Abstract
The principle etiology of leg pain (sciatica) from lumbar disc herniation is mechanical compression of the nerve root. Sciatica is reduced by decompression of the herniated disc, i.e., removing mechanical compression of the nerve root. Decompression surgery typically reduces sciatica more than lumbar back pain (LBP). Decompression surgery reduces mechanical compression of the nerve root. However, decompression surgery does not directly reduce sensitization of the sensory nerves in the epidural space and disc. In addition, sensory nerves in the annulus fibrosus and epidural space are not protected from topical interaction with pain mediators induced by decompression surgery. The secondary etiology of sciatica from lumbar disc herniation is sensitization of the nerve root. Sensitization of the nerve root results from a) mechanical compression, b) exposure to cellular pain mediators, and/or c) exposure to biochemical pain mediators. Although decompression surgery reduces nerve root compression, sensory nerve sensitization often persists. These observations are consistent with continued exposure of tissue in the epidural space, including the nerve root, to increased cellular and biochemical pain mediators following surgery. A potential contributor to lumbar back pain (LBP) is stimulation of sensory nerves in the annulus fibrosus by a) cellular pain mediators and/or b) biochemical pain mediators that accompany annular tears or disruption. Sensory fibers located in the outer one-third of the annulus fibrosus increase in number and depth as a result of disc herniation. The nucleus pulposus is comprised of material that can produce an autoimmune stimulation of the sensory nerves located in the annulus and epidural space leading to LBP. The sensory nerves of the annulus fibrosus and epidural space may be sensitized by topical exposure to cellular and biochemical pain mediators induced by lumbar surgery. Annulotomy or annular rupture allows the nucleus pulposus topical access to sensory nerve fibers, thereby leading to LBP. Coverage of the annulus and adjacent structures in the epidural space by absorbable viscoelastic gels appears to reduce LBP following surgery by protecting sensory fibers from cellular and biochemical pain mediators.
Keywords:
biomaterial; viscoelastic gel; back pain; sciatica; lumbar surgery; fibrosis; cytokines; disc herniation; oxiplex; healon 1. Introduction
Patients with sciatica and severe lumbar back pain (LBP) resulting from lumbar disc herniation comprise a clinically challenging subgroup of patients [1,2,3]. Appendix table 1 summarizes many of the clinical reports that have appeared throughout the medical literature over many years. Most recently, the large, multicenter, NIH-funded SPORT study confirmed that most patients with sciatica from a herniated lumbar disc also have lumbar back pain [4,5]. This review summarizes preclinical and clinical data that provide information regarding the source of LBP in these patients. A hypothesis is developed which may provide direction for the development of surgical procedures and locally applied devices to reduce the post operative LBP which often accompanies successful reduction of sciatic pain following removal of the herniated disc.
Many types of biomaterials have been implanted in the epidural space in an effort to reduce postoperative pain caused by scar formation. None of these have been integrated into lumbar spine surgery as standard practice due to the challenges presented by the biomaterials as well as the unique anatomical space. Massie et al. concluded that one potential mode of action for the reduction of pain following surgery with the use of viscoelastic gels is the decreased migration of inflammatory cells into the epidural space by the viscous environment of the gel [6]. Viscoelastic gels would provide a protective tissue coating that decreases fibrosis and shields the nociceptors present on the exposed sensory nerves from pain mediators. The utilization of a mechanical barrier that coats and separates tissues in the lumbar spine provides some measure of surface protection of the sensory nerve against inflammatory mediators that occur as a result of surgery as well as outpouring from the annulotomy site itself.
diZerega et al. reported on a modern biomaterial for adhesion prevention which can be formulated into a flowable, biologically inert, viscoelastic gel with tissue adherence appropriate for use in minimally invasive surgery [7]. The device coats surgically traumatized tissues and remains at the site of placement even in gravitationally dependent areas. The data demonstrate that polysaccharide gels that coat healing tissues protect the tissues from cellular and biochemical pain mediators and fibrotic bridges that lead to adhesions during the healing process. The results of these studies demonstrated that the gels separated tissues during healing, thereby reducing their interaction by the interposition of a barrier.
2. Discussion
2.1. Sciatica
Decompression surgery for disc herniation typically improves sciatica more than lumbar back pain [4,8]. Sciatica is reduced by removing compression on the exiting root of the sciatic nerve. Decompression further reduces the sensitization of the nerve root to pain mediators by reducing inflammation caused by mechanical pain stimulation [9].
There are several sources of pain generation in disc injury involving an intervertebral disc that is degenerative, bulging, or protruding [10,11,12]. Disc herniation provides direct pressure by disc tissue on the nerve root. Mechanical compression of a nerve alone is not necessarily painful, however, if that nerve is inflamed (irritated, tender, swollen), it can produce severe pain with a small amount of mechanical compression. Nerve root compression is an important factor in generating inflammation and resultant sciatica [13,14,15]. When both nerve compression and inflammation around the nerve root are present, there is more nerve injury and pain perception than after either event alone [16,17].
Spinal nerve root compression does not cause sciatica in all circumstances because more than 50% of “normal,” asymptomatic people who have disc prolapses compressing the nerve roots have no pain [18]. In symptomatic individuals, the nerves are sensitized to compression, probably by biochemical pain mediators [19]. The inflammatory response that occurs as a result of nerve root compression also affects the sensory components of the lower back including the sensory nerves of the adjacent soft tissue. The inflammatory process is believed to sensitize the nerve root to all incoming stimuli. In such a state, even minor mechanical stimulation of the nerve root can evoke severe back pain. These pain mediators interact topically with nociceptors. Limiting the direct interaction of pain mediators with nociceptors was shown to reduce pain in preclinical models [6,20].
The mechanical compression of the nerve root may also lead to a series of intraneural tissue reactions, including edema, demyelination, and fibrosis that sensitize the surface of the nerve to pain mediators [9,13,21,22]; or tether the nerve root to adjacent tissues [23,24]. Mechanical compression increases microvascular permeability of the endoneural capillaries resulting in inflammation within the nerve root. Sensitizing the nerve root by topical exposure to pain mediators contributes to the pathogenesis of sciatica [13].
2.2. Lumbar Back Pain
The intervertebral disc is the main source of lumbar back pain (LBP). Intraoperative findings under local anesthesia showed that LBP was reproduced by stimulation of the outer annulus or the posterior longitudinal ligament (locations of sensory neurons). In contrast, sciatica was induced by mechanical stimulation of nerve roots [25,26,27,28,29,30].
The reduction in LBP that follows decompression surgery results from reduced production of pain mediators (biochemical as well as cellular) in the epidural space (disc, adjacent soft tissues, nerve root), which reduces stimulation of nociceptors in the sensory nerve fibers of the annulus and adjacent soft tissues. Decompression surgery also reduces the sensitization of these sensory nerve fibers to stimulation by pain mediators [31].
Microtrauma damages the annulus fibrosus, allowing blood vessels and nerves a deeper penetration into the annulus fibrosus [21]. Increased vascular and neural in-growth are seen in discs associated with LBP. Malinsky demonstrated a variety of free nerve endings and some button-like terminals exist in the outer few layers of the lumbar annulus and noted partially and fully encapsulated mechanoreceptors confined to the annular surface [32,33]. These free nerve endings contribute to pain transmission from the disc producing LBP [32]. Furthermore, the concentration of nerves and blood vessels in the annulus increases with age [34].
Free nerve endings are present in the annulus fibrosus and epidural space (ligaments, nerve roots and muscles). In patients with sciatica caused by disc herniation, reports of LBP preceding sciatic pain are common. Patients with severe LBP associated with disc herniation and sciatica have greater density of sensory nerves in the annulus fibrosus and epidural space than patients with less severe LBP [13,16,21,35,36,37,38,39], which results in sensitization of more sensory nerves and additional LBP following decompression surgery. It has been suggested that this pain may be caused by topical stimulation of nerve endings in the annulus fibrosus as a result of an annular tear and/or herniation and later by inflammation associated with extrusion of the nucleus pulposus. Chemical sensitization of sensory nerve fibers of the disc is induced by inflammation caused initially by disc herniation and later by the trauma of surgical decompression. Removing the herniating portion of the disc and/or the residual nucleus pulposus reduces inflammation in the epidural space [10,12,40,41,42]. Trauma to an intervertebral disc may damage disc components, resulting in the production of irritants (biochemical mediators), which may drain either into the spinal canal, irritating nerves, or into the vertebral body, setting up an autoimmune reaction resulting in LBP [10,12,21,43,44].
Biochemical mediators of pain are present in disc herniation tissue [10]. Axonal injuries and inflammatory stimulation of nociceptors alter nerve root excitability and thereby play an important role in LBP [45,46]. Local production of chemokines within the epidural space also contributes to LBP [47].
A number of experimental studies demonstrated the negative affects of disc tissue, and in particular the nucleus pulposus, on nerve roots [48,49,50,51,52,53,54,55]. When the substance of the nucleus pulposus comes into contact with sensory nerves of the epidural tissues including the outer annulus it:
- 1)
- induces degeneration of nerve fibers;
- 2)
- increases discharge of nerve fibers;
- 3)
- attracts inflammatory cells (cellular mediators of pain) and;
- 4)
- induces increased intraneural capillary permeability.
2.3. Lumbar Disc: Anatomy
In order to understand how pain is generated in the disc and epidural space, a brief review of the disc, epidural space and associated neuroanatomy is useful. The disc is composed of a central nucleus pulposus surrounded peripherally by the annulus fibrosus (Figure 1). In normal young adults, the nucleus is a semi-fluid mass of mucoid material (glycosaminoglycans, proteoglycans, and collagen). The nucleus is composed of approximately 70-90% water in a young healthy disc, but this percentage generally decreases with age [9]. The annulus fibrosus consists of 10-20 concentric collagen fiber layers that surround the nucleus. The layers are arranged in alternating orientation of parallel fibers.
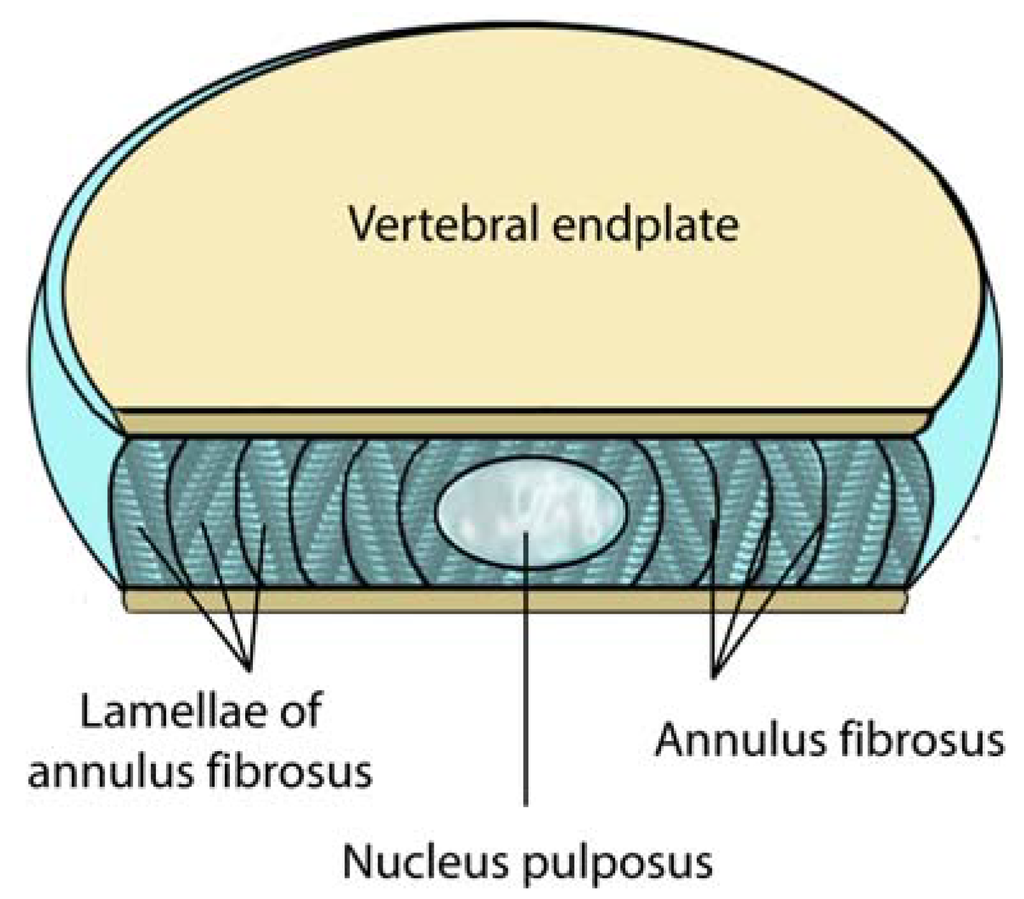
Figure 1.
A sectional view of a normal lumbar disc. Note the locations of the nucleus pulposus, the vertebral end plate and the annulus fibrosus. The intervertebral lumbar disc is typically 4cm wide and 7-10 mm thick (adapted from Raj et al. [34]). The interior of the disc including the nucleus pulposus is avascular.
The vertebral endplate is a thin layer of cartilage located between the vertebral body and the intervertebral disc. Although normally composed of both hyaline and fibrocartilage in youth, older end plates are virtually entirely fibrocartilage exposing the sensory fibers that course through these areas to topical stimulation. The intervertebral disc is the largest avascular structure in the body. As a result, exposure of epidural tissues to the nucleus pulposus produces an autoimmune response increasing the concentration of cellular and biochemical pain mediators.
The central component to any injury involving the lumbosacral discs is the natural aging process and/or trauma [56]. Disc aging typically involves circumferential tears or fissures in the outer annulus. These changes are thought to result from repetitive microtrauma. Brown et al. and Ohtori et al. reported that, in patients with lumbar back pain (LBP), there were increases in the density of sensory nerve fibers in the endplates and defects in the endplate cartilage, strongly suggesting that the endplates and vertebral bodies were sources of pain [57,58].
Sensory nerves in the disc often accompany blood vessels present in the longitudinal ligaments adjacent to the disc, but they can also occur independently, arising as branches of the sinuvertebral nerve or derived from the ventral rami or gray rami communicans [34] (Figure 2). The ventral rami and gray rami communicans form a ventral plexus that supplies the anterior and lateral aspects of the annulus and the anterior longitudinal ligament. The posterior annulus fibrosus and the posterior longitudinal ligament are innervated by the sinuvertebral nerve (contributes branches to the dorsal plexus), which consists of sensory fibers. Each sinuvertebral nerve supplies the disc at its level of entry into the vertebral canal [59,60]. Most of the nerve fibers are sensory in origin and are involved in nociception [33,39,60,61,62].
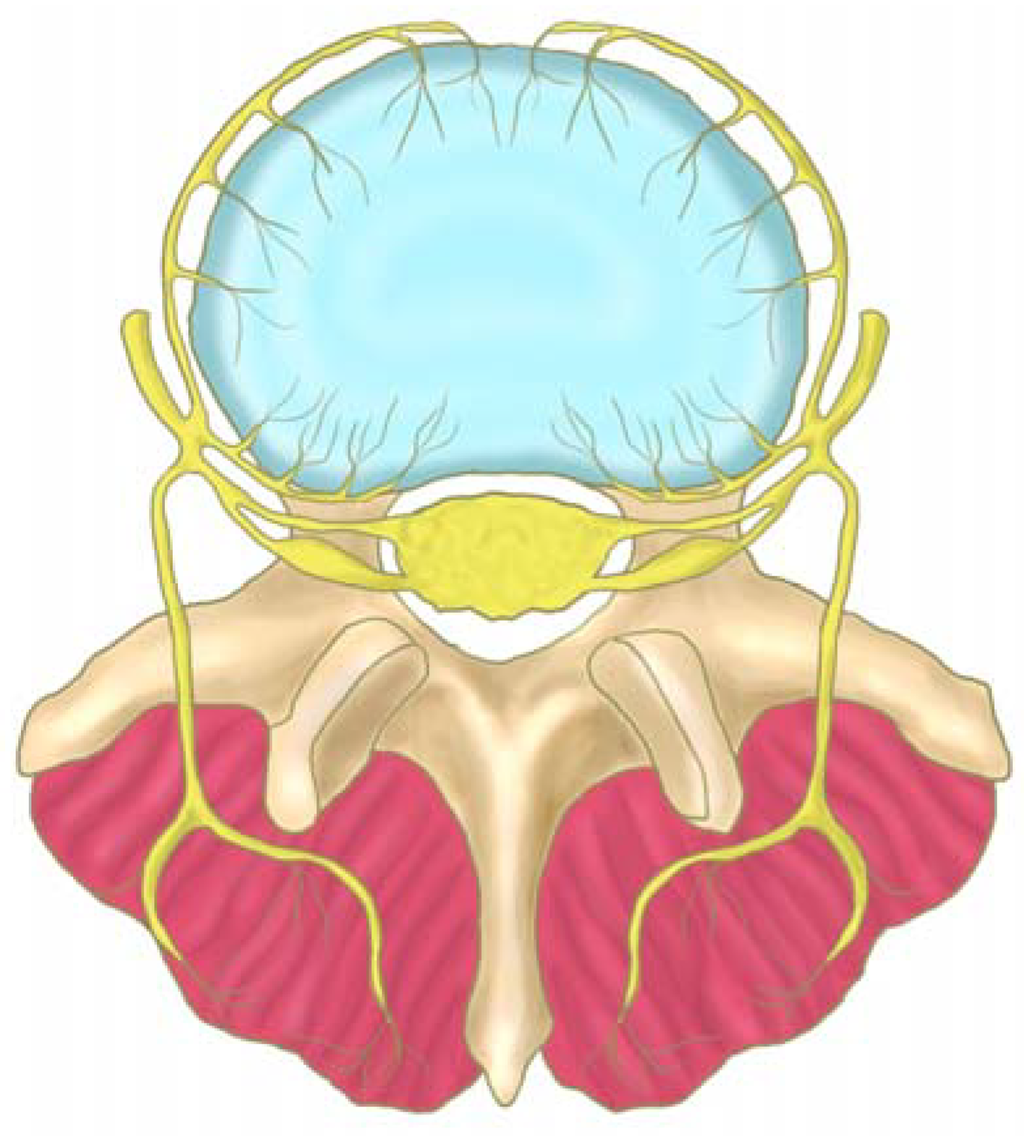
Figure 2.
The course of the sinu-vertebral nerve (yellow), which innervates the postero-lateral region of the disc (blue). The nerve exits from the nerve root and enters the vertebral foramen, where it divides into a major ascending and a lesser descending branch (adapted from Raj et al. [34]).
The anterior longitudinal ligament also receives sensory innervation from branches that originate in the nerve root. The posterior longitudinal ligament (PLL) is richly innervated by nociceptive fibers from the ascending branch of the sinuvertebral nerve (Figure 3). These nerves also provide sensory innervation of the adjacent outer layers of the annulus fibrosus [34]. Herniation and rupture of the disc/longitudinal ligament typically leads to exposure of the epidural space to pain mediators.
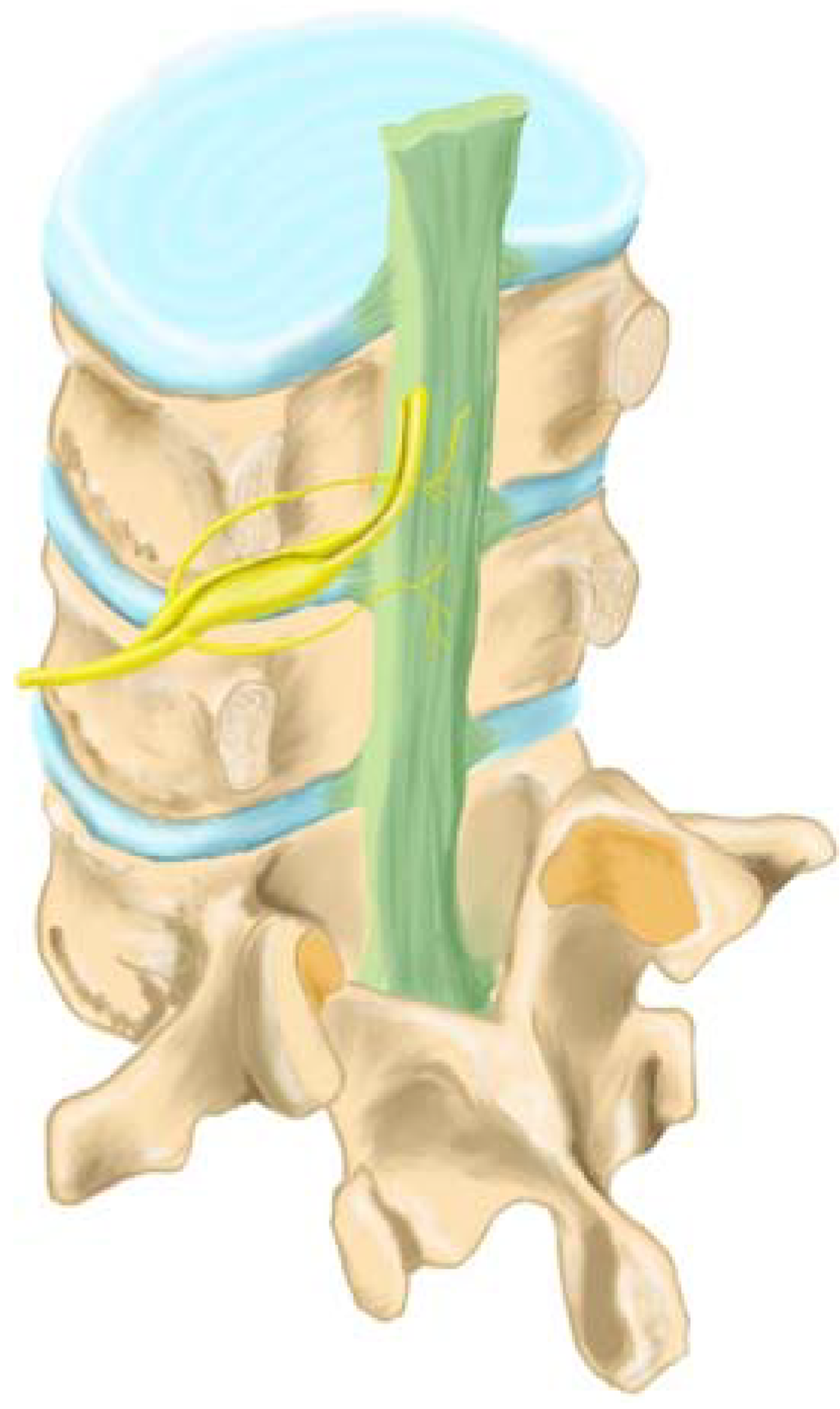
Figure 3.
Sensory innervation of the posterior longitudinal ligament (green) and the disc annulus (blue) occurs by the ascending branch of the sinuvertebral nerve (yellow) (adapted from Raj et al. [34]).
2.4. Sensory Innervation of the Disc
In 1949, Herlihy reported that direct innervation of the intervertebral disc could lead to intrinsic pain stimulation at the disc [59,63]. Subsequently, multiple studies conclusively demonstrated that the intervertebral disc is innervated [59,64]. In a normal disc, the outer one third of the annulus is innervated; the inner two thirds of the annulus and the nucleus pulposus receive no innervation [59]. A high proportion of nociceptive nerve fibers arising from the annulus of the lumbar discs pass through the sympathetic trunks as sympathetic sensory afferents. These pain receptors are sensitized by changes in external pressure (mechanoreceptors) or inflammatory irritation [22,65,66,67].
The lumbar intervertebral disc is innervated by the sinuvertebral nerves (Figure 3) consisting of spinal sensory fibers and postganglionic sympathetic fibers [25,58,68,69,70]. Sensory neurons involved in pain perception (nociceptive) relating to inflammatory pain as occurs with disc herniation or following decompression surgery are typically small, peptide-containing neurons [58,70,71]. As a result, peripheral nerve injuries can lead to pain sensations that are expressed within minutes, days, weeks or months following the actual traumatic event (for example disc herniation or surgical anulotomy) [72,73,74]. Many painful lumbar neuropathies involve subtotal nerve damage including decompression surgery [75]. Patients with painful neuropathies suffer from both spontaneous pain (allodynia) and from a variety of different types of abnormal evoked-pain sensations (hyperalgesia). Application of biochemical pain mediators to the surface of the nerve root produce hyperalgesia [75,76].
The innervation of the disc is concentrated in the outermost part of the annulus fibrosus and endplate [39,68,77,78,79]. Palmgren demonstrated sensory nerve terminals in herniated lumbar disc tissue, in the periphery of the annulus fibrosus, and along deeper annular tears [78]. Ashton et al. and Aoki et al. identified nerve structures in lumbar discs from asymptomatic patients extending 3 mm into the annulus fibrosus [35,71]. In contrast, disc material obtained from patients with LBP showed deeper in-growth of blood vessels and nerves. Freemont examined the innervation of the inner disc using 46 biopsy samples (30 from levels with pain and 16 from levels with no pain) [21]. Innervation of the inner disc was observed more frequently in painful discs than in asymptomatic discs. They further demonstrated the presence of nerve fibers in painful discs demonstrating that nociceptive nerve fibers were growing into the painful disc. Nerve in-growth into the inner disc follows the development of fenestrations resulting from trauma and/or aging (Figure 4). These sensory afferents, which can transmit pain from the disc itself, contribute to LBP with herniation (Figure 5).
The pain receptors of the intervertebral discs are nociceptors, which are activated under inflammatory conditions when their surface comes into contact with biochemical and cellular mediators of pain, which lead to pain perception. Inflammatory changes may cause the silent nociceptors to become responsive to mechanical stimuli, and this nociceptive information is perceived as LBP [27,80]. Takebayashi found that the lumbar intervertebral discs were not responsive to mechanical stimulation under normal conditions, but once inflamed by the topical application of biochemical pain mediators, mechanically insensitive afferents responded to mechanical stimulation [81].
Freemont et al. demonstrated nerves in the annulus with the morphology of nociceptors [21]. Inflammatory cells, mostly macrophages, were found in disc herniation tissue, indicating a topical inflammatory reaction. In contrast, only a few macrophages were observed in normal disc tissue [82]. Histamine produced by macrophages, prostaglandin E2 [82,83,84] and bradykinin all act as chemical mediators of pain. These mediators also sensitize the peripheral nerve endings that evoke pain sensation [85]. The neural structures are susceptible to stimulation by pressure or chemical mediators produced as a result of herniation [79].
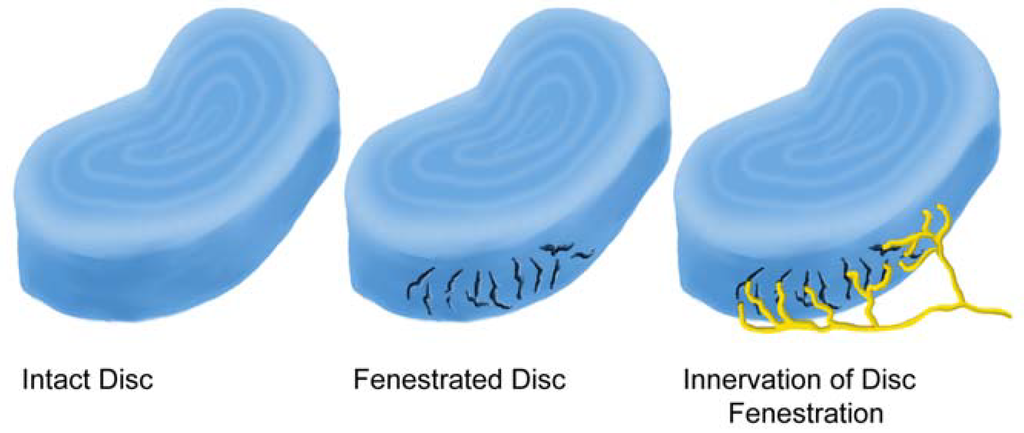
Figure 4.
Fenestrations caused by trauma and/or aging are often the site of nerve ingrowth.
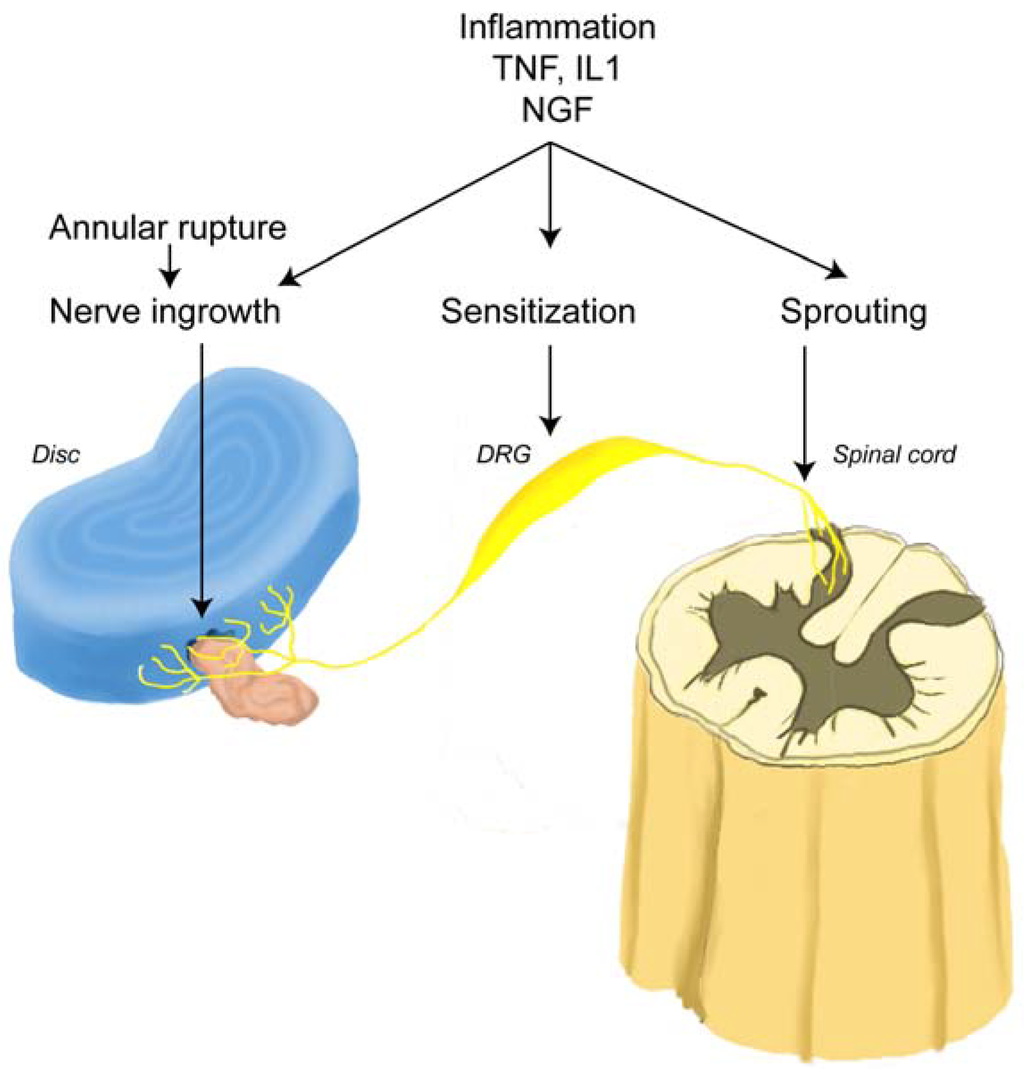
Figure 5.
Schematic representation of a mechanism for LBP. An annular rupture leads to extrusion of the nucleus pulposus (pink) outside annulus fibrosus (blue), which induces nerve injury and nerve in-growth into the disc (yellow). Under inflammatory conditions which may occur following surgery, nerve growth factor (NGF) is induced in the disc and acts on the nerve root (DRG) through the peripheral terminals in the disc. Disc-innervating neurons have a high sensitivity to NGF. NGF may promote nerve in-growth into the disc, sensitize DRG neurons, and cause neuronal sprouting into the dorsal horn (adapted from Takahashi et al. [38]).
2.5. Sensory Innervation of the Epidural Space
The soft tissues (ligaments, muscles) and nerve root adjacent to the disc contribute to lumbar back pain (LBP) by stimulation of sensory nerves [65,86]. Rupture of the posterior longitudinal ligament and extrusion of nucleus pulposus into the epidural space (Figure 5) evokes an autoimmune reaction and infiltration of cellular pain mediators [41,87,88,90]. Chemical mediators throughout the epidural space (especially from the intervertebral disc itself) are a significant source of both LBP and sciatica as well as sensitization of sensory nerves [11,91,92,93]. Burke et al. found that patients with severe LBP generally had higher levels of inflammatory mediators than those patients with lower levels of disc pain [10].
2.6. Sensitization of Sensory Nerves
Mechanical stimuli which are normally innocuous to disc nociceptors can generate an amplified response which has been termed ‘peripheral sensitization’ [47]. This may explain why some herniating discs are painful and others are not [26]. Exposed nuclear material is known to irritate the spinal nerve root and the sinuvertebral nerve endings [26,55,94,95]. In herniated discs the inflammatory granulation tissue present in annular tears and containing sensory nerves [21,44,47,62] behaves in a similar way [10]. This peripheral sensitization has been confirmed clinically [96]. Increased numbers of mechanoreceptors [97] and sensory neurons are found in discs from patients with lumbar back pain (LBP) [57,98].
Exposure of epidural tissue to inflammatory cytokines from the disc nucleus results in both nerve sensitization [12] and nerve injury [42,84]. Franson showed that human PLA2 concentration in the intervertebral disc are 20-10,000 times higher than the PLA2 found in other human tissues [99]. A wide variety of pain mediators that come in contact with the nerve root during and after disc surgery can sensitize neural tissue to postoperative pain and neurological symptoms [10,11,12,19,100] Increase in sensory nerve excitability that can occur following decompression surgery often prolongs sensory nerve sensitization resulting in pain and hyperalgesia long after the surgical procedure [101].
The combination of mechanical compression (mass effect of herniated disc) and chemical irritation by cellular and biochemical mediators (inflammation around nerve root) induces more LBP than either factor alone [16]. As reviewed by Cohen et al., the concept of chemical sensitization may explain the contrasting responses between LBP and asymptomatic discs. Tumor necrosis factor-α (TNF-α) is expressed in the nucleus pulposus and plays a role in generating sciatic pain in patients with disc herniation [102,103,104]. Interleukin-1β (IL-1 β), which is produced in tissues involved in disc herniation, has the capacity to produce hyperalgesia. Also, NGF, which is up regulated by such mediators, has a sensitizing effect on nerve fibers. The levels of inflammatory mediators are higher in painful discs than in asymptomatic discs, suggesting that their interaction with tissues in the epidural space would produce LBP [10,105].
2.7. Post Operative Pain
Surgical dissection and retraction cause nerve root trauma and cellular injury, expose neural tissues to blood and disc substance (nucleus pulposus), filling the epidural space with cellular and biochemical pain mediators. These lead to nerve root irritation, inflammation and fibrin production each of which can trigger additional inflammation, trauma, nerve root compression or tethering and sensory sensitization. Mechanical deformation of nerve tissue resulting from surgical dissection also contributes to prolonged postoperative lumbar back pain (LBP). Laminectomies may also induce an increase in the density of nociceptive neurons in the lumbar disc ascribable to axonal sprouting of fine sensory nerve fibers. Neuronal outgrowth of nociceptive afferents is associated with LBP after lumbar surgery [106]. The leaking "chemical soup" within the nucleus pulposus contains pain mediators that stimulate sensory fibers following annulotomy. Neuropeptides released from peripheral endings of nociceptive afferents are also inflammatory mediators and pain generators [107].
2.8. Pain Mediators during Herniation and Following Decompression Surgery
A large number of cells (principally inflammatory) and biochemicals are present in the disc and epidural space during herniation that are not present in normal disc tissue. They have been shown to interact with nociception and sensory nerve transmission and are collectively referred to here as “pain mediators”. The concentration of these pain mediators increase in these tissues following surgery. As a result, even though mechanical decompression is completed, pain often continues, especially lumbar back pain (LBP) (Appendix Table 2).
2.9. Cellular Pain Mediators
2.9.1. Neutrophils
Inflammatory cells at the surgical site increase in both number and activity. During and immediately following surgical injury, neutrophils enter the surgical field in large numbers. An increase in neutrophil number or concentration within the surgical site can contribute to pain. Neutrophils release a number of products that cause tissue destruction and continued inflammation (oxygen radicals, protease, etc.) that contribute to pain through topical interaction with sensory fibers and nociceptors [11,21,82,94,95,108].
2.9.2. Macrophages
The second cell type to enter the epidural space after surgery is monocytes/macrophages. These cells secrete oxygen free-radicals, along with pro-inflammatory cytokines. Gronblad et al. reported high concentrations of macrophages and biochemical pain mediators in disc material obtained from patients with disc herniation [82]. Doita reported that mononuclear cells infiltrating along the margins of extruded discs expressed inflammatory mediators and appeared to induce neovascularization and persistence of inflammation [41,109]. Macrophages and mast cells are among the chief cellular mediators of inflammatory neuritis. Macrophages can produce a host of inflammatory molecules (e.g., interleukin 1 [110,111,112]) as well as tumor necrosis factor [110,111,113] and can also exert cytotoxic activity by direct physical contact or through the release of toxic by-products (e.g., nitric oxide [114] and proteases [115,116,117]. Macrophages also enhance vascular permeability, provide chemotactic signals and modulate inflammatory cell activities. Release of histamine induced by mast cell degranulation may play an important role in LBP [118]. Intraneural edema and the appearance of macrophages and other inflammatory cells occur at the site of compression leading to sensory nerve sensitization [13].
Haro et al. obtained similar data on the presence of macrophages in painful disc herniations. In addition, they were able to demonstrate statistically significant quantities of factor VII, monocyte chemotactic protein-1, and macrophage inflammatory protein-1 positive cells in symptomatic herniations [119]. Takahashi et al. demonstrated the presence of inflammatory cytokines in human tissue adjacent to nerve roots at the level of a symptomatic herniated disc removed at the time of surgery [120].
2.10. Biochemical Pain Mediators Identified in the Epidural Space in Patients with LBP
A large number of biochemical pain mediators have been identified in the disc and epidural space that contribute to LBP following decompression surgery (Appendix table 2).
Disc rupture/annulotomy exposes epidural tissues to the nucleus pulposus. The nucleus pulposus elicits an immune response by adjacent tissues in the epidural space [121]. Application of disc tissue to a nerve results in nerve fiber injury and pain [11,50,51,53,54,55]. TNF- α is released not only by the herniated disc, but is also released in cases of annular tear [44,122,123,124]. Takahashi reported that inflammatory cytokines were present in disc material removed from patients with herniated discs [120]. Herniated lumbar disc tissue from symptomatic patients contains elevated levels of TNF- α, nitric oxide (NO), prostaglandin E2 (PGE2), IL-1β, IL-6, IL-8, COX-2, and NOS [125,126] as compared with control disc tissue [41,127,128]. These biochemical mediators may be a direct stimulant of LBP as well as sensitize peripheral nociceptors [53,56].
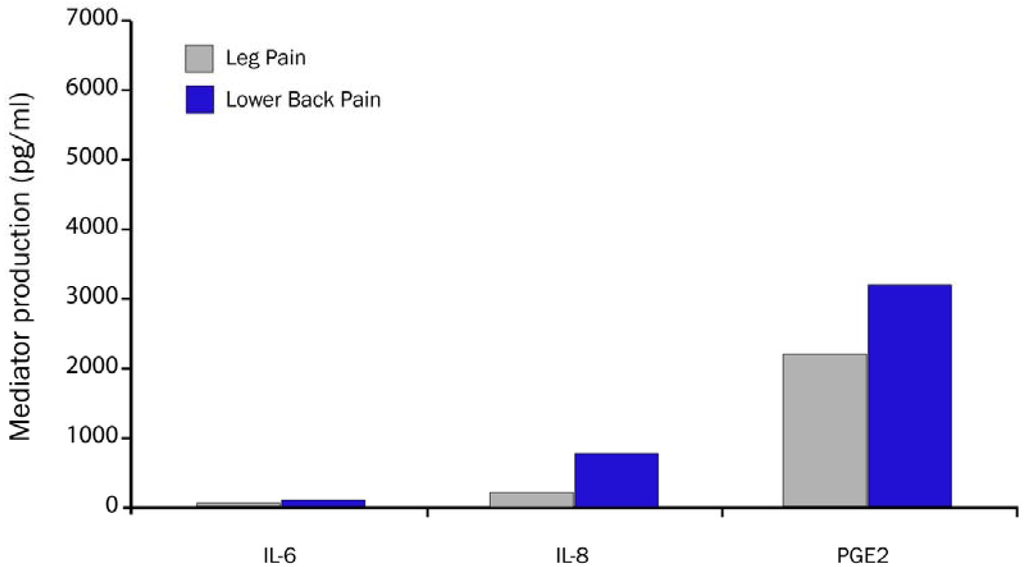
Figure 6.
Biochemical mediator concentration is higher in disc from patients with LBP vs. patients with leg pain and LBP (adapted from Burke et al. [10]).
Burke et al. confirmed and extended these observations by quantitating proinflammatory biochemical pain mediators (IL-6, IL-8 and PGE2) in disc tissue obtained from patients undergoing lumbar surgery for sciatica and LBP (Figure 6) [10]. Those patients with LBP (n = 20) had significantly higher concentrations of biochemical pain mediators than patients with only sciatica (n = 63). Hyperalgesia is induced in an innervated nucleus pulposus by cellular and biochemical pain mediators. The exposure of the nucleus pulposus to the outer annulus fibrosus induces nerve injury. Hyperalgesic responses can be induced by contact of the nociceptors of sensory nerves with nucleus pulposus that signal LBP directly from the disc and epidural space [38].
2.11. Annular Leakage and Pain Sensitization
Disc herniation is typically preceded by one or more attacks of acute lumbar back pain (LBP) [43,129]. Moneta et al. demonstrated that peripheral annular tears in human are the principal source of LBP preceding herniation [130]. These findings were confirmed in clinical studies by Weinstein et al. and Hyodo et al., and animal models by Murata et al. [5,129,131]. LBP may also occur when there is internal disruption of annular fibers but no tearing of the outer layers of the annulus. In this situation, there is local injury to the sensory fibers of the outer one-third of the annulus as well as leakage of the nucleus pulposus into the epidural space. Kayama et al. found that incision of the annulus fibrosus without disc herniation, but with a slow leakage of nucleus pulposus through the incision, induced significant pain and nerve damage in a dog model [37]. These preclinical studies supported their clinical experience that patients suffer from more severe pain when nucleus pulposus is communicating with the epidural space compared with those in whom the herniated nucleus pulposus is contained within the annulus fibrosus [44].
Materials that leak from annular tears induce single level nerve root pain or multi-level nerve root pain through diffusion. Peng et al. found that the nerve injury caused by the combination of mechanical compression (mass effect of herniated nucleus pulposus) and chemical irritation (inflammation around the nerve root or perineural inflammation) may induce more nerve root injury than each factor alone (Figure 7) [44]. The nerve root becomes sensitized by nucleus pulposus material that reaches the epidural space through annular tears [11,37,50,51,52,53,132,133,134,135]. Since the pain threshold has been lowered, the additional mechanical compression on the nerve root may induce an excruciating pain due to the presence of chemical radiculitis resulting from topical exposure of sensory fibers to chemical mediators [44].
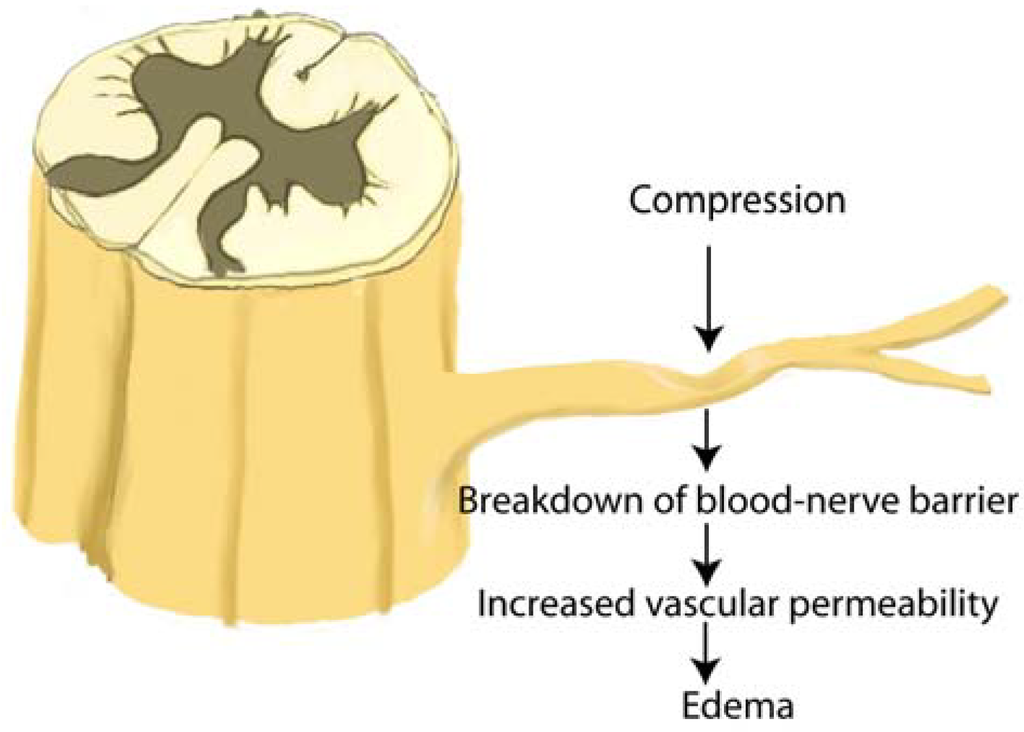
Figure 7.
Nerve root compression produces increased vascular permeability at the site of compression, in the peripheral zone of a compressed anterior nerve root, and in the central zone of a compressed posterior nerve root. After nerve root compression, degeneration of the nerve is found in the area of increased vascular permeability. DR: dorsal root, DRG: dorsal root ganglion, VR: ventral root (adapted from Kobayashi et al. [13]).
2.12. Sensitization by Fibrosis
Fibrosis has received considerable attention as a contributor to sciatica and lumbar back pain (LBP) following decompression surgery. Kuslich et al. found that spinal nerve roots encased in perineural fibrosis were very sensitive to external stimulation in patients with prior laminectomies undergoing repeat procedures under minimal anesthesia [26]. Neuropathic changes in the spinal sensory potential correlated with postoperative perineural fibrosis in a rat model of laminotomy up to 3 months following surgery [136]. These observations provide direct evidence of nerve root sensitization to pain by perineural fibrosis. While epidural fibrosis commonly results following surgical intervention of the spine, leakage of disc material into the epidural space following an annular tear or surgical intervention can also result in the formation of epidural fibrosis [137,138,139,140,141,142,143,144,145,146,147].
Fransen evaluated the reduction of epidural fibrosis in a group of 396 patients following single level disc herniation and presenting with sciatica often associated with LBP [148,149]. All subjects were operated upon by the same surgeon in the same institution (Clinique du Parc Léopold, Brussels, Belgium) between January 1st 2003 and December 31st 2005. Upon completion of a conventional microdiscectomy, in all patients, the decompressed nerve root and epidural space including the annulus fibrosus were systematically covered with a gel composed of carboxymethylcellulose (CMC) and polyethylene oxide (PEO). Five (5) patients needed reoperations for recurrent herniation, two (2) after less than a week, one after one month, and two (2) within the first year after surgery. In perioperative assessment of the reoperations, there was little or no epidural fibrosis. This facilitated dissection and separation of the nerve root from surrounding tissues.
2.13. Epidural Adhesions
Adhesions themselves are not painful. Epidural fibrosis and subsequent tethering of the nerve root to the disc or pedicle (and thereby compression), may contribute to post-surgical sciatica and lumbar back pain (LBP). However, results of clinical outcome studies attempting to correlate adhesion formation with pain have not been consistent [150,151,152]. Most patients with epidural fibrosis do not develop symptomatic complaints [153]. However, fibrous entrapment of nerve roots may cause sciatica as demonstrated by their release, resulting in immediate relief from sciatica [154]. The pain is thought to result from entrapment of the nerve root by fibrosis resulting in enhanced sensitization in contrast to tethering.
Ido reported seven (7) patients with fibrous adhesive entrapment of lumbosacral nerve roots as a cause of sciatica. Radiographic findings (MRI, myelography and CT myelography) of the patients were negative (no disc herniation or nerve root compression). All seven patients complained of sciatica accompanied by LBP. Differential nerve blocks were effective in relieving sciatica and LBP in these patients. Surgical procedures resulted in the release of the nerve root and creation of space around it. All seven patients experienced complete relief from sciatic pain and LBP immediately after the fibrous sheath was released. During the average follow-up period of 7 years and 2 months, no recurrence of sciatic pain accompanied by LBP was observed [154].
2.14. Protection of Epidural Sensory Nerves by Gels
Reduction in fibrin deposition would reduce nerve root entrapment and reduce sensitization to pain [154]. Coating of tissues in the epidural space also reduces postoperative pain following lumbar disc surgery by reducing the interaction of sensory nerves in the lumbar disc and epidural space from cellular and biochemical pain mediators. To be effective, such barrier gels need to be present for a short time to reduce exposure of damaged tissue to macrophages and other inflammatory cells known to stimulate painful nociceptors, and to provide mediators that trigger the development of fibrosis by attracting fibroblasts (Figure 8).
It has been shown in preclinical and clinical studies that coverage of the nerve root with a polyanionic polysaccharide viscoelastic gel such hyaluronic acid (HA), or carboxymethylcellulose (CMC) /polyethylene oxide (PEO) reduces pain and symptoms [155]. Healon® (HA) and Oxiplex® (CMC/PEO) are two such high-molecular-weight polysaccharide gels. The concentration of solids in polysaccharide formulations appears to enhance the mucoadherance and viscoelastic properties that improve tissue coating [7,20,156,157]. A number of preclinical studies demonstrated that HA gel reduces pain and the presence of cellular as well as biochemical pain mediators in rat and canine laminectomy models [6,20,158,159,160,161,162]. Hyaluronic acid gel was also shown to inhibit macrophage migration into the epidural space and release of biochemical pain mediators in the wounds of animals following laminectomy [20,159]. The investigators at the University of California San Diego described a laminectomy model that resulted in a heightened sensitivity to pain [20,158]. Pain reduction by polysaccharide treatment after laminectomy and disc injury in a rat model resulted from reduction in the concentration of the cytokines and inflammatory cell infiltrates that would otherwise occur around the nerve root and the epidural space.
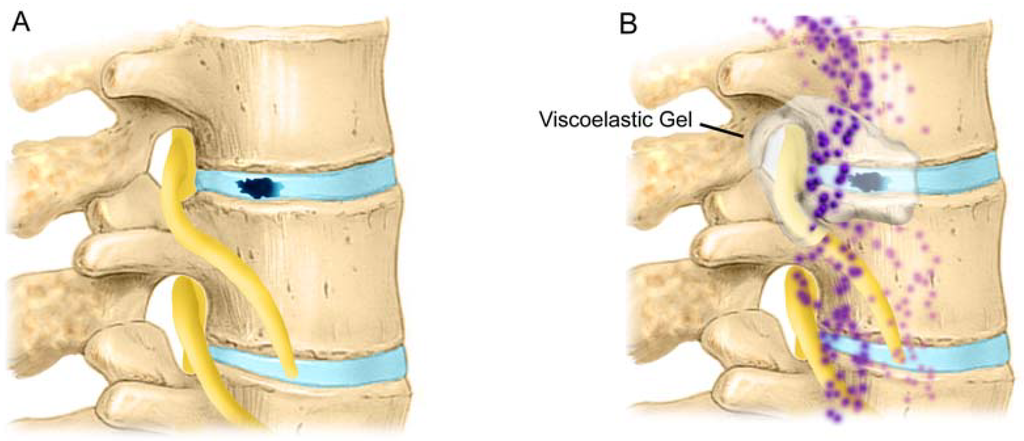
Figure 8.
A) Disc tear/rupture/annulotomy exposes epidural tissues to the nucleus pulposus. The nucleus pulposus elicits an immune response in the epidural space [121]. B) Herniated lumbar disc tissue from symptomatic patients contains elevated levels of TNF- α, nitric oxide (NO), prostaglandin E2 (PGE2); IL-1β, IL-6, IL-8, COX-2, and NOS [103,125,128,179,180] as compared with control disc tissue [41,127,128].
Massie et al. concluded that one mode of action for the reduction of pain following surgery with the use of viscoelastic gels is the decreased migration of inflammatory cells into the epidural space by the viscous environment of the gel [6]. Viscoelastic gels would provide a protective tissue coating that decreases fibrosis and shields the nociceptors present on the exposed sensory nerves from pain mediators. The utilization of a mechanical barrier that coats and separates tissues in the lumbar spine provides some measure of surface protection of the sensory nerve against inflammatory mediators that occur as a result of surgery as well as outpouring from the annulotomy site itself. In this mode of action, the tissue coating properties, molecular weight, concentration, and the rheology of the viscoelastic gel are important [7,163,164].
The components of Oxiplex (carboxymethylcellulose, or CMC, and polyethylene oxide, or PEO) separate tissue by providing a viscous coating that prevents migration and attachment of cellular and biochemical pain mediators to covered tissues cells [165,166,167,168,169]. The CMC component allows for gel adherence to tissues [157,170,171]. The PEO component prevents protein deposition [172,173,174,175,176] on the surface of covered tissues. The combination of CMC and PEO allows the gel to remain at the site of application for a period of time, providing a mechanical barrier to protein and cell deposition that could otherwise lead to pain and adhesion formation during the healing process [156,177,178].
The data demonstrate that polysaccharide gels that coat healing tissues can protect tissues from cellular and biochemical pain mediators and fibrotic bridges that lead to adhesions during the healing process. Coverage of the annulus and adjacent structures in the epidural space by absorbable viscoelastic gels appears to reduce LBP following surgery by protecting sensory fibers from cellular and biochemical pain mediators. The results of these studies demonstrate that gels which protect tissues during healing, thereby reducing their interaction by the interposition of a temporary viscoelastic gel, should provide a useful strategy to reduce both back and leg pain following lumbar disc surgery.
Acknowledgements
The authors would like to acknowledge the contributions of Anthony Freemont, Ronald Ehmsen, John Krelle, Thomas Juarez, Stephanie Cortese and Kathleen Block for their review of this manuscript and insightful suggestions, as well as Deborah Jeffrey for graphical content.
References and Notes
- Koes, B.W. Evidence-based management of acute low back pain. Lancet 2007, 370, 1595–1596. [Google Scholar] [CrossRef] [PubMed]
- Koes, B.W.; van Tulder, M.V.; Peul, W.C. Diagnosis and treatment of sciatica. BMJ 2007, 334, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, K.; Dunn, K. Review of epidemiological studies and prevalence estimates. Spine 2008, 33, 2464–2472. [Google Scholar] [CrossRef] [PubMed]
- Pearson, A.M.; Blood, E.A.; Frymoyer, J.W.; Herkowitz, H.; Abdu, W.A.; Woodward, R.; Longley, M.; Emery, S.E.; Lurie, J.D.; Tosteson, T.D.; Weinstein, J.N. SPORT lumbar intervertebral disk herniation and back pain: does treatment, location, or morphology matter? Spine 2008, 33, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.N.; Lurie, J.D.; Tosteson, T.D.; Skinner, J.S.; Hanscom, B.; Tosteson, A.N.A.; Herkowitz, H.; Fischgrund, J.; Cammisa, F.P.; Albert, T.; Deyo, R.A. Surgical vs. nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT) observational cohort. JAMA 2006, 296, 2451–2459. [Google Scholar] [CrossRef] [PubMed]
- Massie, J.B.; Schimizzi, A.L.; Huang, B.; Kim, C.; Garfin, S.R.; Akeson, W.H. Topical high molecular weight hyaluronan reduces radicular pain post laminectomy in a rat model. Spine J. 2005, 5, 494–502. [Google Scholar] [CrossRef] [PubMed]
- diZerega, G.S.; Cortese, S.; Rodgers, K.E.; Block, K.M.; Falcone, S.J.; Juarez, T.G.; Berg, R. A modern biomaterial for adhesion prevention. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 81, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Toyone, T.; Tanaka, T.; Kato, D.; Kaneyama, R. Low-back pain following surgery for lumbar disc herniation. A prospective study. J. Bone Joint Surg. Am. 2004, 86A, 893–896. [Google Scholar] [PubMed]
- Anderson, D.G.; Albert, T. The Molecular Basis of Intervertebral Disk Degeneration. Semin. Spine Surg. 2003, 15, 352–360. [Google Scholar] [CrossRef]
- Burke, J.G.; Watson, R.W.; McCormack, D.; Dowling, F.E.; Walsh, M.G.; Fitzpatrick, J.M. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J. Bone Joint Surg. Br. 2002, 84, 196–201. [Google Scholar] [CrossRef] [PubMed]
- McCarron, R.F.; Wimpee, M.W.; Hudkins, P.G.; Laros, G.S. The inflammatory effect of nucleus pulposus. A possible element in the pathogenesis of low-back pain. Spine 1987, 12, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Olmarker, K.; Myers, R.R.; Kikuchi, S.; Rydevik, B. Pathophysiology of Nerve Root Pain in Disc Herniation and Spinal Stenosis. In the Lumbar Spine, 3rd ed.; Herkowitz, H.N., Dvorak, J., Bell, G.R., Nordin, M., Grob, D., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2004; Chapter 2; pp. 11–30. [Google Scholar]
- Kobayashi, S.; Yoshizawa, H.; Yamada, S. Pathology of lumbar nerve root compression. Part 1: Intraradicular inflammatory changes induced by mechanical compression. J. Orthop. Res. 2004, 22, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Winkelstein, B.A.; DeLeo, J.A. Nerve root injury severity differentially modulates spinal glial activation in a rat lumbar radiculopathy model: considerations for persistent pain. Brain Res. 2002, 956, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Winkelstein, B.A.; Weinstein, J.N.; DeLeo, J.A. The role of mechanical deformation in lumbar radiculopathy: an in vivo model. Spine 2002, 27, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Yabuki, S.; Aoki, Y.; Kikuchi, S. Pathomechanisms of nerve root injury caused by disc herniation: an experimental study of mechanical compression and chemical irritation. Spine 2003, 28, 435–441. [Google Scholar] [PubMed]
- Wu, G.; Ringkamp, M.; Hartke, T.V.; Murinson, B.B.; Campbell, J.N.; Griffin, J.W.; Meyer, R.A. Early onset of spontaneous activity in uninjured C-fiber nociceptors after injury to neighboring nerve fibers. J. Neurosci. 2001, 21, RC140. [Google Scholar] [PubMed]
- Garfin, S.R.; Glover, M.; Booth, R.E.; Simeone, F.A.; Rothman, R.H. Laminectomy: a review of the Pennsylvania hospital experience. J. Spinal Disord. 1988, 1, 116–133. [Google Scholar] [PubMed]
- Garfin, S.R.; Rydevik, B.; Lind, B.; Massie, J. Spinal nerve root compression. Spine 1995, 20, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Schimizzi, A.L.; Massie, J.B.; Murphy, M.; Perry, A.; Kim, C.W.; Garfin, S.R.; Akeson, W.H. High molecular-weight hyaluronan inhibits macrophage proliferation and cytokine release in the early wound of a preclinical postlaminectomy rat model. Spine J. 2006, 6, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Freemont, A.J. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology 2009, 48, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Sekine, M.; Yamashita, T.; Takebayashi, T.; Sakamoto, N.; Minaki, Y.; Ishii, S. Mechanosensitive afferent units in the lumbar posterior longitudinal ligament. Spine 2001, 26, 1516–1521. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, M.A. and Hernandez-Vaquero, D. Preventing peridural fibrosis with nonsteroidal anti-inflammatory drugs. Eur. Spine J. 2008, 17, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Slipman, C.W.; Shin, C.H.; Patel, R.K.; Isaac, Z.; Huston, C.W.; Lipetz, J.S.; Lenrow, D.A.; Braverman, D.L.; Vresilovic, E.J., Jr. Etiologies of failed back surgery syndrome. Pain Med. 2002, 3, 200–217. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, C.; Ingelmark, B.E.; Miller, M. The anatomical basis for low back pain. Studies on the presence of sensory nerve endings in ligamentous, capsular and intervertebral disc structures in the human lumbar spine. Acta Orthop. Scand. 1963, 33, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kuslich, S.D.; Ulstrom, C.L.; Michael, C.J. The tissue origin of low back pain and sciatica: a report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Orthop. Clin. North Am. 1991, 22, 181–187. [Google Scholar] [PubMed]
- Mooney, V. Where is the pain coming from? Spine 1987, 12, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Nachemson, A.L. The lumbar spine: an orthopaedic challenge. Spine 1976, 1, 59–76. [Google Scholar] [CrossRef]
- Smyth, M.J.; Wright, V. Sciatica and the intervertebral disc; an experimental study. J. Bone Joint Surg. Am. 1958, 40, 1401–1418. [Google Scholar] [PubMed]
- Wiberg, G. Back pain in relation to the nerve supply of the intervertebral disc. Acta Orthop. Scand. 1949, 19, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, J.M.; Ozaktay, A.C.; Yamashita, T.; Avramov, A.; Getchell, T.V.; King, A.I. Mechanisms of low back pain: a neurophysiologic and neuroanatomic study. Clin. Orthop. Relat. Res. 1997, 335, 166–180. [Google Scholar] [PubMed]
- Edgar, M.A. The nerve supply of the lumbar intervertebral disc. J. Bone Joint Surg. Br. 2007, 89, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Malinsky, J. The ontogenetic development of nerve terminations in the intervertebral discs of man. (Histology of intervertebral discs, 11th communication). Acta Anat. 1959, 38, 96–113. [Google Scholar] [CrossRef] [PubMed]
- Raj, P.P. Intervertebral disc: anatomy-physiology-pathophysiology-treatment. Pain Pract. 2008, 8, 18–44. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Takahashi, Y.; Ohtori, S.; Moriya, H.; Takahashi, K. Distribution and immunocytochemical characterization of dorsal root ganglion neurons innervating the lumbar intervertebral disc in rats: a review. Life Sci. 2004, 74, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Freemont, A.J.; Peacock, T.E.; Goupille, P.; Hoyland, J.A.; O’Brien, J.; Jayson, M.I. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet 1997, 350, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Kayama, S.; Konno, S.; Olmarker, K.; Yabuki, S.; Kikuchi, S. Incision of the anulus fibrosus induces nerve root morphologic, vascular, and functional changes. An experimental study. Spine 1996, 21, 2539–2543. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Aoki, Y.; Ohtori, S. Resolving discogenic pain. Eur. Spine J. 2008, 17, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, H.; O'Brien, J.P.; Smith, W.T.; Trumper, M. The neuropathology of intervertebral discs removed for back pain. J. Pathol. 1980, 132, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Akeda, K.; An, H.S.; Aoki, Y.; Pichika, R.; Muehleman, C.; Kimura, T.; Masuda, K. Proinflammatory cytokines stimulate the expression of nerve growth factor by human intervertebral disc cells. Spine 2007, 32, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Doita, M.; Kanatani, T.; Harada, T.; Mizuno, K. Immunohistologic study of the ruptured intervertebral disc of the lumbar spine. Spine 1996, 21, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Inoue, G.; Ohtori, S.; Aoki, Y.; Ozawa, T.; Doya, H.; Saito, T.; Ito, T.; Akazawa, T.; Moriya, H.; Takahashi, K. Exposure of the nucleus pulposus to the outside of the anulus fibrosis induces nerve injury and regeneration of the afferent fibers innervating the lumbar intervertebral discs in rats. Spine 2006, 31, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Takahashi, K.; Ohtori, S.; Moriya, H. Neuropathology of discogenic low back pain: a review. Int. J. Spine Surg. 2005, 2, 1–21. [Google Scholar]
- Peng, B.; Wu, W.; Li, Z.; Guo, J.; Wang, X. Chemical radiculitis. Pain 2007, 127, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Waxman, S.G. The molecular pathophysiology of pain: abnormal expression of sodium channel genes and its contributions to hyperexcitability of primary sensory neurons. Pain 1999, Suppl 6, S133–S140. [Google Scholar] [CrossRef]
- Waxman, S.G.; Cummins, T.R.; Dib-Hajj, S.; Fjell, J.; Black, J.A. Sodium channels, excitability of primary sensory neurons, and the molecular basis of pain. Muscle Nerve 1999, 22, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Brisby, H. Pathology and possible mechanisms of nervous system response to disc degeneration. J. Bone Joint Surg. 2006, 88 (Suppl. 2), 68–71. [Google Scholar] [CrossRef] [PubMed]
- Olmarker, K.; Rydevik, B. Pathophysiology of sciatica. Orthoped. Clin. N. Amer. 1991, 22, 223–234. [Google Scholar]
- Olmarker, K.; Rydevik, B.; Nordborg, C. Autologous nucleus pulposus induces neurophysiologic and histologic changes in porcine cauda equina nerve roots. Spine 1993, 18, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Olmarker, K.; Blomquist, J.; Strömberg, J.; Nannmark, U.; Thomsen, P.; Rydevik, B. Inflammatogenic properties of nucleus pulposus. Spine 1995, 20, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Olmarker, K.; Nordborg, C.; Larsson, K.; Rydevik, B. Ultrastructural changes in spinal nerve roots induced by autologous nucleus pulposus. Spine 1996, 21, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Olmarker, K. and Larsson, K. Tumor necrosis factor alpha and nucleus-pulposus-induced nerve root injury. Spine 1998, 23, 2538–2544. [Google Scholar] [CrossRef] [PubMed]
- Olmarker, K.; Myers, R.R. Pathogenesis of sciatic pain: Role of herniated nucleus pulposus and deformation of spinal nerve root and dorsal root ganglion. Pain 1998, 78, 99–100. [Google Scholar] [CrossRef] [PubMed]
- Olmarker, K.; Rydevik, B. Selective inhibition of tumor necrosis factor-alpha prevents nucleus pulposus-induced thrombus formation, intraneural edema, and reduction of nerve conduction velocity: possible implications for future pharmacologic treatment strategies of sciatica. Spine 2001, 26, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Olmarker, K.; Storkson, R.; Berge, O. Pathogenesis of sciatic pain: A study of spontaneous behavior in rats exposed to experimental disc herniation. Spine 2002, 27, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A. Aging and degeneration of the human intervertebral disc. Spine 1995, 20, 1307–1314. [Google Scholar] [PubMed]
- Brown, M.F.; Hukkanen, M.V.J.; Hukkanen, M.V.J; McCarthy, I.D.; Redfern, D.R.M.; Batten, J.J.; Crock, H.V.; Hughes, S.P.F.; Polak, J.M. Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J. Bone Joint Surg. Br. 1997, 79B, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Ohtori, S.; Inoue, G.; Ito, T.; Kosi, T.; Ozawa, T.; Doya, H.; Saito, T.; Moriya, H.; Takahashi, K. Tumor necrosis factor-immunoreactive cells and PGP 9.5-immunoreactive nerve fibers in vertebral endplates of patients with discogenic low back Pain and Modic Type 1 or Type 2 changes on MRI. Spine 2006, 31, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Bogduk, N. International Spinal Injection Society guidelines for the performance of spinal injection procedures. Part 1: Zygapophysial joint blocks. Clin. J. Pain 1997, 13, 285–302. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Takahashi, K.; Takahashi, Y.; Morinaga, T.; Shimada, Y.; Moriya, H. Origin of nerves supplying the posterior portion of lumbar intervertebral discs in rats. Spine 1996, 21, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Coppes, M.H.; Marani, E.; Thomeer, R.T.; Groen, G.J. Innervation of “painful” lumbar discs. Spine 1997, 22, 2342–2350. [Google Scholar] [CrossRef] [PubMed]
- Lotz, J.C.; Ulrich, J.A. Innervation, inflammation, and hypermobility may characterize pathologic disc degeneration: review of animal model data. J. Bone Joint Surg. Am. 2006, 88, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Herlihy, W.F. The sinuvertebral nerve. N. Z. Med. J. 1949, 48, 214–216. [Google Scholar] [PubMed]
- Coppes, M.H.; Marani, E.; Thomeer, R.T.; Oudega, M.; Groen, G.J. Innervation of annulus fibrosis in low back pain. Lancet 1990, 336, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Gillette, R.G.; Kramis, R.C.; Roberts, W.J. Sympathetic activation of cat spinal neurons responsive to noxious stimulation of deep tissues in the low back. Pain 1994, 56, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Ness, T.J.; Gebhart, G. Visceral pain: a review of experimental studies. Pain 1990, 41, 167–234. [Google Scholar] [CrossRef] [PubMed]
- Suseki, K.; Takahashi, Y.; Takahashi, K.; Chiba, T.; Tanaka, K.; Moriya, H. CGRP-immunoreactive nerve fibers projecting to lumbar facet joints through the paravertebral sympathetic trunk in rats. Neurosci. Lett. 1996, 221, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Bogduk, N.; Tynan, W.; Wilson, A.S. The nerve supply to the human lumbar intervertebral discs. J. Anat. 1981, 132, 39–56. [Google Scholar] [PubMed]
- Jackson, H.C., 2nd; Winkelmann, R.K.; Bickel, W.H. Nerve endings in the human lumbar spinal column and related structures. J. Bone Joint Surg. Am. 1966, 48, 1272–1281. [Google Scholar] [PubMed]
- Ohtori, S.; Inoue, G.; Koshi, T.; Ito, T.; Doya, H.; Moriya, H.; Takahashi, K. Substance P-saporin down-regulates substance P receptor immunoreactive sensory dorsal root ganglion neurons innervating the lumbar intervertebral discs in rats. Spine 2006, 31, 2987–2991. [Google Scholar] [CrossRef] [PubMed]
- Ashton, I.K.; Roberts, S.; Jaffray, D.C.; Polak, J.M.; Eisenstein, S.M. Neuropeptides in the human intervertebral disc. J. Orthop. Res. 1994, 12, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.G.; Winegarner, F.G. Causalgia. A review of twenty-eight treated cases. Am. J. Surg. 1969, 117, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Carlton, S.M.; Lekan, H.A.; Kim, S.H.; Chung, J.M. Behavioral manifestations of an experimental model for peripheral neuropathy produced by spinal nerve ligation in the primate. Pain 1994, 56, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Tahmoush, A.J. Causalgia: redefinition as a clinical pain syndrome. Pain 1981, 10, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G.J. Animal model of neuropathic pain: a review. Muscle Nerve 1993, 10, 1040–1048. [Google Scholar] [CrossRef]
- Meller, S.T.; Gebhart, G.F.; Maves, T.J. Neonatal capsaicin treatment prevents the development of the thermal hyperalgesia produced in a model of neuropathic pain in the rat. Pain 1992, 51, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Averill, S.; McMahon, S.B.; Clary, D.O.; Reichardt, L.F.; Priestly, J.V. Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur. J. Neurosci. 1995, 1484–1494. [Google Scholar] [CrossRef]
- Palmgren, T.; Gronblad, M.; Seitsalo, S.; Ruuskanen, M.; Karaharju, E. Immunohistochemical demonstration of sensory and autonomic nerve terminals in herniated lumbar disc tissue. Spine 1996, 21, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Palmgren, T.; Grönblad, M.; Virri, J.; Kääpä, E; Karaharju, E. An immunohistochemical study of nerve structures in the anulus fibrosus of human normal lumbar intervertebral discs. Spine 1999, 24, 2075–2079. [Google Scholar] [CrossRef] [PubMed]
- Nachemson, A.L. Disc Pressure Measurements. Spine 1981, 6, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Takebayashi, T.; Cavanaugh, J.M.; Kallakuri, S.; Chen, C.; Yamashita, T. Sympathetic afferent units from lumbar intervertebral discs. J. Bone Joint Surg. Br. 2006, 88, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Grönblad, M.; Virri, J.; Tolonen, J.; Seitsalo, S.; Kääpä, E.; Kankare, J.; Myllynen, P.; Karaharju, E.O. A controlled immunohistochemical study of inflammatory cells in disc herniation tissue. Spine 1994, 19, 2744–2751. [Google Scholar] [CrossRef] [PubMed]
- O'Donnell, J.L.; O'Donnell, A.L. Prostaglandin E2 content in herniated lumbar disc disease. Spine 1996, 21, 1653–1656. [Google Scholar] [CrossRef] [PubMed]
- Saal, J.S.; Franson, R.C.; Dobrow, R.; Saal, J.A.; White, A.H.; Goldthwaite, N. High levels of inflammatory phospholipase A2 activity in lumbar disc herniations. Spine 1990, 15, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, K.M.; Swift, J.Q.; Roszkowski, M.T.; Bowles, W.; Garry, M.G.; Jackson, D.L. Pharmacology of peripheral neuropeptide and inflammatory mediator release. Oral Surg. Oral Med. Oral Pathol. 1994, 78, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Echlin, F. MD. Pain responses on stimulation of the lumbar sympathetic chain under local anesthesia; a case report. J. Neurosurg. 1949, 6, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Elves, M.W.; Bucknill, T.; Sullivan, M.F. In vitro inhibition of leukocyte migration in patients with intervertebral disc lesions. Orthop. Clin. North. Am. 1975, 6, 59–65. [Google Scholar] [PubMed]
- Gertzbein, S.D.; Tile, M.; Gross, A.; Falk, R. Autoimmunity in degenerative disc disease of the lumbar spine. Orthop. Clin. North Am. 1975, 6, 67–73. [Google Scholar] [PubMed]
- Takenaka, Y.; Kahan, A.; Amor, B. Experimental autoimmune spondylodiscitis in rats. J. Rheumatol. 1986, 13, 397–400. [Google Scholar] [PubMed]
- Warner, S.J.; Libby, P. Human vascular smooth muscle cells. Target for and source of tumor necrosis factor. J. Immunol. 1989, 142, 100–109. [Google Scholar] [PubMed]
- Cohen, S.P.; Larkin, T.M.; Barna, S.A.; Palmer, W.E.; Hecht, A.C.; Stojanovic, M.P. Lumbar discography: a comprehensive review of outcome studies, diagnostic accuracy, and principles. Reg. Anesth. Pain Med. 2005, 30, 163–813. [Google Scholar] [PubMed]
- Korecki, C.L.; Costi, J.J.; Iatridis, J.C. Needle puncture injury affects intervertebral disc mechanics and biology in an organ culture model. Spine 2008, 33, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T.; Ohtori, S.; Inoue, G.; Aoki, Y.; Moriya, H.; Takahashi, K. The degenerated lumbar intervertebral disc is innervated primarily by peptide-containing sensory nerve fibers in humans. Spine 2006, 31, 2418–2422. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, M.; Weinstein, J.N.; Chantani, K.; Spratt, K.F.; Meller, S.T.; Gebhart, G.F. Experimental lumbar radiculopathy. Behavioral and histologic changes in a model of radicular pain after spinal nerve root irritation with chromic gut ligatures in the rat. Spine 1994, 19, 1795–1802. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, M.; Weinstein, J.N.; Spratt, K.F.; Chantani, K.; Traub, R.J.; Meller, S.T.; Gebhart, G.F. Experimental lumbar radiculopathy. Immunohistochemical and quantitative demonstrations of pain induced by lumbar nerve root irritation of the rat. Spine 1994, 19, 1780–1794. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.; Claverie, W.; Gibson, S. The pain of discography. Spine 1988, 13, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Eisenstein, S.M.; Menage, J.; Evans, E.H.; Ashton, I.K. Mechanoreceptors in intervertebral discs. Morphology, distribution, and neuropeptides. Spine 1995, 20, 2645–2651. [Google Scholar] [CrossRef] [PubMed]
- Morinaga, T.; Takahashi, K.; Yamagata, M.; Chiba, T.; Tanaka, K.; Takahashi, Y.; Nakamura, S.; Suseki, K.; Moriya, H. Sensory innervation to the anterior portion of lumbar intervertebral disc. Spine 1996, 21, 1848–1851. [Google Scholar] [CrossRef] [PubMed]
- Franson, R.; Saal, J.S.; Saal, J.A. Human disc phospholipase A2 is inflammatory. Spine 1992, 17, S129–S132. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Rydevik, B.; Kikuchi, S.; Olmarker, K. Local application of disc-related cytokines on spinal nerve roots. Spine 2002, 27, 1614–1617. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M. Pathobiology of neuropathic pain. Eur. J. Pharmacol. 2001, 429, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Seguin, C.A.; Pilliar, R.; Roughly, P.J.; Kandel, R.A. Tumor necrosis factor-alpha modulates matrix production and catabolism in nucleus pulposus tissue. Spine 2005, 30, 1940–1948. [Google Scholar] [CrossRef] [PubMed]
- Tobinick, E.L.; Britschgi-Davoodifar, S. Perispinal TNF-alpha inhibition for discogenic pain. Swiss Med. Wkly. 2003, 133, 170–177. [Google Scholar] [PubMed]
- Zanella, J.M.; Burright, E.N.; Hildebrand, K.; Hobot, C.; Cox, M.; Christoferson, L.; McKay, W.F. Effect of etanercept, a tumor necrosis factor-alpha inhibitor, on neuropathic pain in the rat chronic constriction injury model. Spine 2008, 33, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Hao, S.; Hou, S.; Wu, W.; Jiang, D.; Fu, X.; Yang, Y. Possible pathogenesis of painful intervertebral disc degeneration. Spine 2006, 31, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Saxler, G.; Brankamp, J.; von Knoch, M.; Löer, F.; Hilken, G.; Hanesch, U. The density of nociceptive SP- and CGRP-immunopositive nerve fibers in the dura mater lumbalis of rats is enhanced after laminectomy, even after application of autologous fat grafts. Eur. Spine J. 2008, 17, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Kosharskyy, B.; Rozen, D. Lumbar discogenic pain. Disk degeneration and minimally invasive interventional therapies. Anasthesiol. Intensivmed Notfallmed Schmerzther 2007, 42, 262–267. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Revel, M.; Loty, B. A quantitative model of post-laminectomy scar formation. Spine 1995, 20, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Doita, M.; Kanatani, T.; Ozaki, T.; Matsui, N.; Kurosaka, M.; Yoshiya, S. Influence of macrophage infiltration of herniated disc tissue on the production of matrix metalloproteinases leading to disc resorption. Spine 2001, 26, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, M.D.; DeLeo, J.A. The Role of Cytokines in the Initiation and Maintenance of Chronic Pain. Drug News Perspect. 2002, 15, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. The biology of interleukin 1 and comparison to tumor necrosis factor. Immunol. Lett. 1987, 16, 227–231. [Google Scholar]
- Rotshenker, S.; Aamar, S.; Barak, V. Interleukin-1 activity in lesioned peripheral nerve. J. Neuroimmunol. 1992, 39, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B.; Greenwald, D.; Hulmes, J.D.; Chang, M.; Pan, Y.C.E.; Mathixon, J.; Ulevitch, R.; Cermai, A. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature 1985, 316, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Palmer, R.M.J.; Higgs, E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar] [PubMed]
- Carlsson, C.A.; Thulin, C.A. Regeneration of feline dorsal roots. Cell. Mol. Life Sci. 1967, 23, 125–126. [Google Scholar] [CrossRef]
- Dayer, J.M.; Graham, R.; Russell, G.; Krance, S.M. Collagenase production by rhematoid synovial cells: stimulation by a human lymphocyte factor. Science 1977, 195, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Essick, C.R. Formation of macrophages by the cells lining the subarachnoid cavity in response to the stimulus of particulate matter. Carnegie Contr. Embry 1920, 42, 379–389. [Google Scholar]
- Lagunoff, D. The mechanism of histamine release from mast cells. Biochem. Pharmacol. 1972, 21, 1889–1896. [Google Scholar] [CrossRef] [PubMed]
- Haro, H.; Kato, T.; Komori, H.; Osada, M.; Shinomiya, K. Vascular endothelial growth factor (VEGF)-induced angiogenesis in herniated disc resorption. J. Orthop. Res. 2002, 20, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Suguro, T.; Okazima, Y.; Motegi, M.; Okada, Y.; Kakiuchi, T. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine 1996, 21, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.S.; Kikuchi, S.; Shubayev, V.; Myers, R.R. 2000 Volvo Award winner in basic science studies: Exogenous tumor necrosis factor-alpha mimics nucleus pulposus-induced neuropathology. Molecular, histologic, and behavioral comparisons in rats. Spine 2000, 25, 2975–2980. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, M.; Kikuchi, S.; Myers, R.R. Experimental spinal stenosis: relationship between degree of cauda equina compression, neuropathology, and pain. Spine 2004, 29, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, A.; Kikuchi, S.; Konno, S.; Olmarker, K. Inflammatory cytokines released from the facet joint tissue in degenerative lumbar spinal disorders. Spine 2004, 29, 2091–2095. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, Y.; Ohtori, S.; Miyagi, M.; Ishikawa, T.; Inoue, G.; Doya, H.; Koshi, T.; Ito, T.; Yamashita, M.; Yamauchi, K.; et al. Up-regulation of p55 TNF alpha-receptor in dorsal root ganglia neurons following lumbar facet joint injury in rats. Eur. Spine J. 2007, 16, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Onda, A.; Hamba, M.; Yabuki, S.; Kikuchi, S. Exogenous tumor necrosis factor-alpha induces abnormal discharges in rat dorsal horn neurons. Spine 2002, 27, 1618–1624. [Google Scholar] [CrossRef] [PubMed]
- Weiler, C.; Nerlich, A.; Bachmeier, B.E.; Boos, N. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs; a study in surgical specimen and autopsy controls. Spine 2004, 30, 44–54. [Google Scholar]
- Nerlich, A.G.; Weiler, C.; Zipperer, J.; Narozny, M.; Boos, N. Immunolocalization of phagocytic cells in normal and degenerated intervertebral discs. Spine 2002, 27, 2484–2490. [Google Scholar] [CrossRef] [PubMed]
- Weiler, C.; Nerlich, A.G.; Zipperer, J.; Bachmeier, B.E.; Boos, N. 2002 SSE Award Competition in Basic Science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur. Spine J. 2002, 11, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, H.; Sato, T.; Sasaki, H.; Tanaka, Y. Discogenic pain in acute nonspecific low-back pain. Eur. Spine J. 2005, 14, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Moneta, G.B.; Videman, T.; Kaivanto, K.; Aprill, C.; Spivey, M.; Vanharanta, H.; Sachs, B.L.; Guyer, R.D.; Hochsculer, S.H.; Raschbaum, R.F.; Mooney, V. Reported pain during lumbar discography as a function of annular ruptures and disc degeneration. A re-analysis of 833 discograms. Spine 1994, 19, 1968–1974. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.B.; Rydevik, B.; Takahashi, K.; Larsson, K.; Olmarker, K. Incision of the intervertebral disc induces disintegration and increases permeability of the dorsal root ganglion capsule. Spine 2005, 30, 1712–1716. [Google Scholar] [CrossRef] [PubMed]
- Kayama, S.; Olmarker, K.; Larsson, K.; Sjögren-Jansson, E.; Lindahl, A.; Rydevik, B. Cultured, autologous nucleus pulposus cells induce functional changes in spinal nerve roots. Spine 1998, 23, 2155–2158. [Google Scholar] [CrossRef] [PubMed]
- Takebayashi, T.; Cavanaugh, J.M.; Ozaktay, A.C.; Kallakuri, S.; Chen, C. Effect of nucleus pulposus on the neural activity of dorsal root ganglion. Spine 2001, 26, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, S.; Kawaguchi, Y.; Nordborg, C.; Kikuchi, S.; Rydevik, B.; Olmarker, K. Effects of lidocaine on nucleus pulposus-induced nerve root injury. A neurophysiologic and histologic study of the pig cauda equina. Spine 1998, 23, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, S.; Kikuchi, S.; Olmarker, K.; Myers, R.R. Acute effects of nucleus pulposus on blood flow and endoneurial fluid pressure in rat dorsal root ganglia. Spine 1998, 23, 2517–2523. [Google Scholar] [CrossRef] [PubMed]
- Jou, I.M.; Tai, T.W.; Tsai, C.L.; Tsai, T.M.; Yung, W.S.; Jung, Y.C. Spinal somatosensory evoked potential to evaluate neurophysiologic changes associated with postlaminotomy fibrosis: an experimental study. Spine 2007, 32, 2111–2118. [Google Scholar] [CrossRef] [PubMed]
- Emmez, H.; Kardes, O.; Dogulu, F.; Kurt, G.; Memis, L.; Baykaner, M.K. Role of antifibrotic cytokine interfern-y in the prevention of postlaminectomy peridural fibrosis in rats. Neurosurgery 2008, 62, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M.R.; Berman, B. γ interferon is the lymphokine and β interferon is the monokine responsible for inhibition of fibroblast collagen production and late but not early fibroblast proliferation. J. Exp. Med. 1985, 162, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Gillery, P.; Serpier, H.; Polette, M.; Bellon, G.; Clavel, C.; Wegrowski, Y.; Birembaut, P.; Kalis, B.; Cariou, R.; Maquart, F.X. Gamma-interferon inhibits extracellular matrix synthesis and remodeling in collagen lattice cultures of normal and scleroderma skin fibroblasts. Eur. J. Cell. Biol. 1992, 57, 244–253. [Google Scholar] [PubMed]
- Goldring, M.B.; Sandell, L.J.; Stephenson, M.L.; Krane, S.M. Immune interferon suppresses levels of procollagen mRNA and type III collagen synthesis in cultured human articular and costal chondrocytes. J. Biol. Chem. 1986, 261, 9049–9055. [Google Scholar] [PubMed]
- Granstein, R.D.; Flotte, T.J.; Amento, E.P. Interferons and collagen production. J. Invest. Dermatol. 1990, 95, S75–S80. [Google Scholar] [CrossRef]
- Jaffe, H.A.; Gao, Z.; Mori, Y.; Varga, J. Selective inhibition of collagen gene expression in fibroblasts by an interferon-gamma transgene. Exp. Lung Res. 1999, 25, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Melin, M.; Hartmann, D.J.; Magloire, H.; Falcoff, E.; Auriaylt, C.; Grimaud, J.A. Human recombinant y-interferon stimulates proliferation and inhibits collagen and fibronectin production by human dental pulp fibroblasts. Cell. Mol. Biol. 1989, 35, 97–110. [Google Scholar] [PubMed]
- Nguyen, K.D.; Hoang, A.T.; Lee, D. Transcriptional control of human Tenon's capsule fibroblast collagen synthesis in vitro by y-interferon. Invest. Ophthalmol. Vis. Sci. 1994, 35, 3064–3070. [Google Scholar] [PubMed]
- Sime, P.J.; O'Reilly, K.M. Fibrosis of the lung and other tissues: New concepts in pathogenesis and treatment. Clin. Immunol. 2001, 99, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Tredget, E.E.; Wang, R.; Shen, Q.; Scott, P.G.; Ghahary, A. Transforming growth factor-beta mRNA and protein in hypertrophic scar tissues and fibroblasts: antagonism by IFN-alpha and IFN-gamma in vitro and in vivo. J. Interferon Cytokine Res. 2000, 20, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Ziesche, R.; Block, L.H. Mechanisms of antifibrotic action of IFN y-1b in pulmonary fibrosis. Wien. Klin. Wochenschr. 2000, 112, 785–790. [Google Scholar] [PubMed]
- Fransen, P. A Prospective Randomized Controlled Study to Evaluate the Use of a Synthetic Fibrosis Inhibitor in the Reduction of Low Back Pain Following Lumbar Microdiscectomy. Spine J. 2008, 8, S56–S57. [Google Scholar] [CrossRef]
- Fransen, P. Safety of carboxymethylcellulose/polyethylene oxide for the prevention of adhesions in lumbar disc herniation – consecutive case series review. Ann. Surg. Innov. Res. 2008, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Atlas, S.J.; Keller, R.B.; Chang, Y.; Deyo, R.A.; Singer, D.E. Surgical and nonsurgical management of sciatica secondary to a lumbar disc herniation: five-year outcomes from the Maine Lumbar Spine Study. Spine 2001, 26, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- North, R.B.; Ewend, M.G.; Lawton, M.T.; Kidd, D.H.; Piantadosi, S. Failed back surgery syndrome: 5-year follow-up after spinal cord stimulator implantation. Neurosurgery 1991, 28, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Vogelsang, J.P.; Finkenstaedt, M.; Vogelsang, M.; Markakis, E. Recurrent pain after lumbar discectomy: the diagnostic value of peridural scar on MRI. Eur. Spine J. 1999, 8, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Coskun, E.; Süzer, T.; Topuz, O.; Zencir, M.; Pakdemirli, E.; Tahta, K. Relationships between epidural fibrosis, pain, disability, and psychological factors after lumbar disc surgery. Eur. Spine J. 2000, 9, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Ido, K.; Urushidani, H. Fibrous adhesive entrapment of lumbosacral nerve roots as a cause of sciatica. Spinal Cord 2001, 39, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Simons, J. Reduction of radiculopathy using MediShield anti-adhesion gel in spinal surgery. In the Congress of Neurological Surgeons, San Francisco, CA, USA, October 2004.
- Liu, L.S.; Berg, R.A. Adhesion barriers of carboxymethylcellulose and polyethylene oxide composite gels. J. Biomed. Mater. Res. Appl. Biomater. 2002, 63, 326–332. [Google Scholar] [CrossRef]
- Madsen, F.; Eberth, K.; Smart, J.D. A rheological examination of the mucoadhesive/mucus interaction: the effect of mucoadhesive type and concentration. J. Control Release 1998, 50, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Akeson, W.H.; Massie, J.B.; Huang, B.; Giurea, A.; Sah, R.; Garfin, S.R.; Kim, C.W. Topical high-molecular-weight hyaluronan and a roofing barrier sheet equally inhibit postlaminectomy fibrosis. Spine J. 2005, 5, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Haro, H.; Komori, H.; Shinomiya, K. Evaluation of hyaluronic acid sheet for the prevention of postlaminectomy adhesions. Spine J. 2005, 5, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Songer, M.N.; Ghosh, L.; Spencer, D.L. Effects of sodium hyaluronate on peridural fibrosis after lumbar laminotomy and discectomy. Spine 1990, 15, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Songer, M.N.; Rauschning, W.; Carson, E.W.; Pandit, S.M. Analysis of peridural scar formation and its prevention after lumbar laminotomy and discectomy in canines. Spine 1995, 20, 571–80. [Google Scholar] [CrossRef]
- Tatsui, C.E.; Martinez, G.; Li, X.; Pattany, P.; Levi, A.D. Evaluation of Duragen in preventing peridural fibrosis. J. Neurosurg. Spine 2006, 4, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Falcone, S.J.; Palmeri, D.M.; Berg, R.A. Biomedical Applications of Hyaluronic Acid. In Polysaccharides for Drug Delivery and Pharmaceutical Applications; Marchessault, R.H., Ravenelle, F., Zhu, X.X., Eds.; American Chemical Society: Washington, DC, USA, 2006; Volume 934, pp. 155–174. [Google Scholar]
- Falcone, S.J.; Palmeri, D.M.; Berg, R.A. Rheological and cohesive properties of hyaluronic acid. J. Biomed. Mater. Res. 2006, 76A, 721–728. [Google Scholar] [CrossRef]
- Assietti, R.; Mora, A.; Brayda-Bruno, M. Use of carboxymethylcellulose/polyethylene oxide gel in microdiscectomy with interlaminectomy: a case series comparison with long-term follow-up. Spine 2008, 33, 1762–1765. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.D.; Wang, J.C.; Roberston, D.P.; Brodke, D.S.; BenDebba, M.; Block, K.M.; diZerega, G.S. Reduction in leg pain and lower-extremity weakness with Oxiplex/SP Gel for 1 year after laminectomy, laminotomy, and discectomy. Neurosurg. Focus 2004, 17, 1–6. [Google Scholar] [CrossRef]
- Kim, K.D.; Wang, J.C.; Robertson, D.P.; Brodke, D.S.; Olson, E.M.; Duberg, A.C.; BenDebba, M.; Block, K.M.; diZerega, G.S. Reduction of radiculopathy and pain with Oxiplex/SP Gel after laminectomy, laminotomy, and discectomy: A pilot clinical study. Spine 2003, 28, 1080–1088. [Google Scholar] [PubMed]
- Rhyne, A.L.; Blumenthol, S.L.; Frank, E.H.; Hsu, K.Y.; Kim, K.D.; Youssef, J.A.; Wang, J.C.; Arnold, P.; BenDebba, M.; Block, K.M.; et al. Oxiplex reduces leg pain, back pain and associated symptoms 6 months following single-level lower lumbar laminectomy for removal of a herniated disc. Spine 2009. submitted . [Google Scholar]
- Zuki, Z. The use of anti-adhesion gel (carboxymethylcellulose+polyethylene oxide) after spinal decompression surgery. Malaysia Orthopedic Association: Selangor, Malaysia, 2006. [Google Scholar]
- Mortazavi, S.A.; Smart, J.D. An investigation of some factors influencing the in vitro assessment of mucoadhesion. Itnl. J. Pharm. 1995, 116, 223–230. [Google Scholar]
- Rossi, S.; Bonferoni, M.C.; Lippoli, G.; Bertoni, M.; Ferrari, F.; Caramella, C.; Conte, U. Influence of mucin type on polymer-mucin rheological interactions. Biomaterials 1995, 16, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Atha, D.H.; Ingham, K.C. Mechanism of precipitation of proteins by polyethylene glycols. Analysis in terms of excluded volume. J. Biol. Chem. 1981, 256, 12108–12117. [Google Scholar] [PubMed]
- Gombotz, W.R.; Wang, G.H.; Horbett, T.A.; Hoffman, A.S. Protein adsorption to poly(ethylene oxide) surfaces. J. Biomed. Mater. Res. 1991, 25, 1547–1562. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.G. (Ed.) Protein Purification Process Engineering; Marcel Decker: New York, NY, USA, 1993; pp. 115–208.
- Lee, J.H.; Kopecek, J.; Andrade, J.D. Protein-resistant surfaces prepared by PEO-containing block copolymer surfactants. J. Biomed. Mater. Res. 1989, 23, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Paleg, L.G.; Stewart, G.R.; Bradbeer, J.W. Proline and Glycine Betaine Influence Protein Solvation. Plant Physiol. 1984, 75, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Kurt, G.; Cemil, B.; Celik, B.; Durdag, E.; Erdem, O.; Ceviker, N. Comparison of Oxiplex and Gore-Tex effectivity in an experimental peridural fibrosis model. Neurocirugía 2009, 20, 360–366. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rodgers, K.E.; Robertson, J.T.; Espinoza, T.; Oppelt, W.; Cortese, S.; diZerega, G.S.; Berg, R.A. Reduction of epidural fibrosis in lumbar surgery with Oxiplex adhesion barriers of carboxymethylcellulose and polyethylene oxide. Spine J. 2003, 3, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Mulleman, D.; Mammou, S.; Griffoul, I.; Watier, H.; Goupille, P. Pathophysiology of disk-related sciatica. I. Evidence supporting chemical component. Joint Bone Spine 2006, 73, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Mulleman, D.; Mammou, S.; Griffoul, I.; Watier, H.; Goupille, P. Pathophysiology of disk-related low back pain and sciatica. II. Evidence supporting treatment with TNF-alpha antagonists. Joint Bone Spine 2006, 73, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Spangfort, E.V. The lumbar disc herniation. A computer-aided analysis of 2,504 operations. Acta Orthop. Scand. 1972, 142 (Suppl.), 1–95. [Google Scholar] [CrossRef]
- Atlas, S.J.; Keller, R.B.; Wu, Y.A.; Deyo, R.A.; Singer, D.E. Long-term outcomes of surgical and nonsurgical management of sciatica secondary to a lumbar disc herniation: 10 year results from the Maine lumbar spine study. Spine 2005, 30, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, J.; Gauchat, M.H.; Valach, L. The outcome of surgery for lumbar disc herniation. I. A 4-17 years' follow-up with emphasis on somatic aspects. Spine 1988, 12, 1418–1422. [Google Scholar] [CrossRef]
- Asch, H.L.; Lewis, P.J.; Moreland, D.B.; Egnatchik, J.G.; Yu, Y.J.; Clabeaux, D.E.; Hyland, A.H. Prospective multiple outcomes study of outpatient lumbar microdiscectomy: should 75 to 80% success rates be the norm? J. Neurosurg. 2002, 96 (Suppl. 1), 34–44. [Google Scholar] [PubMed]
- Loupasis, G.A.; Stamos, K.; Katonis, P.G.; Sapkas, G.; Korres, D.S.; Hartofilakidis, G. Seven- to 20-year outcome of lumbar discectomy. Spine 1999, 24, 2313–2317. [Google Scholar] [CrossRef] [PubMed]
- Silvers, H.R.; Lewis, P.J.; Asch, H.L.; Clabeaux, D. Lumbar microdiscectomy in the elderly patient. Br. J. Neurosurg. 1997, 11, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.J.; Weir, B.K.; Broad, R.W.; Grace, M.G. Long-term prospective study of lumbosacral discectomy. J. Neurosurg. 1987, 67, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Weber, H. Lumbar disc herniation. A controlled, prospective study with ten years of observation. Spine 1983, 8, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, T.; Karppinen, J.; Paimela, L.; Malmivaara, A.; Lindgren, K.A.; Bowman, C.; Hammond, A.; Kirkham, B.; Järvinen, S.; Niinimäki, J.; et al. The treatment of disc-herniation-induced sciatica with infliximab: one-year follow-up results of FIRST II, a randomized controlled trial. Spine 2006, 31, 2759–2766. [Google Scholar] [CrossRef] [PubMed]
- Silvers, H.R.; Lewis, P.J.; Clabeaux, D.; Asch, H.L. Lumbar disc excisions in patients under the age of 21 years. Spine 1994, 19, 2387–2391. [Google Scholar] [CrossRef] [PubMed]
- Osterman, H.; Seitsalo, S.; Karppinen, J.; Malmivaara, A. Effectiveness of microdiscectomy for lumbar disc herniation A randomized controlled trial with 2 years of follow-up. Spine 2006, 31, 2409–2414. [Google Scholar] [CrossRef] [PubMed]
- Peul, W.C.; Brand, R.; Thomeer, R.T.; Koes, B.W. Influence of gender and other prognostic factors on outcome of sciatica. Pain 2008, 138, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Peul, W.C.; van Houwelingen, H.C.; van den Hout, W.B.; Brand, R.; Eekhof, J.A.H.; Tans, J.T.J.; Thomeer, R.T.W.M.; Koes, B.W. for the Leiden–The Hague Spine Intervention Prognostic Study Group. Surgery versus prolonged conservative treatment for sciatica. New Engl. J. Med. 2007, 356, 2245–2256. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.J.; O'neill, C.W.; Lotz, J.C. Glucosamine HCl alters production of inflammatory mediators by rat intervertebral disc cells in vitro. Spine J. 2007, 7, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; An, H.S.; Takahashi, K.; Miyamoto, K.; Lenz, M.E.; Moriya, H.; Masuda, K. Axonal growth potential of lumbar dorsal root ganglion neurons in an organ culture system: response of nerve growth factor-sensitive neurons to neuronal injury and an inflammatory cytokine. Spine 2007, 32, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Omoigui, S. The biochemical origin of pain--proposing a new law of pain: the origin of all pain is inflammation and the inflammatory response. Part 1 of 3--a unifying law of pain. Med. Hypotheses 2007, 69, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Yaksh, T.L.; Hua, X.Y.; Kalcheva, I.; Nozaki-Taguchi, N.; Marsala, M. The spinal biology in humans and animals of pain states generated by persistent small afferent input. Proc. Natl. Acad. Sci. USA 1999, 96, 7680–7686. [Google Scholar] [CrossRef] [PubMed]

Appendix Table 1.
Reports of patients with leg and back pain due to lumbar disc herniation.
| Author | No. of Subjects | Conclusion |
|---|---|---|
| Spangfort, E.V. | 2504 | 30% continued to have lumbar back pain; 23% continued to have sciatica following discectomy [181]. |
| Pearson, A.M.; et al. | 775 | 72% with lumbar back pain after discectomy; sciatica improved more following surgery than lumbar back pain; lumbar back pain improvement only moderately correlated with sciatica improvement [4]. |
| Atlas, S.J.; et al. | 507 | Residual lumbar back pain 1.9; residual sciatica 1.5, 10 years after disc surgery [182]. |
| Dvorak, J.; et al. | 382 | 66% continued to have significant lumbar back pain; 45% continued to have sciatica 2-5 years after discectomy [183]. |
| Asch, H.L.; et al. | 212 | Lumbar back pain relief: 77%; sciatica relief: 80% [184]. |
| Loupasis, G.A.; et al. | 109 | Good-excellent relief of lumbar back pain: 80%; good-excellent relief of sciatica: 86% [185] |
| Silvers, H.R.; et al. | 104 | Relief of lumbar back pain: 76%; relief of sciatica: 89% following discectomy [186]. |
| Lewis, P.J.; et al. | 100 | 13% had greater lumbar back pain than sciatica at baseline [187]. |
| Weber, H. | 45 | 17% of 45 subjects no lumbar back pain; 41% of 36 subjects no sciatica, 4 years after discectomy [188]. |
| Toyone, T.; et al. | 40 | Lumbar disc herniation might be a possible cause of lumbar back pain; potential sensory pathways described for discogenic pain due to disc herniation [8]. |
| Korhonen, T.; et al. | 21 | Lumbar back pain relief 51%; sciatica relief 68% following novel drug therapy [189]. |
| Silvers, H.R.; et al. | 15 | Relief of lumbar back pain: 77%; relief of sciatica: 85% following discectomy [190]. |
| Osterman; et al. | 58 | Leg pain baseline VAS score 9/100, back pain VAS score 7/100. Only leg pain superior at 6 weeks [191]. |
| Peul; et al. | 283 | Baseline VAS score; leg pain 17.7/100, back pain 11.3/100. 81% disappearance of symptoms at 8 weeks [192,193]. |

Appendix Table 2.
Biochemical pain mediators.
| Mediator | Description / Complete Name | Activity | Source / Location | Reference |
|---|---|---|---|---|
| Cytokines/Growth Factors (Signaling Substances) | ||||
| COX | Cyclooxygenase |
|
| Walsh [194] |
| FGF2 or FGFb | Fibroblast growth factor, basic |
|
| Weiler [126] |
| GAP-43 | Growth-associated protein 43 |
|
| Aoki [195] |
| GM-CSF | Granulocyte macrophage colony stimulation factor |
|
| Weiler [126] |
| IGF-1 | Insulin-like growth factor |
|
| Weiler [126] |
| IL-1 | Interleukin-1 |
|
| Ohtori [58] |
| Walsh [194] | |||
| IL-1β | Interleukin-1β |
|
| Abe [40] |
| Brisby [47] | |||
| Omoigui [196] | |||
| IL-6 | Interleukin-6 |
|
| Ohtori [58] |
| Brisby [47] | |||
| Omoigui [196] | |||
| IL-8 | Interleukin-8 |
|
| Brisby [47] |
| IL-10 | Interleukin-10 |
|
| Weiler [126] |
| Omoigui [196] | ||||
| NGF | Nerve growth factor |
|
| Abe [40] |
| NO | Nitric oxide |
|
| Walsh [194] |
| PGE2 | Prostaglandin E2 [23, 24] |
|
| Weiler [126] |
| Walsh [194] | |||
| PLA2 | Phospholipase A2 |
| Weiler [126] | |
| TGF-β | Transforming growth factor- β |
|
| Weiler [126] |
| TNF-α | Tumor necrosis factor-α |
|
| Abe [40] |
| Aoki [195] | |||
|
| Sakuma 2007 [124], Ohtori [58] | ||
|
| Weiler [126] | ||
| Neuropeptides (Ligands) | ||||
| VEGF | Vascular endothelial growth factor |
|
| Haro [119] |
| CGRP | Calcitonin-gene related peptide |
|
| Aoki [195] |
|
| Ohtori [58] | ||
| (PK) C | Protein kinase C |
| Yaksh [197] | |
| Proteoglycans |
|
| Abe [40] | |
| Neurotransmitters (Receptors) | ||||
| AMPA | Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
|
| Yaksh [197] |
| Glutamate | Glutamate |
|
| Yaksh [197] |
| NK-1 | Neurokinin-1 receptor |
|
| Ohtori [58] |
| NMDA | n-methyl-D-aspartate |
|
| Yaksh [197] |
| P (sP) | Substance P |
|
| Ohtori [70] |
| p75NGFR | p75NGFR |
|
| Abe [40] |
| TrkA | Transmembrane tyrosine kinase (“TrackA”) |
|
| Abe [40] |
Inflammation and the inflammatory response is the underlying source of leg pain and back pain. The biochemical mediators of inflammation include cytokines, growth factors, neuropeptides and neurotransmitters [196]. The following table includes biochemical inflammatory pain mediators identified in the epidural space in patients with lumbar back pain [40,58,70,124,195,196].
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).