Utility of Echocardiography for Tailoring Cardiac Resynchronisation Therapy
Summary
Resumé
Introduction
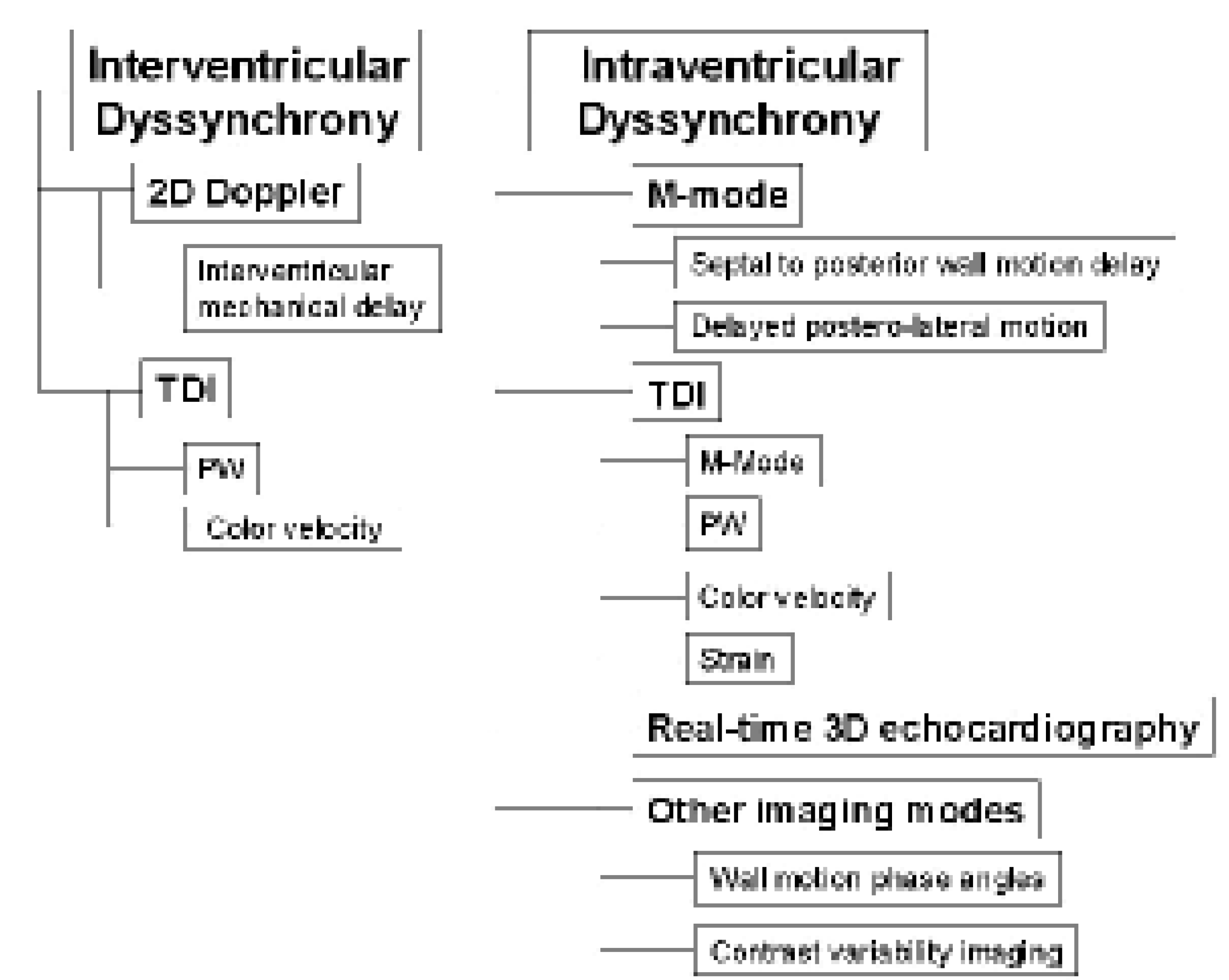
Evaluation of cardiac dyssynchrony for patient selection
M-mode echocardiography
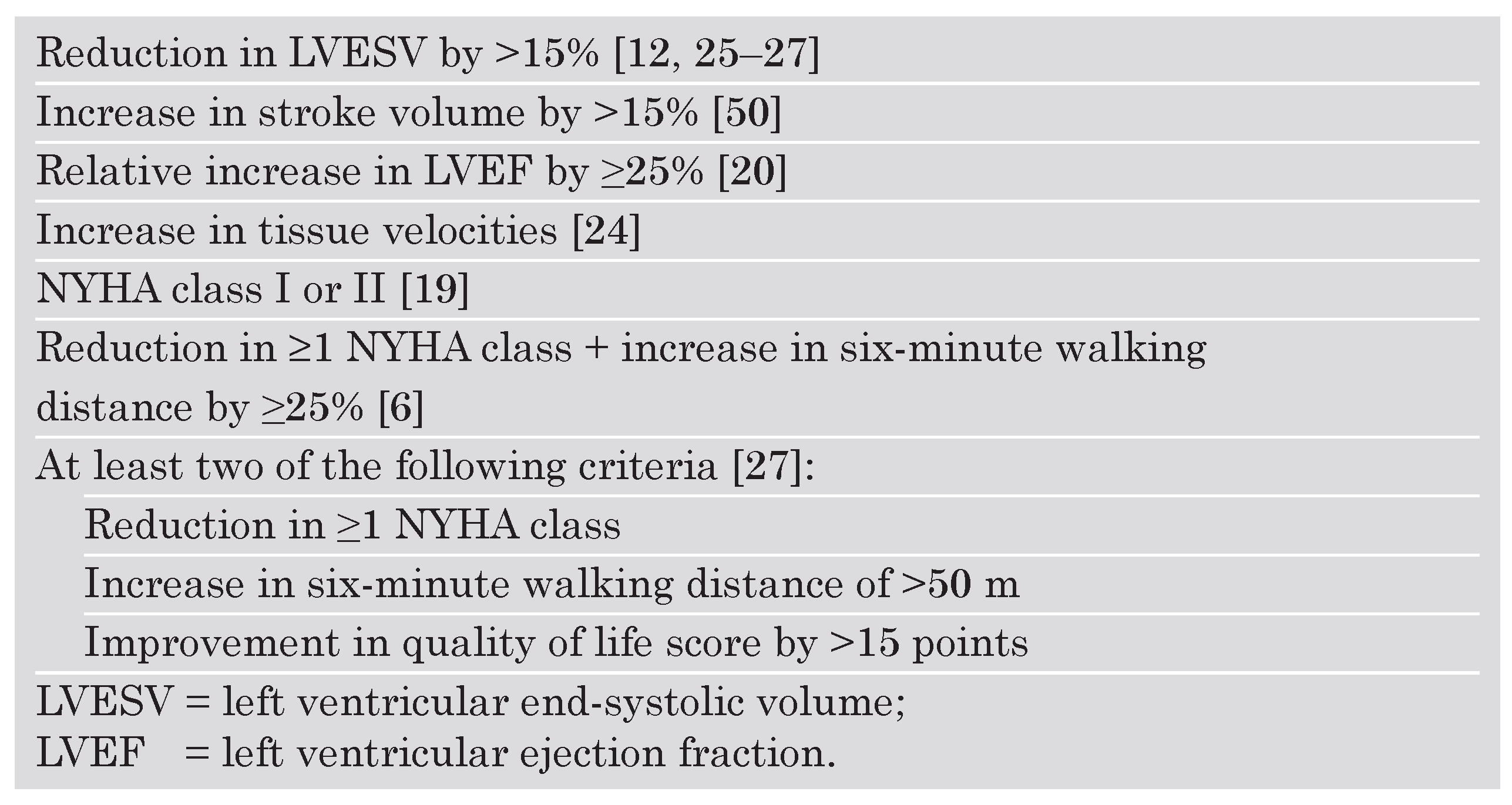
Conventional Doppler echography
 |
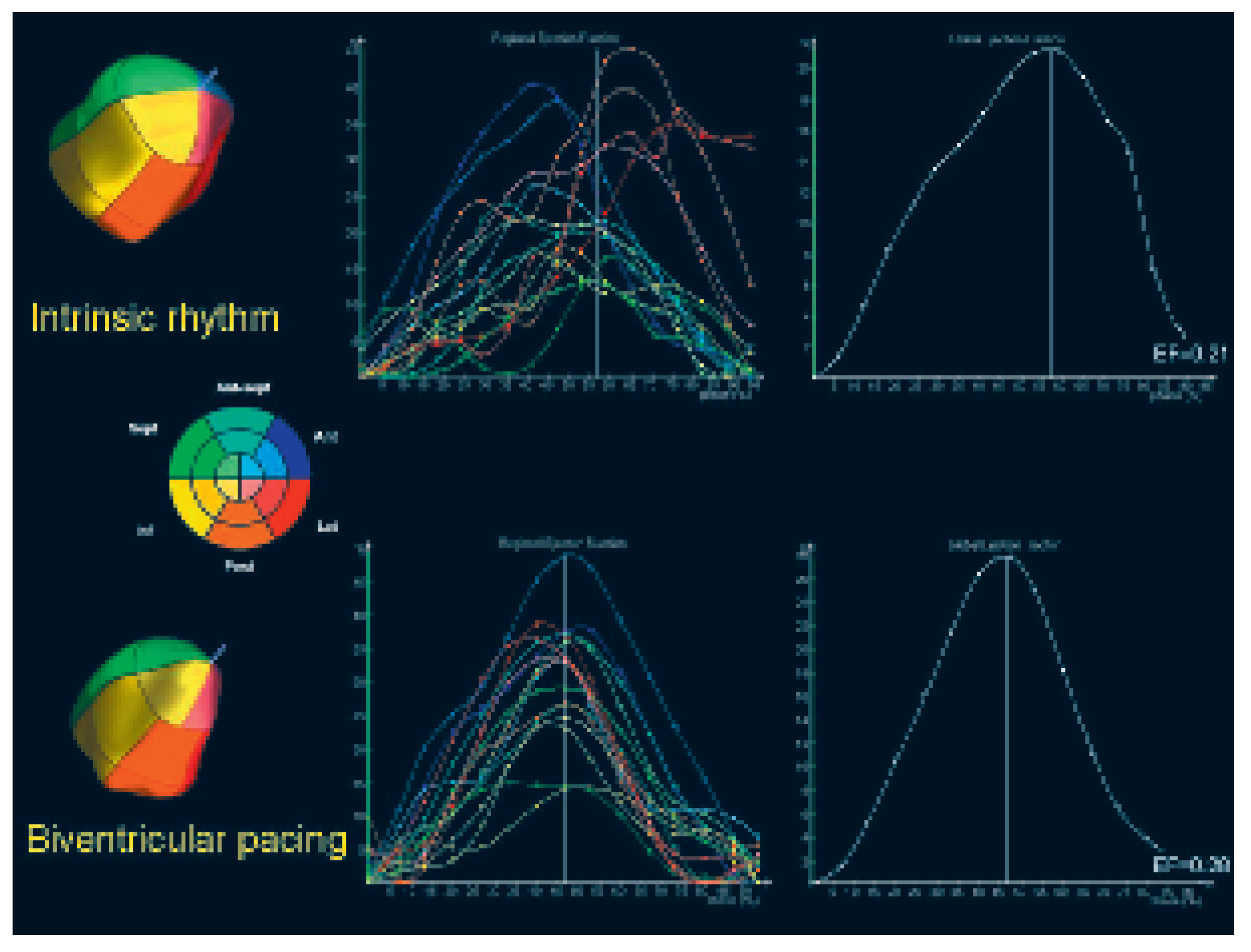
Real-time 3D echocardiography
Other imaging modes
Utility of echocardiography for the implant procedure and for targeting lead placement
Device optimisation by echocardiography
AV interval optimisation
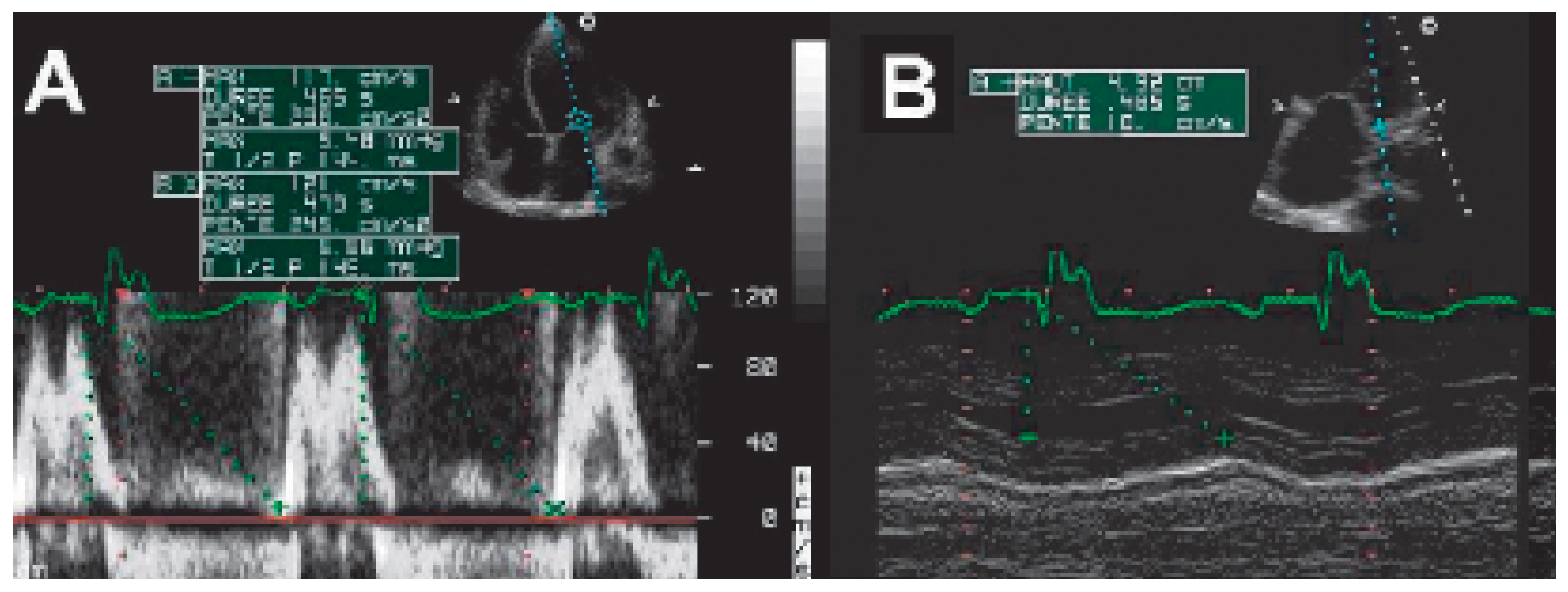
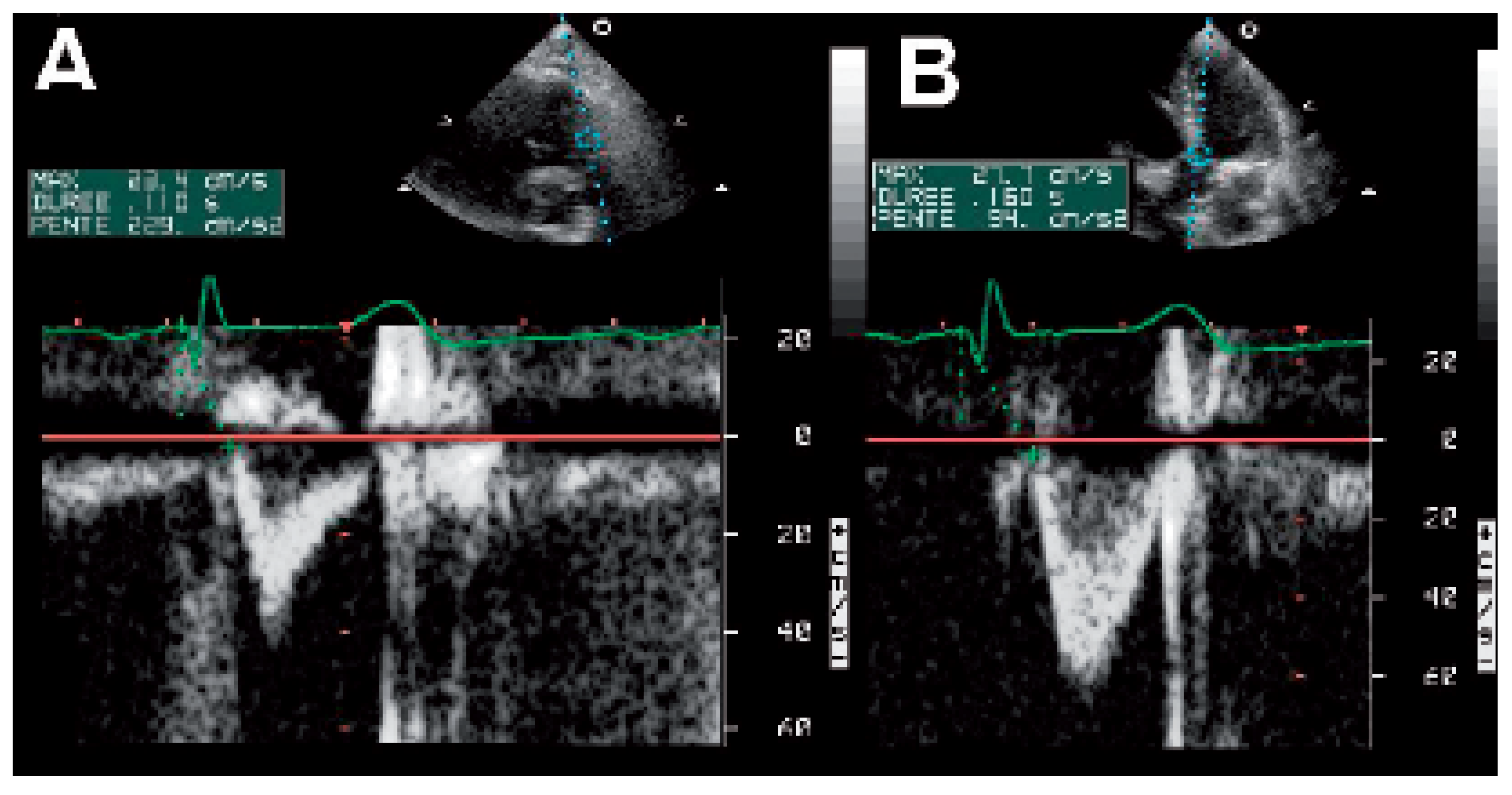
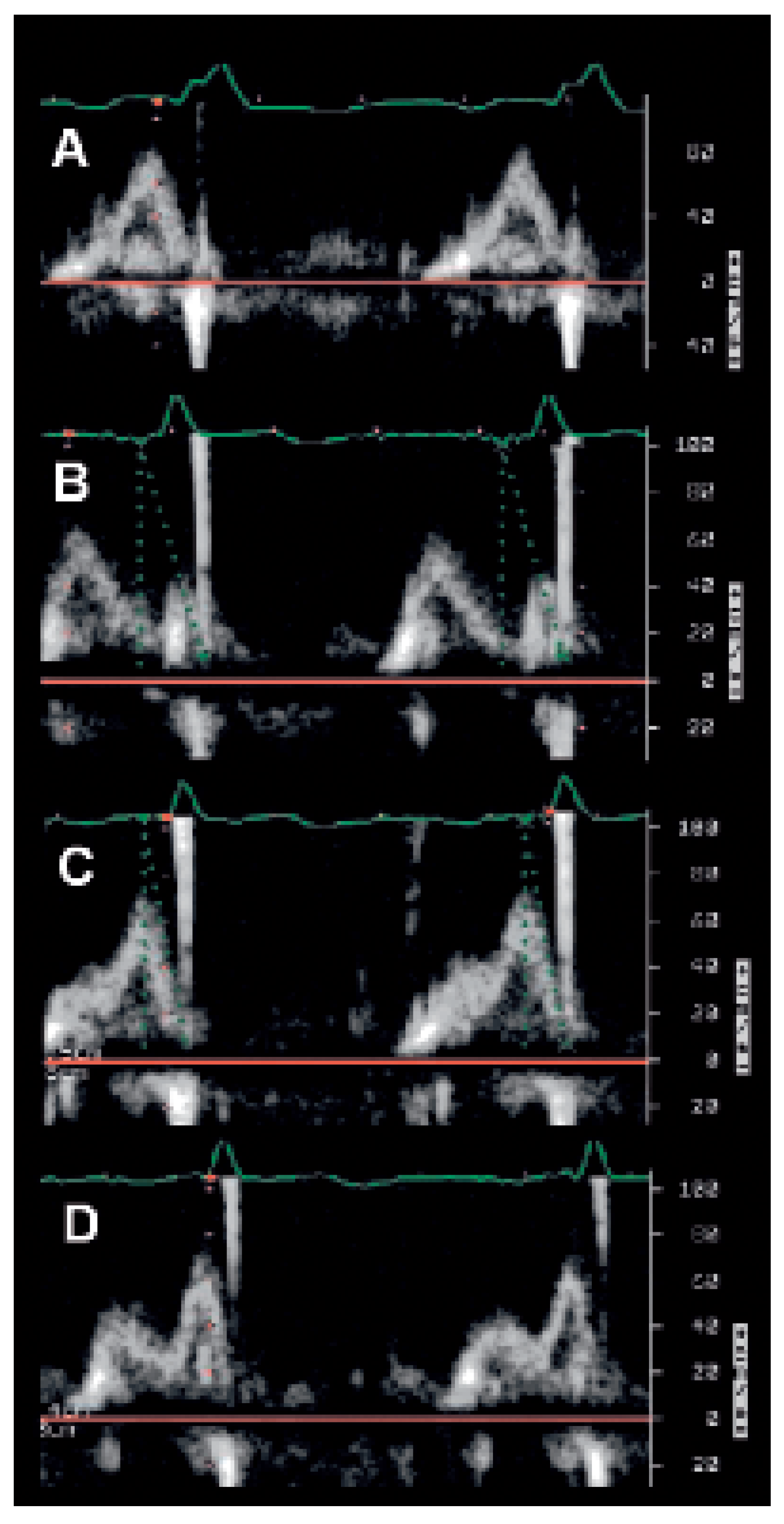
The Ritter method (Figure 6)
The iterative method
The aortic velocity-time integral (VTI) method
VV interval optimisation
Conclusion
References
- Abraham, W.T.; Fisher, W.G.; Smith, A.L.; Delurgio, D.B.; Leon, A.R.; Loh, E.; et al. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002, 346, 1845–1853. [Google Scholar] [CrossRef]
- Bristow, M.R.; Saxon, L.A.; Boehmer, J.; Krueger, S.; Kass, D.A.; De Marco, T.; et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004, 350, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.G.; Daubert, J.C.; Erdmann, E.; Freemantle, N.; Gras, D.; Kappenberger, L.; et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005, 352, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.S.; Curry, C.W.; Wyman, B.T.; Kramer, A.; Declerck, J.; Talbot, M.; et al. Predictors of systolic augmentation from left ventricular preexcitation in patients with dilated cardiomyopathy and intraventricular conduction delay. Circulation 2000, 101, 2703–2709. [Google Scholar] [CrossRef] [PubMed]
- Fauchier, L.; Marie, O.; Casset-Senon, D.; Babuty, D.; Cosnay, P.; Fauchier, J.P. Interventricular and intraventricular dyssynchrony in idiopathic dilated cardiomyopathy: A prognostic study with fourier phase analysis of radionuclide angioscintigraphy. J Am Coll Cardiol 2002, 40, 2022–2030. [Google Scholar] [CrossRef]
- Bax, J.J.; Bleeker, G.B.; Marwick, T.H.; Molhoek, S.G.; Boersma, E.; Steendijk, P.; et al. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol 2004, 44, 1834–1840. [Google Scholar] [CrossRef]
- Yu, C.M.; Chau, E.; Sanderson, J.E.; Fan, K.; Tang, M.O.; Fung, W.H.; et al. Tissue Doppler echocardiographic evidence of reverse remodeling and improved synchronicity by simultaneously delaying regional contraction after biventricular pacing therapy in heart failure. Circulation 2002, 105, 438–445. [Google Scholar] [CrossRef]
- Fauchier, L.; Marie, O.; Casset-Senon, D.; Babuty, D.; Cosnay, P.; Fauchier, J.P. Reliability of QRS duration and morphology on surface electrocardiogram to identify ventricular dyssynchrony in patients with idiopathic dilated cardiomyopathy. Am J Cardiol 2003, 92, 341–344. [Google Scholar] [CrossRef]
- Yu, C.M.; Lin, H.; Zhang, Q.; Sanderson, J.E. High prevalence of left ventricular systolic and diastolic asynchrony in patients with congestive heart failure and normal QRS duration. Heart 2003, 89, 54–60. [Google Scholar] [CrossRef]
- Bader, H.; Garrigue, S.; Lafitte, S.; Reuter, S.; Jais, P.; Haissaguerre, M.; et al. Intra-left ventricular electromechanical asynchrony. A new independent predictor of severe cardiac events in heart failure patients. J Am Coll Cardiol 2004, 43, 248–256. [Google Scholar] [CrossRef]
- Achilli, A.; Sassara, M.; Ficili, S.; Pontillo, D.; Achilli, P.; Alessi, C.; et al. Long-term effectiveness of cardiac resynchronization therapy in patients with refractory heart failure and “narrow” QRS. J Am Coll Cardiol 2003, 42, 2117–2124. [Google Scholar] [CrossRef]
- Pitzalis, M.V.; Iacoviello, M.; Romito, R.; Massari, F.; Rizzon, B.; Luzzi, G.; et al. Cardiac resynchronization therapy tailored by echocardiographic evaluation of ventricular asynchrony. J Am Coll Cardiol 2002, 40, 1615–1622. [Google Scholar] [CrossRef]
- Pitzalis, M.V.; Iacoviello, M.; Romito, R.; Guida, P.; De Tommasi, E.; Luzzi, G.; et al. Ventricular asynchrony predicts a better outcome in patients with chronic heart failure receiving cardiac resynchronization therapy. J Am Coll Cardiol 2005, 45, 65–69. [Google Scholar] [CrossRef]
- Diaz-Infante, E.; Mont Girbau, J.; Vidal, B.; Sitges, M.; Tamborero, D.; Roig, E.; et al. Is septal-to-posterior wall delay measured by M-mode a good predictor of response to resynchronisation therapy? (abstract). Europace 2005, 7, 145. [Google Scholar]
- Burri, H.; Müller, H.; Lerch, R. Measuring ventricular asynchrony using standard echocardiography or by tissue Doppler imaging: Do the results agree? (abstract). Eur Heart J 2005, 26, S211. [Google Scholar]
- Adamson, P.B.; St John Sutton, M.; Plappert, T.; Abraham, W. Echo-defined ventricular dysynchrony predicts magnitude of response to cardiac resynchronization (abstract). Circulation 2002, 106, 381. [Google Scholar]
- St John Sutton, M.; Plappert, T.; Hilpisch, K.E.; Chinchoy, E. Baseline aortic pre-ejection interval (bAPEI) as a predictor of response to cardiac resynchronization therapy (abstract). Circulation 2002, 106, 380. [Google Scholar]
- Bordachar, P.; Garrigue, S.; Lafitte, S.; Reuter, S.; Jais, P.; Haissaguerre, M.; et al. Interventricular and intra-left ventricular electromechanical delays in right ventricular paced patients with heart failure: Implications for upgrading to biventricular stimulation. Heart 2003, 89, 1401–1405. [Google Scholar] [CrossRef][Green Version]
- Garrigue, S.; Reuter, S.; Labeque, J.N.; Jais, P.; Hocini, M.; Shah, D.C.; et al. Usefulness of biventricular pacing in patients with congestive heart failure and right bundle branch block. Am J Cardiol 2001, 88, 1436–1441. [Google Scholar] [CrossRef]
- Penicka, M.; Bartunek, J.; De Bruyne, B.; Vanderheyden, M.; Goethals, M.; De Zutter, M.; et al. Improvement of left ventricular function after cardiac resynchronization therapy is predicted by tissue Doppler imaging echocardiography. Circulation 2004, 109, 978–983. [Google Scholar] [CrossRef]
- Bordachar, P.; Lafitte, S.; Reuter, S.; Sanders, P.; Jaïs, P.; Haïssaguerre, M.; et al. Echocardiographic parameters of ventricular dyssynchrony validation in patients with heart failure using sequential biventricular pacing. J Am Coll Cardiol 2004, 44, 2157–2165. [Google Scholar] [CrossRef]
- Bordachar, P.; Lafitte, S.; Reuter, S.; Garrigue, S.; Sanders, P.; Roudaut, R.; et al. Biventricular pacing and left ventricular pacing in heart failure: Similar hemodynamic improvement despite marked electromechanical differences. J Cardiovasc Electrophysiol 2004, 15, 1342–1347. [Google Scholar] [CrossRef]
- Tada, H.; Toide, H.; Naito, S.; Kurosaki, K.; Ito, S.; Miyaji, K.; et al. Tissue Doppler imaging and strain Doppler imaging as modalities for predicting clinical improvement in patients receiving biventricular pacing. Circ J 2005, 69, 194–200. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sogaard, P.; Egeblad, H.; Kim, W.Y.; Jensen, H.K.; Pedersen, A.K.; Kristensen, B.O.; et al. Tissue Doppler imaging predicts improved systolic performance and reversed left ventricular remodeling during long-term cardiac resynchronization therapy. J Am Coll Cardiol 2002, 40, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.M.; Fung, W.H.; Lin, H.; Zhang, Q.; Sanderson, J.E.; Lau, C.P. Predictors of left ventricular reverse remodeling after cardiac resynchronization therapy for heart failure secondary to idiopathic dilated or ischemic cardiomyopathy. Am J Cardiol 2003, 91, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.M.; Fung, J.W.H.; Zhang, Q.; Chan, C.K.; Chan, Y.S.; Lin, H.; et al. Tissue Doppler imaging is superior to strain rate imaging and postsystolic shortening on the prediction of reverse remodeling in both ischemic and nonischemic heart failure after cardiac resynchronization therapy. Circulation 2004, 110, 66–73. [Google Scholar] [CrossRef]
- Notabartolo, D.; Merlino, J.D.; Smith, A.L.; DeLurgio, D.B.; Vera, F.V.; Easley, K.A.; et al. Usefulness of the peak velocity difference by tissue Doppler imaging technique as an effective predictor of response to cardiac resynchronization therapy. Am J Cardiol 2004, 94, 817–820. [Google Scholar] [CrossRef]
- Yu, C.M.; Lin, H.; Ho, P.C.; Yang, H. Assessment of left and right ventricular systolic and diastolic synchronicity in normal subjects by tissue Doppler echocardiography and the effects of age and heart rate. Echocardiography 2003, 20, 19–27. [Google Scholar] [CrossRef]
- Pai, R.G.; Gill, K.S. Amplitudes, durations, and timings of apically directed left ventricular myocardial velocities: I. Their normal pattern and coupling to ventricular filling and ejection. J Am Soc Echocardiogr 1998, 11, 105–111. [Google Scholar] [CrossRef]
- Breithardt, O.A.; Stellbrink, C.; Herbots, L.; Claus, P.; Sinha, A.M.; Bijnens, B.; et al. Cardiac resynchronization therapy can reverse abnormal myocardial strain distribution in patients with heart failure and left bundle branch block. J Am Coll Cardiol 2003, 42, 486–494. [Google Scholar] [CrossRef]
- Popovic, Z.B.; Grimm, R.A.; Perlic, G.; Chinchoy, E.; Geraci, M.; Sun, J.P.; et al. Noninvasive assessment of cardiac resynchronization therapy for congestive heart failure using myocardial strain and left ventricular peak power as parameters of myocardial synchrony and function. J Cardiovasc Electrophysiol 2002, 13, 1203–1208. [Google Scholar] [CrossRef]
- Yu, C.M.; Fung, W.H.; Lin, H.; Zhang, Q.; Sanderson, J.E.; Lau, C.P. Predictors of left ventricular reverse remodeling after cardiac resynchronization therapy for heart failure secondary to idiopathic dilated or ischemic cardiomyopathy. Am J Cardiol 2003, 91, 684–688. [Google Scholar] [CrossRef]
- El-Chami. Assessment of the dyssynchrony index as a predictor of response to resynchronization therapy. (abstract). Circulation 2004, 110, 724.
- Gorcsan, J., 3rd; Kanzaki, H.; Bazaz, R.; Dohi, K.; Schwartzman, D. Usefulness of echocardiographic tissue synchronization imaging to predict acute response to cardiac resynchronization therapy. Am J Cardiol 2004, 93, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Kapetanakis, S.; Kearney, M.; Siva, A.; Gall, N.; Cooklin, M.; Monaghan, M. Real-time three-dimensional echocardiography: A novel technique to quantify global left ventricular mechanical dyssynchrony. Circulation 2005, 112, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Breithardt, O.A.; Stellbrink, C.; Kramer, A.P.; Sinha, A.M.; Franke, A.; Salo, R.; et al. Echocardiographic quantification of left ventricular asynchrony predicts an acute hemodynamic benefit of cardiac resynchronization therapy. J Am Coll Cardiol 2002, 40, 536–545. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Murabayashi, T.; Fetics, B.J.; Nelson, G.S.; Samejima, H.; Nevo, E.; et al. Quantitation of basal dyssynchrony and acute resynchronization from left or biventricular pacing by novel echo-contrast variability imaging. J Am Coll Cardiol 2002, 39, 2052–2058. [Google Scholar] [CrossRef][Green Version]
- Ansalone, G.; Giannantoni, P.; Ricci, R.; Trambaiolo, P.; Fedele, F.; Santini, M. Doppler myocardial imaging to evaluate the effectiveness of pacing sites in patients receiving biventricular pacing. J Am Coll Cardiol 2002, 39, 489–499. [Google Scholar] [CrossRef]
- Kindermann, M.; Frohlig, G.; Doerr, T.; Schieffer, H. Optimizing the AV delay in DDD pacemaker patients with high degree AV block: Mitral valve Doppler versus impedance cardiography. Pacing Clin Electrophysiol 1997, 20, 2453–2462. [Google Scholar] [CrossRef]
- Dupuis, J.M.; Kobeissi, A.; Vitali, L.; Gaggini, G.; Merheb, M.; Rouleau, F.; et al. Programming optimal atrioventricular delay in dual chamber pacing using peak endocardial acceleration: Comparison with a standard echocardiographic procedure. Pacing Clin Electrophysiol 2003, 26, 210–213. [Google Scholar] [CrossRef]
- Sawhney, N.; Waggoner, A.; Garhwal, S.; Chawla, M.; J; Faddis, M. Randomized prospective trial of atrioventricular delay programming for cardiac resynchronization therapy. Heart Rhythm 2004, 1, 526–567. [Google Scholar] [CrossRef]
- Scharf, C.; Li, P.; Muntwyler, J.; Chugh, A.; Oral, H.; Pelosi, F.; et al. Rate-dependent AV delay optimization in cardiac resynchronization therapy. Pacing Clin Electro 2005, 28, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Burri, H.; Sunthorn, H.; Zaza, S.; Somsen, A.; Fleury, E.; Righetti, A.; et al. Effect of sequential biventricular pacing on interventricular asynchrony (abstract). Circulation 2004, 110, 724. [Google Scholar]
- Sogaard, P.; Egeblad, H.; Pedersen, A.K.; Kim, W.Y.; Kristensen, B.O.; Hansen, P.S.; et al. Sequential versus simultaneous biventricular resynchronization for severe heart failure: Evaluation by tissue Doppler imaging. Circulation 2002, 106, 2078–2084. [Google Scholar] [CrossRef]
- Mortensen, P.T.; Sogaard, P.; Mansour, H.; Ponsaille, J.; Gras, D.; Lazarus, A.; et al. Sequential biventricular pacing: Evaluation of safety and efficacy. Pacing Clin Electrophysiol 2004, 27, 339–345. [Google Scholar] [CrossRef]
- Riedlbauchova, L.; Kautzner, J.; Fridl, P. Influence of different atrioventricular and interventricular delays on cardiac output during cardiac resynchronization therapy. Pacing Clin Electrophysiol 2005, 28, S19–S23. [Google Scholar] [CrossRef]
- Hunt, S.A.; Abraham, W.T.; Chin, M.H.; Feldman, A.M.; Francis, G.S.; Ganiats, T.G.; et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2005, 112, 1825–1852. [Google Scholar]
- Swedberg, K.; Cleland, J.; Dargie, H.; Drexler, H.; Follath, F.; Komajda, M.; et al. Guidelines for the diagnosis and treatment of chronic heart failure: Executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J 2005, 26, 1115–1140. [Google Scholar] [CrossRef]
- Burri, H.; Lerch, R. Visualization of cardiac resynchronization using real-time three-dimensional echocardiography. Heart Rhythm 2005, 2, 447–448. [Google Scholar] [CrossRef]
- Gorcsan, J.; 3rd Kanzaki, H.; Bazaz, R.; Dohi, K.; Schwartzman, D. Usefulness of echocardiographic tissue synchronization imaging to predict acute response to cardiac resynchronization therapy. Am J Cardiol 2004, 93, 1178–1181. [Google Scholar] [CrossRef]

© 2006 by the authors. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Burri, H.; Lerch, R. Utility of Echocardiography for Tailoring Cardiac Resynchronisation Therapy. Cardiovasc. Med. 2006, 9, 188. https://doi.org/10.4414/cvm.2006.01175
Burri H, Lerch R. Utility of Echocardiography for Tailoring Cardiac Resynchronisation Therapy. Cardiovascular Medicine. 2006; 9(5):188. https://doi.org/10.4414/cvm.2006.01175
Chicago/Turabian StyleBurri, Haran, and René Lerch. 2006. "Utility of Echocardiography for Tailoring Cardiac Resynchronisation Therapy" Cardiovascular Medicine 9, no. 5: 188. https://doi.org/10.4414/cvm.2006.01175
APA StyleBurri, H., & Lerch, R. (2006). Utility of Echocardiography for Tailoring Cardiac Resynchronisation Therapy. Cardiovascular Medicine, 9(5), 188. https://doi.org/10.4414/cvm.2006.01175




