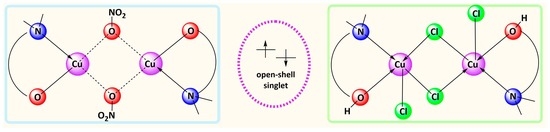
Dinuclear vs. Mononuclear Copper(II) Coordination Species of Tylosin and Tilmicosin in Non-Aqueous Solutions
Abstract
1. Introduction
2. Results and Discussion
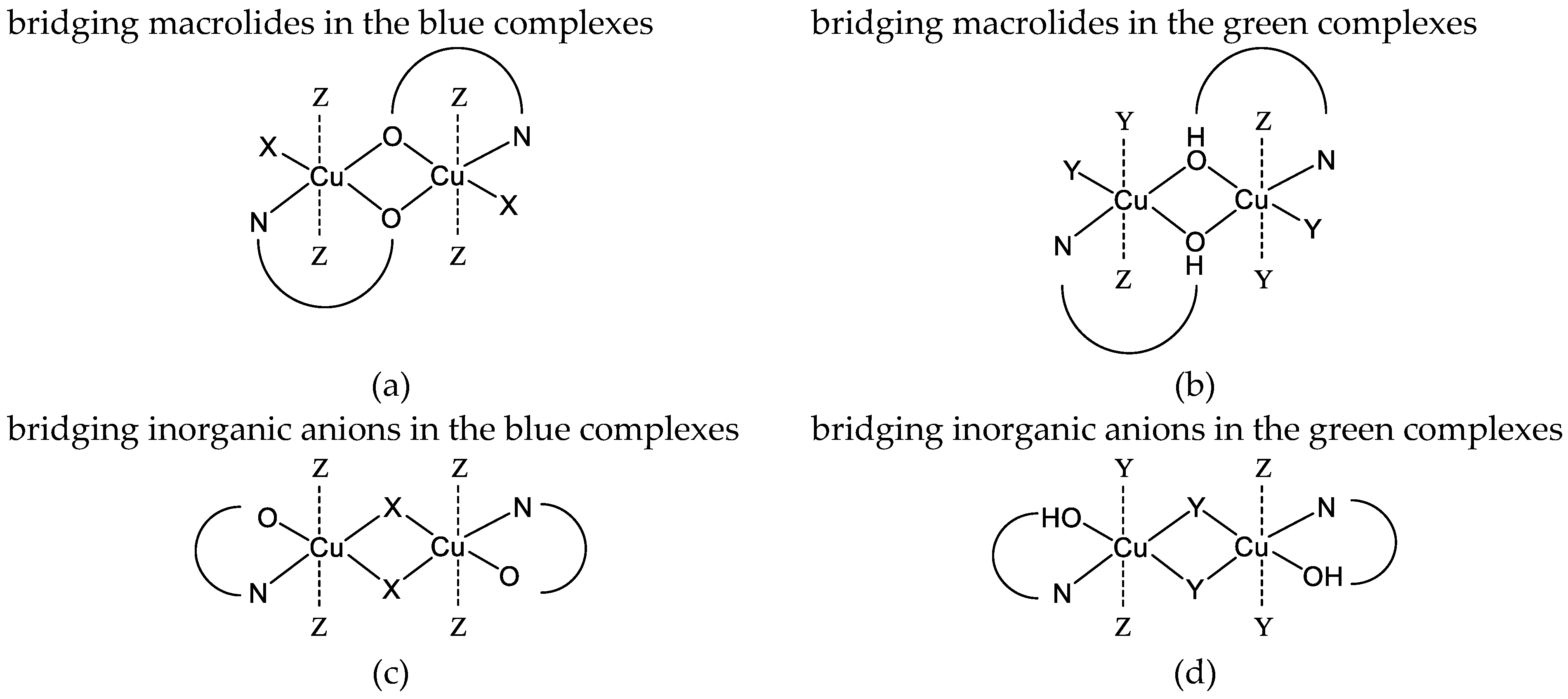
2.1. Physicochemical Properties of the Blue (2) and Green (3) Copper(II) Complexes
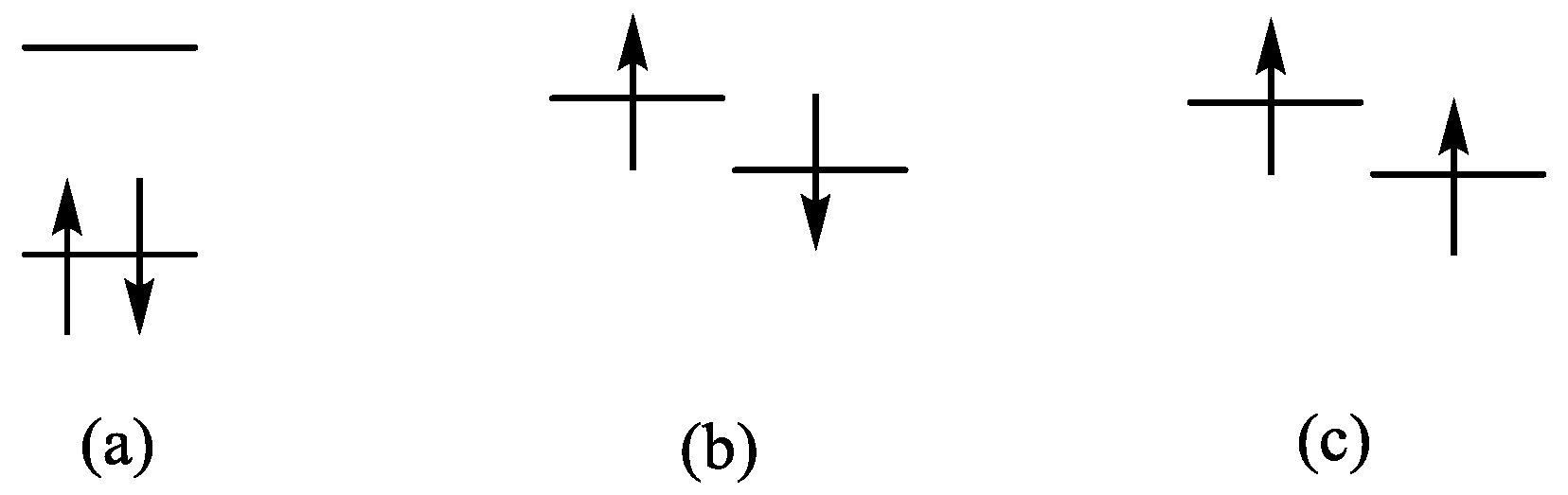
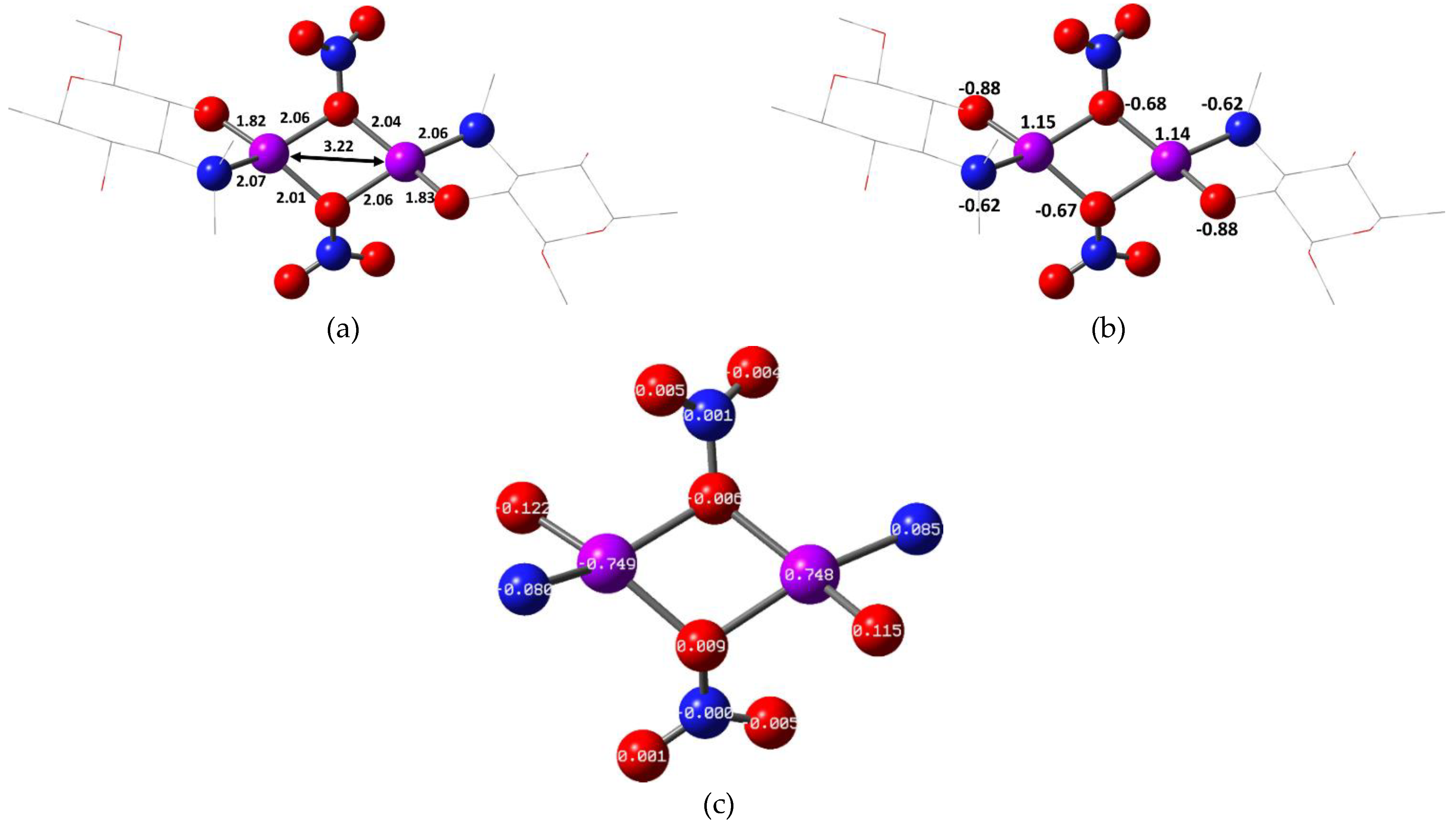
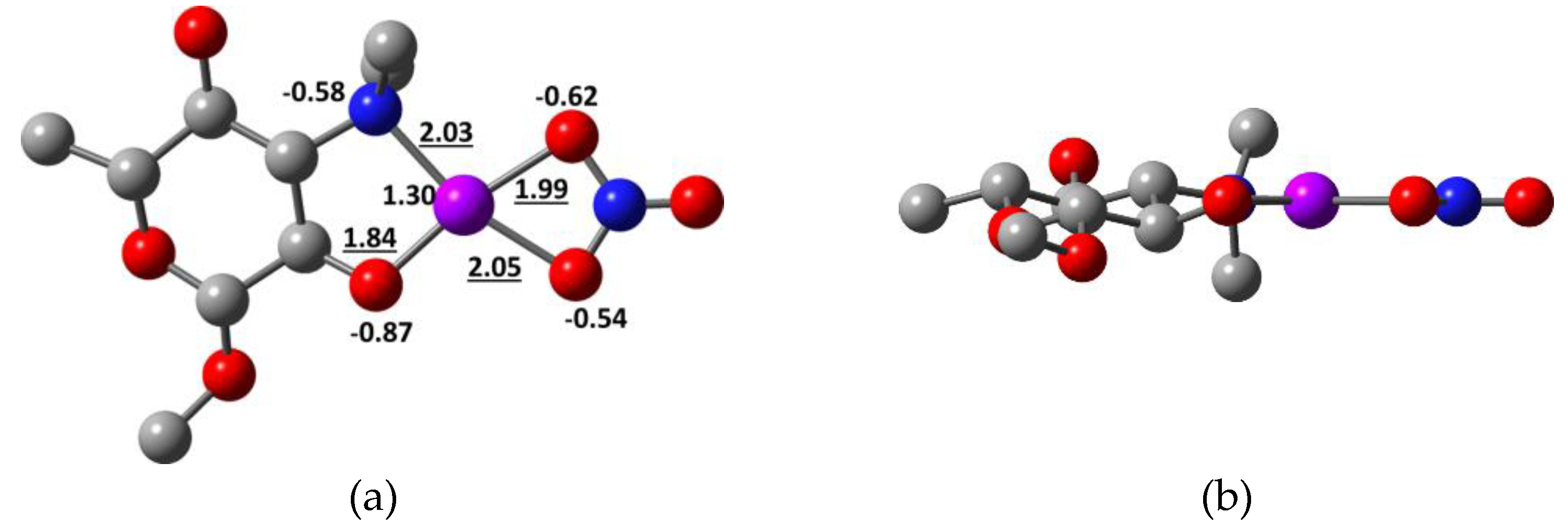
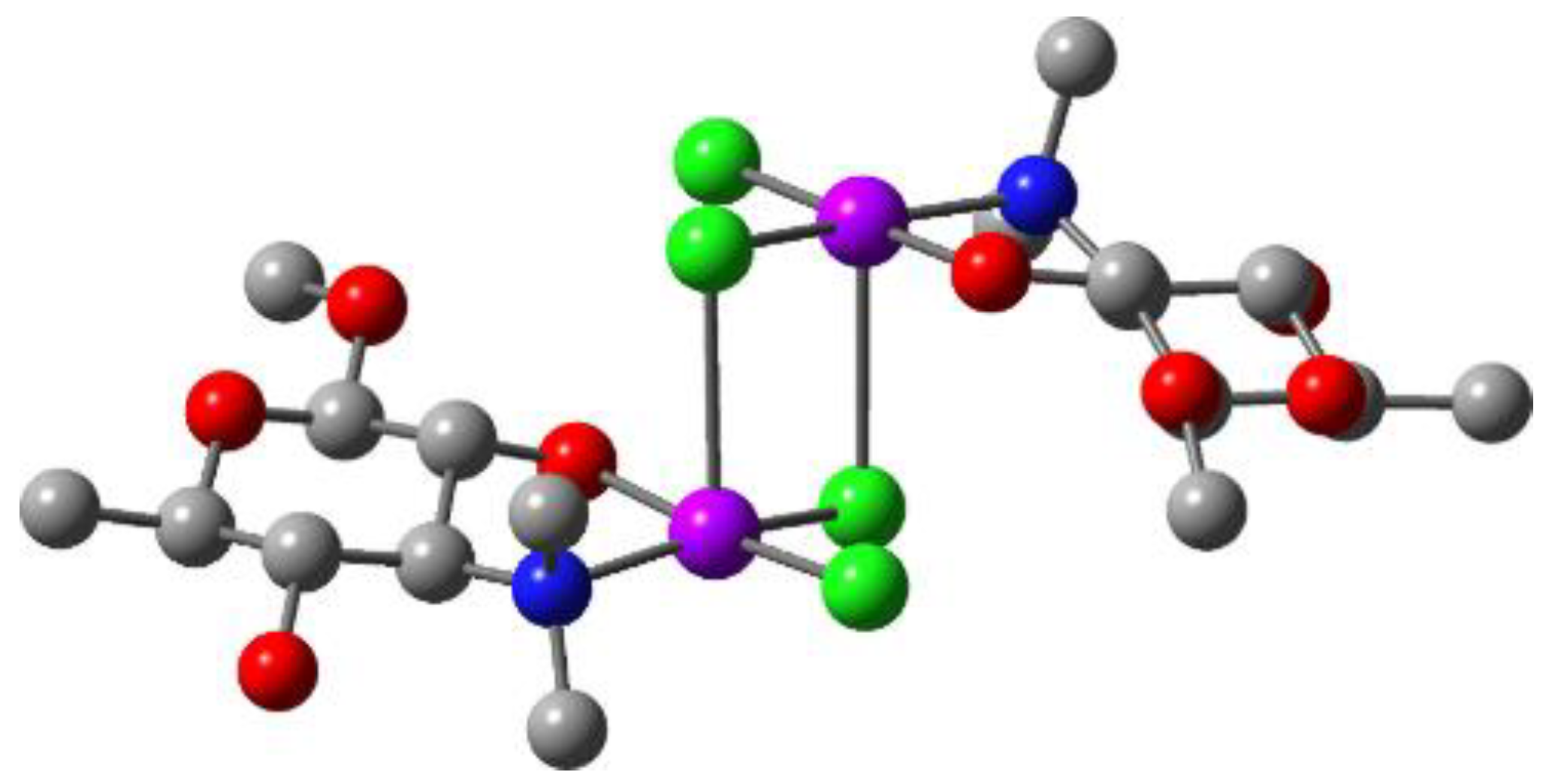
2.2. Quantum Chemical Calculations
3. Experimental Section
3.1. Materials
3.2. Methods
3.3. Synthesis
3.3.1. Synthesis of Nitrate-Containing Cu(II) complexes, 2a–b
3.3.2. Synthesis of Chloride-Containing Cu(II) complexes, 3a–b
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Iakovidis, I.; Delimaris, I.; Piperakis, S.M. Copper and its complexes in medicine: A biochemical approach. Mol. Biol. Int. 2011, 2011, 594529. [Google Scholar] [CrossRef] [PubMed]
- Szymański, P.; Frączek, T.; Markowicz, M.; Mikiciuk-Olasik, E. Development of copper based drugs, radiopharmaceuticals and medical materials. Biometals 2012, 25, 1089–1112. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.; White, A.R. Copper complexes as therapeutic agents. Metallomics 2012, 4, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Krajčiová, D.; Melník, M.; Havránek, E.; Forgácsová, A.; Mikuš, P. Copper compounds in nuclear medicine and oncology. J. Coord. Chem. 2014, 67, 1493–1519. [Google Scholar] [CrossRef]
- Denoyer, D.; Clatworthy, S.A.S.; Cater, M.A. Copper complexes in cancer therapy. In Metallo-Drugs: Development and Action of Anticancer Agents; Sigel, A., Sigel, H., Freisinger, E., Sigel, R.K.O., Eds.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2018; pp. 469–506. [Google Scholar] [CrossRef]
- McGuire, J.M.; Bunch, R.L.; Anderson, R.C.; Boaz, H.E.; Flyn, E.H.; Powell, H.M.; Smith, J.W. Ilotycin, a new antibiotic. Antibiot. Chemother. 1952, 2, 281–283. [Google Scholar]
- Sakakibara, H.; Okekawa, O.; Fujiwara, T.; Aizawa, M.; Omura, S. Acyl derivatives of 16-membered macrolide. II. Antibacterial activities and serum levels of 3′-O-acyl derivatives of leucomycin. J. Antibiot. 1981, 34, 1011–1018. [Google Scholar] [CrossRef]
- Neu, H.C. In Vitro activity of midecamycin, a new macrolide antibiotic. Antimicrob Agents Chemother. 1983, 24, 443–444. [Google Scholar] [CrossRef][Green Version]
- Morimoto, S.; Takahashi, Y.; Watanabe, Y.; Omura, S. Chemical modification of erythromycins. I. Synthesis and antibacterial activity of 6-O-methylerythromycins. J. Antibiot. 1984, 37, 187–189. [Google Scholar] [CrossRef]
- Bright, G.M.; Nagel, A.A.; Bordner, J.; Desai, K.A.; Dibrino, J.N.; Nowakowska, J.; Vincent, L.; Watrous, R.M.; Sciavolino, F.C.; English, A.R.; et al. Synthesis, in vitro and in vivo activity of novel 9-deoxo-9a-AZA-9a-homoerythromycin A derivatives: A new class of macrolide antibiotics, the azalides. J. Antibiot. 1988, 41, 1029–1047. [Google Scholar] [CrossRef]
- Fernandes, P.B.; Baker, W.R.; Freiberg, L.A.; Hardy, D.J.; McDonald, E.J. New macrolides active against Streptococcus pyogenes with inducible or constitutive type of macrolide-lincosamide-streptogramin B resistance. Antimicrob. Agents Chemother. 1989, 33, 78. [Google Scholar] [CrossRef]
- Kirst, H.A. Recent developments with macrolide antibiotics. Expert Opin. Ther. Pat. 1998, 8, 111–120. [Google Scholar] [CrossRef]
- Champney, W.S.; Tober, C.L. Superiority of 11,12 carbonate macrolide antibiotics as inhibitors of translation and 50S ribosomal subunit formation 1 in Staphylococcus aureus cells. Curr. Microbiol. 1999, 38, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Gaynor, M.A.S. Macrolide antibiotics: Binding site, mechanism of action, resistance. Curr. Top. Med. Chem. 2003, 3, 949–961. [Google Scholar] [CrossRef]
- Henninger, T.C. Recent progress in the field of macrolide antibiotics. Expert Opin. Ther. Pat. 2003, 13, 787–805. [Google Scholar] [CrossRef]
- Hammerschlag, M.R.; Sharma, R. Use of cethromycin, a new ketolide, for treatment of community-acquired respiratory infections. Expert Opin. Investig. Drugs 2008, 17, 387–400. [Google Scholar] [CrossRef]
- Woosley, L.N.; Castanheira, M.; Jones, R.N. CEM-101 Activity against Gram-positive organisms. Antimicrob. Agents Chemother. 2010, 54, 2182–2187. [Google Scholar] [CrossRef]
- Cui, W.; Ma, S. Recent advances in the field of 16-membered macrolide antibiotics. MiniRev. Med. Chem. 2011, 11, 1009–1018. [Google Scholar] [CrossRef]
- Zhao, Z.; Jin, L.; Xu, Y.; Zhu, D.; Liu, Y.; Liu, C.; Lei, P. Synthesis and antibacterial activity of a series of novel 9-O-acetyl-4′-substituted 16-membered macrolides derived from josamycin. Bioorg. Med. Chem. Lett. 2014, 2, 480–484. [Google Scholar] [CrossRef]
- Pyörälä, S.; Baptiste, K.E.; Catry, B.; van Duijkeren, E.; Greko, C.; Moreno, M.A.; Pomba, M.C.M.F.; Rantala, M.; Ružauskas, M.; Sanders, P.; et al. Macrolides and lincosamides in cattle and pigs: Use and development of antimicrobial resistance. Vet. J. 2014, 200, 230–239. [Google Scholar] [CrossRef]
- Seppälä, P.; Sillanpää, R.; Lehtonen, A. Structural diversity of copper(II) amino alcoholate complexes. Coord. Chem. Rev. 2017, 347, 98–114. [Google Scholar] [CrossRef]
- Đokič, S.; Vajtner, Z.; Lopotar, N.; Mrvoš-Sermek, D.; Kamenar, B.; Nagl, A. Complexes of azithromycin with some divalent metal ions. Croat. Chem. Acta 1995, 68, 375–381. Available online: https://hrcak.srce.hr/136758 (accessed on 6 March 2021).
- Sher, A.; Rau, H.; Greiner, G.; Haubold, W. Spectroscopic and polarographic investigations of copper(II)-azithromycin interactions under equilibrium conditions. Int. J. Pharm. 1996, 133, 237–244. [Google Scholar] [CrossRef]
- Saeed, A.M.; Najma, S.; Sana, S.; Asia, N. Synthesis, characterization and antimicrobial activities of azithromycin metal complexes. Mod. Chem. Appl. 2014, 2. [Google Scholar] [CrossRef]
- Arsic, B.; Barber, J.; Čikoš, A.; Mladenovic, M.; Stankovic, N.; Novak, P. 16-membered macrolide antibiotics: A review. Int. J. Antimicrob. Agents 2018, 51, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Pantcheva, I.N.; Stambolyiska, R.D.; Petkov, N.N.; Tadjer, A.V.; Simova, S.D.; Stoyanova, R.R.; Dorkov, P.D. Mononuclear complexes of the macrolide antibiotics tylosin A and tilmicosin. Trans. Metal Chem. 2022, 47, 67–76. [Google Scholar] [CrossRef]
- Bontchev, P.R.; Kadum, H.; Evtimova, B.; Nachev, C.; Ivanov, D.; Zhecheva, E.; Mehandjiev, D. Copper(II) complexes of (1-[isopropylamine]-3-[1-naphthyloxy]-2-propanol) (propranolol). J. Inorg. Biochem. 1992, 48, 153–161. [Google Scholar] [CrossRef]
- Bontchev, P.R.; Pantcheva, I.N.; Bontchev, R.P.; Ivanov, D.S.; Danchev, N.D. Copper(II) complexes of the beta-blocker pindolol: Properties, structure, biological activity. BioMetals 2002, 15, 79–85. [Google Scholar] [CrossRef]
- Bontchev, P.R.; Pantcheva, I.N.; Gochev, G.P.; Mehandjiev, D.R.; Ivanov, D.S. Complexes of copper(II) with the β-blocker atenolol. Trans. Metal Chem. 2000, 25, 196–199. [Google Scholar] [CrossRef]
- Bontchev, P.R.; Pantcheva, I.N.; Todorov, T.; Mehandjiev, D.R.; Savov, N.S. Complexation of the antihypertensive drug oxprenolol with copper(II). J. Inorg. Biochem. 2001, 83, 25–30. [Google Scholar] [CrossRef]
- Getova, V.T.; Bontchev, R.P.; Mehandjiev, D.R.; Bontchev, P.R. Copper(II) complexes with 4-amino-a-(t-butylaminomethyl)-3,5-dichlorobenzyl alcohol hydrochloride (Clenbuterol). Crystal structures of the binuclear and mononuclear Cu(II) complexes with Clenbuterol. Polyhedron 2005, 24, 1983–1990. [Google Scholar] [CrossRef]
- Getova, V.; Mehandjiev, D.; Skumryev, V.; Bontchev, P. A binuclear copper(II) complex of the antihypertensive drug Labetalol: Synthesis and properties. J. Uni. Chem. Techn. Met. 2006, 41, 193–198. [Google Scholar]
- Getova, V.T.; Bontchev, R.P.; Mehandjiev, D.R.; Bontchev, P.R. Complexes of 1-[2-[2-hydroxy-3-(propylamino)propoxy]phenyl]-3-phenyl-1-propanone (propafenone) with copper(II): Crystal structure of the mononuclear Cu(II) complex with propafenone. Polyhedron 2006, 25, 2254–2260. [Google Scholar] [CrossRef]
- Hathaway, B.J. Copper. In Comprehensive Coordination Chemistry; Wilkinson, G., McCleverty, J.A., Gillard, R.D., Eds.; Pergamon Press: Oxford, UK, 1987; Volume 5, p. 663. [Google Scholar]
- Harish, S.P.; Sobhanardi, J. EPR and magnetic studies of copper amino carboxylates. Inorg. Chim. Acta 1985, 108, 147–153. [Google Scholar] [CrossRef]
- Hancock, R.D. Molecular mechanics calculations as a tool in coordination chemistry. Prog. Inorg. Chem. 1989, 37, 187–291. [Google Scholar] [CrossRef]
- General, Atomic and Crystallographic Properties and Features of Copper. Available online: https://www.copper.org/resources/properties/atomic_properties.html (accessed on 11 May 2022).
- Hathaway, B.J.; Billing, D.E. The electronic properties and stereochemistry of mononuclear complexes of the copper(II) ion. Coord. Chem. Rev. 1970, 5, 143–207. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley. Interdiscip. Rev. Comput. Mol. Sci. 2017, 8, e1327. [Google Scholar] [CrossRef]
- Wachters, A.J.H. Gaussian basis set for molecular wavefunctions containing third row atoms. J. Chem. Phys. 1970, 52, 1033–1036. [Google Scholar] [CrossRef]


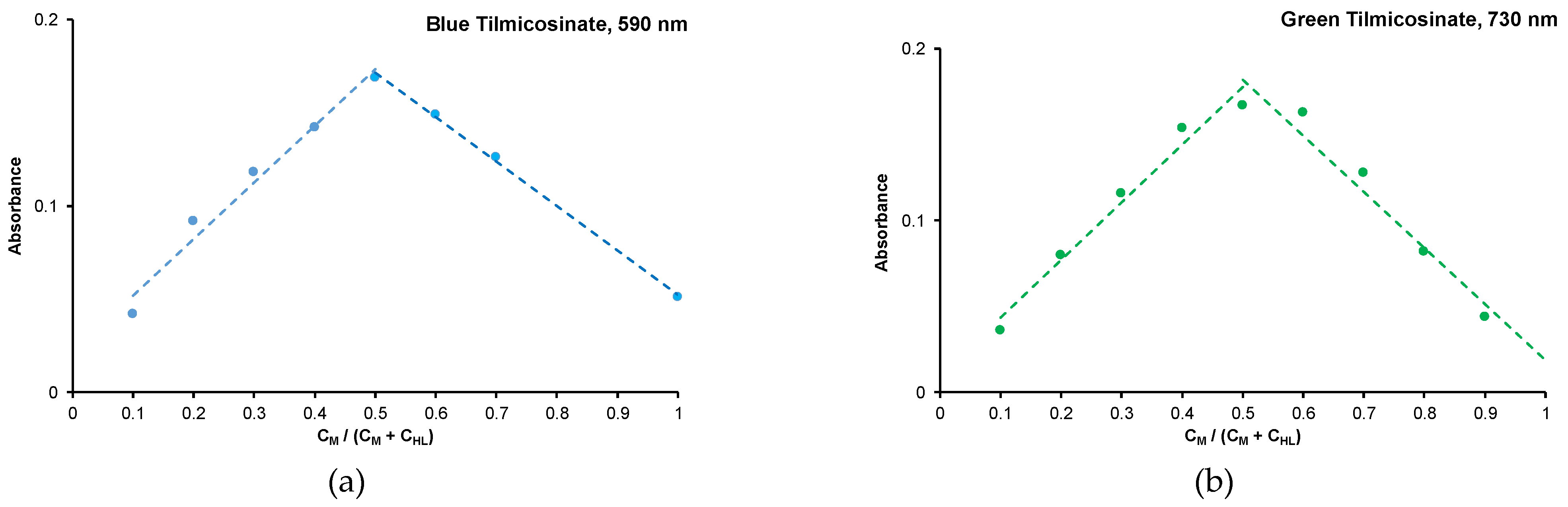
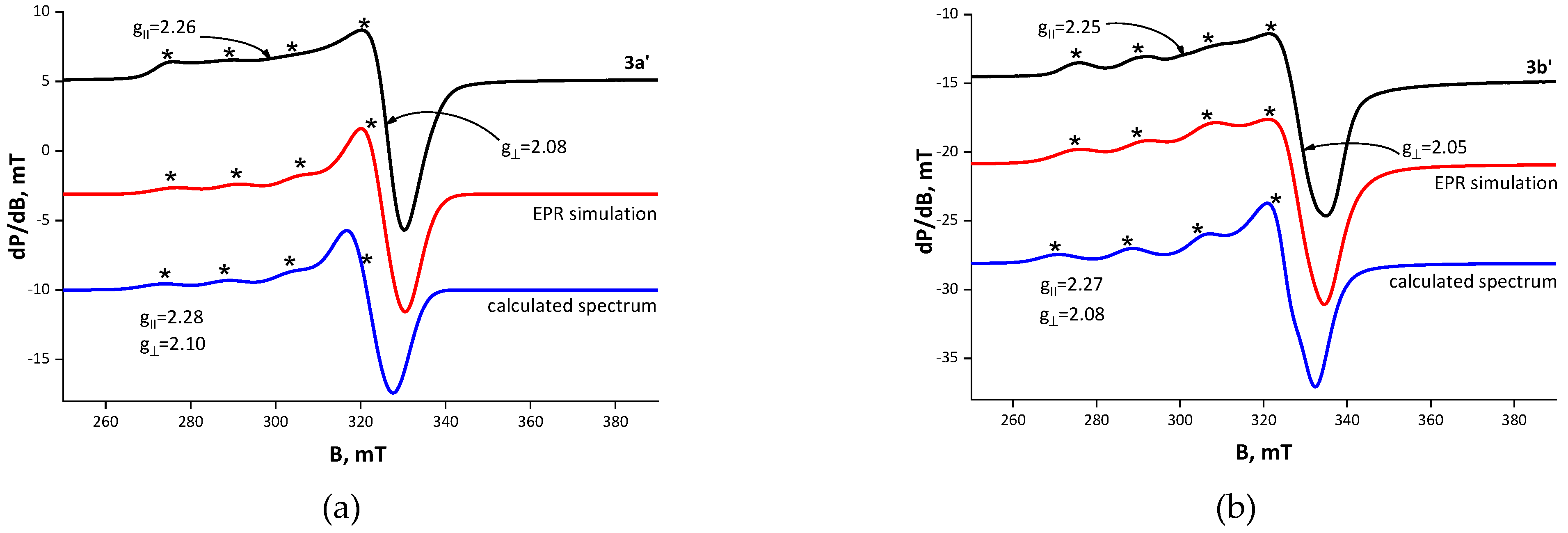
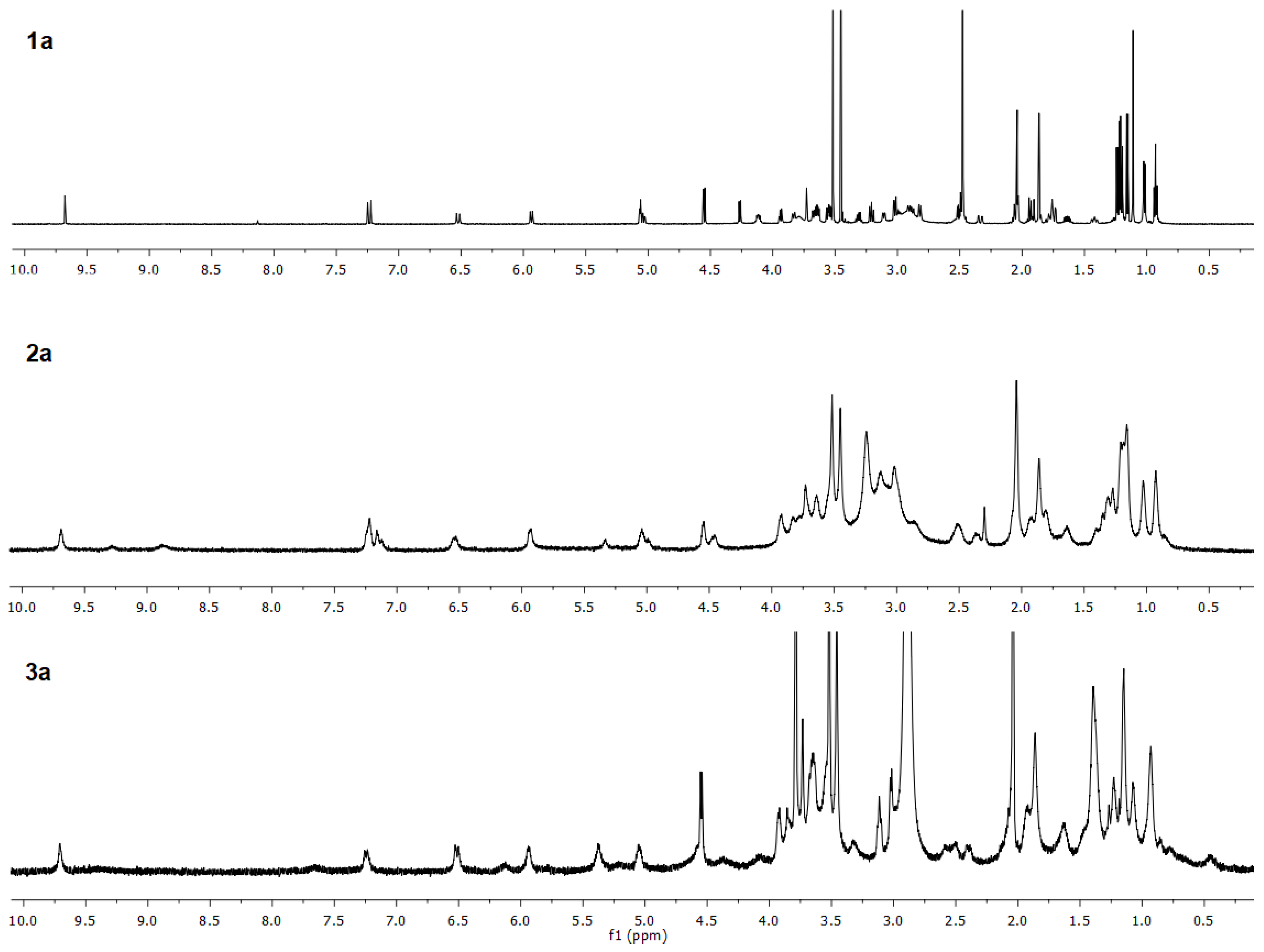
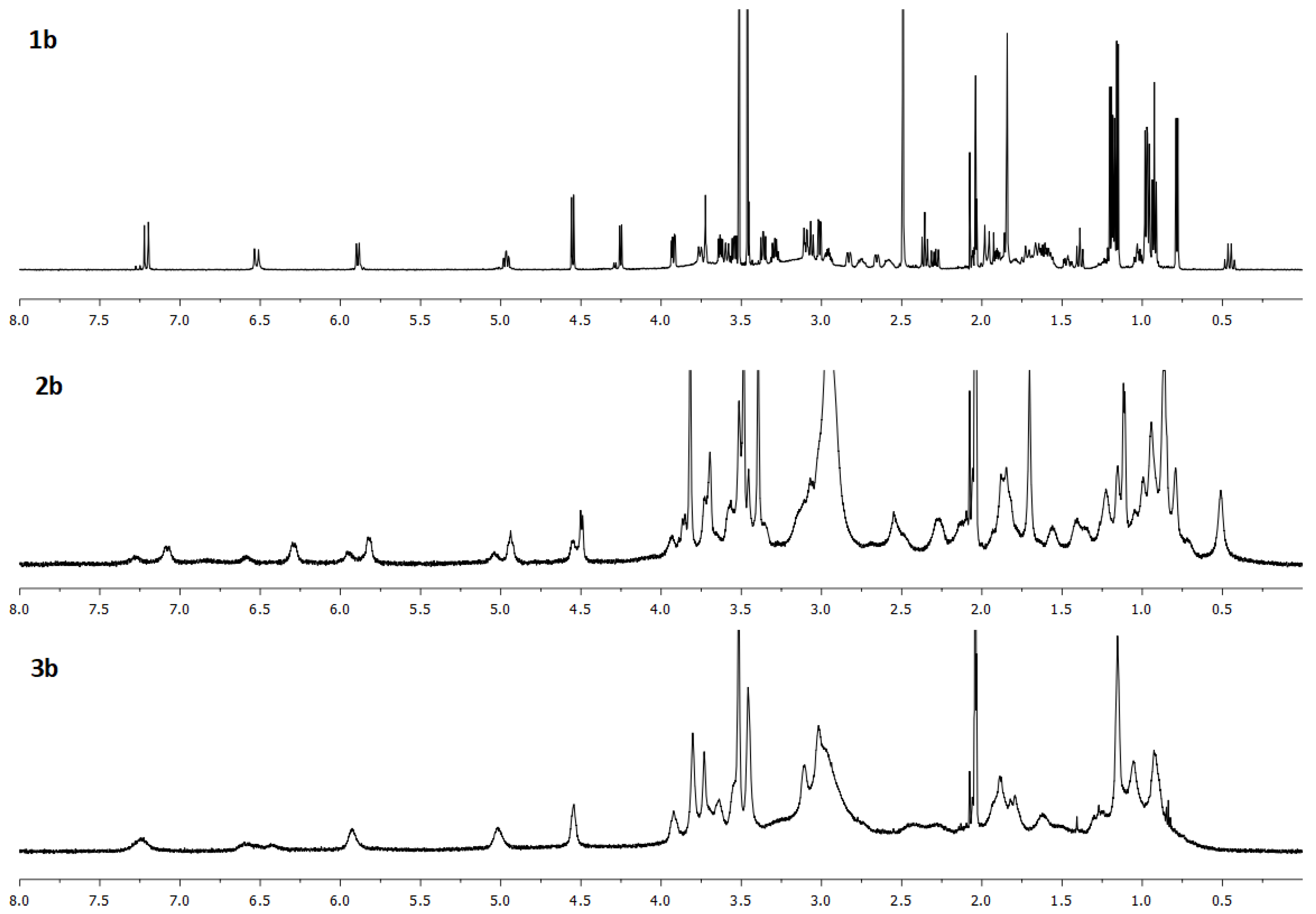
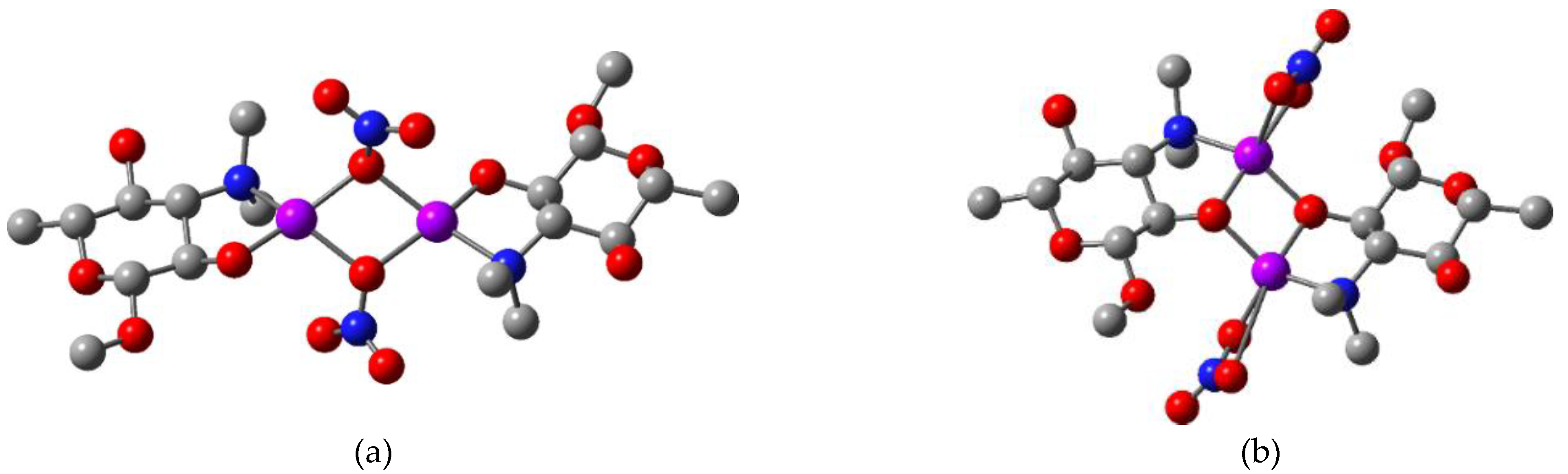

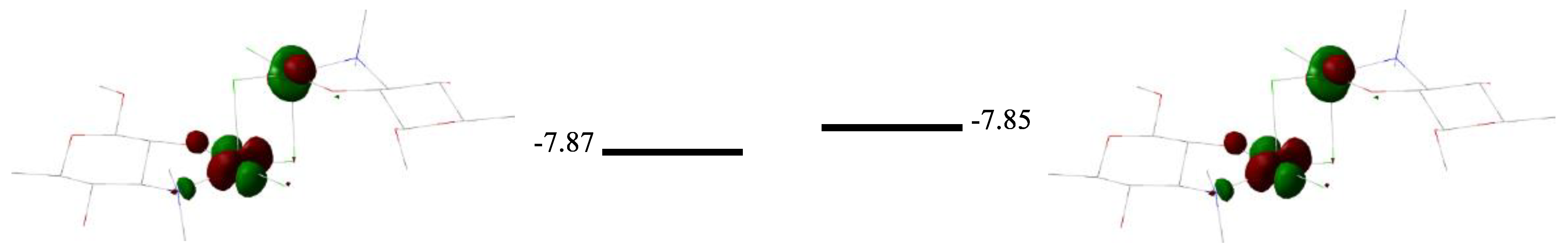
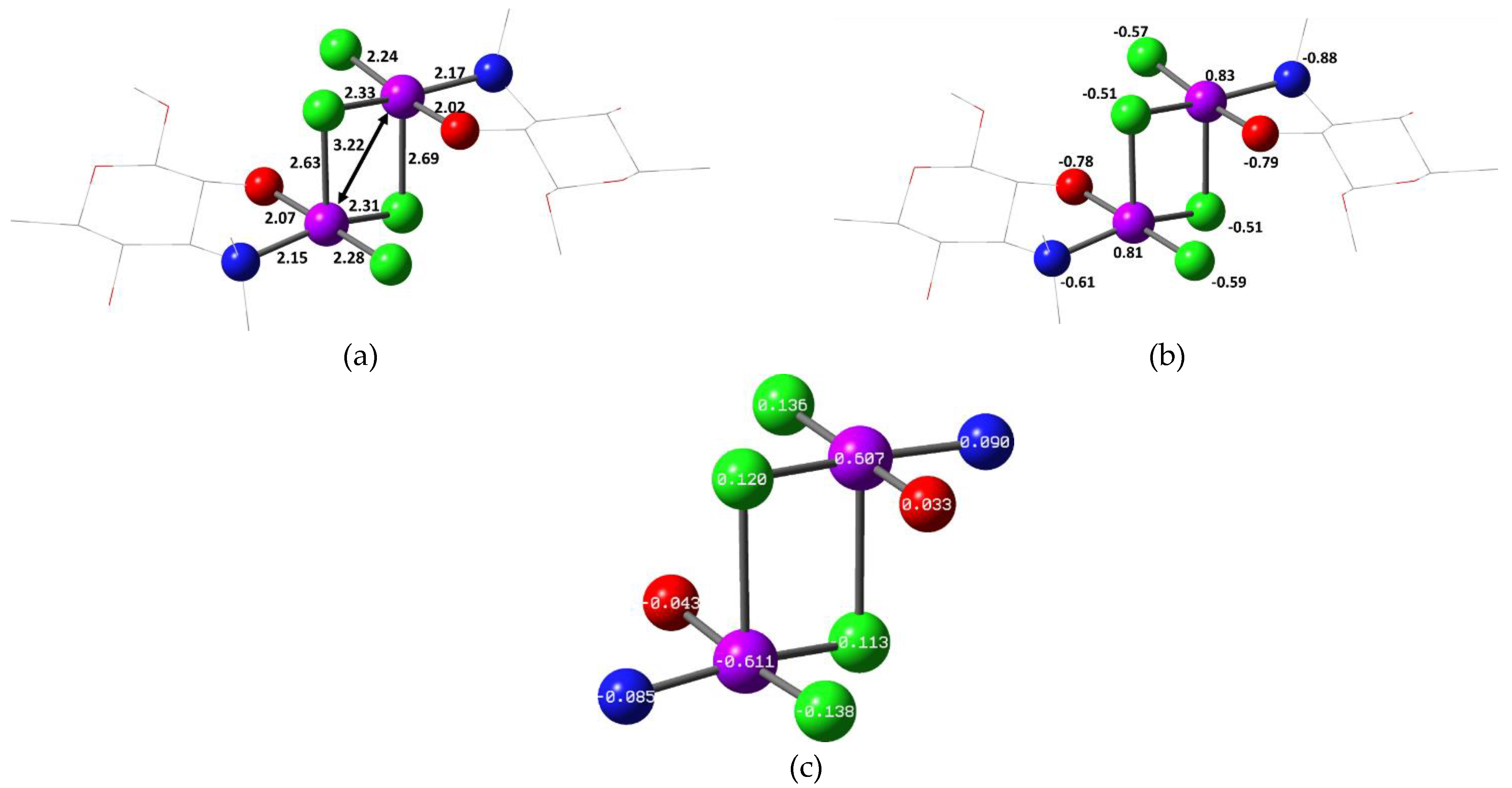
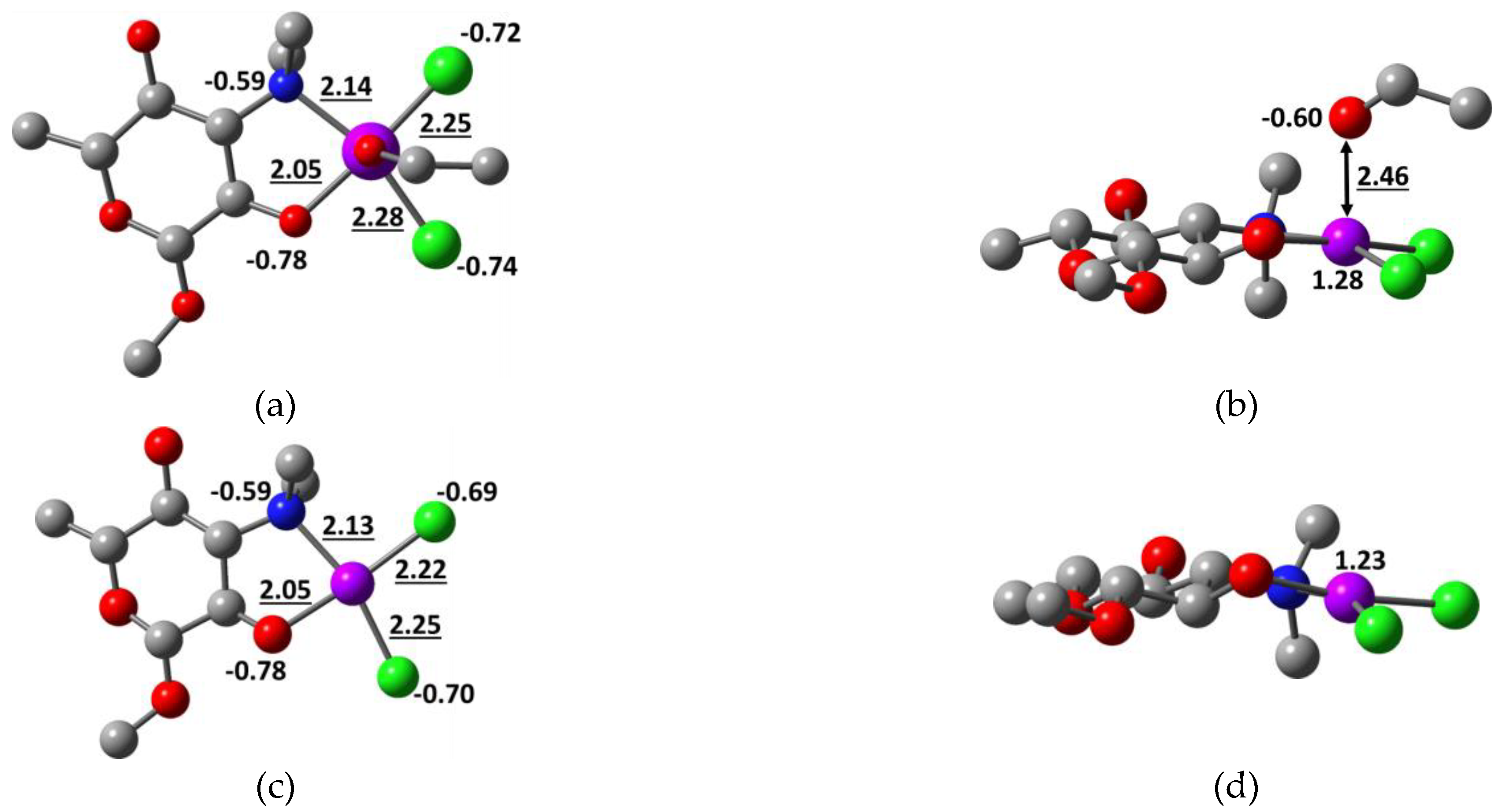
| Compound | Solvent | λ, nm | amax, L·g−1·cm−1 | εmax, M−1·cm−1 |
|---|---|---|---|---|
| HTyl, 1a | EtOH | 285 | 21.24 | 19,460 |
| Blue tylosinate | EtOH | 285 735 | 19.17 0.04 | 39,900 78 |
| acetone | 706 | 0.08 | 160 | |
| Green tylosinate | EtOH | 285 800 | 20.75 0.03 | 42,843 62 |
| acetone | 475 860 | 0.96 0.13 | 1959 239 | |
| HTilm, 1b | EtOH | 285 | 21.32 | 18,530 |
| Blue tilmicosinate | EtOH | 285 677 | 19.14 0.10 | 38,220 192 |
| acetone | 670 | 0.10 | 192 | |
| Green tilmicosinate | EtOH | 285 720 | 20.29 0.06 | 40,161 128 |
| acetone | 480 630 755 | 0.07 0.08 0.11 | 145 158 208 | |
| Cu(NO3)2 × 3H2O | EtOH | 797 | 0.18 | 42 |
| acetone | 770 | 4.59 | 1108 | |
| CuCl2 × 2H2O | EtOH | 870 | 0.42 | 72 |
| acetone | 475 | 11.53 | 1960 | |
| 865 | 3.56 | 612 |
| Complex | [Cu(NO3)Tyl] 2a’ | [Cu(NO3)Tilm] 2b’ | [CuCl2(HTyl)] 3a’ | [CuCl2(HTilm)] 3b’ |
|---|---|---|---|---|
| Composition | C46H76CuN2O20 | C46H79CuN3O16 | C46H77Cl2CuNO17 | C46H80Cl2CuN2O13 |
| MW, g/mol | 1040.65 | 993.69 | 1050.56 | 1003.59 |
| Complex | [Cu2(µ-NO3)2Tyl2] 2a | [Cu2(µ-NO3)2Tilm2] 2b | [Cu2(µ-Cl)2Cl2(HTyl)2] 3a | [Cu2(µ-Cl)2Cl2(HTilm)2] 3b |
| Composition | C92H152Cu2N4O40 | C92H158Cu2N6O32 | C92H154Cl4Cu2N2O34 | C92H160Cl4Cu2N4O26 |
| MW, g/mol | 2081.31 | 1987.38 | 2101.12 | 2007.19 |
| C%, exp. (calcd.) | 51.75 (53.09) | 54.73 (55.60) | 52.65 (52.59) | 54.77 (55.05) |
| H%, exp. (calcd.) | 7.63 (7.36) | 8.66 (8.01) | 7.15 (7.39) | 7.88 (8.04) |
| N%, exp. (calcd.) | 2.29 (2.69) | 4.12 (4.23) | 1.18 (1.33) | 2.56 (2.79) |
| Cl%, exp. (calcd.) | - | - | 6.49 (6.75) | 7.12 (7.06) |
| Cu%, exp. (calcd.) | 5.52 (6.11) | 5.65 (6.39) | 6.25 (6.05) | 6.67 (6.33) |
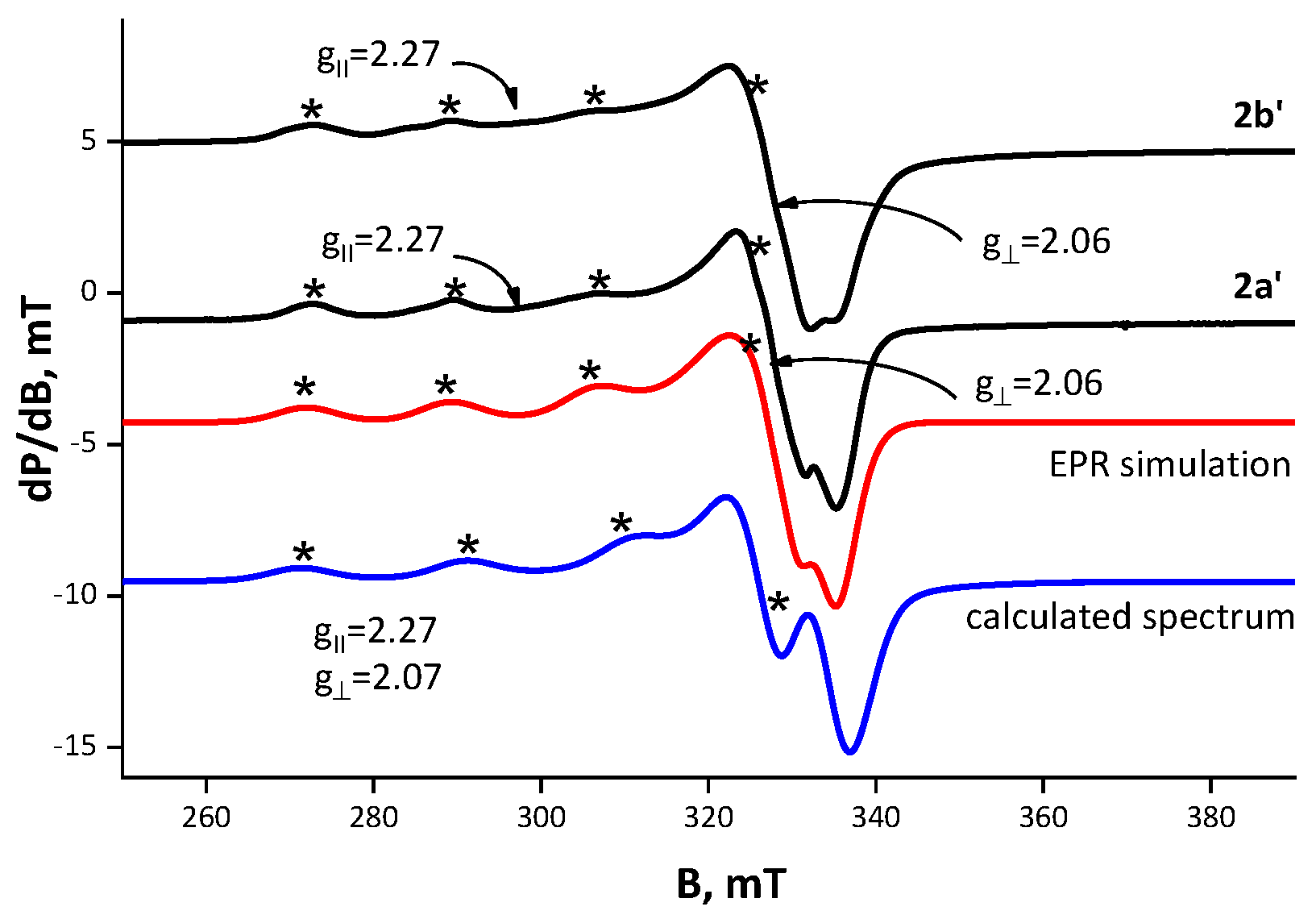
| Parameter | State | B‖ (G) | g‖ | A‖ (G) | B⊥ (G) | g⊥ | giso | α2 | G | |
|---|---|---|---|---|---|---|---|---|---|---|
| Complex | ||||||||||
| [Cu(NO3)Tyl], 2a’ | solid | 2961 | 2.27 | 170 | 3263 | 2.06 | 2.14 | 0.77 | 4.64 | |
| EtOH | 2872 | 2.34 | 140 | 3256 | 2.06 | 2.16 | 0.77 | 5.47 | ||
| acetone | 2824 | 2.38 | 137 | 3233 | 2.08 | 2.18 | 0.81 | 4.92 | ||
| [Cu(NO3)Tilm], 2b’ | solid | 3001 | 2.25 | 175 | 3279 | 2.06 | 2.13 | 0.76 | 4.35 | |
| EtOH | 3012 | 2.24 | 155 | 3290 | 2.06 | 2.11 | 0.69 | 5.21 | ||
| acetone | 2979 | 2.26 | 170 | 3280 | 2.05 | 2.12 | 0.76 | 5.02 | ||
| [CuCl2(HTyl)], 3a’ | solid | 2987 | 2.26 | 146 | 3252 | 2.07 | 2.15 | 0.71 | 3.50 | |
| EtOH | 2.35 2.42 | 128 110 | 2.08 2.08 | 2.17 2.20 | ||||||
| acetone | 2879 | 2.34 | 140 | 3257 | 2.06 | 2.13 | 0.76 | 5.39 | ||
| [CuCl2(HTilm)], 3b’ | solid | 2999 | 2.25 | 164 | 3301 | 2.05 | 2.11 | 0.73 | 5.82 | |
| EtOH | 2991 | 2.25 | 164 | 3294 | 2.05 | 2.11 | 0.73 | 5.85 | ||
| acetone | 2990 | 2.25 | 164 | 3293 | 2.05 | 2.11 | 0.73 | 5.85 | ||
| Cu(NO3)2×3H2O | EtOH | 2.38 2.42 | 130 110 | 2.08 2.08 | 2.18 2.20 | |||||
| acetone | 2.38 | 135 | 2.08 | 2.18 | ||||||
| CuCl2×2H2O | EtOH | 2.36 2.42 | 126 110 | 2.08 2.08 | 2.17 2.20 | |||||
| acetone | 2.33 | 140 | 2.07 | 2.16 | ||||||
| Compound | Theoretical Spin Concentration | Experimentally Determined Spin Concentration | |
|---|---|---|---|
| Powder | Frozen Solution | ||
| [Cu(NO3)Tyl], 2a’ | 5.8 × 1020 | 1.4 × 1019 | 7.0 × 1018 |
| [Cu(NO3)Tilm], 2b’ | 6.0 × 1020 | 1.5 × 1019 | 1.4 × 1019 |
| [CuCl2(HTyl)], 3a’ | 5.7 × 1020 | 8.6 × 1019 | 7.8 × 1019 |
| [CuCl2(HTilm)], 3b’ | 6.0 × 1020 | 2.2 × 1019 | 1.3 × 1019 |
| Cu(NO3)2 × 3H2O | 2.5 × 1021 | 2.4 × 1021 | 3.1 × 1021 |
| CuCl2 × 2H2O | 3.5 × 1021 | 5.3 × 1021 | 5.0 × 1021 |
| E, eV | λ, nm | f |
|---|---|---|
| 1.66 | 747 | 0.001 |
| 1.67 | 744 | 0.001 |
| 1.80 | 688 | 0.001 |
| EPR Parameters | g⊥ | g‖ | Δg | A‖, G |
|---|---|---|---|---|
| Calculated, 6 | 2.07 | 2.25 | 0.18 | 197 |
| Experimental, 2a’–b’ | 2.07/2.06 | 2.27/2.27 | 0.20/0.19 | 170/175 |
| EPR Parameters | g⊥ | g‖ | Δg | A‖, G |
|---|---|---|---|---|
| Calculated, 8a | 2.10 | 2.28 | 0.18 | 151 |
| Calculated, 8b | 2.08 | 2.27 | 0.19 | 176 |
| Experimental, 3a’–b’ | 2.08/2.05 | 2.26/2.25 | 0.18/0.20 | 146/164 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantcheva, I.; Stamboliyska, R.; Petkov, N.; Tadjer, A.; Simova, S.; Stoyanova, R.; Kukeva, R.; Dorkov, P. Dinuclear vs. Mononuclear Copper(II) Coordination Species of Tylosin and Tilmicosin in Non-Aqueous Solutions. Molecules 2022, 27, 3899. https://doi.org/10.3390/molecules27123899
Pantcheva I, Stamboliyska R, Petkov N, Tadjer A, Simova S, Stoyanova R, Kukeva R, Dorkov P. Dinuclear vs. Mononuclear Copper(II) Coordination Species of Tylosin and Tilmicosin in Non-Aqueous Solutions. Molecules. 2022; 27(12):3899. https://doi.org/10.3390/molecules27123899
Chicago/Turabian StylePantcheva, Ivayla, Radoslava Stamboliyska, Nikolay Petkov, Alia Tadjer, Svetlana Simova, Radostina Stoyanova, Rositza Kukeva, and Petar Dorkov. 2022. "Dinuclear vs. Mononuclear Copper(II) Coordination Species of Tylosin and Tilmicosin in Non-Aqueous Solutions" Molecules 27, no. 12: 3899. https://doi.org/10.3390/molecules27123899
APA StylePantcheva, I., Stamboliyska, R., Petkov, N., Tadjer, A., Simova, S., Stoyanova, R., Kukeva, R., & Dorkov, P. (2022). Dinuclear vs. Mononuclear Copper(II) Coordination Species of Tylosin and Tilmicosin in Non-Aqueous Solutions. Molecules, 27(12), 3899. https://doi.org/10.3390/molecules27123899