Primary Signet Ring Cell/Histiocytoid Carcinoma of the Eyelid: Somatic Mutations in CDH1 and Other Clinically Actionable Mutations Imply Early Use of Targeted Agents
Abstract
1. Introduction
2. Materials and Methods
3. Results
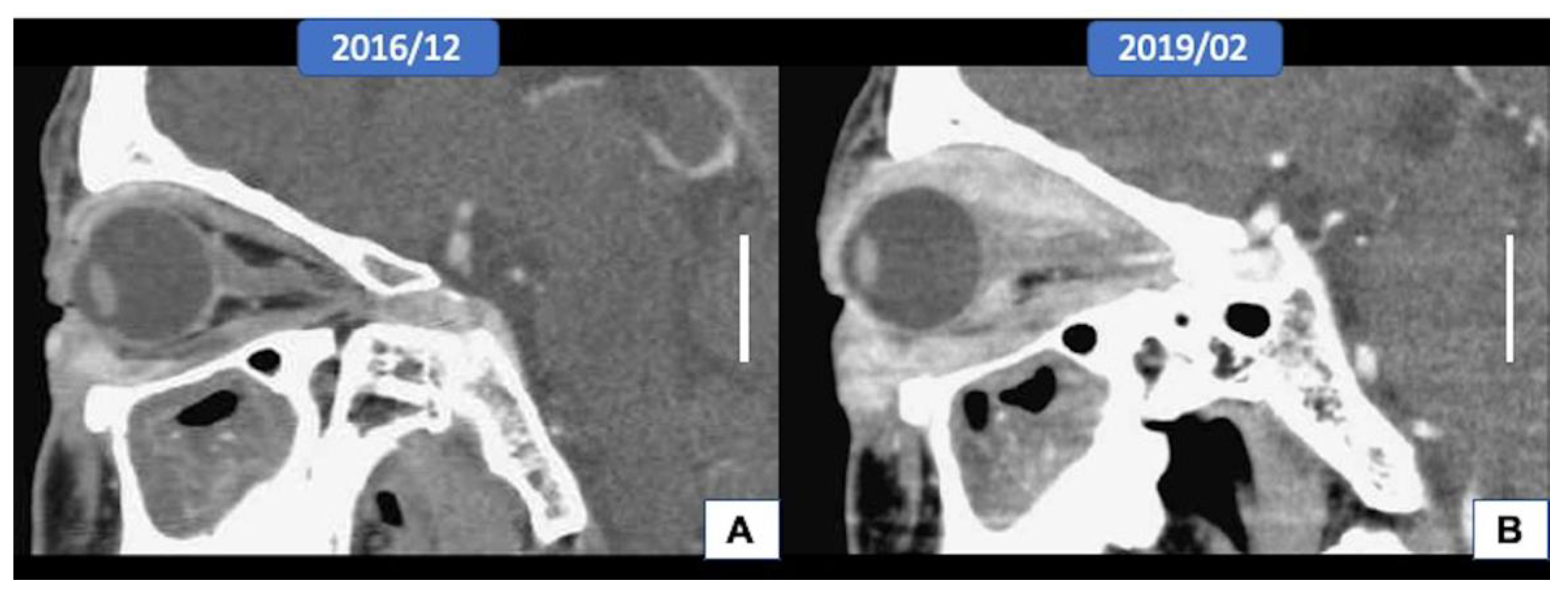
3.1. Clinical Features
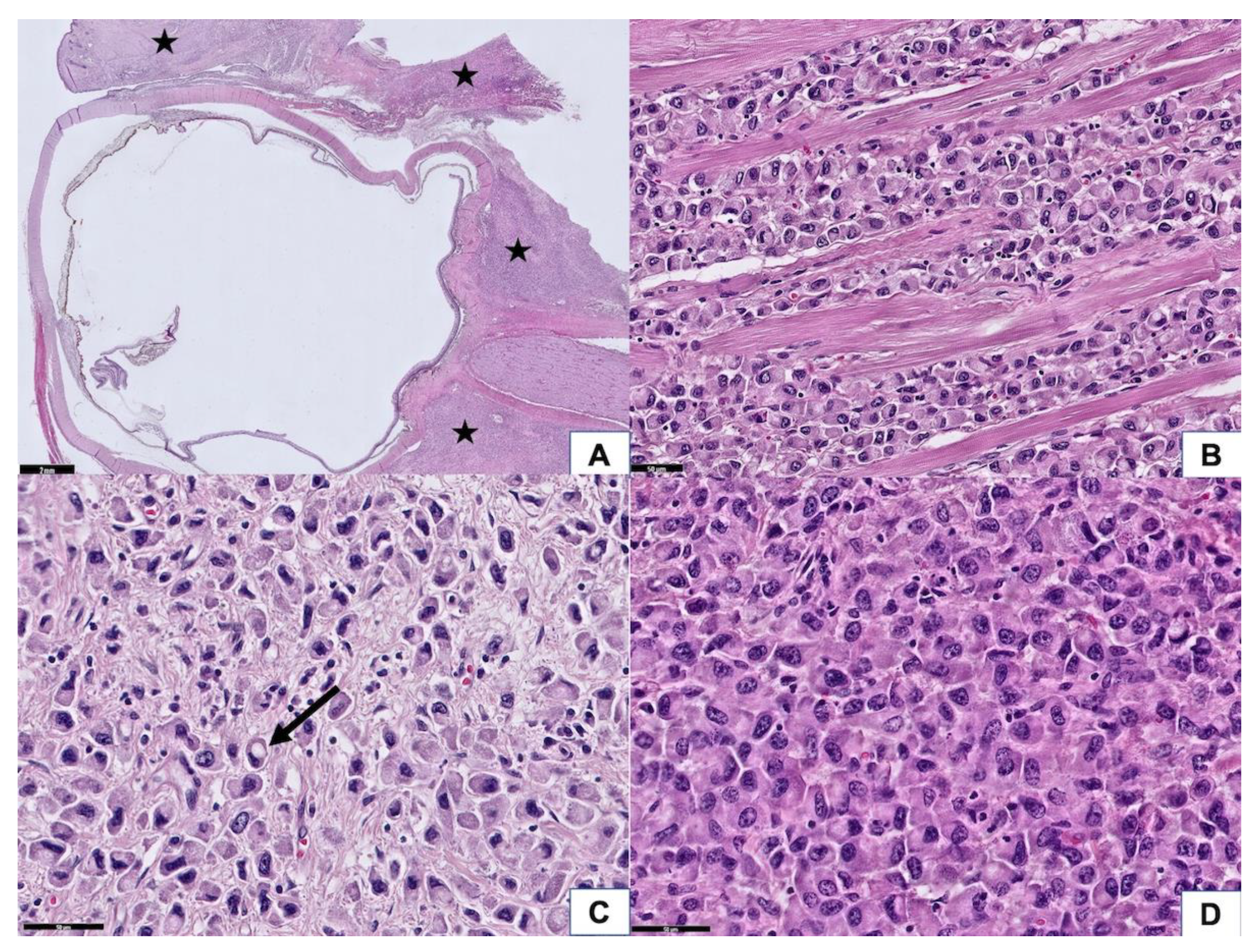
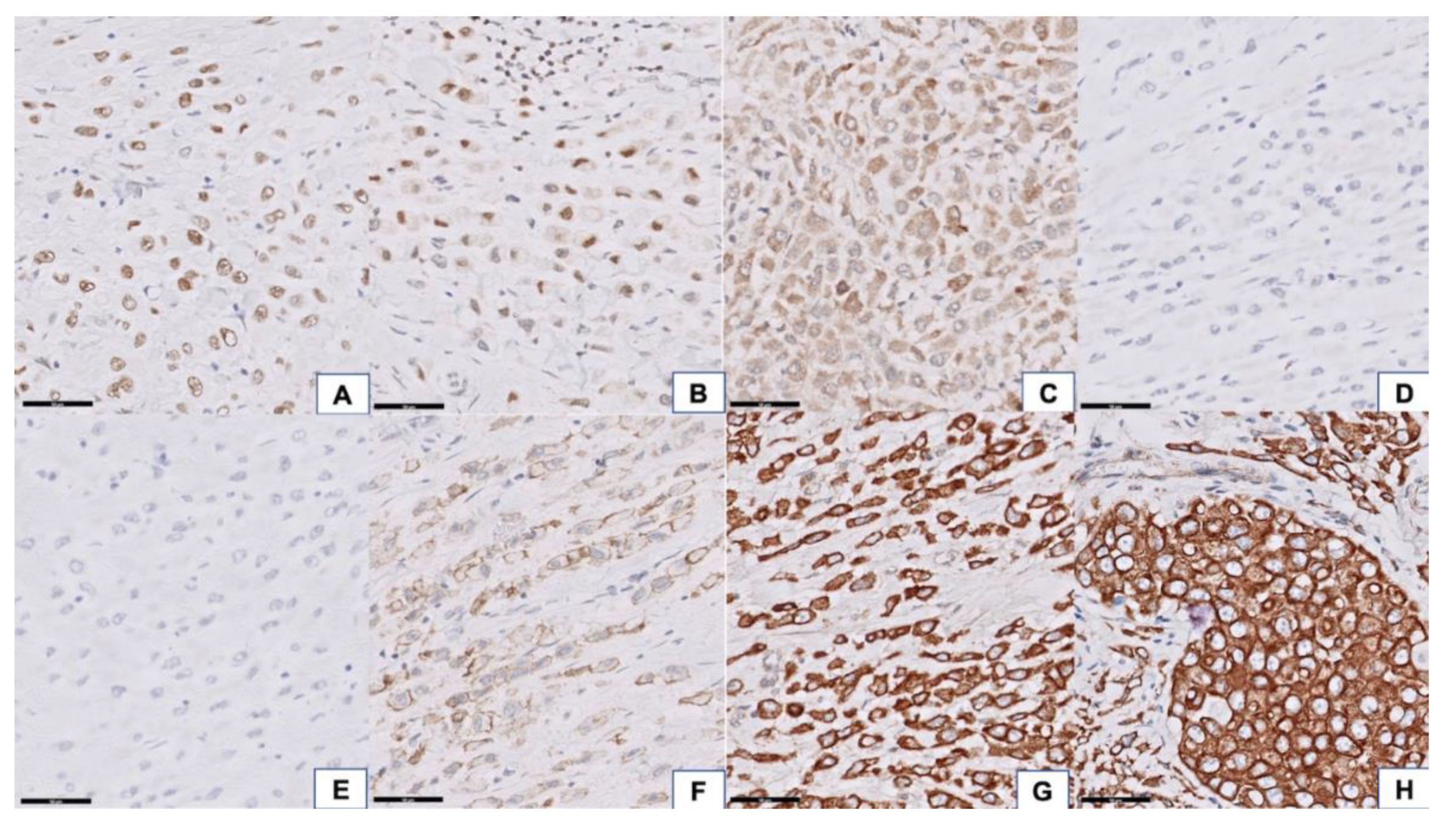
3.2. Histopathologic Findings
3.3. Next-Generation Sequencing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kramer, T.R.; E Grossniklaus, H.; McLean, I.W.; Orcutt, J.; Green, W.R.; Iliff, N.T.; Tressera, F. Histiocytoid variant of eccrine sweat gland carcinoma of the eyelid and orbit: Report of five cases. Ophthalmology 2002, 109, 553–559. [Google Scholar] [CrossRef]
- Requena, L.; Prieto, V.G.; Requena, C.; Requena, C.; Sarasa, J.L.; Manzano, R.; Seco, M.; Rütten, A.; Kazakov, D.V.; Cerroni, L.; et al. Primary signet-ring cell/histiocytoid carcinoma of the eyelid: A clinicopathologic study of 5 cases and review of the literature. Am. J. Surg. Pathol. 2011, 35, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Iwaya, M.; Uehara, T.; Yoshizawa, A.; Kobayashi, Y.; Momose, M.; Honda, T.; Ota, H. A case of primary signet-ring cell/histiocytoid carcinoma of the eyelid: Immunohistochemical comparison with the normal sweat gland and review of the literature. Am. J. Dermatopathol. 2012, 34, e139–e145. [Google Scholar] [CrossRef]
- Bernárdez, C.; Macías del Toro, E.; Ramírez Bellver, J.L.; Martinez Menchón, T.; Martinez Barba, E.; Molina-Ruiz, A.M.; Requena, L. Primary Signet-Ring Cell/Histiocytoid Carcinoma of the Eyelid: A “Binocle” Presentation of the “Monocle Tumor”. Am. J. Dermatopathol. 2016, 38, 623–627. [Google Scholar] [CrossRef]
- Auw-Haedrich, C.; Boehm, N.; Weissenberger, C. Signet ring carcinoma of the eccrine sweat gland in the eyelid, treated by radiotherapy alone. Br. J. Ophthalmol. 2001, 85, 112–113. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bellman, B.; Gregory, N.A.; Silvers, D.; Fountain, K.S. Sweat gland carcinoma with metastases to the skin: Response to 5-fluorouracil chemotherapy. Cutis 1995, 55, 221–224. [Google Scholar] [PubMed]
- Fb, F.B.; Hofman, V.; Lagier, J.; Gastaud, P.; Santini, J.; Hofman, P. Primary signet ring cell carcinoma of the eccrine sweat gland in the eyelid. Immunohistochemical and ultrastructural study of a case. J. Fr. Ophtalmol. 2002, 25, 547–551. [Google Scholar]
- Bhargava, R.; Beriwal, S.; Dabbs, D.J. Mammaglobin vs GCDFP-15: An immunohistologic validation survey for sensitivity and specificity. Am. J. Clin. Pathol. 2007, 127, 103–113. [Google Scholar] [CrossRef]
- González-Lois, C.; Rodríguez-Peralto, J.L.; Serrano-Pardo, R.; Martínez-González, M.A.; López-Ríos, F. Cutaneous signet ring cell carcinoma: A report of a case and review of the literature. Am. J. Dermatopathol. 2001, 23, 325–328. [Google Scholar] [CrossRef]
- Bouza Gonzalo, E.; Luezas Morcuende, J.; Martínez Montero, J.C.; Cuesta Gil, M.; Fernández De Castro Pombo, J. Eccrine gland adenocarcinoma of the eyelid. Arch. Soc. Esp. Oftalmol. 2001, 76, 739–742. [Google Scholar]
- Grizzard, W.S.; Torezynski, E.; Edwards, W.C. Adenocarcinoma of eccrine sweat glands. Arch Ophthalmol. 1976, 94, 2119–2123. [Google Scholar] [CrossRef]
- Jakobiec, F.A.; Austin, P.; Iwamoto, T.; Trokel, S.L.; Marquardt, M.D.; Harrison, W. Primary infiltrating signet ring carcinoma of the eyelids. Ophthalmology 1983, 90, 291–299. [Google Scholar] [CrossRef]
- Kiyohara, T.; Kumakiri, M.; Kouraba, S.; Tokuriki, A.; Ansai, S. Primary cutaneous signet ring cell carcinoma expressing cytokeratin 20 immunoreactivity. J. Am. Acad. Dermatol. 2006, 54, 532–536. [Google Scholar] [CrossRef][Green Version]
- Langel, D.J.; Yeatts, R.P.; White, W.L. Primary signet ring cell carcinoma of the eyelid: Report of a case demonstrating further analogy to lobular carcinoma of the breast with a literature review. Am. J. Dermatopathol. 2001, 23, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A.L.; Heegaard, S.; Clemmensen, O.; Prause, J.U. Signet ring cell carcinoma of the eyelid—The monocle tumour. Apmis 2008, 116, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Rosen, Y.; Kim, B.; Yermakov, V.A. Eccrine sweat gland tumor of clear cell origin involving the eyelids. Cancer 1975, 36, 1034–1041. [Google Scholar] [CrossRef]
- Swinson, B.; Ryan, F.; Barrett, A.W.; Jerjes, W.; Landon, G.; Calonje, E.; Kalavrezos, N. Histiocytoid eccrine sweat gland carcinoma of the eyelid: Report of a case. Clin. Exp. Dermatol. 2006, 31, 786–789. [Google Scholar] [CrossRef]
- Thomas, J.W.; Fu, Y.S.; Levine, M.R. Primary mucinous sweat gland carcinoma of the eyelid simulating metastatic carcinoma. Am. J. Ophthalmol. 1979, 87, 29–33. [Google Scholar] [CrossRef]
- Wollensak, G.; Witschel, H.; Böhm, N. Signet ring cell carcinoma of the eccrine sweat glands in the eyelid. Ophthalmology 1996, 103, 1788–1793. [Google Scholar] [CrossRef]
- Palakkamanil, M.M.; Mahmood, M.N.; Chan, A. Diagnostic and treatment challenges of a case of primary cutaneous signet-ring cell/histiocytoid carcinoma of the eyelid. BMC Ophthalmol. 2020, 20, 410. [Google Scholar] [CrossRef] [PubMed]
- Tanboon, J.; Uiprasertkul, M.; Luemsamran, P. Signet-ring cell/histiocytoid carcinoma of the eyelid: A case report and review of the literature. Am. J. Dermatopathol. 2013, 35, e1–e5. [Google Scholar] [CrossRef]
- Stewart, S.; Houghton, J.; Kamalarajah, S.; Curragh, D. Primary Adnexal Signet-Ring Cell/Histiocytoid Carcinoma. Ophthalmic. Plast Reconstr. Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sayan, A.; Mitchell, O.; Taibjee, S.; Ilankovan, V. Unusual case of primary cutaneous signet-ring cell (histocytoid) carcinoma. Br. J. Oral. Maxillofac. Surg. 2020, 58, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Ito, T.; Tanioka, F.; Fukamizu, H.; Tokura, Y. Primary signet-ring cell/histiocytoid carcinoma of the eyelid expressing androgen receptors and treated with bicalutamide. J. Dermatol. 2017, 44, e230–e231. [Google Scholar] [CrossRef] [PubMed]
- Droubi, D.; Zeitouni, N.C.; Skitzki, J.; Bogner, P.N. Primary signet-ring cell carcinoma of the axilla. J. Cutan. Pathol. 2013, 40, 269–273. [Google Scholar] [CrossRef]
- Tan, J.S.; McKelvie, P.A.; Hardy, T.G. Primary signet ring cell carcinoma of the eyelid. Orbit 2013, 32, 399–401. [Google Scholar] [CrossRef]
- Cameselle-Teijeiro, J.; Alfonsín-Barreiro, N.; Allegue, F.; Caeiro, M. Apocrine carcinoma with signet ring cells and histiocytoid features. A potentially confusing axillary tumor. Pathol. Res. Pract. 1997, 193, 713–720; discussion 721–722. [Google Scholar] [CrossRef]
- Kuno, Y.; Numata, T.; Kanzaki, T. Adenocarcinoma with signet ring cells of the axilla showing apocrine features: A case report. Am. J. Dermatopathol. 1999, 21, 37–41. [Google Scholar] [CrossRef]
- Misago, N.; Shinoda, Y.; Okawa, T.; Aoki, S.; Toda, S.; Koike, K.; Narisawa, Y. Histiocytoid and signet-ring cell carcinoma of the axilla: A type of cutaneous apocrine carcinoma equivalent to histiocytoid lobular carcinoma of the breast? Clin. Exp. Dermatol. 2011, 36, 874–877. [Google Scholar] [CrossRef]
- Raghavan, S.S.; Clark, M.; Louie, C.Y.; Jensen, K.C.; Dietrich, B.; Beadle, B.M.; El-Sawy, T.; Baik, F.; Kunder, C.A.; Brown, R.A. Molecular profiling of a primary cutaneous signet-ring cell/histiocytoid carcinoma of the eyelid. J. Cutan. Pathol. 2020, 47, 860–864. [Google Scholar] [CrossRef]
- Oliveira, C.; Pinheiro, H.; Figueiredo, J.; Seruca, R.; Carneiro, F. E-cadherin alterations in hereditary disorders with emphasis on hereditary diffuse gastric cancer. Prog. Mol. Biol. Transl. Sci. 2013, 116, 337–359. [Google Scholar]
- Da Silva, L.; Parry, S.; Reid, L.; Keith, P.; Waddell, N.; Kossai, M.; Clarke, C.; Lakhani, S.R.; Simpson, P.T. Aberrant expression of E-cadherin in lobular carcinomas of the breast. Am. J. Surg. Pathol. 2008, 32, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Berx, G.; Cleton-Jansen, A.M.; Strumane, K.; Keith, P.; Waddell, N.; Kossai, M.; Catherine, C.; Sunil, R.L.; Peter, T.S.; Simpson, P.T.; et al. E-cadherin is inactivated in a majority of invasive human lobular breast cancers by truncation mutations throughout its extracellular domain. Oncogene 1996, 13, 1919–1925. [Google Scholar]
- Harigopal, M.; Shin, S.J.; Murray, M.P.; Tickoo, S.K.; Brogi, E.; Rosen, P.P. Aberrant E-cadherin staining patterns in invasive mammary carcinoma. World J. Surg. Oncol. 2005, 3, 73. [Google Scholar] [CrossRef] [PubMed]
- Ohshiro, K.; Zaidi, S.; Korkut, A.; Chen, J.; Rao, S.; Gu, S.; Jogunoori, W.; Mishra, B.; Akbani, R.; Mishra, L. A Pan-Cancer Analysis Reveals High-Frequency Genetic Alterations in Mediators of Signaling by the TGF-β Superfamily. Cell Syst. 2018, 7, 422–437.e427. [Google Scholar]
- Al-Ahmadie, H.A.; Iyer, G.; Lee, B.H.; Scott, S.N.; Mehra, R.; Bagrodia, A.; Jordan, E.J.; Gao, S.P.; Ramirez, R.; Cha, E.K.; et al. Frequent somatic CDH1 loss-of-function mutations in plasmacytoid variant bladder cancer. Nat. Genet. 2016, 48, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Johnson, A.M.; Dumbrava, E.E.I.; Raghav, K.; Balaji, K.; Bhatt, M.; Murthy, R.K.; Rodon, J.; Piha-Paul, S.A. Advances in HER2-Targeted Therapy: Novel Agents and Opportunities Beyond Breast and Gastric Cancer. Clin. Cancer Res. 2019, 25, 2033–2041. [Google Scholar] [CrossRef] [PubMed]
- Bose, R.; Kavuri, S.M.; Searleman, A.C.; Shen, W.; Shen, D.; Koboldt, D.C.; Monsey, J.; Goel, N.; Aronson, A.B.; Li, S.; et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013, 3, 224–237. [Google Scholar] [CrossRef]
- Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/208051s004lbl.pdf (accessed on 15 February 2021).
- Volinia, S.; Hiles, I.; Ormondroyd, E.; Nizetic, D.; Antonacci, R.; Rocchi, M.; Waterfield, M.O. Molecular cloning, cDNA sequence, and chromosomal localization of the human phosphatidylinositol 3-kinase p110 alpha (PIK3CA) gene. Genomics 1994, 24, 472–477. [Google Scholar] [CrossRef]
- Weinstein, J.N.; The Cancer Genome Atlas Research Network; Collisson, E.A.; Mills, G.B.; Shaw, K.R.M.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
| Antibody | Clone | Source | Dilution | Case |
|---|---|---|---|---|
| E-cadherin | HECD1 | Invitrogen | 1:300 | +++ |
| β-catenin | D10A8 | Cell Signaling | 1:50 | +++ (membrane and cytoplasm) |
| Estrogen receptor | 6F11 | Leica | 1:100 | - |
| Progesterone receptor | 16 | Leica | 1:200 | - |
| Her2-Neu | A0485 | DAKO | 1:900 | ++ |
| Cytokeratin | AE1/AE3 | Leica | 1:200 | +++ |
| Cytokeratin 7 | RN7 | Leica | 1:100 | +++ |
| GCDFP15 | 23A3 | Novocastra | 1:150 | ++ |
| GATA3 | L50-823 | ZETA | 1:250 | ++ |
| AR | SP107 | ZETA | 1:100 | ++ |
| Gene | Amino Acid Change | Coding | Locus | Variant Effect | Allele Frequency |
|---|---|---|---|---|---|
| TPR | p.(D1348H) | c.4042G>C | chr1:186308883 | missense | 16.82% |
| LRP1B | p.(D41N) | c.121G>A | chr2:142567932 | missense | 4.41% |
| NFE2L2 | p.(F246S) | c.737T>C | chr2:178096594 | missense | 53.28% |
| LTF | p.(R23dup) | c.68_69insAAG | chr3:46501284 | Inframe insertion | 99.95% |
| PIK3CA | p.(K111_L113del) | c.333_341delGATCCTCAA | chr3:178916943 | Inframe deletion | 24.73% |
| TET2 | p.(H863Y) | c.2587C>T | chr4:106157686 | missense | 13.31% |
| WRN | p.(P982S) | c.2944C>T | chr8:30989999 | missense | 45.58% |
| CDKN2B | p.(A38T) | c.112G>A | chr9:22008841 | missense | 4.00% |
| TAF1L | p.(M155I) | c.465G>A | chr9:32635113 | missense | 15.25% |
| RALGDS | p.(T883I) | c.2648C>T | chr9:135974068 | missense | 8.05% |
| SUFU | p.(P18S) | c.52C>T | chr10:104263961 | missense | 5.56% |
| EP400 | p.(P35S) | c.103C>T | chr12:132445267 | missense | 4.97% |
| ERCC5 | p.(D703G) | c.2108A>G | chr13:103518170 | missense | 46.35% |
| CDH1 | unknown | c.687+1G>T | chr16:68842752 | splice donor variant | 27.08% |
| FANCA | p.(R591Q) | c.1772G>A | chr16:89845355 | missense | 89.43% |
| NLRP1 | p.(V1241L) | c.3721G>C | chr17:5424906 | missense | 55.09% |
| NLRP1 | p.(M1119V) | c.3355A>G | chr17:5433966 | missense | 51.70% |
| NLRP1 | p.(T995I) | c.2984C>T | chr17:5437285 | missense | 48.10% |
| NLRP1 | p.(T878M) | c.2633C>T | chr17:5445243 | missense | 47.70% |
| NLRP1 | p.(T782S) | c.2345C>G | chr17:5461671 | missense | 52.24% |
| NLRP1 | p.(T246S) | c.737C>G | chr17:5463279 | missense | 47.73% |
| ERBB2 | p.(V777L) | c.2329G>T | chr17:37881000 | missense | 13.26% |
| RNF213 | p.(A5021V) | c.15062C>T | chr17:78363034 | missense | 49.92% |
| CDH2 | p.(E38K) | c.112G>A | chr18:25727697 | missense | 7.50% |
| STK11 | p.(H168Y) | c.502C>T | chr19:1220409 | missense | 4.72% |
| JAK3 | p.(G936V) | c.2807G>T | chr19:17942208 | missense | 9.55% |
| AXL | p.(I252V) | c.754A>G | chr19:41737174 | missense | 51.61% |
| TAF1 | p.(V803I) | c.2407G>A | chrX:70607231 | missense | 7.46% |
| TAF1 | p.(G804S) | c.2410G>A | chrX:70607234 | missense | 5.97% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.-C.; Lin, T.-C.; Yeh, Y.-C.; Ho, H.-L.; Tsai, C.-C.; Chou, T.-Y. Primary Signet Ring Cell/Histiocytoid Carcinoma of the Eyelid: Somatic Mutations in CDH1 and Other Clinically Actionable Mutations Imply Early Use of Targeted Agents. Curr. Oncol. 2021, 28, 918-927. https://doi.org/10.3390/curroncol28010090
Wang L-C, Lin T-C, Yeh Y-C, Ho H-L, Tsai C-C, Chou T-Y. Primary Signet Ring Cell/Histiocytoid Carcinoma of the Eyelid: Somatic Mutations in CDH1 and Other Clinically Actionable Mutations Imply Early Use of Targeted Agents. Current Oncology. 2021; 28(1):918-927. https://doi.org/10.3390/curroncol28010090
Chicago/Turabian StyleWang, Lei-Chi, Tai-Chi Lin, Yi-Chen Yeh, Hsiang-Ling Ho, Chieh-Chih Tsai, and Teh-Ying Chou. 2021. "Primary Signet Ring Cell/Histiocytoid Carcinoma of the Eyelid: Somatic Mutations in CDH1 and Other Clinically Actionable Mutations Imply Early Use of Targeted Agents" Current Oncology 28, no. 1: 918-927. https://doi.org/10.3390/curroncol28010090
APA StyleWang, L.-C., Lin, T.-C., Yeh, Y.-C., Ho, H.-L., Tsai, C.-C., & Chou, T.-Y. (2021). Primary Signet Ring Cell/Histiocytoid Carcinoma of the Eyelid: Somatic Mutations in CDH1 and Other Clinically Actionable Mutations Imply Early Use of Targeted Agents. Current Oncology, 28(1), 918-927. https://doi.org/10.3390/curroncol28010090







