Current and Future Therapies for Pulmonary Hypertension
Abstract
Zusammenfassung
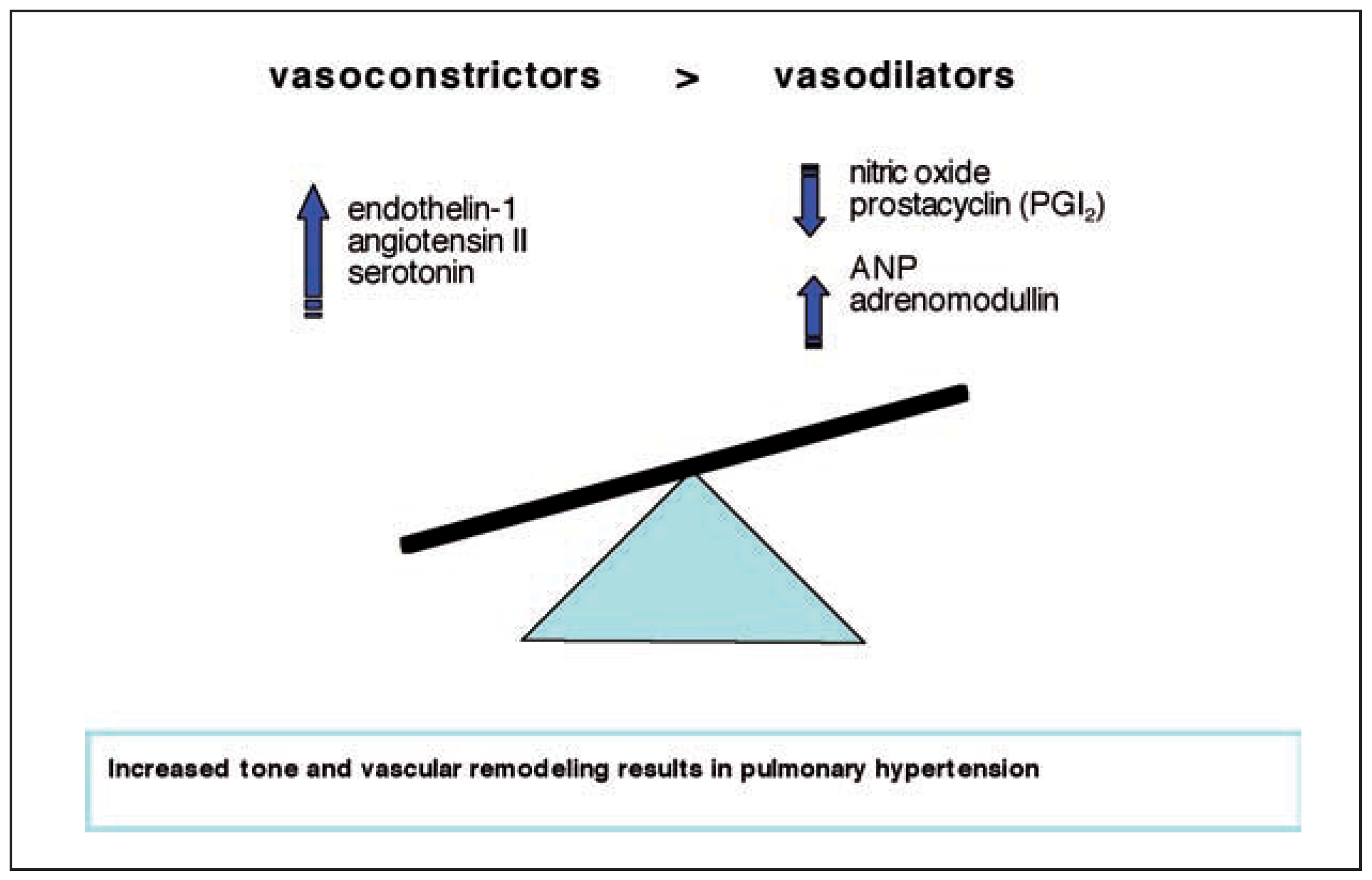
Introduction
Conventional therapy
Anticoagulation
Diuretics
Other drugs for left heart failure
Long-term oxygen therapy
Calcium-channel blocker therapy
Current specific therapy for PAH
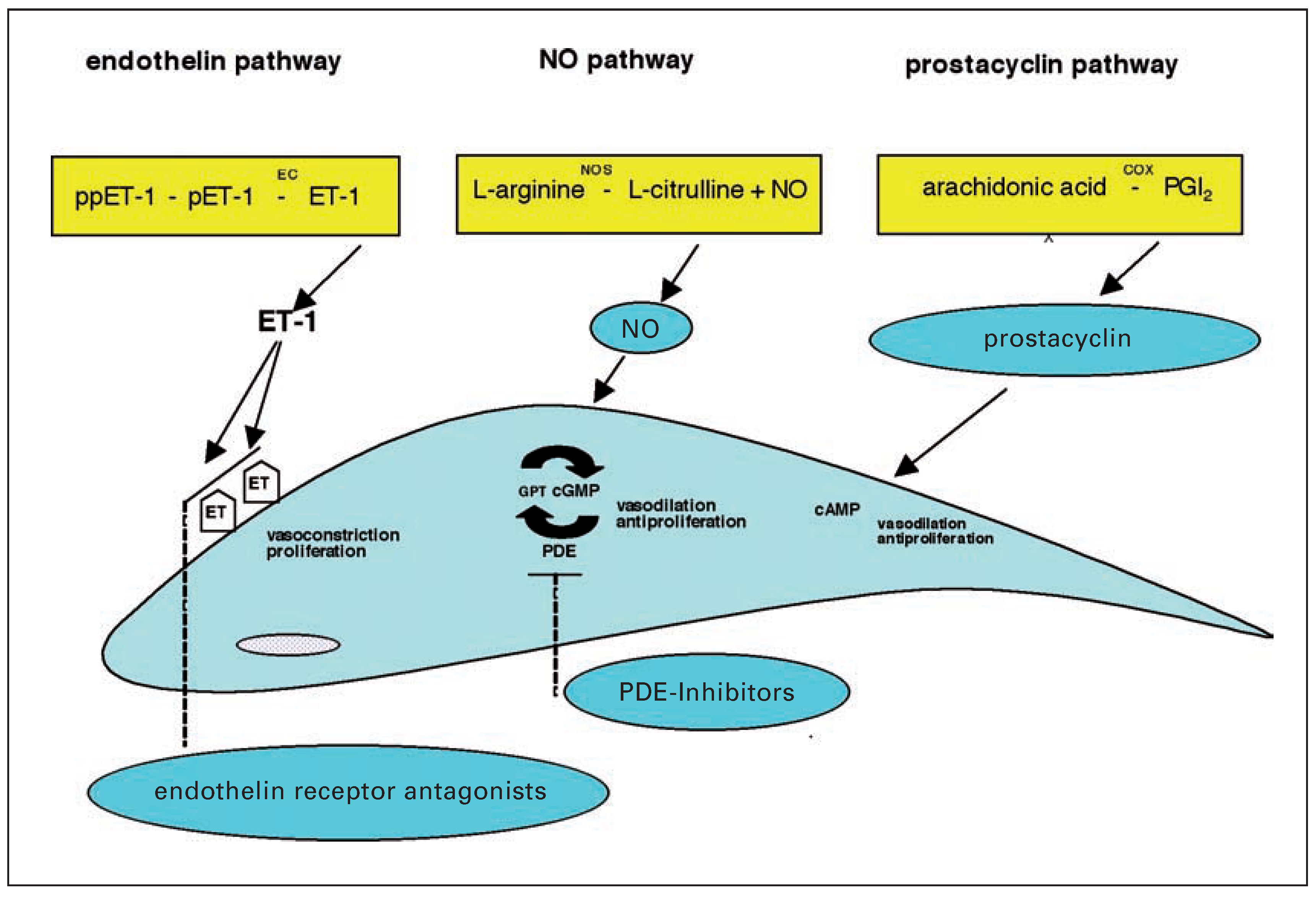
Prostanoids
Endothelin receptor antagonists
Phosphodiesterase inhibitors
Combination therapy
Therapy for chronic thromboembolic pulmonary hypertension
Surgery
Medical and interventional therapy for CTEPH
Lung transplantation in pulmonary hypertension
Future directions in the treatment of pulmonary hypertension
Cell proliferation and angiogenesis
Inflammation and immune response
Conclusion
References
- Simonneau, G.; Galie, N.; Rubin, L.J.; et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004, 43, 5–12S. [Google Scholar] [CrossRef] [PubMed]
- D’Alonzo, G.E.; Barst, R.J.; Ayres, S.M.; et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991, 115, 343–349. [Google Scholar] [CrossRef]
- Humbert, M.; Morrell, N.W.; Archer, S.L.; et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004, 43, 13–24S. [Google Scholar] [CrossRef]
- Rich, S.; Kaufmann, E.; Levy, P.S. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med. 1992, 327, 76–81. [Google Scholar] [CrossRef]
- Pengo, V.; Lensing, A.W.; Prins, M.H.; et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004, 350, 2257–2264. [Google Scholar] [CrossRef]
- Fuster, V.; Steele, P.M.; Edwards, W.D.; Gersh, B.J.; McGoon, M.D.; Frye, R.L. Primary pulmonary hypertension: Natural history and the importance of thrombosis. Circulation. 1984, 70, 580–587. [Google Scholar] [CrossRef]
- Bjornsson, J.; Edwards, W.D. Primary pulmonary hypertension: A histopathologic study of 80 cases. Mayo Clin Proc. 1985, 60, 16–25. [Google Scholar] [CrossRef]
- Robbins, I.M.; Kawut, S.M.; Yung, D.; et al. A study of aspirin and clopidogrel in idiopathic pulmonary arterial hypertension. Eur Respir J. 2006, 27, 578–584. [Google Scholar] [CrossRef]
- Escudero, J.; Navarro, J.; Padua, A.; Betancourt, L.; Nava, G. [Use of enalapril, an angiotensin-converting enzyme inhibitor, in pulmonary artery hypertension]. Arch Inst Cardiol Mex. 1986, 56, 467–473. [Google Scholar] [PubMed]
- Rich, S.; Seidlitz, M.; Dodin, E.; et al. The short-term effects of digoxin in patients with right ventricular dysfunction from pulmonary hypertension. Chest. 1998, 114, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Velez-Roa, S.; Ciarka, A.; Najem, B.; Vachiery, J.L.; Naeije, R.; van de Borne, P. Increased sympathetic nerve activity in pulmonary artery hypertension. Circulation 2004, 110, 1308–1312. [Google Scholar] [CrossRef]
- Provencher, S.; Herve, P.; Jais, X.; et al. Deleterious effects of beta-blockers on exercise capacity and hemodynamics in patients with portopulmonary hypertension. Gastroenterology. 2006, 130, 120–126. [Google Scholar] [CrossRef]
- Doherty, D.E.; Petty, T.L.; Bailey, W.; et al. Recommendations of the 6th long-term oxygen therapy consensus conference. Respir Care. 2006, 51, 519–525. [Google Scholar] [PubMed]
- Petty, T.L. Long-term outpatient oxygen therapy in advanced chronic obstructive pulmonary disease. Chest. 1980, 77, 304. [Google Scholar] [CrossRef] [PubMed]
- Petty, T.L.; Nett, L.M. The history of long-term oxygen therapy. Respir Care. 1983, 28, 859–865. [Google Scholar]
- Weitzenblum, E.; Sautegeau, A.; Ehrhart, M.; Mammosser, M.; Pelletier, A. Long-term oxygen therapy can reverse the progression of pulmonary hypertension in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1985, 131, 493–498. [Google Scholar] [CrossRef]
- Wuertemberger, G.; Zielinsky, J.; Sliwinsky, P.; Auw-Haedrich, C.; Matthys, H. Survival in chronic obstructive pulmonary disease after diagnosis of pulmonary hypertension related to long-term oxygen therapy. Lung. 1990, 168, 762–769. [Google Scholar] [CrossRef]
- Flenley, D.C.; Muir, A.L. Cardiovascular effects of oxygen therapy for pulmonary arterial hypertension. Clin Chest Med. 1983, 4, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Gluskowski, J.; Jedrzejewska-Makowska, M.; Hawrylkiewicz, I.; Vertun, B.; Zielinski, J. Effects of prolonged oxygen therapy on pulmonary hypertension and blood viscosity in patients with advanced cor pulmonale. Respiration. 1983, 44, 177–183. [Google Scholar] [CrossRef]
- Johansson, B.W.; Torp, A.; Trell, E. Prolonged ambulatory oxygen therapy in pulmonary hypertension of various etiology. Acta Med Scand. 1971, 189, 155–159. [Google Scholar] [CrossRef]
- Roberts, D.H.; Lepore, J.J.; Maroo, A.; Semigran, M.J.; Ginns, L.C. Oxygen therapy improves cardiac index and pulmonary vascular resistance in patients with pulmonary hypertension. Chest. 2001, 120, 1547–1555. [Google Scholar] [CrossRef][Green Version]
- O’Donohue WJ, Jr. Home oxygen therapy. Clin Chest Med. 1997, 18, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Sitbon, O.; Humbert, M.; Jais, X.; et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005, 111, 3105–3111. [Google Scholar] [CrossRef]
- Badesch, D.B.; Abman, S.H.; Ahearn, G.S.; et al. Medical therapy for pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004, 126, 35–62S. [Google Scholar] [CrossRef]
- Barst, R.J.; Rubin, L.J.; Long, W.A.; et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group. N Engl J Med. 1996, 334, 296–302. [Google Scholar] [CrossRef]
- Badesch, D.B.; Tapson, V.F.; McGoon, M.D.; et al. Continuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease. A randomized, controlled trial. Ann Intern Med. 2000, 132, 425–434. [Google Scholar] [CrossRef]
- Badesch, D.B.; McLaughlin, V.V.; Delcroix, M.; et al. Prostanoid therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2004, 43, 56–61S. [Google Scholar] [CrossRef]
- Simonneau, G.; Barst, R.J.; Galie, N.; et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: A doubleblind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2002, 165, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Olschewski, H.; Ghofrani, H.A.; Schmehl, T.; et al. Inhaled iloprost to treat severe pulmonary hypertension. An uncontrolled trial. German PPH Study Group. Ann Intern Med. 2000, 132, 435–443. [Google Scholar] [CrossRef]
- Olschewski, H.; Simonneau, G.; Galie, N.; et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002, 347, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Voelkel, N.F.; Cool, C. Pathology of pulmonary hypertension. Cardiol Clin. 2004, 22, 343–351. [Google Scholar] [CrossRef]
- Voelkel, N.F.; Tuder, R.M. Cellular and molecular mechanisms in the pathogenesis of severe pulmonary hypertension. Eur Respir J. 1995, 8, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Channick, R.; Badesch, D.B.; Tapson, V.F.; et al. Effects of the dual endothelin receptor antagonist bosentan in patients with pulmonary hypertension: A placebo-controlled study. J Heart Lung Transplant. 2001, 20, 262–263. [Google Scholar] [CrossRef]
- Rubin, L.J.; Badesch, D.B.; Barst, R.J.; et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002, 346, 896–903. [Google Scholar] [CrossRef]
- Benigni, A.; Remuzzi, G. Endothelin antagonists. Lancet. 1999, 353, 133–138. [Google Scholar] [CrossRef]
- Michelakis, E.; Tymchak, W.; Lien, D.; Webster, L.; Hashimoto, K.; Archer, S. Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension: Comparison with inhaled nitric oxide. Circulation. 2002, 105, 2398–2403. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Mason, N.A.; Morrell, N.W.; et al. Sildenafil inhibits hypoxia-induced pulmonary hypertension. Circulation. 2001, 104, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, H.A.; Voswinckel, R.; Reichenberger, F.; et al. Differences in hemodynamic and oxygenation responses to three different phosphodiesterase-5 inhibitors in patients with pulmonary arterial hypertension: A randomized prospective study. J Am Coll Cardiol. 2004, 44, 1488–1496. [Google Scholar]
- Hoeper, M.M.; Faulenbach, C.; Golpon, H.; Winkler, J.; Welte, T.; Niedermeyer, J. Combination therapy with bosentan and sildenafil in idiopathic pulmonary arterial hypertension. Eur Respir J. 2004, 24, 1007–1010. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Taha, N.; Bekjarova, A.; Gatzke, R.; Spiekerkoetter, E. Bosentan treatment in patients with primary pulmonary hypertension receiving nonparenteral prostanoids. Eur Respir J. 2003, 22, 330–334. [Google Scholar] [CrossRef]
- Beyer, S.; Speich, R.; Fischler, M.; Maggiorini, M.; Ulrich, S. Longterm experience with oral or inhaled vasodilator combination therapy in patients with pulmonary hypertension. Swiss Med Wkly. 2006, 136, 114–118. [Google Scholar] [CrossRef]
- Ghofrani, H.A.; Rose, F.; Schermuly, R.T.; et al. Oral sildenafil as long-term adjunct therapy to inhaled iloprost in severe pulmonary arterial hypertension. J Am Coll Cardiol. 2003, 42, 158–164. [Google Scholar] [CrossRef]
- Ghofrani, H.A.; Wiedemann, R.; Rose, F.; et al. Combination therapy with oral sildenafil and inhaled iloprost for severe pulmonary hypertension. Ann Intern Med. 2002, 136, 515–522. [Google Scholar] [CrossRef]
- Humbert, M.; Barst, R.J.; Robbins, I.M.; et al. Combination of bosentan with epoprostenol in pulmonary arterial hypertension: BREATHE-2. Eur Respir J. 2004, 24, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Stiebellehner, L.; Petkov, V.; Vonbank, K.; et al. Long-term treatment with oral sildenafil in addition to continuous IV epoprostenol in patients with pulmonary arterial hypertension. Chest. 2003, 123, 1293–1295. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, M.; Satoh, T.; Manabe, T.; et al. Oral sildenafil improves primary pulmonary hypertension refractory to epoprostenol. Circ J. 2005, 69, 461–465. [Google Scholar] [CrossRef]
- Becattini, C.; Agnelli, G.; Pesavento, R.; et al. Incidence of chronic thromboembolic pulmonary hypertension after a first episode of pulmonary embolism. Chest. 2006, 130, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Fedullo, P.F.; Auger, W.R.; Kerr, K.M.; Rubin, L.J. Chronic thromboembolic pulmonary hypertension. N Engl J Med. 2001, 345, 1465–1472. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Mayer, E.; Simonneau, G.; Rubin, L.J. Chronic thromboembolic pulmonary hypertension. Circulation. 2006, 113, 2011–2020. [Google Scholar] [CrossRef]
- Jamieson, S.W.; Kapelanski, D.P.; Sakakibara, N.; et al. Pulmonary endarterectomy: Experience and lessons learned in 1,500 cases. Ann Thorac Surg. 2003, 76, 1457–1462, discussion 1462–1464. [Google Scholar] [CrossRef]
- Moser, K.M.; Auger, W.R.; Fedullo, P.F. Chronic major-vessel thromboembolic pulmonary hypertension. Circulation. 1990, 81, 1735–1743. [Google Scholar] [CrossRef]
- Klepetko, W.; Mayer, E.; Sandoval, J.; et al. Interventional and surgical modalities of treatment for pulmonary arterial hypertension. J Am Coll Cardiol. 2004, 43, 73–80S. [Google Scholar] [CrossRef]
- Dartevelle, P.; Fadel, E.; Mussot, S.; et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J. 2004, 23, 637–648. [Google Scholar] [CrossRef]
- Bresser, P.; Fedullo, P.F.; Auger, W.R.; et al. Continuous intravenous epoprostenol for chronic thromboembolic pulmonary hypertension. Eur Respir J. 2004, 23, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Kerr, K.M.; Rubin, L.J. Epoprostenol therapy as a bridge to pulmonary thromboendarterectomy for chronic thromboembolic pulmonary hypertension. Chest. 2003, 123, 319–320. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, N.; Sasaki, N.; Ando, M.; et al. Prostacyclin therapy before pulmonary thromboendarterectomy in patients with chronic thromboembolic pulmonary hypertension. Chest. 2003, 123, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, H.A.; Schermuly, R.T.; Rose, F.; et al. Sildenafil for long-term treatment of nonoperable chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2003, 167, 1139–1141. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Kramm, T.; Wilkens, H.; et al. Bosentan therapy for inoperable chronic thromboembolic pulmonary hypertension. Chest 2005, 128, 2363–2367. [Google Scholar] [CrossRef]
- Bonderman, D.; Nowotny, R.; Skoro-Sajer, N.; et al. Bosentan therapy for inoperable chronic thromboembolic pulmonary hypertension. Chest. 2005, 128, 2599–2603. [Google Scholar] [CrossRef]
- Feinstein, J.A.; Goldhaber, S.Z.; Lock, J.E.; Ferndandes, S.M.; Landzberg, M.J. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation. 2001, 103, 10–13. [Google Scholar]
- Pitton, M.B.; Herber, S.; Mayer, E.; Thelen, M. Pulmonary balloon angioplasty of chronic thromboembolic pulmonary hypertension (CTEPH) in surgically inaccessible cases. Rofo. 2003, 175, 631–634. [Google Scholar] [CrossRef]
- International guidelines for the selection of lung transplant candidates. The American Society for Transplant Physicians (ASTP)/American Thoracic Society(ATS)/European Respiratory Society(ERS)/International Society for Heart and Lung Transplantation (ISHLT). Am J Respir Crit Care Med. 1998, 158, 335–339.
- Mendeloff, E.N.; Meyers, B.F.; Sundt, T.M.; et al. Lung transplantation for pulmonary vascular disease. Ann Thorac Surg. 2002, 73, 209–217. [Google Scholar] [CrossRef]
- Voelkel, N.F.; Cool, C.; Taraceviene-Stewart, L.; et al. Janus face of vascular endothelial growth factor: The obligatory survival factor for lung vascular endothelium controls precapillary artery remodeling in severe pulmonary hypertension. Crit Care Med. 2002, 30, S251–6. [Google Scholar] [CrossRef]
- Lee, S.D.; Shroyer, K.R.; Markham, N.E.; Cool, C.D.; Voelkel, N.F.; Tuder, R.M. Monoclonal endothelial cell proliferation is present in primary but not secondary pulmonary hypertension. J Clin Invest. 1998, 101, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Lane, K.B.; Machado, R.D.; Pauciulo, M.W.; et al. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. The International PPH Consortium. Nat Genet. 2000, 26, 81–84. [Google Scholar] [CrossRef]
- Deng, Z.; Morse, J.H.; Slager, S.L.; et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet. 2000, 67, 737–744. [Google Scholar] [CrossRef]
- Kwak, B.; Mulhaupt, F.; Myit, S.; Mach, F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000, 6, 1399–1402. [Google Scholar] [CrossRef] [PubMed]
- Kao, P.N. Simvastatin treatment of pulmonary hypertension: An observational case series. Chest. 2005, 127, 1446–1452. [Google Scholar] [CrossRef]
- Hu, H.; Sung, A.; Zhao, G.; et al. Simvastatin enhances bone morphogenetic protein receptor type II expression. Biochem Biophys Res Commun 2006, 339, 59–64. [Google Scholar] [CrossRef]
- Vasa, M.; Fichtlscherer, S.; Adler, K.; et al. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001, 103, 2885–2890. [Google Scholar] [CrossRef]
- MacLean, M.R. Pulmonary hypertension, anorexigens and 5-HT: Pharmacological synergism in action? Trends Pharmacol Sci. 1999, 20, 490–495. [Google Scholar] [CrossRef]
- MacLean, M.R.; Herve, P.; Eddahibi, S.; Adnot, S. 5-hydroxytryptamine and the pulmonary circulation: Receptors, transporters and relevance to pulmonary arterial hypertension. Br J Pharmacol. 2000, 131, 161–168. [Google Scholar] [CrossRef]
- Herve, P.; Launay, J.M.; Scrobohaci, M.L.; et al. Increased plasma serotonin in primary pulmonary hypertension. Am J Med. 1995, 99, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Eddahibi, S.; Morrell, N.; d’Ortho, M.P.; Naeije, R.; Adnot, S. Pathobiology of pulmonary arterial hypertension. Eur Respir J. 2002, 20, 1559–1572. [Google Scholar] [CrossRef] [PubMed]
- Eddahibi, S.; Raffestin, B.; Hamon, M.; Adnot, S. Is the serotonin transporter involved in the pathogenesis of pulmonary hypertension? J Lab Clin Med. 2002, 139, 194–201. [Google Scholar] [CrossRef]
- Eddahibi, S.; Fabre, V.; Boni, C.; et al. Induction of serotonin transporter by hypoxia in pulmonary vascular smooth muscle cells. Relationship with the mitogenic action of serotonin. Circ Res. 1999, 84, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Marcos, E.; Adnot, S.; Pham, M.H.; et al. Serotonin transporter inhibitors protect against hypoxic pulmonary hypertension. Am J Respir Crit Care Med. 2003, 168, 487–493. [Google Scholar] [CrossRef]
- Kawut, S.M.; Horn, E.M.; Berekashvili, K.K.; et al. Selective serotonin reuptake inhibitor use and outcomes in pulmonary arterial hypertension. Pulm Pharmacol Ther. 2006, 19, 370–374. [Google Scholar] [CrossRef]
- Schermuly, R.T.; Dony, E.; Ghofrani, H.A.; et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005, 115, 2811–2821. [Google Scholar] [CrossRef]
- Ghofrani, H.A.; Seeger, W.; Grimminger, F. Imatinib for the treatment of pulmonary arterial hypertension. N Engl J Med. 2005, 353, 1412–1413. [Google Scholar] [CrossRef]
- Simonneau, G.; Galiè, N.; Rubin, L.J.; et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004, 43, 5–12S. [Google Scholar] [CrossRef] [PubMed]
- Voelkel, N.F.; Cool, C.; Lee, S.D.; Wright, L.; Geraci, M.W.; Tuder, R.M. Primary pulmonary hypertension between inflammation and cancer. Chest. 1998, 114, S225–30. [Google Scholar] [CrossRef]
- Dorfmuller, P.; Perros, F.; Balabanian, K.; Humbert, M. Inflammation in pulmonary arterial hypertension. Eur Respir J. 2003, 22, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Monti, G.; Brenot, F.; et al. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med. 1995, 151, 1628–1631. [Google Scholar] [CrossRef]
- Nicolls, M.R.; Taraseviciene-Stewart, L.; Rai, P.R.; Badesch, D.B.; Voelkel, N.F. Autoimmunity and pulmonary hypertension: A perspective. Eur Respir J. 2005, 26, 1110–1118. [Google Scholar] [CrossRef]
- Mehta, N.J.; Khan, I.A.; Mehta, R.N.; Sepkowitz, D.A. HIV-Related pulmonary hypertension: Analytic review of 131 cases. Chest. 2000, 118, 1133–1141. [Google Scholar] [CrossRef]
- Speich, R.; Jenni, R.; Opravil, M.; Pfab, M.; Russi, E.W. Primary pulmonary hypertension in HIV infection. Chest. 1991, 100, 1268–1271. [Google Scholar] [CrossRef]
- Zuber, J.P.; Calmy, A.; Evison, J.M.; et al. Pulmonary arterial hypertension related to HIV infection: Improved hemodynamics and survival associated with antiretroviral therapy. Clin Infect Dis. 2004, 38, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Karmochkine, M.; Cacoub, P.; Dorent, R.; et al. High prevalence of antiphospholipid antibodies in precapillary pulmonary hypertension. J Rheumatol. 1996, 23, 286–290. [Google Scholar]
- Morelli, S.; Giordano, M.; De Marzio, P.; Priori, R.; Sgreccia, A.; Valesini, G. Pulmonary arterial hypertension responsive to immunosuppressive therapy in systemic lupus erythematosus. Lupus. 1993, 2, 367–369. [Google Scholar] [CrossRef]
- Groen, H.; Bootsma, H.; Postma, D.S.; Kallenberg, C.G. Primary pulmonary hypertension in a patient with systemic lupus erythematosus: Partial improvement with cyclophosphamide. J Rheumatol. 1993, 20, 1055–1057. [Google Scholar] [PubMed]
- Goupille, P.; Fauchier, L.; Babuty, D.; Fauchier, J.P.; Valat, J.P. Precapillary pulmonary hypertension dramatically improved with high doses of corticosteroids during systemic lupus erythematosus. J Rheumatol. 1994, 21, 1976–1977. [Google Scholar] [PubMed]
- Dahl, M.; Chalmers, A.; Wade, J.; Calverley, D.; Munt, B. Ten year survival of a patient with advanced pulmonary hypertension and mixed connective tissue disease treated with immunosuppressive therapy. J Rheumatol. 1992, 19, 1807–1809. [Google Scholar] [PubMed]

 |
 |
© 2007 by the authors. Attribution—Non-Commercial—NoDerivatives 4.0.
Share and Cite
Ulrich, S.; Fischler, M.; Speich, R., 1,2. Current and Future Therapies for Pulmonary Hypertension. Cardiovasc. Med. 2007, 10, 192. https://doi.org/10.4414/cvm.2007.01251
Ulrich S, Fischler M, Speich R 1,2. Current and Future Therapies for Pulmonary Hypertension. Cardiovascular Medicine. 2007; 10(6):192. https://doi.org/10.4414/cvm.2007.01251
Chicago/Turabian StyleUlrich, Silvia, Manuel Fischler, and Rudolf Speich, 1,2. 2007. "Current and Future Therapies for Pulmonary Hypertension" Cardiovascular Medicine 10, no. 6: 192. https://doi.org/10.4414/cvm.2007.01251
APA StyleUlrich, S., Fischler, M., & Speich, R., 1,2. (2007). Current and Future Therapies for Pulmonary Hypertension. Cardiovascular Medicine, 10(6), 192. https://doi.org/10.4414/cvm.2007.01251




