Cannabinoids for Therapeutic Use in Atherosclerosis †
Summary
Zusammenfassung
- Atherosklerose (Arteriosklerose) stellt nach wie vor die Hauptursache für Herzerkrankungen und Schlaganfall dar und ist für etwa 50% aller Todesfälle in der westlichen Gesellschaft verantwortlich. Ein grosses Interesse für die Medizin besteht daher in der Entwicklung neuer anti-atherosklerotischer Therapien.
- Cannabinoide, wie zum Beispiel die in Marijuana hauptsächlich enthaltene psychaktive Substanz D9-Tetrahydrocannabinol (THC), erzielen ihre biologische Wirkung durch Interaktion mit spezifischen Rezeptoren. Im Mausmodel konnten wir kürzlich zeigen, dass THC das Fortschreiten der Atherosklerose mittels vielseitiger Effekte auf inflammatorische Zellen verlangsamt. Durch Blockierung des vorwiegend auf Immunzellen vorhandenen Cannabinoid-Rezeptors CB2 wurden all diese Effekte verhindert. Der potentielle therapeutische Nutzen steht im Konflikt mit dem bekannten Gesundheitsrisiko, welches mit dem Konsum von Marijuana verbunden ist. Denn es ist bekannt, dass THC auch an den neuronalen Cannabinoid-Rezeptor CB1 bindet und somit aktiviert. Neben den bekannten bewusstseinsverändernden Auswirkungen verursacht THC auch kardiovaskuläre Effekte wie Vasodilatation und Hypotension. Die Entwicklung neuer Cannabinoid-Rezeptor-Liganden ohne die ungewünschten Nebenwirkungen könnte helfen, dieses Problem zu überwinden. Zudem könnte die pharmakologische Manipulation des Endocannabinoidsystems eine neue Therapiestrategie zur Behandlung von Atherosklerose darstellen. Verschiedene Studien, welche auf eine Beteiligung des Endocannabinoidsystems in verschiedenen inflammatorischen Situationen hinweisen, unterstützen diese Hypothese.
- Schlüsselwörter: Atherosclerosis; chronische Entzündung, Cannabinoide
Introduction
Cannabinoid receptors
Cannabinoids and immunomodulation
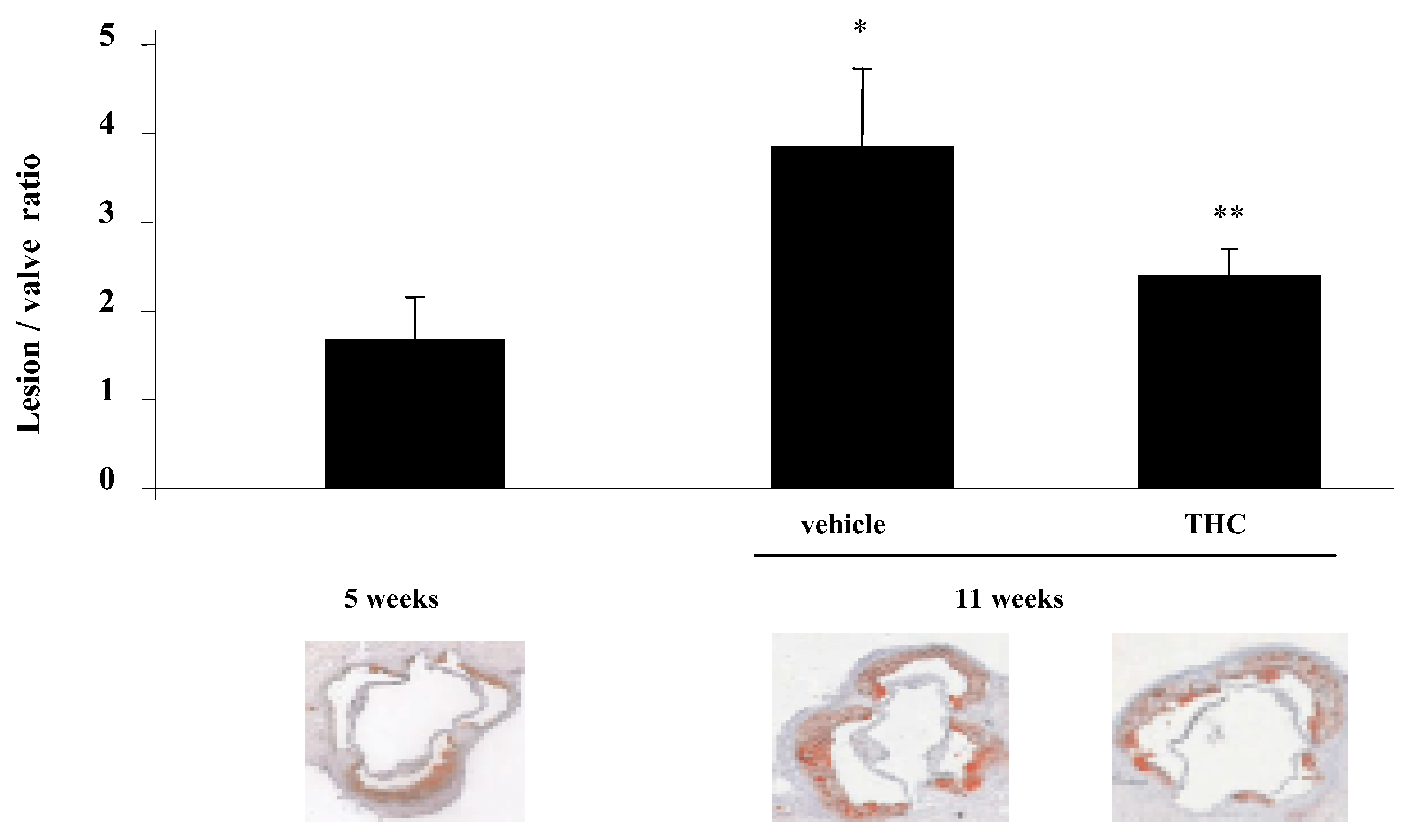
Effects of THC on atherosclerosis
Potential role of endocannabinoids in atherosclerosis
Cardiovascular effects of cannabinoids
Non-psychoactive cannabinoid receptor ligands for therapeutic use
Conclusion
References
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 1995, 50, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K.; et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun 1995, 215, 89–97. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Cascio, M.G.; Di Marzo, V. The endocannabinoid system: a general view and latest additions. Br J Pharmacol 2004, 141, 765–774. [Google Scholar] [CrossRef]
- McAllister, S.D.; Glass, M. CB(1) and CB(2) receptor-mediated signalling: a focus on endocannabinoids. Prostaglandins Leukot Essent Fatty Acids 2002, 66, 161–171. [Google Scholar] [CrossRef]
- Howlett, A.C.; Johnson, M.R.; Melvin, L.S.; Milne, G.M. Nonclassical cannabinoid analgetics inhibit adenylate cyclase: development of a cannabinoid receptor model. Mol Pharmacol 1988, 33, 297–302. [Google Scholar] [CrossRef]
- Bouaboula, M.; Poinot-Chazel, C.; Marchand, J.; Canat, X.; Bourrie, B.; Rinaldi-Carmona, M.; et al. Signaling pathway associated with stimulation of CB2 peripheral cannabinoid receptor. Involvement of both mitogen-activated protein kinase and induction of Krox-24 expression. Eur J Biochem 1996, 237, 704–711. [Google Scholar] [CrossRef]
- Daaka, Y.; Zhu, W.; Friedman, H.; Klein, T.W. Induction of interleukin-2 receptor alpha gene by delta 9-tetrahydrocannabinol is mediated by nuclear factor kappaB and CB1 cannabinoid receptor. DNA Cell Biol 1997, 16, 301–309. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Yang, K.H.; Pulaski, J.T.; Kaminski, N.E. Attenuation of inducible nitric oxide synthase gene expression by delta 9-tetrahydrocannabinol is mediated through the inhibition of nuclear factor-kappa B/Rel activation. Mol Pharmacol 1996, 50, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Lutz, B. Molecular biology of cannabinoid receptors. Prostaglandins Leukot Essent Fatty Acids 2002, 66, 123–142. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.W.; Newton, C.; Larsen, K.; Lu, L.; Perkins, I.; Nong, L.; et al. The cannabinoid system and immune modulation. J Leukoc Biol 2003, 74, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Van Sickle, M.D.; Duncan, M.; Kingsley, P.J.; Mouihate, A.; Urbani, P.; Mackie, K.; et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 2005, 310, 329–332. [Google Scholar] [CrossRef]
- Batkai, S.; Jarai, Z.; Wagner, J.A.; Goparaju, S.K.; Varga, K.; Liu, J.; et al. Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat Med 2001, 7, 827–832. [Google Scholar] [CrossRef]
- Bensaid, M.; Gary-Bobo, M.; Esclangon, A.; Maffrand, J.P.; Le Fur, G.; Oury-Donat, F.; et al. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol 2003, 63, 908–14. [Google Scholar] [CrossRef]
- Osei-Hyiaman, D.; DePetrillo, M.; Pacher, P.; Liu, J.; Radaeva, S.; Batkai, S.; et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest 2005, 115, 1298–1305. [Google Scholar] [CrossRef]
- Roche, R.; Hoareau, L.; Bes-Houtmann, S.; Gonthier, M.P.; Laborde, C.; Baron, J.F.; et al. Presence of the cannabinoid receptors, CB1 and CB2, in human omental and subcutaneous adipocytes. Histochem Cell Biol, Springer: Berlin/Heidelberg, 2006; 1–11. [Google Scholar]
- Cota, D.; Marsicano, G.; Tschop, M.; Grubler, Y.; Flachskamm, C.; Schubert, M.; et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest 2003, 112, 423–431. [Google Scholar] [CrossRef]
- Juan-Pico, P.; Fuentes, E.; Javier Bermudez-Silva, F.; Javier Diaz-Molina, F.; Ripoll, C.; Rodriguez de Fonseca, F.; et al. Cannabinoid receptors regulate Ca(2+) signals and insulin secretion in pancreatic beta-cell. Cell Calcium 2006, 39, 155–162. [Google Scholar] [CrossRef]
- Nakajima, Y.; Furuichi, Y.; Biswas, K.K.; Hashiguchi, T.; Kawahara, K.; Yamaji, K.; et al. Endocannabinoid, anandamide in gingival tissue regulates the periodontal inflammation through NF-kappaB pathway inhibition. FEBS Lett 2006, 580, 613–619. [Google Scholar] [CrossRef]
- Idris, A.I.; van ‘t Hof, R.J.; Greig, I.R.; Ridge, S.A.; Baker, D.; Ross, R.A.; et al. Regulation of bone mass, bone loss and osteoclast activity by cannabinoid receptors. Nat Med 2005, 11, 774–9. [Google Scholar] [CrossRef] [PubMed]
- Karsak, M.; Cohen-Solal, M.; Freudenberg, J.; Ostertag, A.; Morieux, C.; Kornak, U.; et al. Cannabinoid receptor type 2 gene is associated with human osteoporosis. Hum Mol Genet 2005, 14, 3389–3396. [Google Scholar] [CrossRef] [PubMed]
- Ofek, O.; Karsak, M.; Leclerc, N.; Fogel, M.; Frenkel, B.; Wright, K.; et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc Natl Acad Sci USA 2006, 103, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Begg, M.; Pacher, P.; Batkai, S.; Osei-Hyiaman, D.; Offertaler, L.; Mo, F.M.; et al. Evidence for novel cannabinoid receptors. Pharmacol Ther 2005, 106, 133–145. [Google Scholar] [CrossRef]
- Zygmunt, P.M.; Petersson, J.; Andersson, D.A.; Chuang, H.; Sorgard, M.; Di Marzo, V.; et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 1999, 400, 452–7. [Google Scholar] [CrossRef]
- Burstein, S. PPAR-gamma: a nuclear receptor with affinity for cannabinoids. Life Sci 2005, 77, 1674–1684. [Google Scholar] [CrossRef]
- O’Sullivan, S.E.; Tarling, E.J.; Bennett, A.J.; Kendall, D.A.; Randall, M.D. Novel time-dependent vascular actions of Delta 9tetrahydrocannabinol mediated by peroxisome proliferatoractivated receptor gamma. Biochem Biophys Res Commun 2005, 337, 824–831. [Google Scholar] [CrossRef]
- Srivastava, M.D.; Srivastava, B.I.; Brouhard, B. Delta 9-tetrahydrocannabinol and cannabidiol alter cytokine production by human immune cells. Immunopharmacology 1998, 40, 179–185. [Google Scholar] [CrossRef]
- Yuan, M.; Kiertscher, S.M.; Cheng, Q.; Zoumalan, R.; Tashkin, D.P.; Roth, M.D. Delta 9-tetrahydrocannabinol regulates Th1/Th2 cytokine balance in activated human T cells. J Neuroimmunol 2002, 133, 124–131. [Google Scholar] [CrossRef]
- Buckley, N.E.; McCoy, K.L.; Mezey, E.; Bonner, T.; Zimmer, A.; Felder, C.C.; et al. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur J Pharmacol 2000, 396, 141–149. [Google Scholar] [CrossRef]
- Malfait, A.M.; Gallily, R.; Sumariwalla, P.F.; Malik, A.S.; Andreakos, E.; Mechoulam, R.; et al. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci U S A 2000, 97, 9561–9566. [Google Scholar] [CrossRef]
- Croxford, J.L.; Miller, S.D. Immunoregulation of a viral model of multiple sclerosis using the synthetic cannabinoid R+WIN55,212. J Clin Invest 2003, 111, 1231–1340. [Google Scholar] [CrossRef] [PubMed]
- Arevalo-Martin, A.; Vela, J.M.; Molina-Holgado, E.; Borrell, J.; Guaza, C. Therapeutic action of cannabinoids in a murine model of multiple sclerosis. J Neurosci 2003, 23, 2511–2516. [Google Scholar] [CrossRef] [PubMed]
- Mestre, L.; Correa, F.; Arevalo-Martin, A.; Molina-Holgado, E.; Valenti, M.; Ortar, G.; et al. Pharmacological modulation of the endocannabinoid system in a viral model of multiple sclerosis. J Neurochem 2005, 92, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Gutierrez, S.; Molina-Holgado, E.; Arevalo-Martin, A.; Correa, F.; Viso, A.; Lopez-Rodriguez, M.L.; et al. Activation of the endocannabinoid system as therapeutic approach in a murine model of multiple sclerosis. Faseb J 2005, 19, 1338–1340. [Google Scholar] [CrossRef]
- Steffens, S.; Veillard, N.R.; Arnaud, C.; Pelli, G.; Burger, F.; Staub, C.; et al. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature 2005, 434, 782–786. [Google Scholar] [CrossRef]
- Rinaldi-Carmona, M.; Barth, F.; Millan, J.; Derocq, J.M.; Casellas, P.; Congy, C.; et al. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J Pharmacol Exp Ther 1998, 284, 644–650. [Google Scholar] [CrossRef]
- McGilveray, I.J. Pharmacokinetics of cannabinoids. Pain Res Manag 2005, 10, 15A–22A. [Google Scholar] [CrossRef]
- Nahas, G.; Leger, C.; Tocque, B.; Hoellinger, H. The kinetics of cannabinoid distribution and storage with special reference to the brain and testis. J Clin Pharmacol 1981, 21, 208S–214S. [Google Scholar] [CrossRef]
- Nahas, G.G. The pharmacokinetics of THC in fat and brain: resulting functional responses to marihuana smoking. Hum Psychopharmacol 2001, 16, 247–255. [Google Scholar] [CrossRef]
- Massa, F.; Marsicano, G.; Hermann, H.; Cannich, A.; Monory, K.; Cravatt, B.F.; et al. The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest 2004, 113, 1202–1209. [Google Scholar] [CrossRef]
- Ueda, Y.; Miyagawa, N.; Matsui, T.; Kaya, T.; Iwamura, H. Involvement of cannabinoid CB(2) receptor-mediated response and efficacy of cannabinoid CB(2) receptor inverse agonist, JTE-907, in cutaneous inflammation in mice. Eur J Pharmacol 2005, 520, 164–171. [Google Scholar] [CrossRef]
- Randall, M.D.; Kendall, D.A.; O’Sullivan, S. The complexities of the cardiovascular actions of cannabinoids. Br J Pharmacol 2004, 142, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.J. Endocannabinoids and vascular function. J Pharmacol Exp Ther 2000, 294, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Kunos, G.; Jarai, Z.; Batkai, S.; Goparaju, S.K.; Ishac, E.J.; Liu, J.; et al. Endocannabinoids as cardiovascular modulators. Chem Phys Lipids 2000, 108, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Batkai, S.; Kunos, G. Blood pressure regulation by endocannabinoids and their receptors. Neuropharmacology 2005, 48, 1130–1138. [Google Scholar] [CrossRef]
- Batkai, S.; Pacher, P.; Osei-Hyiaman, D.; Radaeva, S.; Liu, J.; Harvey-White, J.; et al. Endocannabinoids acting at cannabinoid1 receptors regulate cardiovascular function in hypertension. Circulation 2004, 110, 1996–2002. [Google Scholar] [CrossRef]
- Van Gaal, L.F.; Rissanen, A.M.; Scheen, A.J.; Ziegler, O; Rossner, S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 2005, 365, 1389–1397. [Google Scholar] [CrossRef]
- S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 2005, 365, 1389–1397. [CrossRef]
- Despres, J.P.; Golay, A.; Sjostrom, L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med 2005, 353, 2121–2134. [Google Scholar] [CrossRef]
- Gebremedhin, D.; Lange, A.R.; Campbell, W.B.; Hillard, C.J.; Harder, D.R. Cannabinoid CB1 receptor of cat cerebral arterial muscle functions to inhibit L-type Ca2+ channel current. Am J Physiol 2085, 276, H2085–H2093. [Google Scholar] [CrossRef]
- Liu, J.; Gao, B.; Mirshahi, F.; Sanyal, A.J.; Khanolkar, A.D.; Makriyannis, A.; et al. Functional CB1 cannabinoid receptors in human vascular endothelial cells. Biochem J 2000, 346 Pt 3, 835–840. [Google Scholar] [CrossRef]
- Sugiura, T.; Kodaka, T.; Nakane, S.; Kishimoto, S.; Kondo, S.; Waku, K. Detection of an endogenous cannabimimetic molecule, 2-arachidonoylglycerol, and cannabinoid CB1 receptor mRNA in human vascular cells: is 2-arachidonoylglycerol a possible vasomodulator? Biochem Biophys Res Commun 1998, 243, 838–843. [Google Scholar] [CrossRef]
- Klein, T.W. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol 2005, 5, 400–411. [Google Scholar] [CrossRef]
© 2006 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Steffens, S. Cannabinoids for Therapeutic Use in Atherosclerosis. Cardiovasc. Med. 2006, 9, 268. https://doi.org/10.4414/cvm.2006.01188
Steffens S. Cannabinoids for Therapeutic Use in Atherosclerosis. Cardiovascular Medicine. 2006; 9(7):268. https://doi.org/10.4414/cvm.2006.01188
Chicago/Turabian StyleSteffens, Sabine. 2006. "Cannabinoids for Therapeutic Use in Atherosclerosis" Cardiovascular Medicine 9, no. 7: 268. https://doi.org/10.4414/cvm.2006.01188
APA StyleSteffens, S. (2006). Cannabinoids for Therapeutic Use in Atherosclerosis. Cardiovascular Medicine, 9(7), 268. https://doi.org/10.4414/cvm.2006.01188



