Case report
An 83 year old hypertensive patient with severe, symptomatic aortic stenosis in NYHA III heart failure and a history of syncope was referred for valve replacement to our centre. His previous history was remarkable for prostate cancer, hospitalisation for decompensated heart failure, paroxysmal atrial fibrillation (for which he was on vitamin K antagonist) and a previous cerebrovascular accident. His STS score was calculated as 3.3% and the EuroScore II was 4.6%. The ECG showed atrial fibrillation at a normal heart rate (74 bpm) and a left bundle branch block. On transthoracic echocardiography a mean gradient of 39 mm Hg was found and the indexed aortic valve area was 0.5 cm/m2. The left ventricle was mildly dilated with a left ventricular ejection fraction of 35%. Significant coronary artery disease could be excluded by coronary angiography.
Due to his age and his metastasising prostate cancer, the patient refused to undergo open-heart surgery. Technical eligibility for transcatheter valve implantation was therefore evaluated. The angiography showed that the peripheral arteries were large enough for a transfemoral procedure, in that they could accommodate an 18French sheath. Annulus sizing was performed by CT-angiography revealing sinuses of Valsalva of 41 mm, a mean annulus diameter of 31.5 mm (min. 26 mm, max. 37 mm), a perimeter of 102 mm and an annulus area of 720 mm2. The largest device currently available is the 31 mm CoreValve prosthesis with a device area of 755 mm2 and a perimeter of 97 mm. For the use of this valve type, a maximum annular area of 660 mm2 and a maximum perimeter of 91 mm to achieve reasonable 7–14% over-sizing is recommended. Despite the above-mentioned measurements on CT, it was decided to proceed with balloon-sizing of the annulus based on the angiographic impression (suggesting feasibility of TAVI) and if this showed good sealing to proceed ad-hoc with the valve implantation. The procedure was performed with local anaesthesia and with fluoroscopic guidance only. An 18French Cook sheath (Cook Medical Inc., Bloomington, USA) was introduced via the right femoral artery after preclosure with two Perclose ProGlides (Abbott Vascular, Abbott Park, USA). Balloon-sizing was performed using a 26-mm Z-Med Balloon (NuMED Inc., Hopkinton, USA). Aortic balloon sizing was performed under rapidpacing using simultaneous contrast injection to the aortic root. Minimal aortic regurgitation was seen during valvuloplasty and the decision was taken to pursue with implantation of a CoreValve 31-mm prosthesis (Medtronic, Minneapolis, USA). After valve implantation, a large pulse-pressure and a high left ventricular end-diastolic pressure were evident. Reusing the 26-mm Z-Med balloon, a postdilatation was performed which improved haemodynamics (LVEDP 13 mm Hg, diastolic blood pressure 43 mm Hg, systolic blood pressure 106 mm). The final aortogram showed mild aortic regurgitation.
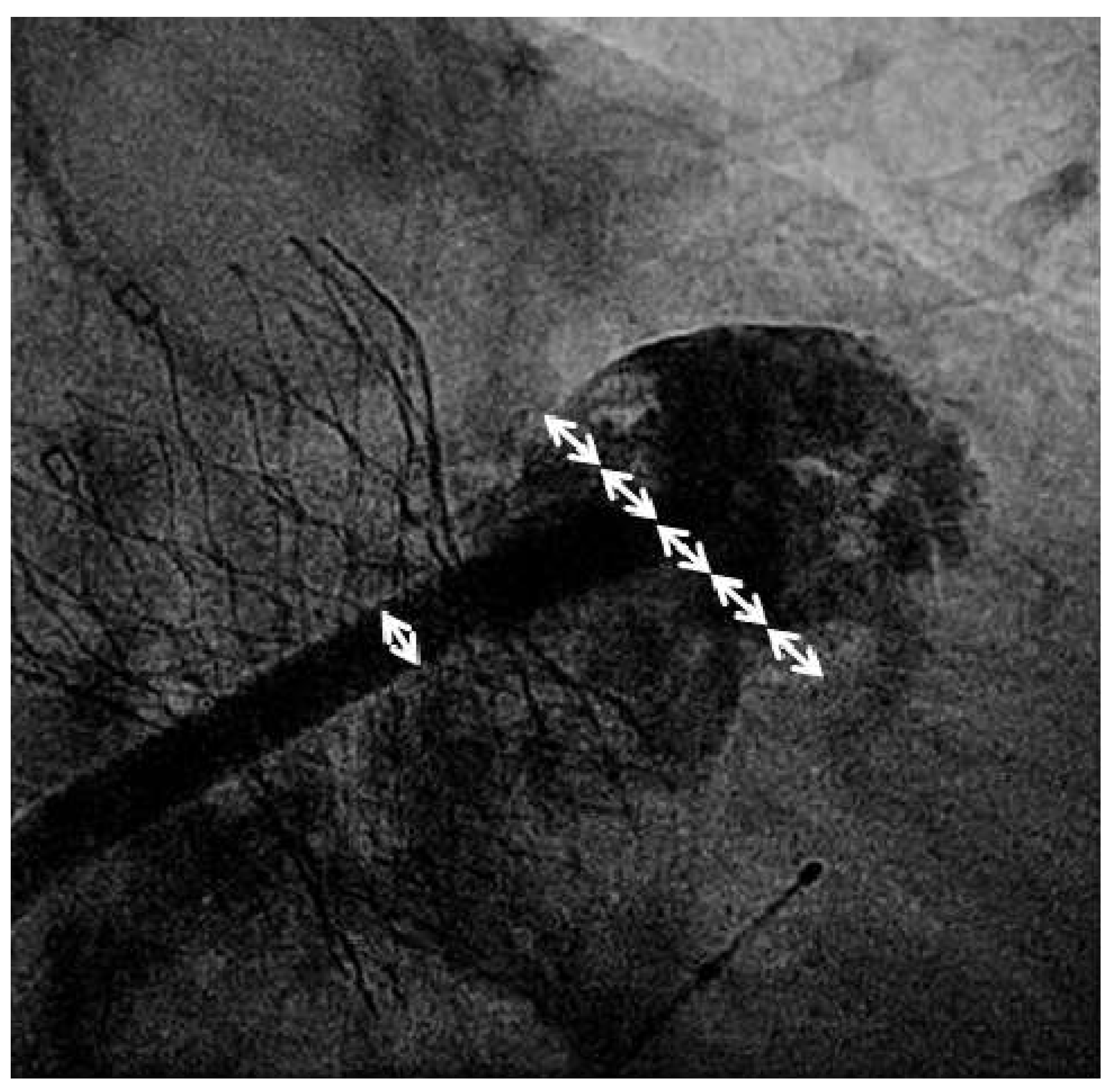
Valve implantation was followed by transseptal puncture through a right femoral venous access using an 8French Mullins transseptal sheath and the Brockenbrough Curved Needle (Medtronic, Minneapolis, USA). Using contrast injections through the transseptal sheath, the left atrial appendage (LAA) was visualised and a thrombus within the left atrial appendage was not detected. The transseptal sheath was replaced by the Amplatzer TorqueVue 45×45 sheath (St. Jude Medical, St. Paul, USA). Sizing of the left atrial appendage was performed using the outer diameter of the delivery sheath as a reference (14F; 5.4 mm;
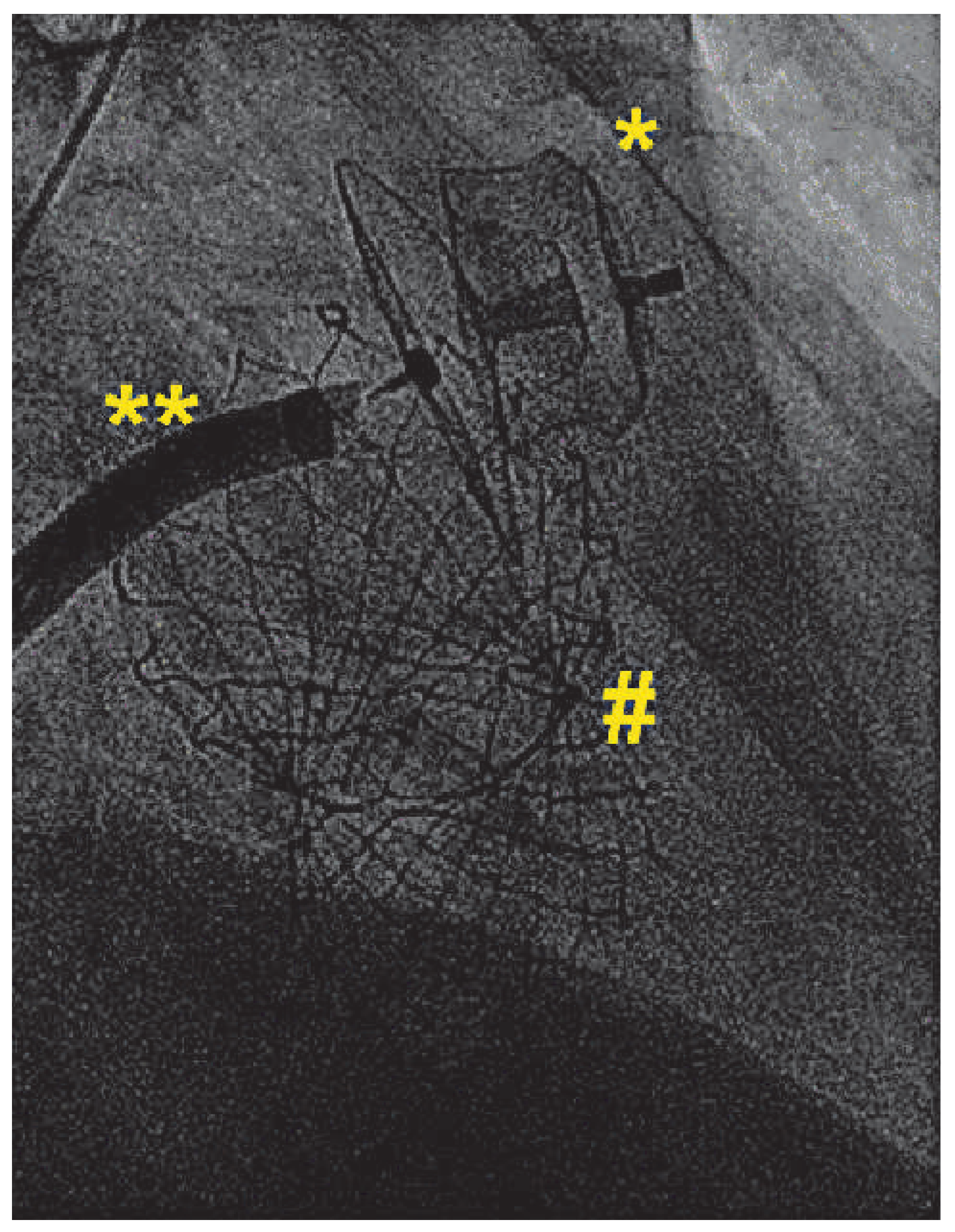
Figure 1). An Amplatzer Amulet 28 mm LAA-Occluder (St. Jude Medical, St. Paul, USA) was deployed in the left atrial appendage in a stable position with good sealing (
Figure 2).
The following day, a transthoracic echocardiography confirmed a well-functioning CoreValve prosthesis with only trivial paravalvular regurgitation and a mean transvalvular gradient of 5 mm Hg as well as the correct position of the Amplatzer Amulet 28-mm Occluder.
Discussion
In most centres, CT-scans are routinely performed prior to transcatheter aortic valve implantation (TAVI) for annulus sizing and to depict the annular coronary distance [
1]. Standard protocols facilitate patient management and standardised investigations also help to feed registry databases for future scientific analyses. Furthermore, multimodality imaging and heart-team approaches allow sharing of responsibilities, also in case a complication occurs. Routine work-up consists of transthoracic, followed by transoesophageal echocardiography, a coronary angiogram, aortogram and angiogram of the peripheral vessels, followed by a CT-angiogram. Balloon-sizing of the aortic annulus is only performed in borderline cases. In our patient, CT-angiogram measurements might have prevented us from performing TAVI and balloon-sizing allowed us to proceed with TAVI with an excellent result.
Therefore, the question arises, why not bypass some previous investigations and proceed to balloonsizing right away? Currently, there are no data published comparing CT-sizing with balloon-sizing, however, if in borderline cases we rely most on balloon-sizing, the technique may be considered as a “gold-standard”. Balloon-sizing not only gives excellent anatomical and functional information about the annular size, but also allows the assessment of the position of the native valve leaflets in relation to the left main and right coronary arteries and to determine their patency. With the concept of balloon-sizing, ad-hoc TAVI becomes a reality in selected patients. A sophisticated pressure-based technique of balloon-sizing was proposed by Babaliaros and colleagues [
2], but a simplified version relying on fluoroscopic assessment of contrast injections is equally reliable in our experience (assessment of aortic regurgitation and coronary flow with a fully inflated balloon).
As already demonstrated, a combined approach with catheter-based aortic valve implantation and occlusion of the left atrial appendage is technically feasible [
3,
4,
5], as is ad-hoc left atrial appendage occlusion without previous and intraprocedural transoesophageal echocardiography [
6]. Combined procedures allow for a patient-friendly treatment, despite contrary financial incentives by our health care system. Since elderly and multimorbid patients are at increased risk for intracranial and gastrointestinal bleeding as recognised by elevated HAS-BLED Scores (in our patient the HAS-BLED Score summed up to 3), combined interventions are not only patient-friendly, but may also be safer. Left atrial appendage closure represents an alternative to oral anticoagulants for patients with atrial fibrillation and is probably one of the most under-performed procedures in cardiology. Long-term data from the PROTECT AF trial have now shown not only superiority in terms of stroke prevention, but also a significant 60% reduction in cardiovascular mortality and a 34% reduction in all-cause mortality as compared to oral anticoagulation (4-year F/U PROTECT AF trial; D. Holmes, presented at EuroPCR 2013). Given these data together with the latest long-term results on left atrial appendage closure with Amplatzer occluders [
7], left atrial appendage closure should be considered an attractive alternative to systemic oral anticoagulation in suitable patients with atrial fibrillation.