Chronic Thromboembolic Pulmonary Hypertension
Abstract
Introduction
Epidemiology
Pathogenesis
Clinical presentation
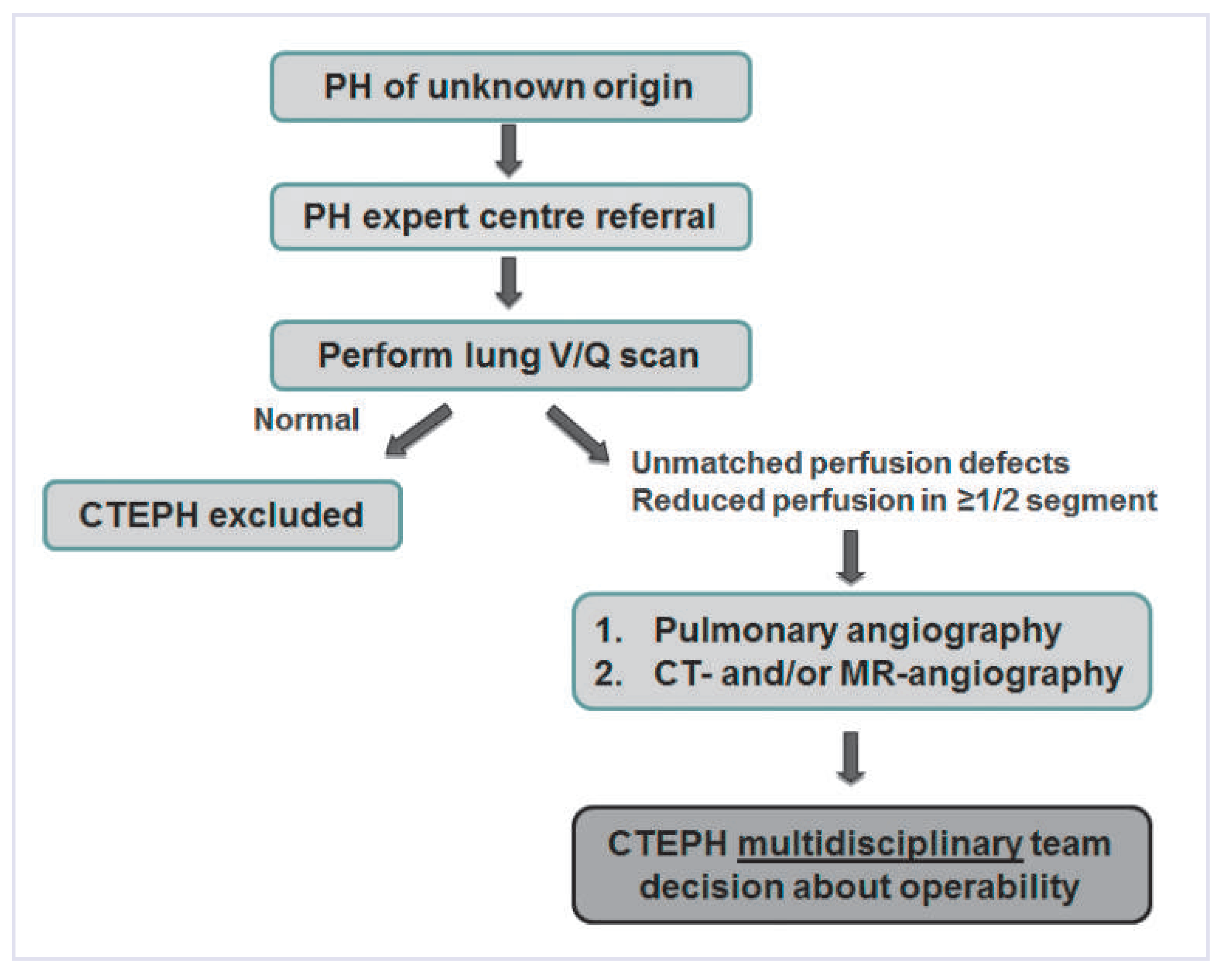
CTEPH diagnosis
- –
- VQ scan is recommended as screening tool for chronic thromboembolic disease in all patients with no obvious reason for precapillary PH.
- –
- Pulmonary angiography remains the gold standard for confirmation of chronic thromboembolic disease and evaluation of operability.
- –
- High-quality multidetector CTPA may be a suitable alternative to pulmonary angiography in centres with experience in CTEPH.
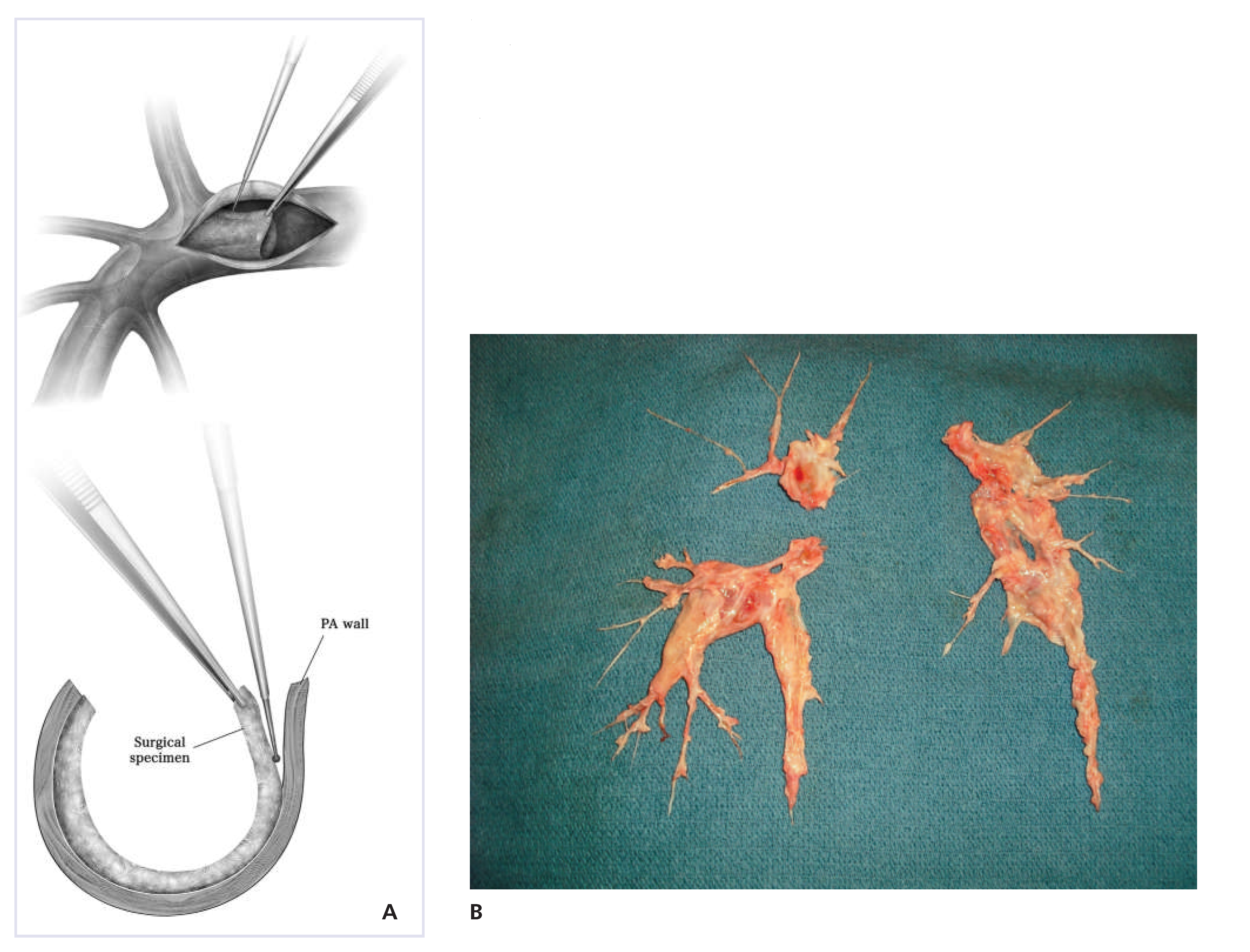
Surgical therapy
Medical therapy
Funding/potential competing interests:
References
- Fedullo, P.F.; Auger, W.R.; Kerr, K.M.; Rubin, L.J. Chronic thromboembolic pulmonary hypertension. N Engl J Med. 2001, 345, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Pengo, V.; Lensing, A.W.; Prins, M.H.; Marchiori, A.; Davidson, B.L.; Tiozzo, F.; et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004, 350, 2257–2264. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.P.; Madani, M.; Mayer, E.; Kerr, K.; Kim, N.; Klepetko, W.; et al. Surgical treatment of chronic thromboembolic pulmonary hypertension. Eur Respir J. 2013, 41, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, H.A.; D’Armini, A.M.; Grimminger, F.; Hoeper, M.M.; Jansa, P.; Kim, N.H.; et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013, 369, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Delcroix, M.; Jenkins, D.P.; Channick, R.; Dartevelle, P.; Jansa, P.; et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol. 2013, 62 (Suppl. 25), D92–D99. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.M.; Pesavento, R.; Bonderman, D.; Yuan, J.X. Risk factors and basic mechanisms of chronic thromboembolic pulmonary hypertension: A current understanding. Eur Respir J. 2013, 41, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Miniati, M.; Monti, S.; Bottai, M.; Scoscia, E.; Bauleo, C.; Tonelli, L.; et al. Survival and restoration of pulmonary perfusion in a long-term follow-up of patients after acute pulmonary embolism. Medicine 2006, 85, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Dentali, F.; Donadini, M.; Gianni, M.; Bertolini, A.; Squizzato, A.; Venco, A.; et al. Incidence of chronic pulmonary hypertension in patients with previous pulmonary embolism. Thromb Res. 2009, 124, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Lindmarker, P.; Johnsson, H.; Juhlin-Dannfelt, A.; Jorfeldt, L. Pulmonary embolism: One-year follow-up with echocardiography doppler and five-year survival analysis. Circulation 1999, 99, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Bonderman, D.; Jakowitsch, J.; Adlbrecht, C.; Schemper, M.; Kyrle, P.A.; Schonauer, V.; et al. Medical conditions increasing the risk of chronic thromboembolic pulmonary hypertension. Thromb Haemost. 2005, 93, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Bonderman, D.; Skoro-Sajer, N.; Jakowitsch, J.; Adlbrecht, C.; Dunkler, D.; Taghavi, S.; et al. Predictors of outcome in chronic thromboembolic pulmonary hypertension. Circulation 2007, 115, 2153–2158. [Google Scholar] [CrossRef] [PubMed]
- Morris, T.A.; Marsh, J.J.; Chiles, P.G.; Auger, W.R.; Fedullo, P.F.; Woods, V.L., Jr. Fibrin derived from patients with chronic thromboembolic pulmonary hypertension is resistant to lysis. Am J Respir Crit Care Med. 2006, 173, 1270–1275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonderman, D.; Turecek, P.L.; Jakowitsch, J.; Weltermann, A.; Adlbrecht, C.; Schneider, B.; et al. High prevalence of elevated clotting factor VIII in chronic thromboembolic pulmonary hypertension. Thromb Haemost. 2003, 90, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R.; Mears, J.G.; Barst, R.J. Continuous infusion of prostacyclin normalizes plasma markers of endothelial cell injury and platelet aggregation in primary pulmonary hypertension. Circulation 1997, 96, 2782–2784. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.L.; Szydlo, R.; Gibbs, S.; Laffan, M. Hereditary and acquired thrombotic risk factors for chronic thromboembolic pulmonary hypertension. Blood Coagul Fibrinolysis 2010, 21, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Lewczuk, J.; Piszko, P.; Jagas, J.; Porada, A.; Wojciak, S.; Sobkowicz, B.; et al. Prognostic factors in medically treated patients with chronic pulmonary embolism. Chest 2001, 119, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Jaff, M.R.; McMurtry, M.S.; Archer, S.L.; Cushman, M.; Goldenberg, N.; Goldhaber, S.Z.; et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: A scientific statement from the American Heart Association. Circulation 2011, 123, 1788–1830. [Google Scholar] [CrossRef] [PubMed]
- McNeil, K. Assessment of patients with pulmonary hypertension. Respiration 2008, 76, 373–374. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E. Surgical and post-operative treatment of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2010, 19, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Simonneau, G. Vasodilators in patients with chronic obstructive pulmonary disease and pulmonary hypertension: Not ready for prime time! Am J Respir Crit Care Med. 2010, 181, 202–203. [Google Scholar] [CrossRef] [PubMed]
- Waltham, M.; Burnand, K.G.; Collins, M.; Smith, A. Vascular endothelial growth factor and basic fibroblast growth factor are found in resolving venous thrombi. Journal of vascular surgery 2000, 32, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Distler, J.H.; Hirth, A.; Kurowska-Stolarska, M.; Gay, R.E.; Gay, S.; Distler, O. Angiogenic and angiostatic factors in the molecular control of angiogenesis. Q. J. Nucl. Med. Off. Publ. Ital. Assoc. Nucl. Med. 2003, 47, 149–161. [Google Scholar] [PubMed]
- Sakao, S.; Hao, H.; Tanabe, N.; Kasahara, Y.; Kurosu, K.; Tatsumi, K. Endothelial-like cells in chronic thromboembolic pulmonary hypertension: Crosstalk with myofibroblast-like cells. Respir Res. 2011, 12, 109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kimura, H.; Okada, O.; Tanabe, N.; Tanaka, Y.; Terai, M.; Takiguchi, Y.; et al. Plasma monocyte chemoattractant protein-1 and pulmonary vascular resistance in chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2001, 164, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Moser, K.M.; Auger, W.R.; Fedullo, P.F. Chronic major-vessel thromboembolic pulmonary hypertension. Circulation 1990, 81, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Wallis, J.D.; Anderson, K.C.; Miller, E.K. Single neurons in prefrontal cortex encode abstract rules. Nature 2001, 411, 953–956. [Google Scholar] [CrossRef] [PubMed]
- Kapitan, K.S.; Buchbinder, M.; Wagner, P.D.; Moser, K.M. Mechanisms of hypoxemia in chronic thromboembolic pulmonary hypertension. Am Rev Respir Dis. 1989, 139, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, V.V.; Langer, A.; Tan, M.; Clements, P.J.; Oudiz, R.J.; Tapson, V.F.; et al. Contemporary trends in the diagnosis and management of pulmonary arterial hypertension: An initiative to close the care gap. Chest 2013, 143, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Opitz, I.; de Perrot, M. Technique of pulmonary thromboendarterectomy. Oper. Tech. Thorac. Cardiovasc. Surg. 2012, 17, 168–180. [Google Scholar] [CrossRef]
- Mayer, E.; Jenkins, D.; Lindner, J.; D’Armini, A.; Kloek, J.; Meyns, B.; et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: Results from an international prospective registry. J Thorac Cardiovasc Surg. 2011, 141, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Madani, M.M.; Auger, W.R.; Pretorius, V.; Sakakibara, N.; Kerr, K.M.; Kim, N.H.; et al. Pulmonary endarterectomy: Recent changes in a single institution’s experience of more than 2,700 patients. Ann Thorac Surg. 2012, 94, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Pepke-Zaba, J.; Hoeper, M.M.; Humbert, M. Chronic thromboembolic pulmonary hypertension: Advances from bench to patient management. Eur Respir J. 2013, 41, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Vuylsteke, A.; Sharples, L.; Charman, G.; Kneeshaw, J.; Tsui, S.; Dunning, J.; et al. Circulatory arrest versus cerebral perfusion during pulmonary endarterectomy surgery (PEACOG): A randomised controlled trial. Lancet 2011, 378, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Mydin, M.; Berman, M.; Klein, A.; Tsui, S.; Dunning, J.; Valchanov, K.; et al. Extracorporeal membrane oxygenation as a bridge to pulmonary endarterectomy. Ann Thorac Surg. 2011, 92, e101–3. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, J.A.; Goldhaber, S.Z.; Lock, J.E.; Ferndandes, S.M.; Landzberg, M.J. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation 2001, 103, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, R.; Kataoka, M.; Inami, T.; Shimura, N.; Ishiguro, H.; Fukuda, K.; et al. Safety and efficacy of percutaneous transluminal pulmonary angioplasty in elderly patients. Int J Cardiol. 2014, 175, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, K.; Fukumoto, Y.; Satoh, K.; Nochioka, K.; Miura, Y.; Aoki, T.; et al. Percutaneous transluminal pulmonary angioplasty markedly improves pulmonary hemodynamics and long-term prognosis in patients with chronic thromboembolic pulmonary hypertension. Circ J. 2012, 76, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, M.; Satoh, T.; Manabe, T.; Anzai, T.; Yoshikawa, T.; Mitamura, H.; et al. Oral sildenafil improves primary pulmonary hypertension refractory to epoprostenol. Circ J. 2005, 69, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, H.; Ogawa, A.; Munemasa, M.; Mikouchi, H.; Ito, H.; Matsubara, H. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ. Cardiovasc. Interv. 2012, 5, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Rollins, B.M.; Silva, M.A.; Donovan, J.L.; Kanaan, A.O. Evaluation of Oral Anticoagulants for the Extended Treatment of Venous Thromboembolism Using a Mixed-Treatment Comparison, Meta-Analytic Approach. Clin. Ther. 2014. [CrossRef] [PubMed]
- Goldhaber, S.Z.; Bounameaux, H. Pulmonary embolism and deep vein thrombosis. Lancet 2012, 379, 1835–1846. [Google Scholar] [CrossRef] [PubMed]
- Ogino, H.; Ando, M.; Matsuda, H.; Minatoya, K.; Sasaki, H.; Nakanishi, N.; et al. Japanese single-center experience of surgery for chronic thromboembolic pulmonary hypertension. Ann Thorac Surg. 2006, 82, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Jais, X.; D’Armini, A.M.; Jansa, P.; Torbicki, A.; Delcroix, M.; Ghofrani, H.A.; et al. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT (Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. J Am Coll Cardiol. 2008, 52, 2127–2134. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Müller-Mottet, S.; Hasler, E.; Opitz, I.; Weder, W.; Schuepbach, R.A.; Speich, R.; Ulrich, S. Chronic Thromboembolic Pulmonary Hypertension. Cardiovasc. Med. 2014, 17, 328. https://doi.org/10.4414/cvm.2014.00285
Müller-Mottet S, Hasler E, Opitz I, Weder W, Schuepbach RA, Speich R, Ulrich S. Chronic Thromboembolic Pulmonary Hypertension. Cardiovascular Medicine. 2014; 17(11):328. https://doi.org/10.4414/cvm.2014.00285
Chicago/Turabian StyleMüller-Mottet, Séverine, Elisabeth Hasler, Isabelle Opitz, Walter Weder, Reto A. Schuepbach, Rudolf Speich, and Silvia Ulrich. 2014. "Chronic Thromboembolic Pulmonary Hypertension" Cardiovascular Medicine 17, no. 11: 328. https://doi.org/10.4414/cvm.2014.00285
APA StyleMüller-Mottet, S., Hasler, E., Opitz, I., Weder, W., Schuepbach, R. A., Speich, R., & Ulrich, S. (2014). Chronic Thromboembolic Pulmonary Hypertension. Cardiovascular Medicine, 17(11), 328. https://doi.org/10.4414/cvm.2014.00285



