Fluorescent Immunoassay with a Copper Polymer as the Signal Label for Catalytic Oxidation of O-Phenylenediamine
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Reagents
2.2. Voltammetric Characterization of Peptide-Cu2+
2.3. Absorption Measurement of AA
2.4. Absorption Measurement of AA
2.5. PSA Detection
3. Results and Discussion
3.1. Redox Behaviors of Cu2+ Complexes
3.2. Catalytic Activity of Cu2+ Complexes
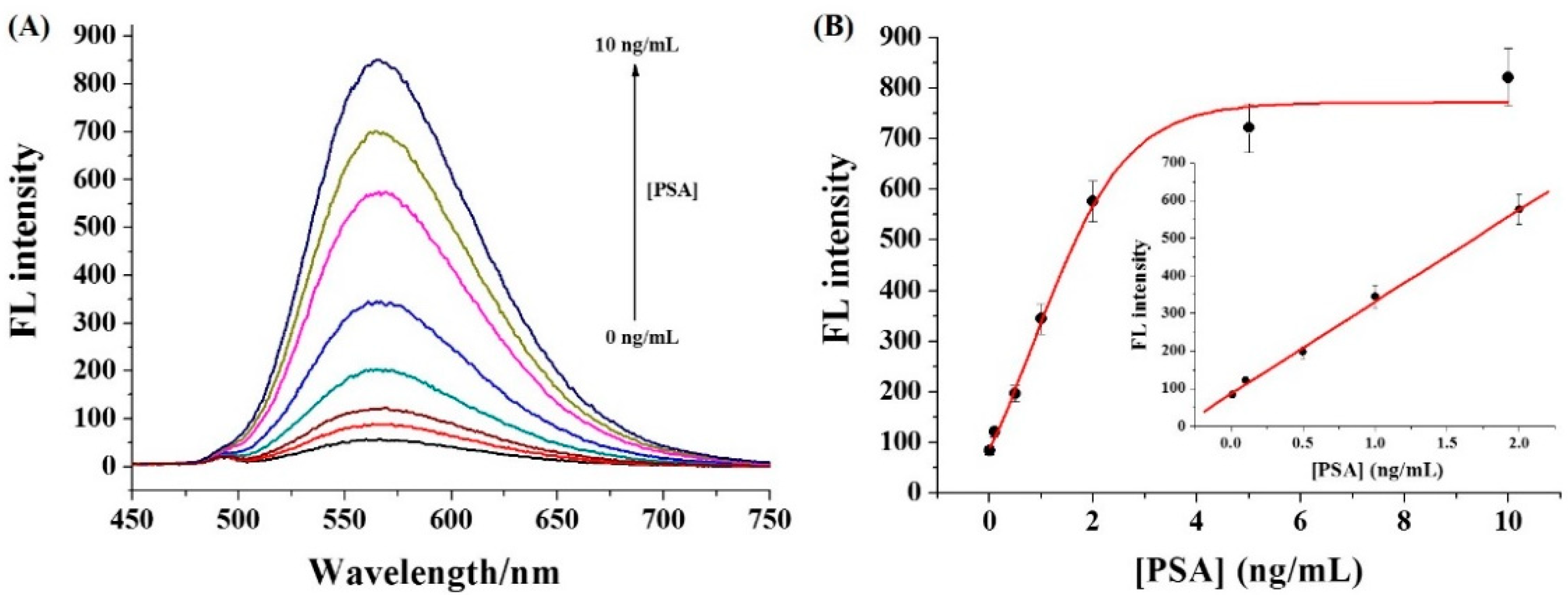
3.3. Sensitivity of Immunoassays
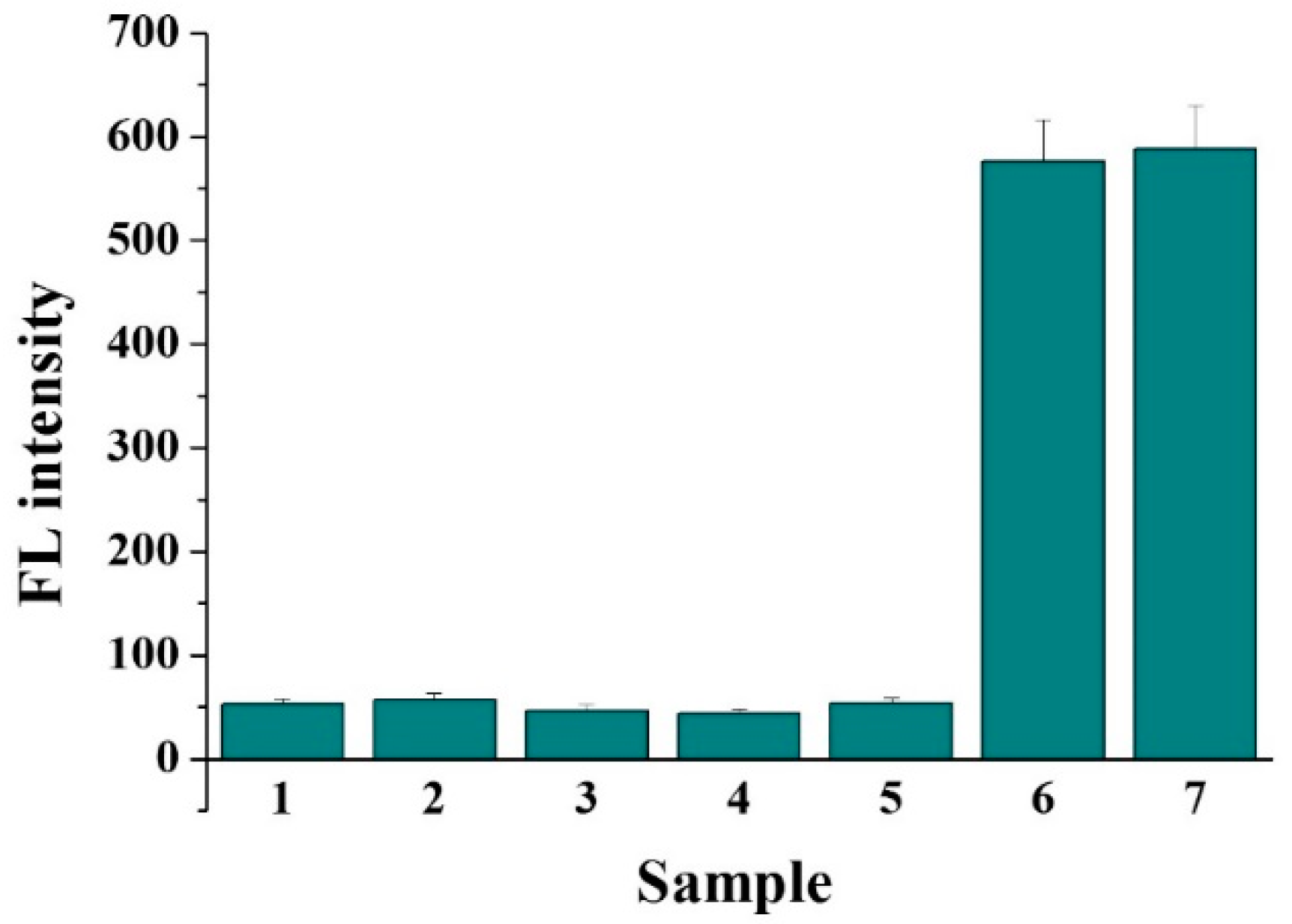
3.4. Selectivity and Real Sample Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Radha, R.; Shahzadi, S.K.; Al-Sayah, M.H. Fluorescent immunoassays for detection and quantification of cardiac troponin I: A short review. Molecules 2021, 26, 4812. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xianyu, Y. Nanobody and nanozyme-enabled immunoassays with enhanced specificity and sensitivity. Small Methods 2022, 6, 2101576. [Google Scholar] [CrossRef]
- Zhao, Q.; Lu, D.; Zhang, G.; Zhang, D.; Shi, X. Recent improvements in enzyme-linked immunosorbent assays based on nanomaterials. Talanta 2021, 223, 121722. [Google Scholar] [CrossRef]
- Gao, L.; Yang, Q.; Wu, P.; Li, F. Recent advances in nanomaterial-enhanced enzyme-linked immunosorbent assays. Analyst 2020, 145, 4069–4078. [Google Scholar] [CrossRef]
- Liang, M.; Yan, X. Nanozymes: From new concepts, mechanisms, and standards to applications. Acc. Chem. Res. 2019, 52, 2190–2200. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Tian, S.; Zeng, K.; Yang, A.; Wang, Q.; Yang, M. A copper based enzyme-free fluorescence ELISA for HER2 detection. J. Immunol. Methods 2017, 451, 78–82. [Google Scholar] [CrossRef]
- Drommi, M.; Rulmont, C.; Esmieu, C.; Hureau, C. Hybrid bis-histidine phenanthroline-based ligands to lessen Aβ-bound Cu ROS production: An illustration of Cu(I) significance. Molecules 2021, 26, 7630. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Prakash, D.; Rajabimoghadam, K.; Wawrzak, Z.; Prasad, P.; Wu, T.; Misra, S.K.; Sharp, J.S.; Garcia-Bosch, I.; Chakraborty, S. De novo design of a self-assembled artificial copper peptide that activates and reduces peroxide. ACS Catal. 2021, 11, 10267–10278. [Google Scholar] [CrossRef]
- Liang, H.; Lin, F.; Zhang, Z.; Liu, B.; Jiang, S.; Yuan, Q.; Liu, J. Multicopper laccase mimicking nanozymes with nucleotides as ligands. ACS Appl. Mater. Interfaces 2017, 9, 1352–1360. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, D.; Wu, Y.; Li, G. Bioinspired nanozyme for portable immunoassay of allergenic proteins based on A smartphone. Biosens. Bioelectron. 2021, 172, 112776. [Google Scholar] [CrossRef]
- Chen, W.-H.; Vázquez-González, M.; Kozell, A.; Cecconello, A.; Willner, I. Cu2+-modified metal–organic framework nanoparticles: A peroxidase-mimicking nanoenzyme. Small 2018, 14, 1703149. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-González, M.; Liao, W.-C.; Cazelles, R.; Wang, S.; Yu, X.; Gutkin, V.; Willner, I. Mimicking horseradish peroxidase functions using Cu2+-modified carbon nitride nanoparticles or Cu2+-modified carbon dots as heterogeneous catalysts. ACS Nano 2017, 11, 3247–3253. [Google Scholar] [CrossRef]
- Wang, S.; Cazelles, R.; Liao, W.-C.; Vázquez-González, M.; Zoabi, A.; Abu-Reziq, R.; Willner, I. Mimicking horseradish peroxidase and NADH peroxidase by heterogeneous Cu2+-modified graphene oxide nanoparticles. Nano Lett. 2017, 17, 2043–2048. [Google Scholar] [CrossRef]
- Ren, X.; Liu, J.; Ren, J.; Tang, F.; Meng, X. One-pot synthesis of active copper-containing carbon dots with laccase-like activities. Nanoscale 2015, 7, 19641–19646. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, J.; Wu, W.; Fang, Y.; Dong, S. Oxidase-like MOF-818 nanozyme with high specificity for catalysis of catechol oxidation. J. Am. Chem. Soc. 2020, 142, 15569–15574. [Google Scholar] [CrossRef]
- Wang, D.; Wu, H.; Wang, C.; Gu, L.; Chen, H.; Jana, D.; Feng, L.; Liu, J.; Wang, X.; Xu, P.; et al. Self-assembled single-site nanozyme for tumor-specific amplified cascade enzymatic therapy. Angew. Chem. Int. Ed. 2021, 60, 3001–3007. [Google Scholar] [CrossRef] [PubMed]
- Sóvágó, I.; Várnagy, K.; Lihi, N.; Grenács, Á. Coordinating properties of peptides containing histidyl residues. Coord. Chem. Rev. 2016, 327–328, 43–54. [Google Scholar] [CrossRef]
- May, N.V.; Jancsó, A.; Enyedy, É.A. Binding models of copper(II) thiosemicarbazone complexes with human serum albumin: A speciation study. Molecules 2021, 26, 2711. [Google Scholar] [CrossRef]
- Portelinha, J.; Duay, S.S.; Yu, S.I.; Heilemann, K.; Libardo, M.D.J.; Juliano, S.A.; Klassen, J.L.; Angeles-Boza, A.M. Antimicrobial peptides and copper(II) ions: Novel therapeutic opportunities. Chem. Rev. 2021, 121, 2648. [Google Scholar] [CrossRef] [PubMed]
- Frączyk, T. Cu(II)-binding N-terminal sequences of human proteins. Chem. Biodivers. 2021, 18, e2100043. [Google Scholar] [CrossRef]
- Mena, S.; Mirats, A.; Caballero, A.B.; Guirado, G.; Barrios, L.A.; Teat, S.J.; Rodriguez-Santiago, L.; Sodupe, M.; Gamez, P. Drastic effect of the peptide sequence on the copper-binding properties of tripeptides and the electrochemical behaviour of their copper(II) complexes. Chem. Eur. J. 2018, 24, 5153–5162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harford, C.; Sarkar, B. Amino terminal Cu(II)- and Ni(II)-binding (ATCUN) motif of proteins and peptides: Metal binding, DNA cleavage, and other properties. Acc. Chem. Res. 1997, 30, 123–130. [Google Scholar] [CrossRef]
- Gonzalez, P.; Bossak, K.; Stefaniak, E.; Hureau, C.; Raibaut, L.; Bal, W.; Faller, P. N-Terminal Cu-binding motifs (Xxx-Zzz-His, Xxx-His) and their derivatives: Chemistry, biology and medicinal applications. Chem. Eur. J. 2018, 24, 8029–8041. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Deng, D.; Yang, S.; Hao, Y.; Wang, L.; Liu, Y.; An, C.; Han, Q.; Liu, L. Electrochemical immunosensors with protease as the signal label for the generation of peptide-Cu(II) complexes as the electrocatalysts toward water oxidation. Sens. Actuators B Chem. 2019, 291, 113–119. [Google Scholar] [CrossRef]
- Liu, L.; Deng, D.; Wang, Y.; Song, K.; Shang, Z.; Wang, Q.; Xia, N.; Zhang, B. A colorimetric strategy for assay of protease activity based on gold nanoparticle growth controlled by ascorbic acid and Cu(II)-coordinated peptide. Sens. Actuators B Chem. 2018, 266, 246–254. [Google Scholar] [CrossRef]
- Deng, D.; Liu, L.; Bu, Y.; Liu, X.; Wang, X.; Zhang, B. Electrochemical sensing devices using ATCUN-Cu(II) complexes as electrocatalysts for water oxidation. Sens. Actuators B Chem. 2018, 269, 189–194. [Google Scholar] [CrossRef]
- Özyurt, C.; Uludağ, İ.; İnce, B.; Sezgintürk, M.K. Biosensing strategies for diagnosis of prostate specific antigen. J. Pharmaceut. Biomed. Anal. 2022, 209, 114535. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Jiang, D.; McDonald, A.; Hao, Y.; Millhauser, G.L.; Zhou, F. Copper redox cycling in the prion protein depends critically on binding mode. J. Am. Chem. Soc. 2011, 133, 12229–12237. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Li, Q.; Tammina, S.K.; Gao, Z.; Yang, Y. Cu-CDs/H2O2 system with peroxidase-like activities at neutral pH for the co-catalytic oxidation of o-phenylenediamine and inhibition of catalytic activity by Cr(III). Sens. Actuators B Chem. 2020, 319, 128273. [Google Scholar] [CrossRef]
- Sun, J.; Wang, B.; Zhao, X.; Li, Z.-J.; Yang, X. Fluorescent and colorimetric dual-readout assay for inorganic pyrophosphatase with Cu2+-triggered oxidation of ophenylenediamine. Anal. Chem. 2016, 88, 1355–1361. [Google Scholar] [CrossRef]
- Yang, X.; Wang, E. A nanoparticle autocatalytic sensor for Ag+ and Cu2+ ions in aqueous solution with high sensitivity and selectivity and its application in test paper. Anal. Chem. 2011, 83, 5005–5011. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Cao, H.; Yan, X.; Sun, J.; Qiu, J.; Qu, X.; Zuo, Y.-N.; Wang, X.; Zhao, X.-E. A convenient fluorescent assay for quinolones based on their inhibition towards the oxidase-like activity of Cu2+. New J. Chem. 2019, 43, 3707–3712. [Google Scholar] [CrossRef]
- Yang, Y.; Li, L.; Lin, L.; Wang, X.; Li, J.; Liu, H.; Liu, X.; Huo, D.; Hou, C. A dualsignal sensing strategy based on ratiometric fluorescence and colorimetry for determination of Cu2+ and glyphosate. Anal. Bioanal. Chem. 2022, 414, 2619–2628. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Liu, X.; Zhang, Y.; Liu, Y.; Wang, W.; Su, R.; Sun, Y.; Huang, Y.; Song, D.; et al. Ratiometric fluorescence and colorimetric dual-mode sensing platform based on carbon dots for detecting copper(II) ions and D-penicillamine. Anal. Bioanal. Chem. 2022, 414, 1651–1662. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Tian, M.; Luo, X.; Jiang, Y.; He, N.; Liao, X.; Liu, Y. A dual-mode fluorescent and colorimetric immunoassay based on in situ ascorbic acid-induced signal generation from metal-organic frameworks. Sens. Actuators B Chem. 2020, 302, 127180. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Hu, H.; Feng, Y.; Zhu, S.; Li, C.; Feng, N. A dual-readout sandwich immunoassay based on biocatalytic perovskite nanocrystals for detection of prostate specific antigen. Biosens. Bioelectron. 2022, 203, 113979. [Google Scholar] [CrossRef]
- Sun, C.; Shi, Y.; Tang, M.; Hu, X.; Long, Y.; Zheng, H. A signal amplification strategy for prostate specific antigen detection via releasing oxidase-mimics from coordination nanoparticles by alkaline phosphatase. Talanta 2020, 213, 120827. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, D.; Wang, B.; Ni, P.; Jiang, Y.; Zhang, C.; Yang, F.; Lu, Y.; Sun, J. Alkaline phosphatase-triggered in situ formation of silicon-containing nanoparticles for a fluorometric and colorimetric dual-channel immunoassay. Anal. Chem. 2020, 92, 4639–4646. [Google Scholar] [CrossRef]
- Hu, R.; Liu, T.; Zhang, X.-B.; Yang, Y.; Chen, T.; Wu, C.; Liu, Y.; Zhu, G.; Huan, S.; Fu, T.; et al. DLISA: A DNAzyme-based ELISA for protein enzyme-free immunoassay of multiple analytes. Anal. Chem. 2015, 87, 7746–7753. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-D.; Peng, J.; Jiang, L.-P.; Zhu, J.-J. Fluorescent immunosensor based on CuS nanoparticles for sensitive detection of cancer biomarker. Analyst 2014, 139, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gao, W.; Ge, X.; Li, S.; Du, D.; Yang, H. CdTe@SiO2 signal reporters-based fluorescent immunosensor for quantitative detection of prostate specific antigen. Anal. Chim. Acta 2019, 1057, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Li, C.-Y.; Qi, C.-B.; Chen, H.-L.; Pang, D.-W.; Tang, H.-W. Multiple optical trapping assisted bead-array based fluorescence assay of free and total prostate-specific antigen in serum. Sens. Actuators B Chem. 2018, 269, 143–150. [Google Scholar] [CrossRef]
- Pei, H.; Zhu, S.; Yang, M.; Kong, R.; Zheng, Y.; Qu, F. Graphene oxide quantum dots@silver core–shell nanocrystals as turn-on fluorescent nanoprobe for ultrasensitive detection of prostate specific antigen. Biosens. Bioelectron. 2015, 74, 909–914. [Google Scholar] [CrossRef]
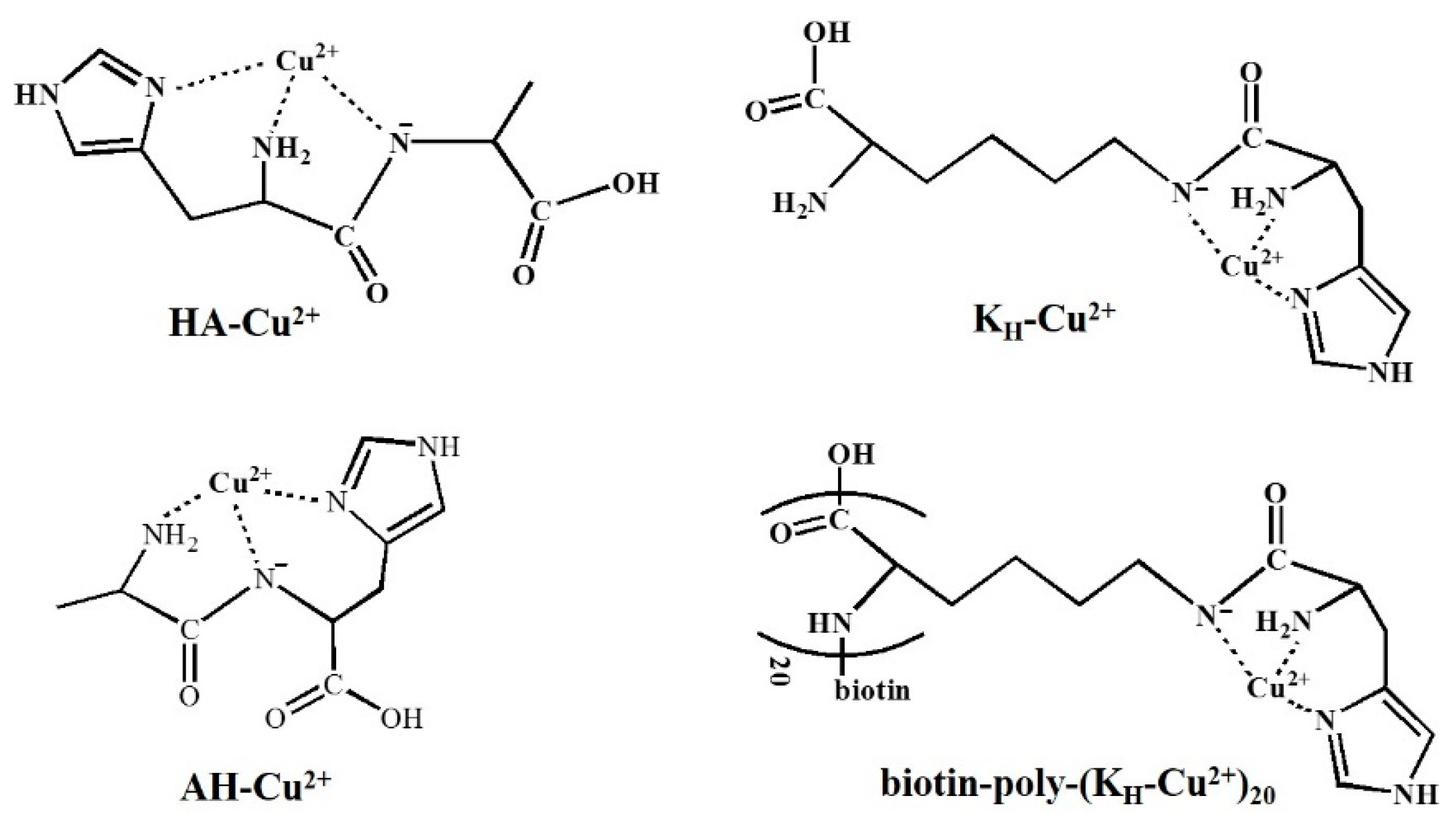
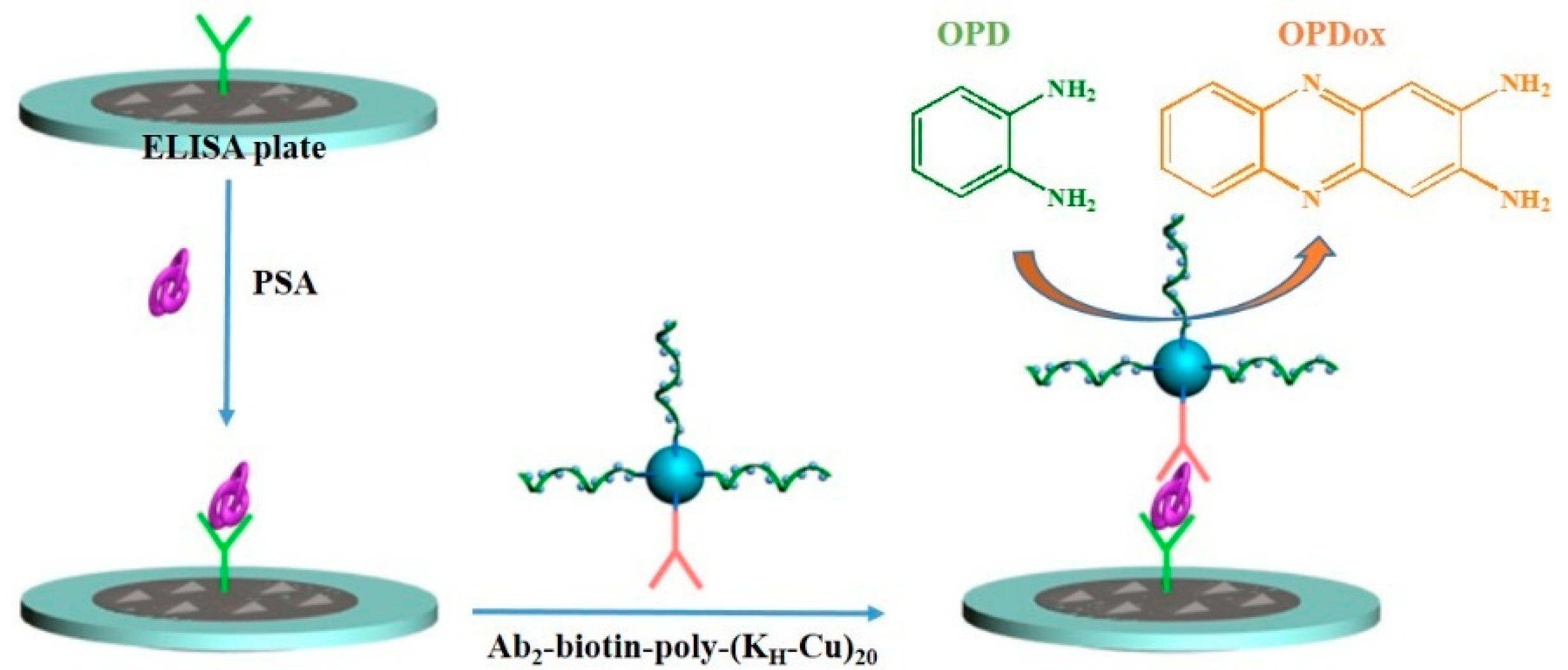
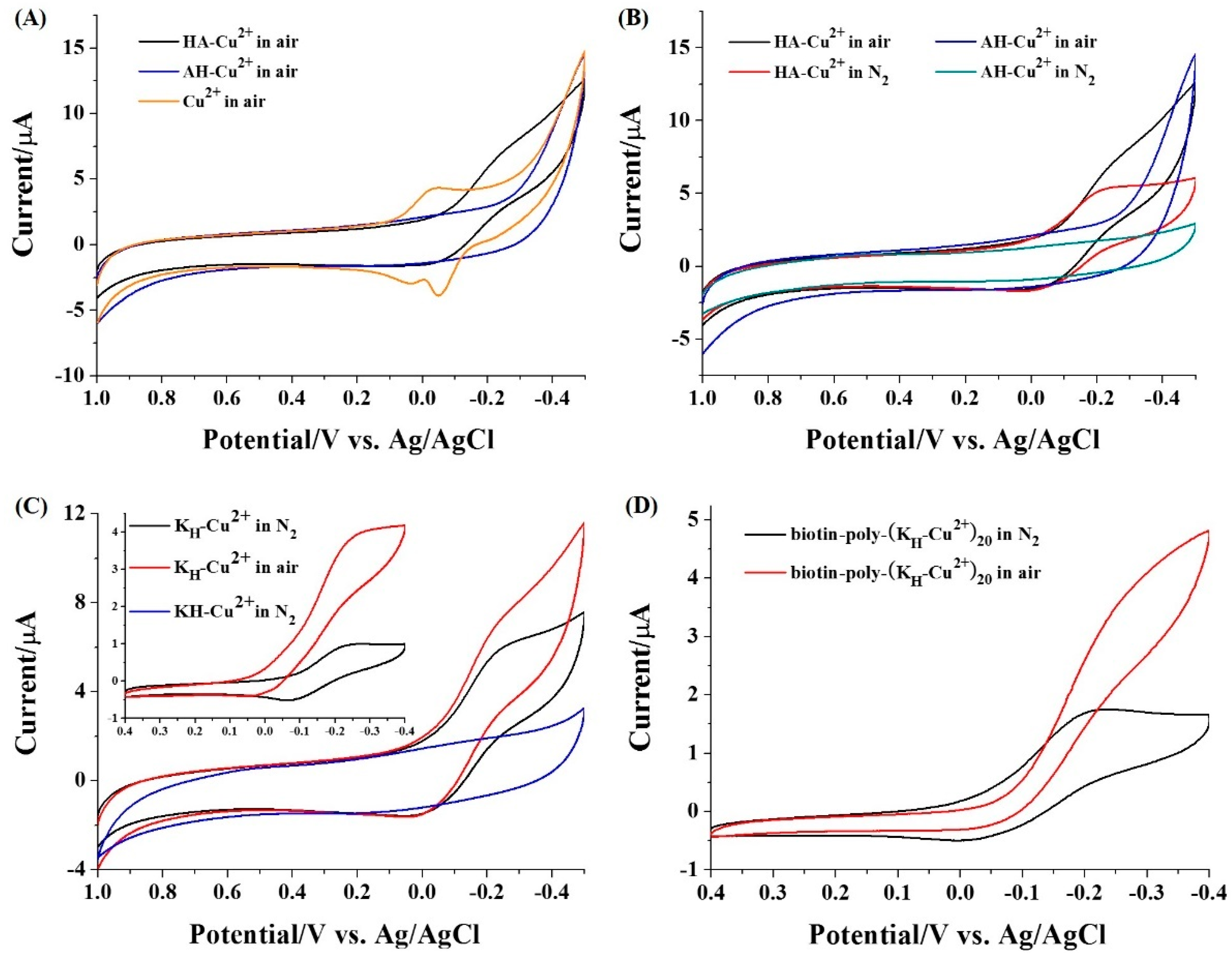
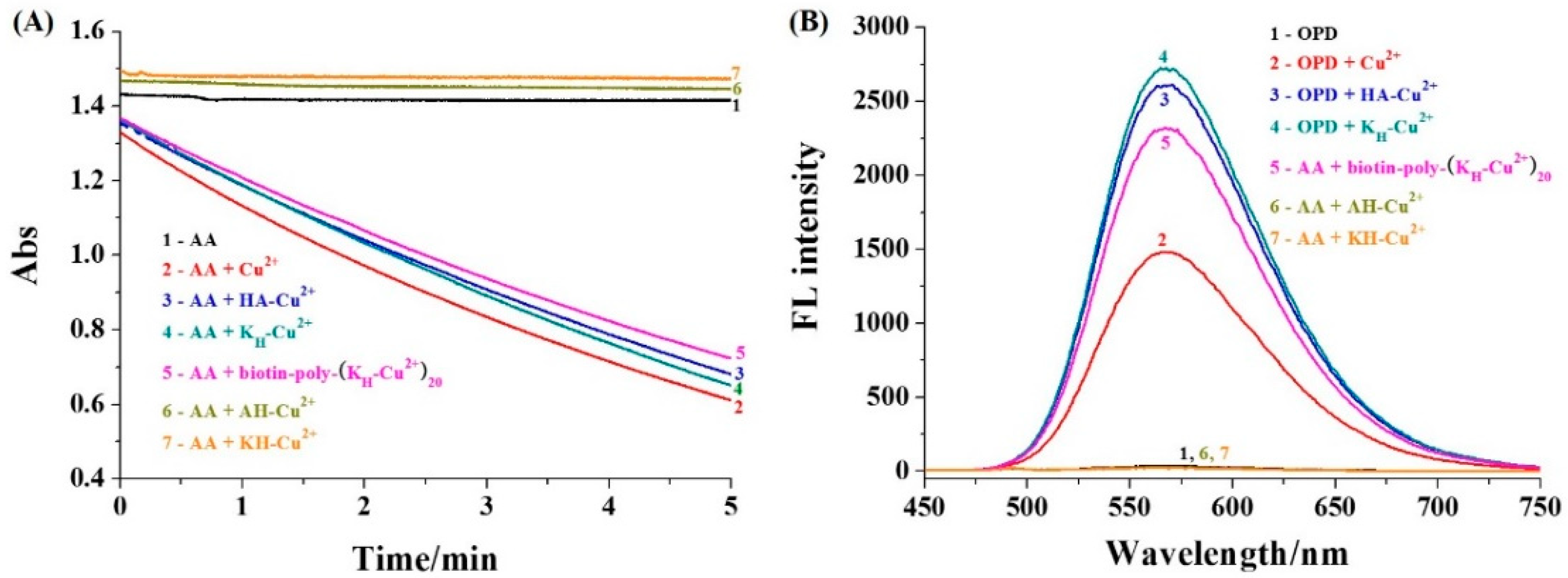
| Supporter | Signal Reporter | Linear Range (ng/mL) | Detection Limit (ng/mL) | Refs. |
|---|---|---|---|---|
| Microplate | Fe-MOFs/ALP/TMB | 1~20 | 0.18 | [36] |
| Microplate | CsPbBr3 NCs/TMB | 1 × 10−2~80 | 8.1 × 10−2 | [37] |
| Microplate | ALP/F@CNPs | 0.1~50 | 4 × 10−2 | [38] |
| Microplate | ALP/Si CNPs | 2 × 10−2~28 | 9.6 × 10−3 | [39] |
| Microplate | ZnS NCs | 1 × 10−5~1 × 103 | 5 × 10−6 | [40] |
| Microplate | CuS/OPD | 5 × 10−4~50 | 1 × 10−4 | [41] |
| Magnetic bead | CdTe@SiO2 | 1 × 10−2~5 | 3 × 10−3 | [42] |
| Magnetic bead | QDs | 1 × 10−2~40 | 2.5 × 10−5 | [43] |
| Magnetic bead | GQDs@Ag | 1 × 10−3~20 | 3 × 10−4 | [44] |
| Microplate | biotin-poly-(KH-Cu)20 | 1 × 10−2~2 | 8 × 10−3 | This work |
| Sample No. | Found (ng/mL) | ELISA (ng/mL) |
|---|---|---|
| 1 | 0.182 ± 0.012 | 0.171 ± 0.018 |
| 2 | 0.087 ± 0.007 | 0.094 ± 0.011 |
| 3 | 0.154 ± 0.013 | 0.132 ± 0.015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Liu, G.; Zhang, C.; Li, J.; Li, Y.; Liu, L. Fluorescent Immunoassay with a Copper Polymer as the Signal Label for Catalytic Oxidation of O-Phenylenediamine. Molecules 2022, 27, 3675. https://doi.org/10.3390/molecules27123675
Feng Y, Liu G, Zhang C, Li J, Li Y, Liu L. Fluorescent Immunoassay with a Copper Polymer as the Signal Label for Catalytic Oxidation of O-Phenylenediamine. Molecules. 2022; 27(12):3675. https://doi.org/10.3390/molecules27123675
Chicago/Turabian StyleFeng, Yunxiao, Gang Liu, Chunhuan Zhang, Jinrui Li, Yuanyuan Li, and Lin Liu. 2022. "Fluorescent Immunoassay with a Copper Polymer as the Signal Label for Catalytic Oxidation of O-Phenylenediamine" Molecules 27, no. 12: 3675. https://doi.org/10.3390/molecules27123675
APA StyleFeng, Y., Liu, G., Zhang, C., Li, J., Li, Y., & Liu, L. (2022). Fluorescent Immunoassay with a Copper Polymer as the Signal Label for Catalytic Oxidation of O-Phenylenediamine. Molecules, 27(12), 3675. https://doi.org/10.3390/molecules27123675







