Abstract
The vast majority of cardiac tumours are metastases, with primary cardiac tumours being extremely rare. Lung cancer is the leading primary tumour involving the heart. Cardiac manifestations of extracardiac tumours usually arise late in the course of the neoplastic disease and are only rarely encountered as the first manifestation of a neoplasm. Depending upon localisation, type and degree of cardiac involvement, cancer patients may present with heart failure, arrhythmia, pericarditis or pericardial effusion. Emergency situations related to extracardiac tumours include cardiac tamponade, myocardial infarction, and superior vena cava syndrome due to tumour compression. Prognosis depends upon the underlying disease but is generally poor. With the availability of better systemic tumour therapies some paraneoplastic cardiac tumour manifestations such as carcinoid heart disease have become rare. However, increasing concern exists over drug therapy or radiotherapy-related cardiopathies in the long-term follow-up of cancer patients—particularly with the rise of innovative new drugs with yet unknown cardiac safety profiles.
Introduction
An oncologist’s referral of a patient to a cardiologist is rarely for cardiac tumours. Of all cardiac tumours only 2–5% are primary, of which 28% are malign [1]. A frequency of less than 0.1% has been reported for primary cardiac tumours in autopsies [2]. Cardiac complications by extracardiac tumours are more frequent and often happen in advanced palliative situations. A close collaboration between oncologists/radio-oncologists and cardiologists is required for the diagnosis and treatment of therapy related cardiopathies due to chemotherapy or radiotherapy. Beginning with a brief case report of cardiac complications in a patient with Hodgkin Lymphoma, this review aims to give an overview of cardiac complications due to extracardiac tumours with an emphasis on incidence and clinical manifestation. These complications can rise from the tumour’s invasion of the heart per continuitatem or by metastases often causing malignant pericardial effusion, by tumour compression, by paraneoplastic phenomena, amyloidosis, and drug-related or post-radiogenic cardiopathy.
Cardiac complications in a patient with Hodgkin lymphoma—a case report
A 47-year old woman presented with a rash, pruritus and a right-axillary lymphadenopathy. A lymph node biopsy revealed the diagnosis of classical Hodgkin Lymphoma with nodular sclerosis. A PET CT scan showed a 9 cm tumour bulk, a conglomerate tumour in the right axilla, involvement of cervical, retroclavicular, cardiophrenic and left axillary lymph nodes as well as splenic and nodular involvement of the pericardium which made the diagnosis of a stage IVB Hodgkin lymphoma (Figure 1). Echocardiography at the initial diagnosis showed a normal left-ventricular function with a small haemodynamically-compensated atrial septum secundum defect. After 3 cycles of chemotherapy of the escalated BEACOPP regimen (doxorubicin, cyclophosphamide, vincristine, procarbazine and prednisone), a metabolically complete remission was seen on the follow-up PET CT scan. After 6 cycles of chemotherapy, the patient presented with progressive respiratory deterioration, exercise intolerance as well as weight gain and ankle swelling as signs of right heart failure. Auscultation revealed a known systolic murmur but was otherwise clinically insignificant. Echocardiography showed a significant left-right shunt through the atrial septum defect, a dilatation of the pulmonary vein, and no pericardial effusion. Chemotherapy was stopped. Further cardiac diagnosis with trans-oesophageal echocardiography was performed and the atrial septum defect was occluded using an AMPLATZER device. Following this procedure another PET CT scan showed a metabolically complete remission with a metabolically inactive 2 cm mediastinal mass (Figure 2). Due to this metabolic complete remission and the cardiac anamnesis it was decided to skip consolidation radiotherapy. In the one year follow-up period the patient remained free of symptoms without signs of cardiac decompensation.
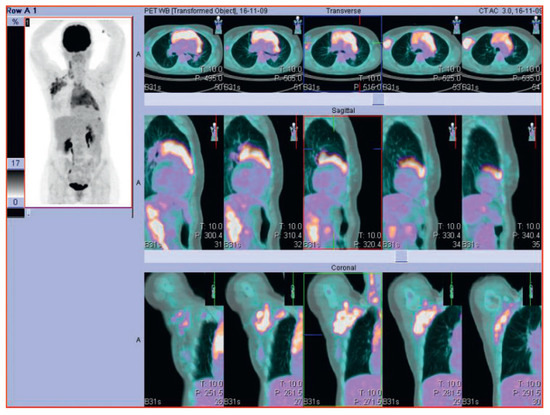
Figure 1.
PET-CT scan of a 47-year old woman with Hodgkin lymphoma at the first diagnosis.
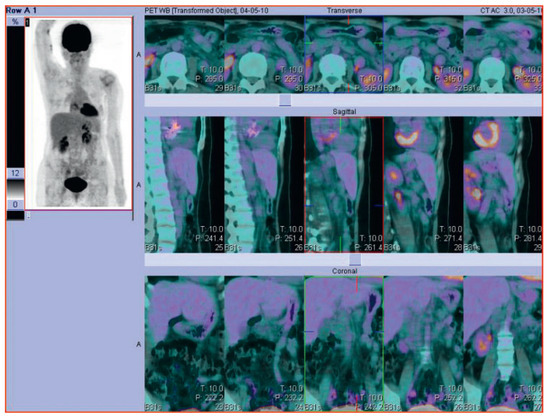
Figure 2.
Follow-up PET-CT scan of the same patient with Hodgkin Lymphoma after successful treatment.
Complications due to tumour invasion—cardiac metastases, direct tumour growth via vena cava veins
The vast majority of cardiac tumours are cardiac metastases which have been found in autopsies with a highly variable incidence of 8 to 21% of all cancer patients [3,4,5]. In a series of 1029 autopsies tu- mours of the lung, the bronchus and pleura were the most frequent primary sites accounting for 37.3% of all metastases to the heart, followed by nonsolid primaries (lymphomas, Kaposi’s sarcomas and leukemias) with 20%, female breast carcinomas (7.3%), carcinomas of the oesophagus (6.4%) and skin (4.5%), tumours of the uterus/cervix, head and neck, pancreas and kidney (3.6% each), tumours of the colon/rectum, stomach, liver, bladder, adenocarcinoma of unknown primary site (1.8% each), ovary (0.9%) and other sites (14.8%) [4]. Of these neoplasms, melanomas showed the highest tendency towards cardiac involvement in more than 50% of all autopsies of patients with a melanoma [6,7,8]. In general, cardiac metastases should be suspected when a patient with a neoplastic disease shows cardiovascular symptoms.

Table 1.
Differential diagnosis for cardiac complications of non-cardiac malignancies.
Table 1.
Differential diagnosis for cardiac complications of non-cardiac malignancies.
| Cardiac metastases |
| Neoplastic pericardial disease |
| Superior vena cava syndrome |
| Carcinoid heart disease |
| Amyloid cardiomyopathy |
| Drug-related cardiopathy |
| Post-radiogenic cardiopathy |
Cardiac tumours can reach the heart haematogenously, via the lymphatic system, or by direct invasion through the cava or pulmonary veins [9]. Direct tumour invasion via the cava veins has been described for hepatocellular carcinoma, testicular teratoma, smooth muscle sarcoma and renal carcinoma [10,11,12]. The clinical manifestation of cardiac tumours depends upon their localisation and size and is thus highly variable including pericardial involvement (as discussed separately below), arrhythmias, diastolic or systolic heart failure and occasionally myocardial infarction [9]. Besides, for renal cell carcinoma for example, large ventricular metastases of the right ventricle, but even rarer also of the left ventricle, have been described without inferior vena cava or right atrium involvement [13,14].
Neoplastic pericardial disease
The incidence of neoplastic diseases of the pericardium is rare. Clinically, neoplastic pericardial disease can manifest as pericarditis, pericardial effusion, cardiac tamponade or pericardial constriction. Pericarditis clinically presents as pleuritic chest pain, a pericardial friction rub and with typical global concave ST segment elevation. In a series of 100 patients presenting with acute pericarditis or pericardial effusion, a specific aetiology could be found in 22 patients, seven of whom had a previously undiagnosed neoplastic disease [15]. In another series of 173 patients undergoing pericardiocentesis, a neoplastic pericardial effusion was found in 58 (33%) patients, of whom 13 had a previously unknown malignancy. After exclusion of patients with easily attributable causes for pericardial effusion (traumatic, uremic, post-pericardiotomy, rheumatic, known neoplasia), 18% of the remaining 74 patients had newly diagnosed cancer as the underlying disease. The most frequent primary tumour leading to a pericardial effusion was lung cancer accounting for approximately one-third of all cases [16]. A malign pericardial effusion occurs late in the neoplastic disease and is associated with a poor prognosis. If the pressure of the accumulating pericardial effusion excels the intra-cardiac pressures, this results in the emergency situation of a cardiac tamponade. Usually, cardiac tamponade from malignant disease is sub-acute. Pericardial constriction results from scarred, thickened pericardium, impairing the cardiac diastolic filling of the ventricles. Pericardial constriction is characterised by brisk early diastolic filling and may clinically present with the Kussmaul’s sign, a lack of an inspiratory decline in the jugular pressure [17].
Complications due to tumour compression–superior vena cava syndrome
Tumour compression leading to the occlusion of the superior vena cava vein can causes one of the feared emergency cases in oncology, referred to as superior vena cava syndrome (SVCS). Typically, patients with a history of cancer will present with distention of the jugular or thoracic veins through formation of collaterals, oedema of the face, neck and upper chest, and occasionally cerebral oedema [18]. Although life-threatening, SVCS was reported to only have caused death in 1 out of 1986 patients [19]. With the increased use of intravascular catheters and pacemakers causing thrombosis, the percentage of malignancy as the underlying condition for SVCS has decreased to approximately 60% [20]. The leading malignancy causing SVCS is lung cancer accounting for approximately two-thirds of all cases. Of these, non-small-cell lung cancer accounted for approximately two-thirds of the cases, and small-cell lung cancer accounted for the remaining onethird. Apart from lung cancer, SVCS can be caused by lymphoma, metastases, germ-cell cancer, thymoma, mesothelioma, and by other cancers in less than 2% of cases [18]. Therapy options for SVCS depend upon the underlying malignancy and their response to systemic therapy. Thus, in patients with generally chemo-sensitive tumours such as small-cell lung cancer, germ-cell cancer or lymphoma, this treatment is the first choice whereas in certain situations (e.g., in non-small-cell carcinoma) a faster relief of symptoms will be achieved by radiotherapy or radio-chemotherapy. Immediate treatment by radiotherapy or stent placement is reserved for patients with stridor due to central airway obstruction or laryngeal oedema, or for patients with cerebral oedema.
Complications due to paraneoplastic phenomena—carcinoid heart disease
Carcinoid syndrome refers to a set of typical symptoms which are induced by humoral factors secreted from carcinoid tumours arising in the gastrointestinal tract and the bronchi, but also occasionally in other sites such as the urogenital tract. The typical symptoms of carcinoid syndrome include cutaneous flushing and venous teleangiectasia, diarrhoea, bronchospasms, and cardiac involvement which is referred to as Carcinoid Heart Disease (CHD). CHD has been reported to arise in >50% of patients with carcinoid syndrome [21] and is characterised by a dysfunction predominantly of the right heart valves. CHD is caused by vasoactive substances produced by neuroendocrine tumour cells typically metastasising to the liver which allows the vasoactive substances to reach the heart without prior hepatic metabolisation and to induce fibrotic endocardial plaques which causes valve dysfunction, usually exclusively of the right side of the heart [22,23,24,25,26,27]. Occasionally, CHD develops in ovarian carcinoma without hepatic metastases. In a retrospective analysis of 265 patients with carcinoid syndrome, CHD developed in 4 patients (1.5%) without hepatic metastases or ovarian cancer who had retroperitoneal lymph nodes metastases [28]. Although the pathogenesis causing CHD is not fully understood, serotonin is considered to be a major stimulator of fibrogenesis and high levels of urinary excretion of the serotonin metabolite, 5-hydroxyindoleacetic acid (5-HIAA), and of plasma atrial natriuretic peptide (ANP) were found to be associated with CHD in 37 consecutive carcinoid patients, nine of whom (24%) developed CHD during a two-year cardiac ultrasound follow-up [29]. In a recent report of 252 patients with carcinoid syndrome, 44 (17%) had developed CHD after a median follow-up of 29 months, and a 5-HIAA level of ≥300 μmol/24 hours and ≥3 flushing episodes per day were found to be predictors of the development or progression of CHD [30]. Since the introduction of somatostatin analogues (octreotide) as a milestone of systemic carcinoid tumour treatment, severe CHD has become rare. Besides systematic tumour control, the treatment of CHD is multidisciplinary including resection or chemo-embolisation of hepatic metastases, treatment of heart failure, and valve replacement surgery for severe CHD [23].
Amyloid cardiomyopathy
Amyloidosis is characterised by the extracellular deposition of specific proteins predominantly in the heart, liver, kidney and autonomic nervous system. Amyloid cardiomyopathy is an important differential diagnosis of restrictive cardiomyopathy characterised by impaired filling of non-dilated ventricles. Its incidence depends upon the type of amyloidosis ranging from less than 5% for AA amyloidosis to approximately 50% for patients with AL amyloidosis, a plasma cell dyscrasia [31,32]. Apart from AL and AA amyloidosis, heart involvement has also been documented for familial amyloidosis and senile systemic amyloidosis [33]. Amyloidosis can be suspected in echocardiography in a patient with ventricular wall thickening without a dilatation of the left ventricle. For a definite diagnosis, the presence of amyloid deposits in an endomyocardial biopsy or other biopsies (abdominal fat tissue, rectum, or kidney) is required. In the heart, amyloid deposits can be found in the coronary arteries, atrial myocardium, heart valves, pericardium, and of clinically utmost importance in the ventricular myocardium and conducting system. Thus clinically, patients with cardiac amyloidosis will most probably present with congestive heart failure though amyloid infiltration of small vessels can also occasionally cause angina pectoris, and involvement of the atria and the conduction system might induce atrial arrhythmias possibly leading to thromboembolism as the first clinical manifestation of amyloidosis [33]. The prognosis of cardiac amyloidosis depends upon the type of amyloid but is generally poor with a reported mortality of 80% within 2 years for patients with AL amyloidosis [34]. Treatment options consist of systematic therapy for amyloidosis as reviewed elsewhere and treatment of heart failure with a usually poor response.
Post-radiogenic cardiopathy
Improvements in radiation therapy (RT) treatment particularly for Hodgkin Lymphoma and breast cancer, but also for other thoracic tumours have resulted in a large cohort of long-term cancer survivors for whom late toxicity from RT becomes relevant. Besides breast cancer and Hodgkin Lymphoma, oesophageal cancer and lung cancer are frequent tumour entities causing cardiotoxicity from radiation therapy. By inducing inflammation and secondary fibrosis, radiation therapy can involve the myocardium, the pericardium, the valves, the conduction system as well as the coronary arteries causing restrictive cardiomyopathy manifesting as congestive heart failure, myocarditis, neoplastic pericardial disease (see above), valvular disease, arrhythmias or premature coronary artery disease [38]. Acute cardiotoxicity from radiotherapy is a rarity. Long-term cardiological follow-ups are required particularly for cancer survivors who received a mediastinal/heart dose of >30 Gy since cardiac manifestations from radiotherapy can occur as late as years or even decades after radiation therapy [38].
Conclusions
Cardiac complications of extracardiac tumours are rarely of immediate clinical relevance usually occurring late in the course of malignant disease. The main primary site of tumours affecting the heart is the lung. Of clinical significance is neoplastic pericardial disease leading to large pericardial effusions and potentially to fatal cardiac tamponade. Complications of carcinoid heart disease have significantly diminished due to effective treatment with somatostatin analogues. With the improvement of curative therapies, the clinical relevance of drug-related and post-radiogenic cardiopathy is possibly still underestimated, and long-term cardiological follow-up programmes of cancer survivors need to be established. Cardio-oncology is a young and dynamic field with high potential.
Acknowledgments
We thank Giannicola D’Addario for providing us with clinical data and images of the case presented.
Funding/Potential Competing Interests
No financial support and no other potential conflict of interest relevant to this article were reported.
References
- Burke, A.; Virmani, R. Tumors of the Heart and Great Vessels; Atlas of tumor pathology, Ser 3; Armed Forces Institute of Pathology: Wahington, DC, USA, 1996. [Google Scholar]
- Reynen, K. Frequency of primary tumors of the heart. Am J Cardiol. 1996, 77, 107. [Google Scholar] [CrossRef] [PubMed]
- Bisel, H.F.; Wroblewski, F.; Ladue, J.S. Incidence and clinical manifestations of cardiac metastases. J Am Med Assoc. 1953, 153, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Klatt, E.C.; Heitz, D.R. Cardiac metastases. Cancer. 1990, 65, 1456–1459. [Google Scholar] [CrossRef]
- Silvestri, F.; Bussani, R.; Pavletic, N.; Mannone, T. Metastases of the heart and pericardium. G Ital Cardiol. 1997, 27, 1252–1255. [Google Scholar]
- Deloach, J.F.; Haynes, J.W. Secondary tumors of heart and pericardium; review of the subject and report of one hundred thirty-seven cases. AMA Arch Intern Med. 1953, 91, 224–249. [Google Scholar] [CrossRef]
- Prichard, R.W. Tumors of the heart; review of the subject and report of 150 cases. AMA Arch Pathol. 1951, 51, 98–128. [Google Scholar]
- Glancy, D.L.; Roberts, W.C. The heart in malignant melanoma. A study of 70 autopsy cases. Am J Cardiol. 1968, 21, 555–571. [Google Scholar] [CrossRef]
- Bussani, R.; De-Giorgio, F.; Abbate, A.; Silvestri, F. Cardiac metastases. J Clin Pathol. 2007, 60, 27–34. [Google Scholar] [CrossRef]
- Chua, S.O.; Chiang, C.W.; Lee, Y.S.; Lin, S.H.; Liaw, Y.F. Moving right atrial mass associated with hepatoma. Two cases detected by echocardiography. Chest. 1986, 89, 148–150. [Google Scholar] [CrossRef]
- Van Camp, G.; Abdulsater, J.; Cosyns, B.; Liebens, I.; Vandenbossche, J.L. Transesophageal echocardiography of right atrial metastasis of a hepatocellular carcinoma. Chest. 1994, 105, 945–947. [Google Scholar] [CrossRef] [PubMed]
- Longo, R.; Mocini, D.; Santini, M.; Giannantoni, P.; Carillio, G.; Torino, F.; et al. Unusual sites of metastatic malignancy: Case 1. Cardiac metastasis in hepatocellular carcinoma. J Clin Oncol. 2004, 22, 5012–5014. [Google Scholar] [CrossRef]
- Atik, F.A.; Navia, J.L.; Krishnamurthi, V.; Singh, G.; Shiota, T.; Pitas, G.; et al. Solitary massive right ventricular metastasis of renal cell carcinoma without inferior vena cava or right atrium involvement. J Card Surg. 2006, 21, 304–306. [Google Scholar] [CrossRef]
- Aburto, J.; Bruckner, B.A.; Blackmon, S.H.; Beyer, E.A.; Reardon, M.J. Renal cell carcinoma, metastatic to the left ventricle. Tex Heart Inst J. 2009, 36, 48–49. [Google Scholar] [PubMed]
- Zayas, R.; Anguita, M.; Torres, F.; Giménez, D.; Bergillos, F.; Ruiz, M.; et al. Incidence of specific etiology and role of methods for specific etiologic diagnosis of primary acute pericarditis. Am J Cardiol. 1995, 75, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Ben-Horin, S.; Bank, I.; Guetta, V.; Livneh, A. Large symptomatic pericardial effusion as the presentation of unrecognized cancer: A study in 173 consecutive patients undergoing pericardiocentesis. Medicine (Baltimore) 2006, 85, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Little, W.C.; Freeman, G.L. Pericardial disease. Circulation. 2006, 113, 1622–1632. [Google Scholar] [CrossRef]
- Wilson, L.D.; Detterbeck, F.C.; Yahalom, J. Clinical practice. Superior vena cava syndrome with malignant causes. N Engl J Med. 2007, 356, 1862–1869. [Google Scholar] [CrossRef]
- Ahmann, F.R. A reassessment of the clinical implications of the superior vena caval syndrome. J Clin Oncol. 1984, 2, 961–969. [Google Scholar] [CrossRef]
- Rice, T.W.; Rodriguez, R.M.; Light, R.W. The superior vena cava syndrome: Clinical characteristics and evolving etiology. Medicine (Baltimore). 2006, 85, 37–42. [Google Scholar] [CrossRef]
- Lundin, L.; Norheim, I.; Landelius, J.; Oberg, K.; Theodorsson-Norheim, E. Carcinoid heart disease: Relationship of circulating vasoactive substances to ultrasound-detectable cardiac abnormalities. Circulation. 1988, 77, 264–269. [Google Scholar] [CrossRef]
- Roberts, W.C. A unique heart disease associated with a unique cancer: Carcinoid heart disease. Am J Cardiol. 1997, 80, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Bernheim, A.M.; Connolly, H.M.; Pellikka, P.A. Carcinoid heart disease. Curr Treat Options Cardiovasc Med. 2007, 9, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, B.I.; Hauso, O.; Drozdov, I.; Kidd, M.; Modlin, I.M. Carcinoid heart disease. Int J Cardiol. 2008, 129, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Dero, I.; De Pauw, M.; Borbath, I.; Delaunoit, T.; Demetter, P.; Demolin, G.; et al. Carcinoid heart disease—a hidden complication of neuroendocrine tumours. Acta Gastroenterol Belg. 2009, 72, 34–38. [Google Scholar]
- Smith, S.A.; Waggoner, A.D.; de las Fuentes, L.; Davila-Roman, V.G. Role of serotoninergic pathways in drug-induced valvular heart disease and diagnostic features by echocardiography. J Am Soc Echocardiogr. 2009, 22, 883–889, Epub 2009 Jun 23. [Google Scholar] [CrossRef]
- Dumoulein, M.; Verslype, C.; van Cutsem, E.; Meuris, B.; Herijgers, P.; Flameng, W.; Herregods, M.C. Carcinoid heart disease: Case and literature review. Acta Cardiol. 2010, 65, 261–264. [Google Scholar] [CrossRef]
- Bernheim, A.M.; Connolly, H.M.; Pellikka, P.A. Carcinoid heart disease in patients without hepatic metastases. Am J Cardiol. 2007, 99, 292–294. [Google Scholar] [CrossRef]
- Zuetenhorst, J.M.; Bonfrer, J.M.; Korse, C.M.; Bakker, R.; van Tinteren, H.; Taal, B.G. Carcinoid heart disease: The role of urinary 5-hydroxyindoleacetic acid excretion and plasma levels of atrial natriuretic peptide, transforming growth factor-beta and fibroblast growth factor. Cancer. 2003, 97, 1609–1615. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Toumpanakis, C.; Chilkunda, D.; Caplin, M.E.; Davar, J. Risk Factors for the Development and Progression of Carcinoid Heart Disease. Am J Cardiol. 2011. [Google Scholar] [CrossRef]
- Dubrey, S.W.; Cha, K.; Simms, R.W.; Skinner, M.; Falk, R.H. Electrocardiography and Doppler echocardiography in secondary (AA) amyloidosis. Am J Cardiol. 1996, 77, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Dubrey, S.W.; Cha, K.; Anderson, J.; Chamarthi, B.; Reisinger, J.; Skinner, M.; Falk, R.H. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM. 1998, 91, 141–157. [Google Scholar] [CrossRef]
- Falk, R.H.; Dubrey, S.W. Amyloid heart disease. Prog Cardiovasc Dis. 2010, 52, 347–361, Erratum in Prog Cardiovasc Dis. 2010, 52, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Kyle, R.A.; Gertz, M.A.; Greipp, P.R.; Witzig, T.E.; Lust, J.A.; Lacy, M.Q.; Therneau, T.M. A trial of three regimens for primary amyloidosis: Colchicine alone, melphalan and prednisone, and melphalan, prednisone, and colchicine. N Engl J Med. 1997, 336, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Wojnowski, L.; Kulle, B.; Schirmer, M.; Schlüter, G.; Schmidt, A.; Rosenberger, A.; et al. NAD(P)H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity. Circulation. 2005, 112, 3754–3762. [Google Scholar] [CrossRef] [PubMed]
- Berliner, S.; Rahima, M.; Sidi, Y.; Teplitsky, Y.; Zohar, Y.; Nussbaum, B.; Pinkhas, J. Acute coronary events following cisplatin-based chemotherapy. Cancer Invest. 1990, 8, 583–586. [Google Scholar] [CrossRef]
- Paiva, C.E.; Michelin, O.C.; Okoshi, K. Acute Coronary Syndrome during Chemotherpy: Report of Three Cases. Revista Brasileira de Cancerologia. 2009, 55, 55–58. [Google Scholar] [CrossRef]
- Bovelli, D.; Plataniotis, G.; Roila, F.; ESMO Guidelines Working Group. Cardiotoxicity of chemotherapeutic agents and radiotherapy-related heart disease: ESMO Clinical Practice Guidelines. Ann Oncol. 2010, 21 (Suppl. S5), v277–v282. [Google Scholar] [CrossRef]
- Chu, T.F.; Rupnick, M.A.; Kerkela, R.; Dallabrida, S.M.; Zurakowski, D.; Nguyen, L.; et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007, 370, 2011–2019. [Google Scholar] [CrossRef]
- Khakoo, A.Y.; Kassiotis, C.M.; Tannir, N.; Plana, J.C.; Halushka, M.; Bickford, C.; Trent, J., 2nd; Champion, J.C.; Durand, J.B.; Lenihan, D.J. Heart failure associated with sunitinib malate: A multitargeted receptor tyrosine kinase inhibitor. Cancer. 2008, 112, 2500–2508. [Google Scholar] [CrossRef]
© 2011 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.