Stereotactic Radiotherapy in the Treatment of Paraneoplastic Vasculitis in Oligometastatic Renal Cell Carcinoma
Abstract
1. Introduction
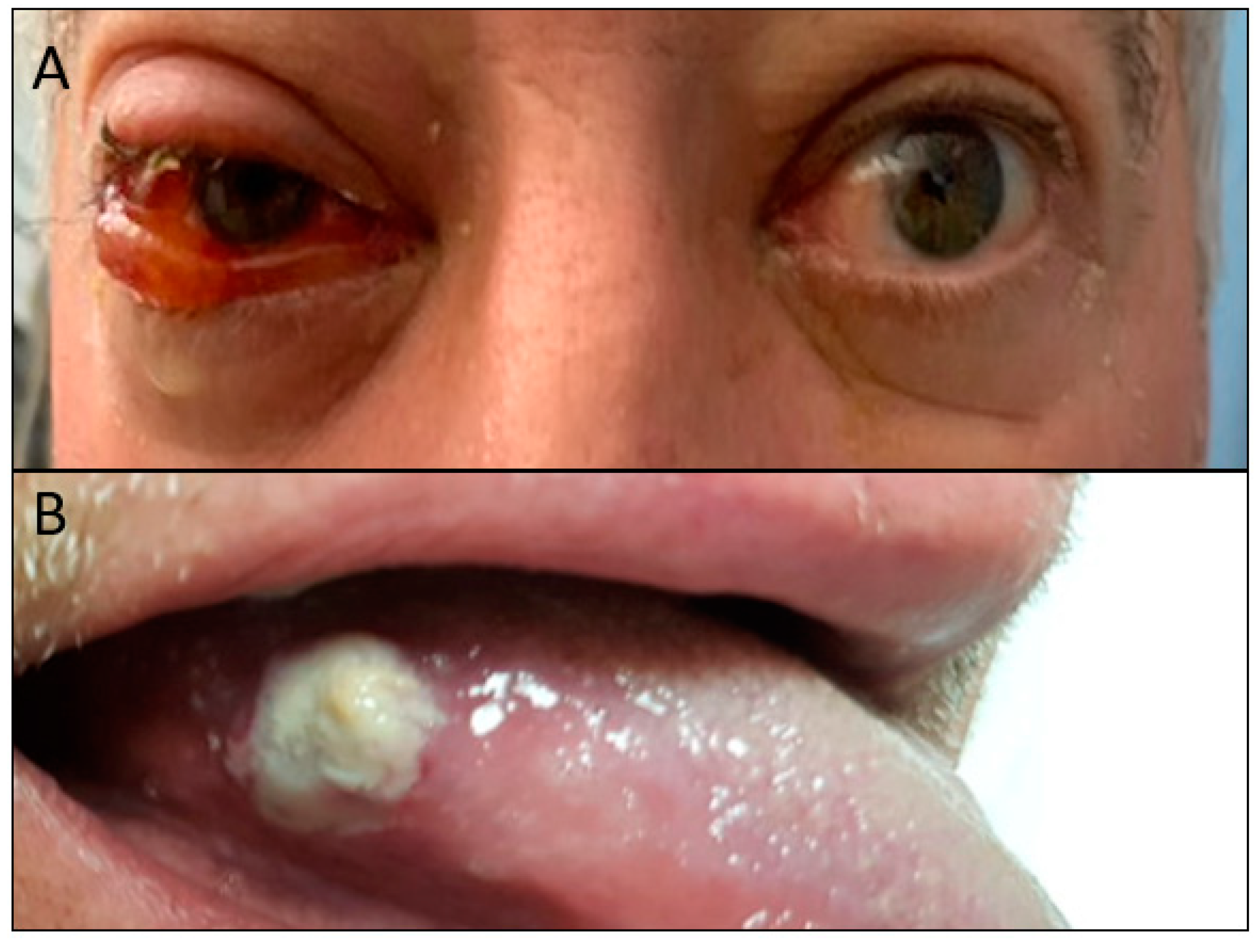
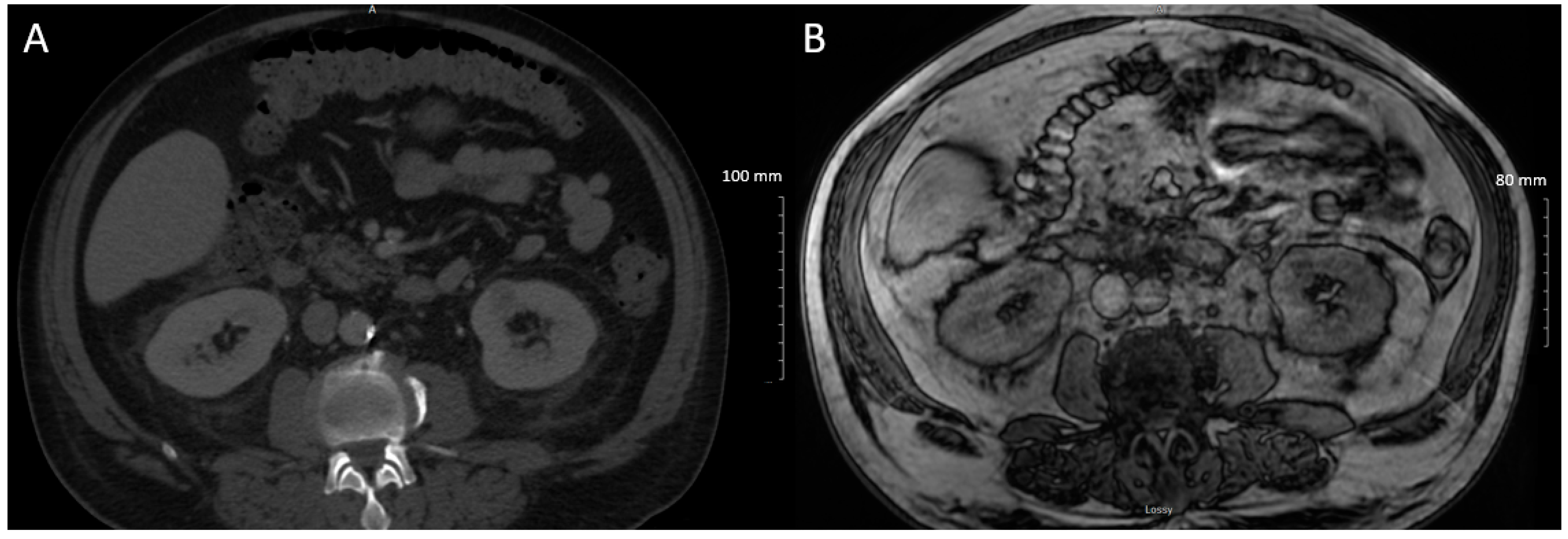
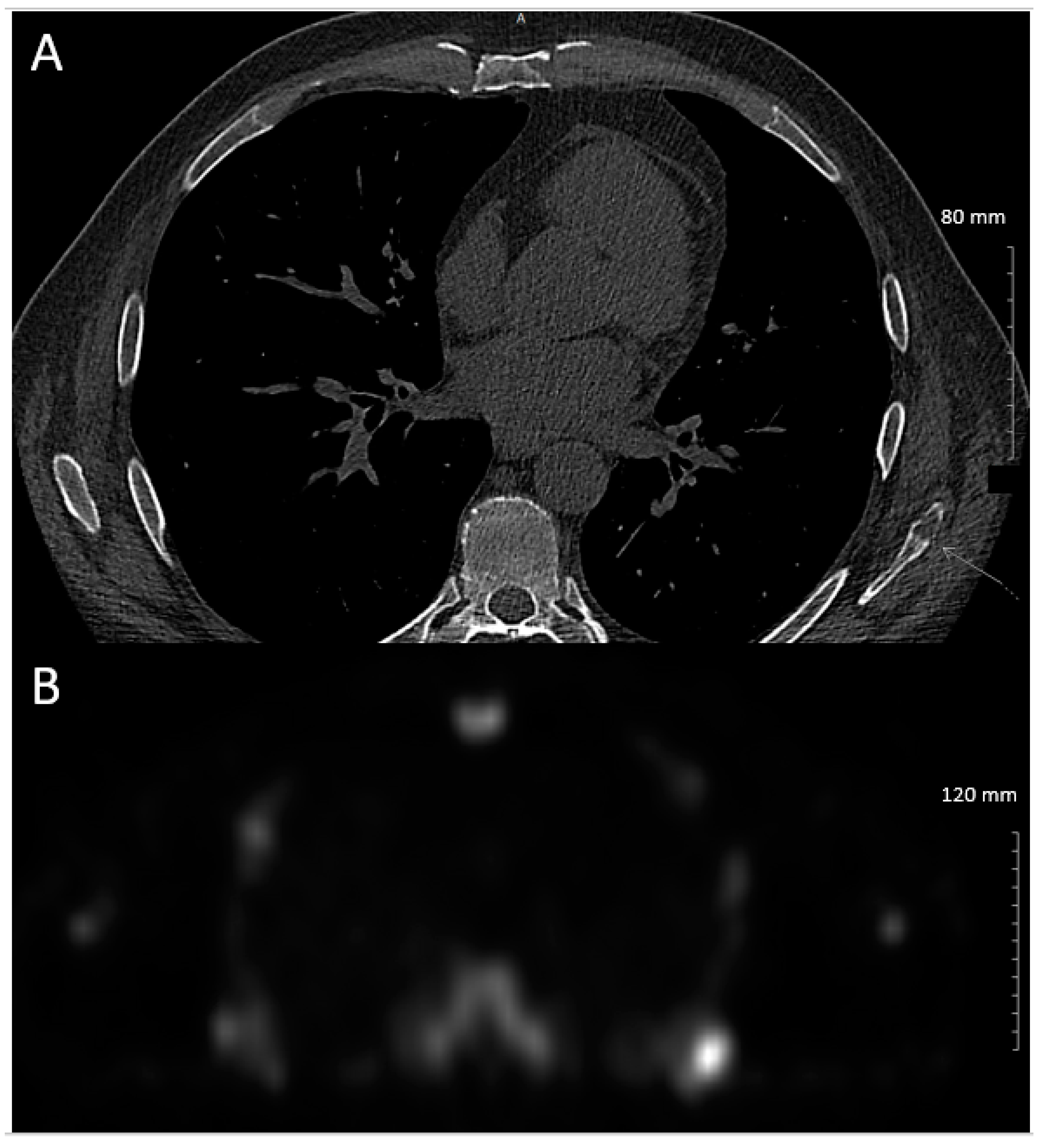
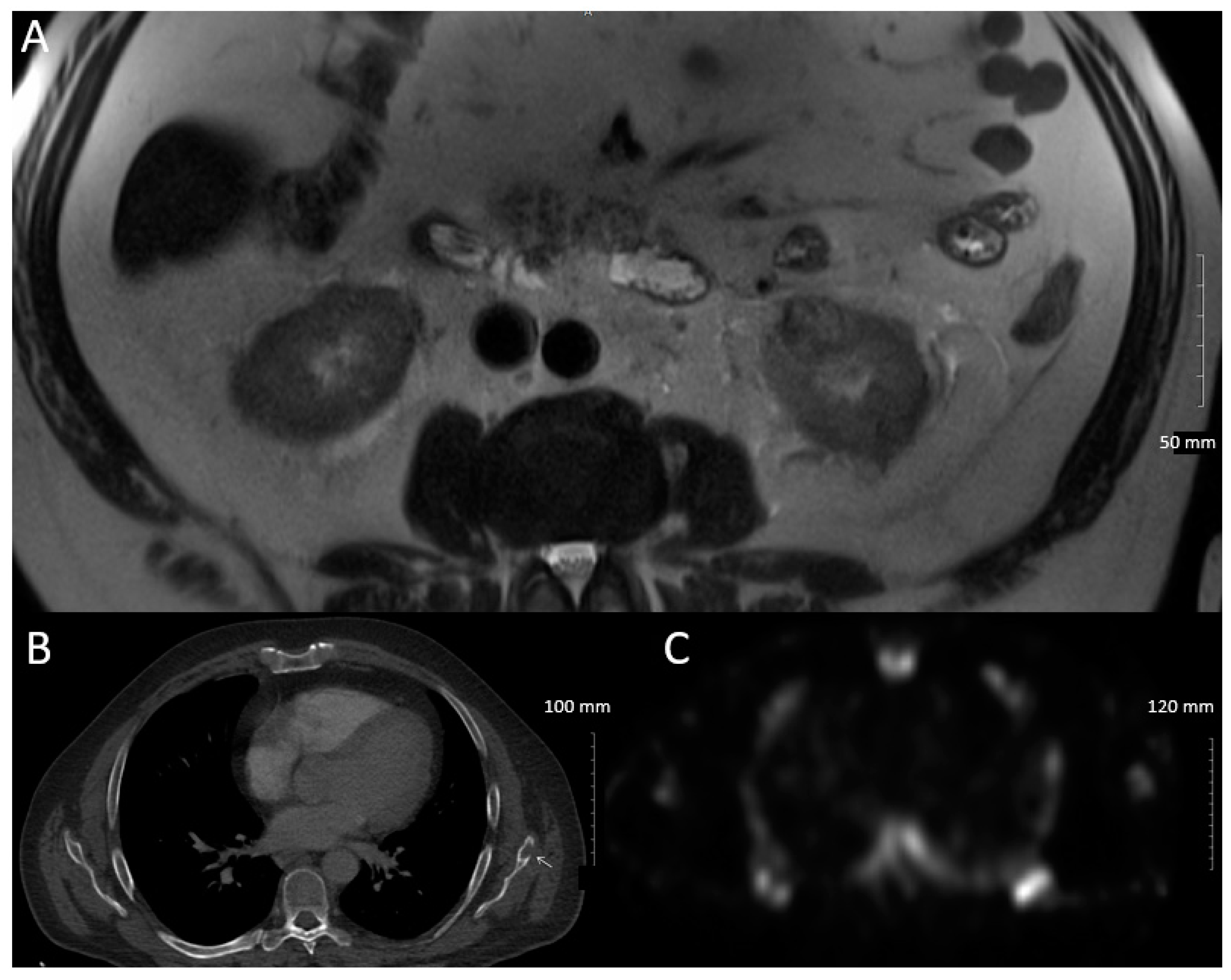
2. Case
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lee, C.T.; Katz, J.; Fearn, P.A.; Russo, P. Mode of presentation of renal cell carcinoma provides prognostic information. Urol. Oncol. 2002, 7, 135–140. [Google Scholar] [CrossRef]
- Kim, H.L.; Belldegrun, A.S.; Freitas, D.G.; Bui, M.H.T.; Han, K.; Dorey, F.J.; Figlin, R.A. Paraneoplastic signs and syptoms of renal cell carcinoma: Implications for prognosis. J. Urol. 2003, 170, 1742–1746. [Google Scholar] [CrossRef] [PubMed]
- Moreira, D.M.; Gershman, B.; Lohse, C.M.; Boorjian, S.A.; Cheville, J.C.; Leibovich, B.C.; Thompson, R.H. Paraneoplastic syndromes are associated with adverse prognosis among patients with renal cell carcinoma undergoing nephrectomy. World J. Urol. 2016, 34, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Sacco, E.; Pinto, F.; Sasso, F.; Racioppi, M.; Gulino, G.; Volpe, A.; Pierfrancesco, B. Paraneoplastic syndromes in patients with urological malignancies. Urol. Int. 2009, 83, 1–11. [Google Scholar] [CrossRef]
- Palapattu, G.S.; Kristo, B.; Rajfer, J. Paraneoplastic Syndromes in Urologic Malignancy: The Many Faces of Renal Cell Carcinoma. Rev. Urol. 2002, 4, 163–170. [Google Scholar]
- Hegemann, M.; Kroeger, N.; Stenzl, A.; Bedke, J. Rare and changeable as a chameleon: Paraneoplastic syndromes in renal cell carcinoma. World J. Urol. 2018, 36, 849–854. [Google Scholar] [CrossRef]
- Hoag, G.N. Renal Cell Carcinoma and Vasculitis: Report of Two Cases. J. Surg. Oncol. 1987, 35, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Solans-Laqué, R.; Bosch-Gil, J.A.; Pérez-Bocanegra, C.; Selva-O’Callaghan, A.; Simeón-Aznar, C.P.; Vilardell-Tarres, M. Paraneoplastic Vasculitis in Patients with Solid Tumors: Report of 15 Cases. J. Rheumatol. 2008, 35, 294–304. [Google Scholar] [PubMed]
- Buggiani, G.; Krysenka, A.; Grazzini, M.; Vasku, V.; Hercogova, J.; Lotti, T. Paraneoplastic vasculitis and paraneoplastic vascular syndromes. Dermatol. Ther. 2010, 23, 597–605. [Google Scholar] [CrossRef]
- Loricera, J.; Calvo-Río, V.; Ortiz-Sanjua, F.; Gonza, M.A.; Rueda-Gotor, J.; Gonzalez-Vela, M.C.; Alvarez, L.; Mata, C.; Martı, V.M.; Gonza, M.A.; et al. The Spectrum of Paraneoplastic Cutaneous Vasculitis in a Defined Population Incidence and Clinical Features. Medicine 2013, 92, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Tsimafeyeu, I.; Leonenko, V.; Kuznetsov, V.; Semenkova, E.; Bondarenko, A.; Demidov, L. Paraneoplastic vasculitis in patients with metastatic renal cell carcinoma. Cancer Rep. 2019, 2. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, U.; Chatterjee, T.; Kandula, M. Polyarteritis Nodosa: An unusual case of paraneoplastic process in renal cell carcinoma. J. Community Hosp. Intern. Med. Perspect. 2020, 10, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Massucci, M.; Mollica, V.; Rizzo, A.; Ventrella, L.; Maggio, I.; Manuzzi, L.; Gatto, L.; Brandi, G.; Massari, F. Encephalic Leukocytoclastic Vasculitis during Treatment with Sunitinib for Renal Cell Carcinoma: A Case Report. Medicines 2021, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Mautner, G.; Roth, J.S.; Grossman, M.E. Leulcocytoclastic vasculitis in association with cryoglobulinemia and renal cell carcinoma. Nephron 1993, 63, 356–357. [Google Scholar] [CrossRef] [PubMed]
- Çurgunlu, A.; Karter, Y.; Uyanik, Ö.; Tunçkale, A.; Çurgunlu, S. Leukocytoclastic Vasculitis and Renal Cell Carcinoma. Intern. Med. 2004, 43, 256–257. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hong, Y.H. Renal cell carcinoma presenting as Henoch-Schönlein purpura with leukocytoclastic vasculitis, hematuria, proteinuria and abdominal pain. Rheumatol. Int. 2010, 30, 1373–1376. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burgess, L.; Keenan, M.; Zhou, A.L.; Lypka, K.; Hasimja Saraqini, D.; Yao, J.; Martin, S.; Morash, C.; Watterson, J.; Canil, C.; et al. Stereotactic Radiotherapy in the Treatment of Paraneoplastic Vasculitis in Oligometastatic Renal Cell Carcinoma. Curr. Oncol. 2021, 28, 1744-1750. https://doi.org/10.3390/curroncol28030162
Burgess L, Keenan M, Zhou AL, Lypka K, Hasimja Saraqini D, Yao J, Martin S, Morash C, Watterson J, Canil C, et al. Stereotactic Radiotherapy in the Treatment of Paraneoplastic Vasculitis in Oligometastatic Renal Cell Carcinoma. Current Oncology. 2021; 28(3):1744-1750. https://doi.org/10.3390/curroncol28030162
Chicago/Turabian StyleBurgess, Laura, Marissa Keenan, Alan Liang Zhou, Kiefer Lypka, Delvina Hasimja Saraqini, Jeff Yao, Samuel Martin, Christopher Morash, James Watterson, Christina Canil, and et al. 2021. "Stereotactic Radiotherapy in the Treatment of Paraneoplastic Vasculitis in Oligometastatic Renal Cell Carcinoma" Current Oncology 28, no. 3: 1744-1750. https://doi.org/10.3390/curroncol28030162
APA StyleBurgess, L., Keenan, M., Zhou, A. L., Lypka, K., Hasimja Saraqini, D., Yao, J., Martin, S., Morash, C., Watterson, J., Canil, C., & MacRae, R. (2021). Stereotactic Radiotherapy in the Treatment of Paraneoplastic Vasculitis in Oligometastatic Renal Cell Carcinoma. Current Oncology, 28(3), 1744-1750. https://doi.org/10.3390/curroncol28030162




