilot study of different dosing durations [18] |
| Palliative/Supportive Care: bone-modifying agents (BMAs) | |||||||||
| Hutton et al., 2013 [85] | Assess current clinical practice regarding the use of BMAs in patients with metastatic breast and prostate cancer. | Survey respondents were medical oncologists (71.1%), radiation oncologists (21.1%) and urologists (7.8%) | 90/193 (49%) | N/A | Participants from previous national annual meetings related to this study | Invited by email | Emailed web-based survey | Significant areas of clinical equipoise with respect to use of BMAs exist. Physicians are interested in de-escalated therapy for breast and prostate cancer patients. | Led to systematic review [62,63] guidelines [64,65] and clinical trials [16,66,67] |
| AlZahrani et al., 2021 [86] | Identify current practices, as well as perceptions around long-term BMA use, BMA de-escalation, and further BMA de-escalation after 2 years of use. | Canadian oncologists treating BC or CRPC | 65/295 (22%) | 4 weeks | Collection of publicly available email addresses used by the research team in previous surveys | Invited by email | Emailed web-based survey | Most physicians are de-escalating BMAs. There is equipoise re: continuing BMA beyond 2 years. Survey gave favoured study endpoints for future prospective studies. | Led to clinical trials [16,72,87,88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saunders, D.; Liu, M.; Vandermeer, L.; Alzahrani, M.J.; Hutton, B.; Clemons, M. The Rethinking Clinical Trials (REaCT) Program. A Canadian-Led Pragmatic Trials Program: Strategies for Integrating Knowledge Users into Trial Design. Curr. Oncol. 2021, 28, 3959-3977. https://doi.org/10.3390/curroncol28050337
Saunders D, Liu M, Vandermeer L, Alzahrani MJ, Hutton B, Clemons M. The Rethinking Clinical Trials (REaCT) Program. A Canadian-Led Pragmatic Trials Program: Strategies for Integrating Knowledge Users into Trial Design. Current Oncology. 2021; 28(5):3959-3977. https://doi.org/10.3390/curroncol28050337
Chicago/Turabian StyleSaunders, Deanna, Michelle Liu, Lisa Vandermeer, Mashari Jemaan Alzahrani, Brian Hutton, and Mark Clemons. 2021. "The Rethinking Clinical Trials (REaCT) Program. A Canadian-Led Pragmatic Trials Program: Strategies for Integrating Knowledge Users into Trial Design" Current Oncology 28, no. 5: 3959-3977. https://doi.org/10.3390/curroncol28050337
APA StyleSaunders, D., Liu, M., Vandermeer, L., Alzahrani, M. J., Hutton, B., & Clemons, M. (2021). The Rethinking Clinical Trials (REaCT) Program. A Canadian-Led Pragmatic Trials Program: Strategies for Integrating Knowledge Users into Trial Design. Current Oncology, 28(5), 3959-3977. https://doi.org/10.3390/curroncol28050337