Pulmonary Hypertension: Classification and Pathobiology
Abstract
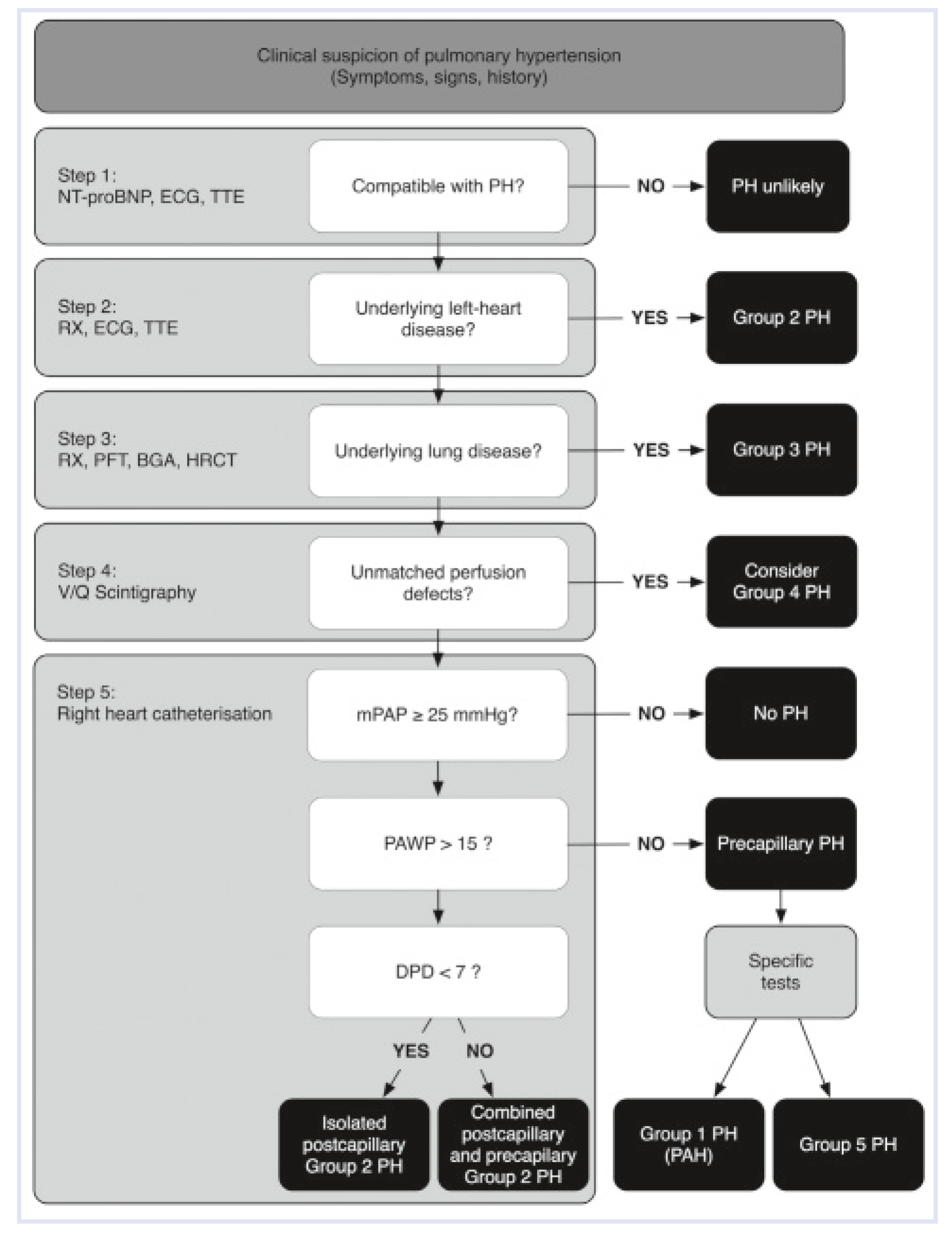
Definition and Haemodynamics
Classification and Epidemiology
Hereditary Pulmonary Arterial Hypertension
Connective Tissue Disease
Congenital Heart Disease/GUCH (Grown-Up Congenital Heart Disease)
Portopulmonary Hypertension
Drugs and Toxins
Human Immunodeficiency Virus
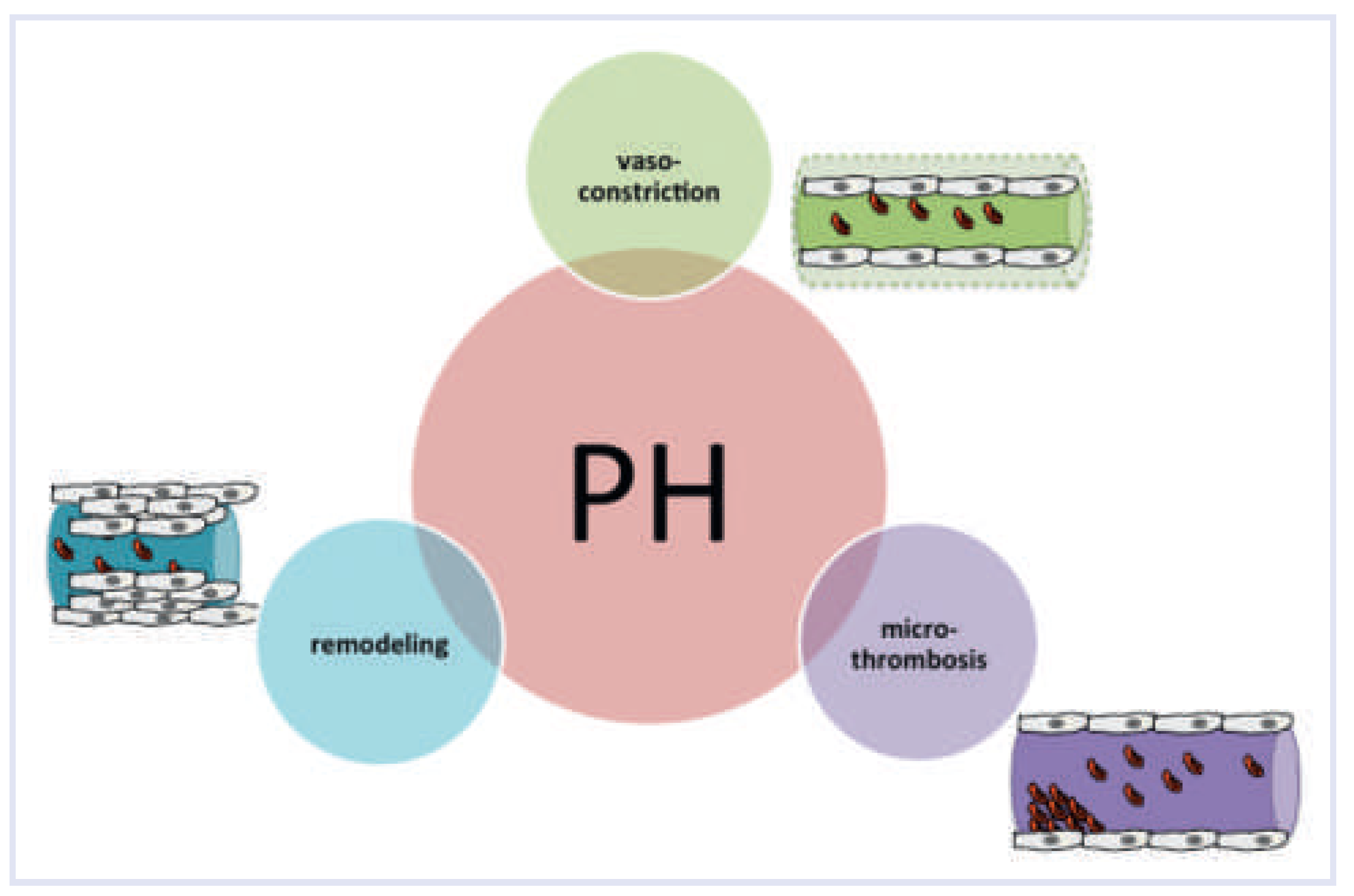
Pathobiology
Vasoconstriction
Microthrombosis
Vascular Remodelling
Acknowledgement
Funding/Potential Competing Interests
References
- Wilkins, L.W.; West, J.B. Respiratory Physiology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Bae, S.; Saggar, R.; Bolster, M.B.; Chung, L.; Csuka, M.E.; Derk, C.; et al. Baseline characteristics and follow-up in patients with normal haemodynamics versus borderline mean pulmonary arterial pressure in systemic sclerosis: Results from the PHAROS registry. Ann Rheum Dis. 2012, 71, 1335–1342. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Bogaard, H.J.; Condliffe, R.; Frantz, R.; Khanna, D.; Kurzyna, M.; et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013, 62, D42–50. [Google Scholar] [CrossRef]
- Lam, C.S.P.; Borlaug, B.A.; Kane, G.C.; Enders, F.T.; Rodeheffer, R.J.; Redfield, M.M. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation. 2009, 119, 2663–2670. [Google Scholar] [CrossRef]
- McQuillan, B.M.; Picard, M.H.; Leavitt, M.; Weyman, A.E. Clinical Correlates and Reference Intervals for Pulmonary Artery Systolic Pressure Among Echocardiographically Normal Subjects. Circulation. 2001, 104, 2797–2802. [Google Scholar] [CrossRef] [PubMed]
- Janda, S.; Shahidi, N.; Gin, K.; Swiston, J. Diagnostic accuracy of echocardiography for pulmonary hypertension: A systematic review and meta-analysis. Heart. 2011, 97, 612–622. [Google Scholar] [CrossRef]
- Knowlton, F.P.; Starling, E.H. The influence of variations in temperature and blood-pressure on the performance of the isolated mammalian heart. J Physiol. 1912, 44, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Halpern, S.D. Misclassification of Pulmonary Hypertension Due to Reliance on Pulmonary Capillary Wedge Pressure Rather Than Left Ventricular End-Diastolic Pressure. Chest. 2009, 136, 37. [Google Scholar] [CrossRef]
- Vachiéry, J. L.; Adir, Y.; Barberà, J.A.; Champion, H.; Coghlan, J.G.; Cottin, V.; et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013, 62, D100–D108. [Google Scholar] [CrossRef] [PubMed]
- Sitbon, O. Long Term Response to Calcium Channel Blockers in Idiopathic Pulmonary Arterial Hypertension. Circulation. 2005, 111, 3105–3111. [Google Scholar] [CrossRef]
- Robbins, I.M.; Hemnes, A.R.; Pugh, M.E.; Brittain, E.; Zhao, D.X.; Piana, R.N.; et al. High Prevalence of Occult Pulmonary Venous Hypertension Re vealed by Fluid Challenge in Pulmonary Hypertension. Circulation: Heart Failure. 2013. [Google Scholar]
- Authors/Task Force Members; Galie, N.; Hoeper, M.M.; Humbert, M.; Torbicki, A.; Vachiery, J.L.; et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treat ment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009, 30, 2493–2537. [Google Scholar] [PubMed]
- Hansdottir, S.; Groskreutz, D.J.; Gehlbach, B.K. WHO’s in Second? Chest. 2013, 144, 638. [Google Scholar] [CrossRef]
- Arrigo, M.; Huber, L.C. Passive Pulmonary Hypertension. Chest. 2014, 145, 413. [Google Scholar] [CrossRef]
- Ling, Y.; Johnson, M.K.; Kiely, D.G.; Condliffe, R.; Elliot, C.A.; Gibbs, J.S.R.; et al. Changing Demographics, Epidemiology, and Survival of Incident Pulmonary Arterial Hypertension. Am J Respir Crit Care Med. 2012, 186, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Sitbon, O.; Chaouat, A.; Bertocchi, M.; Habib, G.; Gressin, V.; et al. Pulmonary Arterial Hypertension in France. Am J Respir Crit Care Med. 2006, 173, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Simonneau, G.; Gatzoulis, M.A.; Adatia, I.; Celermajer, D.; Denton, C.; Ghofrani, A.; et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013, 62, D34–D41. [Google Scholar] [CrossRef]
- International PPH Consortium; Lane, K.B.; Machado, R.D.; Pauciulo, M.W.; Thomson, J.R.; Phillips, J.A.; et al. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000, 26, 81–84. [Google Scholar] [CrossRef]
- Hervier, B.; Meyer, A.; Dieval, C.; Uzunhan, Y.; Devilliers, H.; Launay, D.; et al. Pulmonary hypertension in antisynthetase syndrome: Prevalence, aetiology and survival. Eur Respir J. 2013, 42, 1271–1282. [Google Scholar] [CrossRef]
- Mukerjee, D.; St George, D.; Coleiro, B.; Knight, C.; Denton, C.P.; Davar, J.; et al. Prevalence and outcome in systemic sclerosis associated pulmonary arterial hypertension: Application of a registry approach. Ann Rheum Dis. 2003, 62, 1088–1093. [Google Scholar] [CrossRef]
- Coghlan, G. Does left heart disease cause most systemic sclerosis associated pulmonary hypertension? Eur Respir J. 2013, 42, 888–890. [Google Scholar] [CrossRef]
- Fox, B.D.; Shimony, A.; Langleben, D.; Hirsch, A.; Rudski, L.; Schlesinger, R.; et al. High prevalence of occult left heart disease in scleroderma-pulmonary hypertension. Eur Respir J. 2013, 42, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Wood, P. The Eisenmenger syndrome or pulmonary hypertension with reversed central shunt. I. Br Med J. 1958, 2, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Marelli, A.J.; Mackie, A.S.; Ionescu Ittu, R.; Rahme, E.; Pilote, L. Congenital Heart Disease in the General Population: Changing Prevalence and Age Distribution. Circulation. 2006, 115, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Beghetti, M. Fontan and the pulmonary circulation: A potential role for new pulmonary hypertension therapies. Heart. 2010, 96, 911–916. [Google Scholar] [CrossRef]
- Hervé, P.; Lebrec, D.; Brenot, F.; Simonneau, G.; Humbert, M.; Sitbon, O.; et al. Pulmonary vascular disorders in portal hypertension. Eur Respir J. 1998, 11, 1153–1166. [Google Scholar] [CrossRef]
- Pellicelli, A.M.; Barbaro, G.; Puoti, C.; Guarascio, P.; Lusi, E.A.; Bellis, L.; et al. Plasma Cytokines and Portopulmonary Hypertension in Patients With Cirrhosis Waiting for Orthotopic Liver Transplantation. Angiology. 2010, 61, 802–806. [Google Scholar] [CrossRef]
- Kawut, S.M.; Krowka, M.J.; Trotter, J.F.; Roberts, K.E.; Benza, R.L.; Badesch, D.B.; et al. Clinical risk factors for portopulmonary hypertension. Hepatology. 2008, 48, 196–203. [Google Scholar] [CrossRef]
- Savale, L.; Chaumais, M.C.; Cottin, V.; Bergot, E.; Frachon, I.; Prevot, G.; et al. Pulmonary hypertension associated with benfluorex exposure. European Respiratory Journal. 2012, 40, 1164–1172. [Google Scholar] [CrossRef]
- Montani, D.; Bergot, E.; Gunther, S.; Savale, L.; Bergeron, A.; Bourdin, A.; et al. Pulmonary Arterial Hypertension in Patients Treated by Dasatinib. Circulation. 2012, 125, 2128–2137. [Google Scholar] [CrossRef]
- Ghofrani, H.A.; Seeger, W.; Grimminger, F. Imatinib for the treatment of pulmonary arterial hypertension. N Engl J Med. 2005, 353, 1412–1413. [Google Scholar] [CrossRef] [PubMed]
- Speich, R.; Treder, U.; Domenighetti, G.; Huber, L.C.; Ulrich, S. Weaning from intravenous prostanoids and normalization of hemodynamics by long term imatinib therapy in severe idiopathic pulmonary arterial hypertension. Int J Clin Pharm. 2013. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoeper, M.M.; Barst, R.J.; Bourge, R.C.; Feldman, J.; Frost, A.E.; Galie, N.; et al. Imatinib Mesylate as Add-on Therapy for Pulmonary Arterial Hypertension: Results of the Randomized IMPRES Study. Circulation. 2013, 127, 1128–1138. [Google Scholar] [CrossRef]
- Schiess, R. Tobacco Smoke: A Risk Factor for Pulmonary Arterial Hypertension? Chest. 2010, 138, 1086. [Google Scholar] [CrossRef]
- Santos, S.; Peinado, V.I.; Ramirez, J.; Melgosa, T.; Roca, J.; Rodriguez-Roisin, R.; et al. Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur Respir J. 2002, 19, 632–638. [Google Scholar] [CrossRef]
- Trip, P.; Nossent, E.J.; de Man, F.S.; van den Berk, I.A.H.; Boonstra, A.; Groepenhoff, H.; et al. Severely reduced diffusion capacity in idiopathic pulmonary arterial hypertension: Patient characteristics and treatment responses. Eur Respir J. 2013, 42, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Speich, R. Primary pulmonary hypertension in HIV infection. Chest. 1991, 100, 1268. [Google Scholar] [CrossRef] [PubMed]
- Cool, C.D.; Rai, P.R.; Yeager, M.E.; Hernandez-Saavedra, D.; Serls, A.E.; Bull, T.M.; et al. Expression of human herpesvirus 8 in primary pulmonary hypertension. N Engl J Med. 2003, 349, 1113–1122. [Google Scholar] [CrossRef]
- Zuber, J.P.; Calmy, A.; Evison, J.M.; Hasse, B.; Schiffer, V.; Wagels, T.; et al. Pulmonary arterial hypertension related to HIV infection: Improved hemo dynamics and survival associated with antiretroviral therapy. Clin Infect Dis. 2004, 38, 1178–1185. [Google Scholar]
- Sommer, N.; Dietrich, A.; Schermuly, R.T.; Ghofrani, H.A.; Gudermann, T.; Schulz, R.; et al. Regulation of hypoxic pulmonary vasoconstriction: Basic mechanisms. Eur Respir J. 2008, 32, 1639–1651. [Google Scholar] [CrossRef]
- Rubens, C.; Ewert, R.; Halank, M.; Wensel, R.; Orzechowski, H.D.; Schultheiss, H.P.; et al. Big endothelin-1 and endothelin-1 plasma levels are correlated with the severity of primary pulmonary hypertension. Chest. 2001, 120, 1562–1569. [Google Scholar] [CrossRef]
- Giaid, A.; Saleh, D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995, 333, 214–221. [Google Scholar] [PubMed]
- Tuder, R.M.; Cool, C.D.; Geraci, M.W.; Wang, J.; Abman, S.H.; Wright, L.; et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med. 1999, 159, 1925–1932. [Google Scholar]
- Arrigo, M.; Huber, L.C. Eponyms in cardiopulmonary reflexes. Am J Cardiol. 2013, 112, 449–453. [Google Scholar]
- Wagenvoort, C.A.; Mulder, P.G. Thrombotic lesions in primary plexogenic arteriopathy. Similar pathogenesis or complication? Chest. 1993, 103, 844–849. [Google Scholar] [PubMed]
- Welsh, C.H.; Hassell, K.L.; Badesch, D.B.; Kressin, D.C.; Marlar, R.A. Coagulation and fibrinolytic profiles in patients with severe pulmonary hypertension. Chest. 1996, 110, 710–717. [Google Scholar] [PubMed]
- Hoeper, M.M.; Sosada, M.; Fabel, H. Plasma coagulation profiles in patients with severe primary pulmonary hypertension. Eur Respir J. 1998, 12, 1446–1449. [Google Scholar] [CrossRef]
- Johnson, S.R.; Granton, J.T.; Tomlinson, G.A.; Grosbein, H.A.; Hawker, G.A.; Feldman, B.M. Effect of warfarin on survival in scleroderma-associated pulmonary arterial hypertension (SSc-PAH) and idiopathic PAH. Belief elicitation for Bayesian priors. J Rheumatol. 2011, 38, 462–469. [Google Scholar] [CrossRef]
- Dorfmuller, P.; Humbert, M. Progress in pulmonary arterial hypertension pathology: Relighting a torch inside the tunnel. Am J Respir Crit Care Med. 2012, 186, 210–212. [Google Scholar] [CrossRef]
- Guignabert, C.; Dorfmuller, P. Pathology and Pathobiology of Pulmonary Hypertension. Semin Respir Crit Care Med. 2013, 34, 551–559. [Google Scholar] [CrossRef]
- Pietra, G.G.; Capron, F.; Stewart, S.; Leone, O.; Humbert, M.; Robbins, I.M.; et al. Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol. 2004, 43, S25–S32. [Google Scholar]
- Dorfmüller, P.; Humbert, M.; Capron, F. Update on the pathomorphological assessment of vasculopathies in pulmonary arterial hypertension. Pathologe. 2006, 27, 140–146. [Google Scholar] [CrossRef]
- Atkinson, C. Primary Pulmonary Hypertension Is Associated With Reduced Pulmonary Vascular Expression of Type II Bone Morphogenetic Protein Receptor. Circulation. 2002, 105, 1672–1678. [Google Scholar] [CrossRef]
- Ishida, H.; Kogaki, S.; Takahashi, K.; Ozono, K. Attenuation of bone morphogenetic protein receptor type 2 expression in the pulmonary arteries of patients with failed Fontan circulation. The Journal of Thoracic and Cardiovascular Surgery. 2012, 143, e24–e26. [Google Scholar] [CrossRef]
- Dalvi, P.; O’Brien Ladner, A.; Dhillon, N.K. Downregulation of Bone Morphogenetic Protein Receptor Axis During HIV-1 and Cocaine-Mediated Pulmonary Smooth Muscle Hyperplasia: Implications for HIV-Related Pulmonary Arterial Hypertension. Arterioscler Thromb Vasc Biol. 2013, 33, 2585–2595. [Google Scholar] [CrossRef]
- Takahashi, H. Downregulation of type II bone morphogenetic protein receptor in hypoxic pulmonary hypertension. AJP Lung Cellular and Molecular Physiology. 2005, 290, L450–8. [Google Scholar] [CrossRef] [PubMed]
- Morty, R.E.; Nejman, B.; Kwapiszewska, G.; Hecker, M.; Zakrzewicz, A.; Kouri, F.M.; et al. Dysregulated Bone Morphogenetic Protein Signaling in Monocrotaline-Induced Pulmonary Arterial Hypertension. Arterioscler Thromb Vasc Biol. 2007, 27, 1072–1078. [Google Scholar] [CrossRef]
- Brock, M.; Trenkmann, M.; Gay, R.E.; Michel, B.A.; Gay, S.; Fischler, M.; et al. Interleukin-6 Modulates the Expression of the Bone Morphogenic Protein Receptor Type II Through a Novel STAT3–microRNA Cluster 17/92 Pathway. Circ Res. 2009, 104, 1184–1191. [Google Scholar]
- Reynolds, A.M.; Xia, W.; Holmes, M.D.; Hodge, S.J.; Danilov, S.; Curiel, D.T.; et al. Bone morphogenetic protein type 2 receptor gene therapy attenuates hypoxic pulmonary hypertension. AJP: Lung Cellular and Molecular Physiology. 2007, 292, L1182–92. [Google Scholar] [PubMed]
- Reynolds, A.M.; Holmes, M.D.; Danilov, S.M.; Reynolds, P.N. Targeted gene delivery of BMPR2 attenuates pulmonary hypertension. Eur Respir J. 2012, 39, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Pullamsetti, S.S.; Doebele, C.; Fischer, A.; Savai, R.; Kojonazarov, B.; Dahal, B.K.; et al. Inhibition of MicroRNA-17 Improves Lung and Heart Function in Experimental Pulmonary Hypertension. Am J Respir Crit Care Med. 2012, 185, 409–419. [Google Scholar] [CrossRef]
- Brock, M.; Samillan, V.J.; Trenkmann, M.; Schwarzwald, C.; Ulrich, S.; Gay, R.E.; et al. AntagomiR directed against miR-20a restores functional BMPR2 signalling and prevents vascular remodelling in hypoxia-induced pulmonary hypertension. Eur Heart J. 2012. [Google Scholar]
- Swan, H.J.; Ganz, W.; Forrester, J.; Marcus, H.; Diamond, G.; Chonette, D. Catheterization of the heart in man with use of a flow-directed balloon-tipped catheter. N Engl J Med. 1970, 283, 447–451. [Google Scholar] [CrossRef]
- Bonderman, D.; Wexberg, P.; Martischnig, A.M.; Heinzl, H.; Lang, M.B.; Sadushi, R.; et al. A noninvasive algorithm to exclude pre-capillary pulmonary hypertension. Eur Respir J. 2011, 37, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Aduen, J.F.; Castello, R.; Lozano, M.M.; Hepler, G.N.; Keller, C.A.; Alvarez, F.; et al. An Alternative Echocardiographic Method to Estimate Mean Pulmonary Artery Pressure: Diagnostic and Clinical Implications. Journal of the American Society of Echocardiography. 2009, 22, 814–819. [Google Scholar] [CrossRef]
- Chemla, D. New Formula for Predicting Mean Pulmonary Artery Pressure Using Systolic Pulmonary Artery Pressure. Chest. 2004, 126, 1313–1317. [Google Scholar] [CrossRef]
- Rajaram, S.; Swift, A.J.; Telfer, A.; Hurdman, J.; Marshall, H.; Lorenz, E.; et al. 3D contrast-enhanced lung perfusion MRI is an effective screening tool for chronic thromboembolic pulmonary hypertension: Results from the ASPIRE Registry. Thorax. 2013, 68, 677–678. [Google Scholar] [CrossRef]
- Paulus, W.J.; Tschope, C.; Sanderson, J.E.; Rusconi, C.; Flachskampf, F.A.; Rademakers, F.E.; et al. How to diagnose diastolic heart failure: A consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007, 28, 2539–2550. [Google Scholar] [CrossRef] [PubMed]
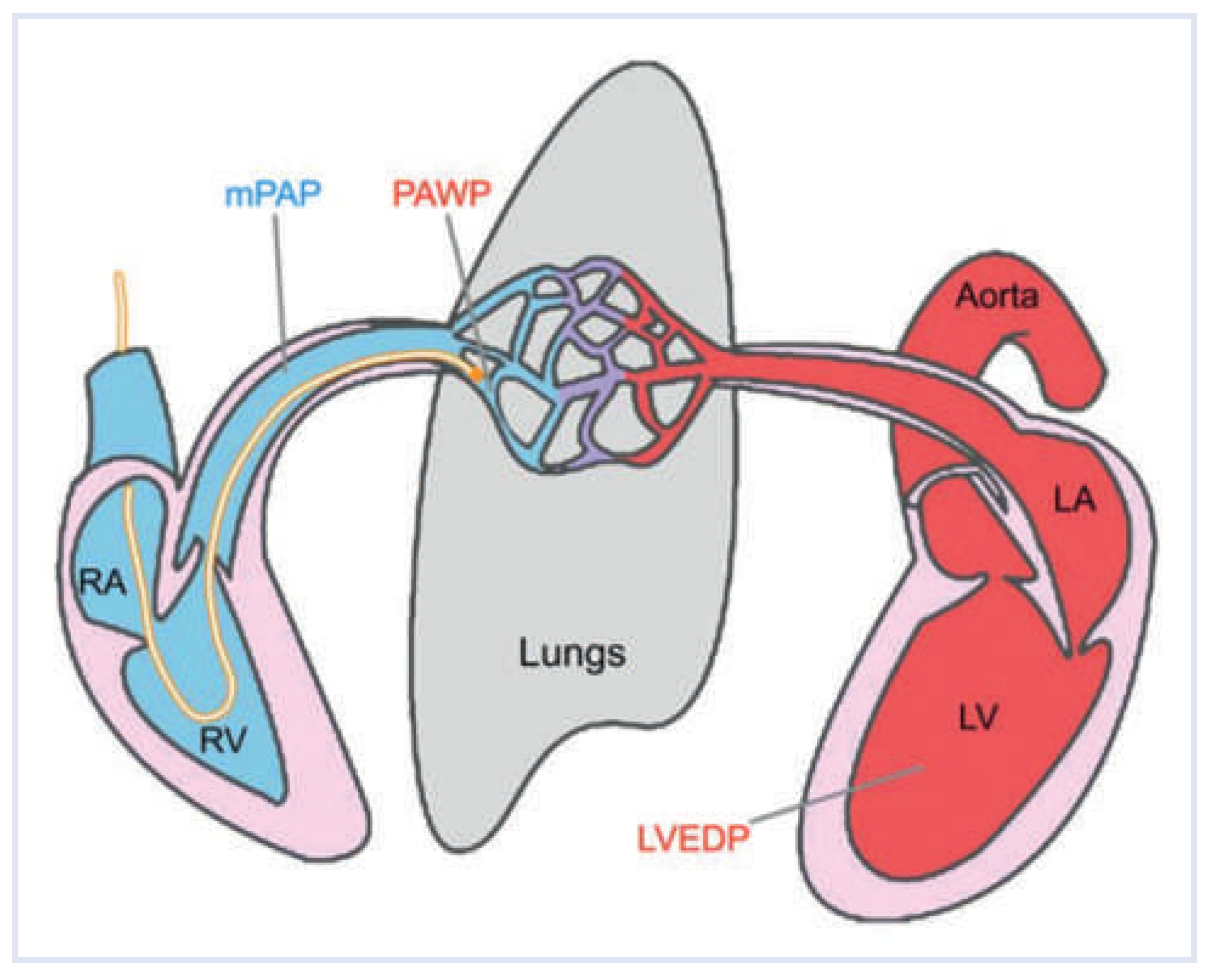


| Abbreviation | Definition | Calculation | Reference Value |
|---|---|---|---|
| CO | Cardiac output | 4–8 L/min | |
| CI | Cardiac index | 2.5–4.2 L/min/m2 | |
| mPAP | Mean pulmonary artery pressure | 9–20 mm Hg | |
| dPAP | Diastolic pulmonary artery pressure | ||
| PAWP | Pulmonary artery wedge pressure | ≤15 mm Hg | |
| LVEDP | Left ventricular end-diastolic pressure | ≤15 mm Hg [12,68] | |
| TPG | Transpulmonary gradient | mPAP—PAWP | ≤12 mm Hg |
| DPD | Diastolic pressure difference | dPAP—PAWP | ≤7 mm Hg |
| PVR | Pulmonary vascular resistance | TPG/CO 80 × TPG/CO | <2 WU (Wood units) <160 dyn × s/cm5 |
| 1. Pulmonary arterial hypertension |
| 1.1 Idiopathic PAH |
| 1.2 Heritable PAH |
| 1.3 Drugand toxin-induced |
| 1.4 Associated with connective tissue disease, HIV infection, portal hypertension, congenital heart diseases, schistosomiasis |
| 2. Pulmonary hypertension due to left heart disease |
| 3. Pulmonary hypertension due to lung diseases and/or hypoxia |
| 4. Chronic thromboembolic pulmonary hypertension (CTEPH) |
| 5. Pulmonary hypertension with unclear multifactorial mechanisms |
© 2014 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Huber, L.C.; Vrugt, B.; Arrigo, M. Pulmonary Hypertension: Classification and Pathobiology. Cardiovasc. Med. 2014, 17, 312. https://doi.org/10.4414/cvm.2014.00286
Huber LC, Vrugt B, Arrigo M. Pulmonary Hypertension: Classification and Pathobiology. Cardiovascular Medicine. 2014; 17(11):312. https://doi.org/10.4414/cvm.2014.00286
Chicago/Turabian StyleHuber, Lars C., Bart Vrugt, and Mattia Arrigo. 2014. "Pulmonary Hypertension: Classification and Pathobiology" Cardiovascular Medicine 17, no. 11: 312. https://doi.org/10.4414/cvm.2014.00286
APA StyleHuber, L. C., Vrugt, B., & Arrigo, M. (2014). Pulmonary Hypertension: Classification and Pathobiology. Cardiovascular Medicine, 17(11), 312. https://doi.org/10.4414/cvm.2014.00286




