Abstract
The enteric nervous system (ENS) interacts bidirectionally with the local immune system, responding to inflammation within the gastrointestinal (GI) tract. In a previous study using the same samples, several gene targets were identified as being differentially expressed in the inflamed colonic tissue of pigs challenged with dextran sodium sulphate (DSS). Additionally, animals in the basal DSS group, exhibited reduced growth and increased fecal and pathology scores, while the relative abundance of beneficial taxa was reduced and harmful bacteria increased. While changes in the innate immune response and barrier function are widely cited regarding inflammatory bowel disease (IBD), the effects of inflammation on the local structures of the enteric nervous system (ENS) are less well understood. Hence, the objectives of this study were to: (1) evaluate the expression of a range of functionally diverse neuroactive receptors, transporters and neurotrophic factors in RNA derived from the colonic tissue from the same pigs; (2) examine associations with these neuroactive components and inflammatory, barrier function and matrix remodeling targets. Mature pigs were split into two experimental groups: (1) basal diet (n = 10); (2) basal diet + DSS (n = 11). The pigs were orally challenged with DSS once daily for four days and sacrificed humanely. Colonic tissue was collected for gene expression analysis. Most of the targets evaluated in this study were present at low levels or in some cases were undetectable by QPCR, including the dopamine receptor DRD5 and the serotonin receptor HTR3A. The dopamine receptors (DRD1, DRD3, DRD4), serotonin receptor (HTR4), and other selected neuroactive receptors (GRM7, GABRA2) were down-regulated in the DSS-challenged animals relative to the basal group (p < 0.05). Most notably, DRD2, was up-regulated four-fold, suggesting an active process involving this receptor (p < 0.05). Relationships with (previously published) gene expression data from the same samples suggest that DRD1 and DRD2 are influenced by different pathways and may also be interlinked with matrix remodeling and, more specifically, genes relevant to the epithelial to mesenchymal transition (CDH1, CDH2, IL6, IL13, IL10, MMP1, MMP2) an important fibrotic process in the pathogenesis of IBD.
1. Introduction
The enteric nervous system (ENS) is an essential part of the communication network between the brain and the gut, and is composed of two ganglionated plexuses (myenteric and submucosal), which regulate key functions such as gut motility, secretion, and absorption [1]. Within this, neuroactive molecules and their receptors mediate communication and facilitate motor, sensory, absorptive, and secretory functions [2], in addition to responding to the extraneous stimuli within the gut, including inflammation, diet, and the microbiome [3,4]. These same neuroactive molecules are present in some foods [5] and can be expressed both by the host and the microbiome, creating an “interkingdom communication system” within the gut [6]. Neuroactive receptors and transporters form part of the first stage of localized ENS signaling cascades in the gut and therefore when viewed in the context of other immune processes, can give insight into the complex dynamics between the ENS and inflammatory processes within the gut.
Inflammatory bowel disease (IBD) is a heterogenous set of inflammatory conditions mediated by the immune system, including ulcerative colitis (UC) and Crohn’s disease (CD) [7,8]. IBDs negatively affect the ENS [9] and regulation and resolution of inflammation in the gut influenced both by inflammatory mediators and neuroactive peptides and hormones [10]. The nervous system and the immune system are intrinsically linked in terms of the regulation of inflammation [11]. This reciprocal relationship is not simple, given the extensive cell types and neuroactive mediators that contribute to it including dopamine and serotonin (5-HT), brain-derived neurotrophic factor (BDNF) [12], acetylcholine [13], gamma-aminobutyric acid (GABA) [14], noradrenaline [15], and glutamate (Glu) [16].
Dopamine is an endogenous catecholamine, which is key to neuronal and non-neuronal processes, and acts as an immune modulator both within and outside the central nervous system [17]. An impaired dopaminergic system has been proposed as a feature of IBD pathogenesis [18] and there is considerable interest in how gut-derived dopamine affects the wider CNS and related pathologies such as Parkinson’s, schizophrenia, and Alzheimer’s disease [19]. Gut inflammation reduces levels of dopamine, and alters cells expressing its receptors, including Treg and effector T-cells [20]. Over 50% of dopamine is synthesized in the human gut [21] and both dopamine and its five receptors are distributed in the intestinal tract, influencing gastric secretion, motility, and mucosal blood flow [22,23]. Within the gut, dopamine is derived from the enteric nervous system [24], mucosa [25] as well as immune cells (dendritic, Treg, B cells, and macrophages) [26], and also by the microbiome [27]. All five classes of D1-like dopamine receptors (DRD) have been detected throughout the gut—DRD1, DRD3, and DRD5 are expressed both in nerve-containing layers and mucosa, DRD4 in the mucosal layer, and DRD2 in the nerve-containing layers only [28]. Detection and exploration of mechanistic effects of these receptors have been explored using a range of techniques including, QPCR, Western blots, immunofluorescence histochemistry [24], knockout studies, bioluminescence resonance energy transfer (BRET) [29], and dopamine receptor agonists [18,30]. These methods are variable in their capacity to detect and quantify the components of the ENS and/or elucidate the mechanisms of action. Hence, the evolving story surrounding the contribution of the ENS to gut health and inflammation that has yet to unfold fully.
Serotonin, also known as 5-hydroxytryptamine (5-HT) is a key neurotransmitter in the central nervous system that mediates a range of physiological functions, influencing mood, stress, feeding, cognition, and sexual behavior [31]. Serotonin produced in the brain acts as a neurotransmitter, while in the periphery it acts as a hormone, paracrine factor, and intracellular signaling molecule. Peripheral serotonin regulates gut function, including intestinal motility and secretion [32] and metabolic homeostasis [33]. Peripheral serotonin acts independently of brain-derived serotonin, as this monoamine neurotransmitter cannot cross the blood–brain barrier [34]. Over 90% of peripheral 5-HT is synthesized by the enterochromaffin cells distributed throughout the gut [35] and, with regards to inflammation, is proposed to play a role in the maintenance of the inflammatory state in Crohn’s disease, mediated through the recruitment and promotion of subepithelial myofibroblasts [36]. Serotonin can promote inflammation through activation of dendritic cell 5-HT7 receptors and can reverse inflammation through epithelial 5-HT4 receptors in the intestinal mucosa [37]. A total of five serotonin (5-hydroxytryptamine, 5-HT) receptors are expressed in the gut within smooth muscle, enteric neurons, enterocytes, and immune cells [38]. Of the five serotonin receptors, 5-HTR4 is the most exposed receptor to the lumen of the gut and has roles in gut motility and control of intestinal secretions [39,40]. Receptors 5-HTR3 and 5-HTR4 have multiple roles in secretory, motility, and immune functions in the GI tract. These receptors are important for the re-establishment of homeostasis in piglets following the post-weaning diarrhea that often occurs during the abrupt weaning that occurs in commercial enterprises [38], with serotonin being a key mediator of the diarrheal response to enterotoxins [41].
DSS-induced acute colitis exhibits ulceration, mucosal damage, reduced crypt depth, up-regulation of inflammatory mediators, and leukocyte infiltration [42]. Dextran sodium sulfate (DSS) is a sulfated polysaccharide that is directly toxic to colonic epithelium, causing epithelial cell injury with subsequent innate immune responses altering mucosal barrier function. This inflammatory response develops in the absence of T-cells and hence is a good model for the study of the innate immune mechanisms involved in the development of intestinal inflammation [43]. Differences in gene expression indicative of inflammatory processes had been observed previously between the control and DSS-exposed colonic samples used in this study [44]. More specifically, genes relating to matrix remodeling and barrier function (MMP1, MMP2, CDH1, MUC4) and inflammatory cytokines (IL6, IL23, IL13) were differentially expressed (Table S1).
Hence, the objectives of this study were: (1) to determine the gene expression pattern of a selection of functionally diverse and representative neuroactive receptors and factors reported to be relevant to IBD pathogenesis; (2) to evaluate the expression of the detected neuroactive receptors relative to markers of gut health and inflammation in the colonic tissue of a DSS porcine model of colitis.
2. Materials and Methods
Porcine colonic RNA samples, described in Rattigan et al., 2020 [44], were used to evaluate the targets in this study. All experimental procedures described in this work were approved under University College Dublin Animal Research Ethics Committee (AREC-14-14-O’Doherty) and were conducted in accordance with Irish legislation (SI no. 534/2012) and the EU directive 2010/63/EU for animal experimentation.
Pigs (63 days of age) were split into two experimental groups and housed separately: (1) basal diet (n = 10); (2) basal diet + DSS (n = 11). The basal diet was the same for both groups and was formulated to contain 14.5 MJ/kg of digestible energy, 210 g/kg crude protein, and a lysine content of 14.5 g/kg. All amino acid requirements were met relative to lysine [45].
The basal diet + DSS pigs were orally challenged with DSS once daily for four days. Pigs in this group were administered DSS solution (0.75 g/kg body weight; molecular weight 47.9 kDa; (TdB Consultancy AB, Uppsala, Sweden) via a single daily oral dose, sufficient to induce epithelial erosion and mucosal inflammation. This dose was based on previous optimizations [46]. Following the four-day DSS challenge and one-day recovery, the animals were sacrificed humanely and sections of proximal colon (1 cm2) were collected and stored in RNALater® (Sigma-Aldrich, St. Louis, MO, USA) overnight at 4 °C for gene expression analysis. The RNALater® was removed prior to storage at −80 °C. Total RNA was extracted using TRIreagent (Sigma-Aldrich) followed by purification with a GenElute™ Mammalian Total RNA miniprep kit. This was followed by an on-column DNase digestion (Sigma-Aldrich). Total RNA (2 μg) was reverse transcribed using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) and oligo (dT) primers in a final reaction volume of 40 μL, in accordance with manufacturer’s guidelines. The synthesized cDNA was made up to a volume of 200 μL with nuclease-free water.
The expression of a panel of genes relating to a range of neuroactive receptors, transporters, and neurotrophic factors (Table 1) was evaluated using quantitative PCR (QPCR). Furthermore, normalized relative quantities (RQ) of genes relating to immune and barrier function in addition to matrix remodeling, from previously published data Rattigan et al., 2020 [44], were reanalyzed to: (1) establish differential expression as only two of the four original experimental groups were used in this study and additional immune and matrix remodeling genes; CXCL8, CDH2, MMP7 were added to this study; (2) to evaluate correlations between the selected neuroactive receptors and factors and inflammatory/matrix remodeling genes.

Table 1.
Porcine oligonucleotide primers used for QPCR.
Primers (Table 1) were designed on Primer Express™ (Applied Biosystems, Foster City, CA, USA), synthesized by Eurofins (Ebersberg, Germany). Primers were validated using cDNA derived from archived RNA from the paraventricular nucleus region of the porcine brain [47], where the targets are abundantly expressed. The QPCR reactions were prepared in a total volume of 20 μL, containing 10 μL FastStart Universal SYBR Green Master Mix (Roche, Basel, Switzerland), 1.8 μL forward and reverse primer mix (300 nm), 3.2 μL nuclease-free water, and 5 μL cDNA. QPCR was performed in duplicate on the 7500 ABI Prism Sequence Detection System (Applied Biosystems, Foster City, CA, USA). Thermocycling conditions were as follows: 95 °C for 10 min for one cycle, followed by 95 °C for 15 s and 60 °C for 1 min for 40 cycles. The efficiency of each assay was determined by plotting the cycling threshold (CT) from four-fold serial dilutions of cDNA against their arbitrary quantities. Primer pairs that exhibited specificity for a single product and >90% efficiency were further evaluated on the colonic cDNA where the CT for each gene was determined. Normalized relative quantities were obtained using the software, qBase PLUS 2.0 (Biogazelle, Ghent, Belgium) from reference genes ACTB and GAPDH.
All gene expression data were checked for normality using the univariate procedure of Statistical Analysis Software (SAS) 9.4 (SAS Institute, Cary, NC, USA) and transformed, if required and outliers removed. The general linearized model (GLM) procedure within SAS was used to analyze the data (Bonferroni adjusted p < 0.05). The results are presented as least square means with standard errors. The probability level that denotes significance is p < 0.05. The Corrplot package [48] within R was used to visualize the correlation matrix of selected genes ordered to the first principal component. The package GGally was used to generate density plots and pairwise plot matrices within R [49].
3. Results
3.1. Gene Expression
3.1.1. Differential Expression
Many neuroactive receptors evaluated in this study were down-regulated in the DSS group compared to the controls and are presented in Table 2; these included the dopamine receptors DRD1 (p < 0.0001), DRD3 (p = 0.025), DRD4 (p = 0.031), serotonin receptor HTR4 (p = 0.002), GRM7 (p = 0.015), and GABRA2 (p = 0.005). In contrast, DRD2 (p = 0.002) was up-regulated four-fold in the DSS group compared to controls (Table 2).

Table 2.
The effects of DSS on expression of a panel of genes in the colon compared to controls including; least square means (LSMean), standard error of mean (SEM). Significance is denoted by p-Value: * p < 0.05, ** p < 0.01, *** p < 0.001 (highlighted in bold).
Concurrent to this, several differentially expressed (DE) genes relating to matrix remodeling (MMP1, MMP2), tight junctions (CDH1), and immune targets (IL13, IL23, IL6), all increased, and mucin (MUC4) decreased, between the basal and basal + DSS groups (p < 0.05) (Table S1).
3.1.2. Co-Expression with Immune and Matrix Remodeling Related Targets
The co-expression patterns of the neuroactive receptors (evaluated in this study) with a range of immune, matrix remodeling, and barrier function genes (reported previously [44]) are represented in Figure 1a for: (a) the Basal group; and Figure 1b for: (b) the Basal + DSS group. Genes are ordered according to their loadings to the first principal component. The change in co-expression dynamics is notable for a few key targets. DRD1 is correlated with HTR4, CDH1, SLC6A3, SLC6A4, FFAR1, ADRB2, MMP2, and MMP9 in the control group, while it is correlated with MUC4 in the DSS group. DRD2 is negatively correlated with IL10, IL13, and GABRA2 in the control samples, while it is positively correlated with CDH2, IL6, SLC6A3, BDNF, and in the DSS group. CDH1 is positively correlated with TGFB, CXCL8, MMP9, TJP1, DRD1, and ADRB2 in the controls. However, in the DSS group, CDH1 is negatively correlated with TGFB, CXCL8, INFG, IL17, IL1B, IL10, IL6, MMP1, MMP9, HTR7, SLC6A3, and ADRB2. Selected associations are illustrated in more detail in Figure 2.
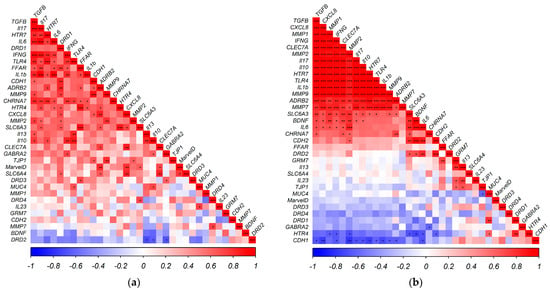
Figure 1.
Correlogram illustrating Pearson correlations, ordered by loadings to the Principal Component in the: (a) Basal group; (b) Basal + DSS group. Positive (Red) and negative (Blue) correlations are represented in color strength on a scale of −1 to 1. * p < 0.05, ** p < 0.01, *** p < 0.001.
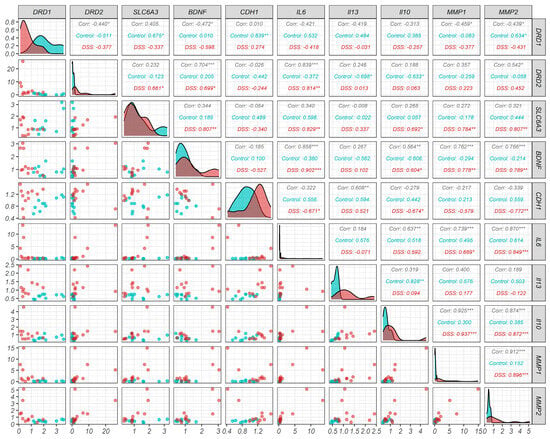
Figure 2.
Graphical representation of the relationship of selected genes across the two experimental groups: Basal group (Blue) and Basal + DSS group (Red). Scatter plots (illustrating relationship between the expression of gene pairs), density plots (illustrating distribution of expression levels), and Pearson correlations (on a scale of −1 to 1. * p < 0.05, ** p < 0.01, *** p < 0.001). Selected targets include dopamine receptors (DRD1 and DRD2), dopamine transporter (SLC6A3), targets relevant to the EMT (CDH1, IL6, IL13, IL10, MMP1, MMP2) and brain-derived neurotrophic factor (BDNF).
3.1.3. Comparison of Target Expressions of Colonic Tissue from Different Data Sources
Two of the targets (DRD5 and HTR3A) were below the limits of detection in colonic tissue from both control and DSS animals. Several targets were barely detectable with CT values > 30 within the selected sample set including DRD1, DRD2, DRD3, DRD4, SLC6A3, HTR7, SLC6A4, GRM7, ADRB2, FFAR1, GABRA2. Genes with a low level of detection is consistent with other data sources including baseline RNA-Seq and protein expression data (Table 3).

Table 3.
Comparison of target expressions of colonic tissue from different data sources. (1) Current study: Mean Cycle Threshold (CT) including maximum and minimum CT derived from QPCR for all samples used in this study; (2) RNA-Seq: mean normalized transcripts per million (nTPM) for human colonic tissue; (3) Protein expression: Immunohistochemical staining pattern and knowledge-based annotation for human colonic tissue.
The expression levels of all the targets evaluated in this study were compared using qPCR (generated in this study), transcriptome analysis, and immunohistochemistry (protein atlas only), Table 3. The immunohistochemistry and transcriptomic data were derived from the protein atlas source only, as this database uses standard measurements and protocols that are comparable across all genes evaluated. These three sources have very high concordance for some targets, including DRD5, HTR4, HTR7, and CHRNA7. While in the remaining targets, the protein data are either lacking or undetectable compared to the transcriptomic and qPCR data.
4. Discussion
This study illustrates how the expression of key neuroactive receptors in the colonic transcriptome of DSS-exposed pigs has been affected, with a significant down-regulation of DRD1, DRD3, DRD4, HTR4, GABRA2, and GRM7, and a four-fold up-regulation of DRD2 compared to the controls. This study also demonstrates the relationship between the expression of neuroactive receptors and other inflammatory and matrix remodeling genes as a notable shift in the patterns of gene co-expression of inflammatory cytokines, MMPs, tight junction proteins, mucin, and neuroactive receptors were observed between the DSS-exposed pigs compared to the controls. Furthermore, the associations between DRD1 and DRD2 and the other targets suggest that these dopamine receptors have differing pathways in the control versus DSS-challenged tissues. Many of the differentially expressed neurotransmitters and receptors have been reported to be influenced by the inflammatory state of the gut, including serotonin [51] and dopamine [52]. The serotonin receptor HTR4 was down-regulated in the DSS group, an important receptor that has been reported previously to regulate the inflammatory response in experimentally induced colitis [53], while activation of HTR4 has been shown to reduce inflammation in colons of mice with colitis [54]. Other receptors reported to be involved in the gut inflammatory processes including, glutamate receptors [55] and GABA receptors [56] were down-regulated in the DSS group. Finally, we discuss how the low or undetectable levels of expression exhibited by many of the receptors evaluated could impede their exploration in colonic tissue from a transcriptomic perspective.
The four-fold up-regulation of DRD2 and a similar magnitude of down-regulation of DRD1 and GABRA2 in the DSS-exposed animals suggest an active and coordinated response to inflammation involving pathways specific to each receptor. This is supported by the differences in the correlations (Figure 2) between both DRD1 and DRD2 and inflammatory and barrier function genes, suggesting that DRD1 and DRD2 exert alternative downstream effects in healthy versus inflamed colonic tissue. In the control group, DRD1 is positively correlated with CDH1, HTR4, SLC6A3, SLC6A4, ADRB2, FFAR1, MMP2, MMP9, and CXCL8, while DRD2 is negatively correlated with GABRA2, IL10, and IL13. In contrast, in the DSS group, DRD1 is down-regulated and positively correlated with MUC4, while DRD2 is up-regulated and positively correlated with CDH2, IL6, SLC6A3, and BDNF compared to the controls. At a localized level in the gut, dopamine signaling events influence IBD development, and mucosal dopamine has been shown to decrease in the intestine of animal models of inflammatory colitis [26], while evidence from animal models of inflammation indicate that high dopamine levels exert an anti-inflammatory effect via stimulation of low-affinity dopamine receptors, including DRD1 and DRD2 [18]. DRD2 has been widely cited as having a role in gut health and inflammation [57]. It has been proposed that the up-regulation of DRD2 in IBD models is a compensatory reaction to decreased local dopamine levels [58]. Polymorphisms within the gene encoding DRD2 are associated with susceptibility to refractory Crohn’s disease [59]. More unconventionally, DRD2 confers colonization resistance via microbial metabolites, by controlling actin cytoskeletal organization within the gut epithelium [60].
While dopamine receptors have been studied in different animal models, tissue types, and disease states, these data have not yet converged to an unequivocal understanding of the signaling mechanisms in the gut. Dopamine signaling is mechanistically complex and is, to date, best defined in neuronal cells [19,22,23,61,62]. In terms of second messenger effects, the simplistic downstream effect is that D1-like dopamine receptors, including DRD1 and DRD5 activate adenylyl cyclase, whereas D2-like receptors including DRD2, DRD3, and DRD4 inhibit adenylyl cyclase [22,26]. Understanding the signaling mechanisms of these receptors at a local level in the gut could expand their potential as therapeutic targets regarding IBD.
The epithelial to mesenchymal transition (EMT) is a process where epithelial cells transform to a mesenchymal cell phenotype, with associated down-regulation of epithelial markers such as adherens and tight junctions and also an up-regulation of extracellular matrix (ECM)-related genes, resulting in the abnormal deposition of ECM, in particular collagen [63]. The epithelial to mesenchymal transition can be classified into three subtypes: type-1; type-2; type-3. Type-1 EMT occurs during embryogenesis. Type-2 EMT is associated with tissue repair and organ fibrosis in response to chronic inflammation. Type-3 EMT is associated with cancer progression [64]. The chronic gut inflammation that occurs in ulcerative colitis can progress to Type-2 EMT and is correlated with tissue regeneration and fibrosis, which can result in excess accumulation of extracellular matrix (ECM), resulting in fistula formation, a complication in IBD patients [63,65]. In this study, the association of neuroactive receptors with key elements of the EMT process is interesting and warrants further consideration, including the evaluation of a wider range of EMT markers and transcription factors [66] including longer and more time points of DSS exposure. In this study, however, the focus is on the cadherins, a group of transmembrane proteins that facilitate cell–cell adhesion and have an important role in tissue morphogenesis and homeostasis [67]. A hallmark of EMT is the down-regulation of Cadherin-1 (CDH1), which is described as “a gatekeeper of the epithelial state” [68], followed by the up-regulation of Cadherin-2 (CDH2), which is a marker of the mesenchymal state, a process regulated by a complex network of signaling factors [69]. In this short-term study, CDH1 was down-regulated in the DSS animals compared to the controls. While CDH2 was expressed at a similar level between the two groups, it exhibited substantial variation across the samples (Table S2) and changed in its association with other genes (Figure 2). In the controls, CDH1 was positively correlated with DRD1, TJP1, MMP9, ADRB2, and CXCL8, while in the DSS group it is negatively correlated with a wide range of inflammatory cytokines (TGFB, CXCL8, INFG, IL17, IL10, IL1b, IL6), matrix metalloproteinases (MMP1 and MMP9), and neuroactive receptors (HTR7, SLC6A3, ADRB2). CDH2 has no significant correlations with any of the other genes in the control group. However, in the DSS samples, CDH2 was positively correlated with DRD2 along with IL6, SLC6A3, and BDNF. This shift in dynamics is interesting and illustrates the involvement of the neuroactive receptors and transporters in a process suggestive of an early stage in the transition from an epithelial to mesenchymal phenotype.
The dopamine receptor DRD5 and the serotonin receptor HTR3A were undetectable using QPCR in this study. However, DRD5 has been detected using qPCR in colonic macrophages in mice and DA-DRD5 signaling was deemed important in controlling the development of colitis through the regulation of M1/M2 macrophage polarization [70]. In addition to this, HTR3A has been confirmed to be expressed at low levels based on RNA-Seq data in the colon (Table 3). Despite evidence of the expression of DRD5 and HTR3A, they were not detected in this study and many of the other targets were present at low levels, as evidenced by high CT values across the sample set. Transcriptomic data presented from the Human Protein Atlas (Table 3) support these observations, where several of the receptors (including DRD2, DRD4, and DRD5) are classified as “undetected” in the colon. This may reflect the technical constraints of RNA-Seq, including sequencing depth and cellular complexity of the starting material [71]. However, on a wider level, it is an obvious impediment to the study of neuroactive receptors and wider exploration of their role in the pathogenesis of IBD.
The gut is a complex multicellular organ with large regional variations, influenced dynamically by the microbiome and diet, and already presents challenges from a transcriptomic perspective. Hence, the local effects of neurotransmitters on the mucosa, the first line of communication between gut and the wider CNS, are difficult to capture. Coordinated biobanking of clinical samples with associated metadata is an important step in the advancement of basic research in Human IBD, using technologies such as transcriptomics [72]. However, existing gaps in the literature regarding the role of the ENS in inflammation may be confounded by the low levels of expression of all the neuroactive receptor genes examined here. Hence, genome-wide transcriptomic strategies may not capture the important contributions made by these receptors to gut inflammatory processes.
5. Conclusions
Several receptors, including dopamine, serotonin, γ-aminobutyric acid, and glutamatergic receptors, were differentially expressed in this porcine colitis model compared to the controls. Furthermore, the relationship between these receptors, immune processes, and matrix remodeling in the colon shifts dramatically between the control and the DSS group, supporting the role of these neurotransmitter receptors’ wider involvement in the inflammatory and fibrotic process and suggest that the initial phase of the epithelial to mesenchymal transition process is captured in this study. The fact that many of these targets exhibit low levels of expression is a challenge from a transcriptomic perspective, particularly in relation to inflammation in the colon. Hence the important contribution of the ENS and its components to the wider narrative of gut inflammation may not be fully captured using widely used transcriptomic approaches and warrants further exploration. While this DSS model has delivered some useful insights, further exploration of these receptors across a range of inflammatory states, contexts, and cell types is essential if we are to fully understand their role with regard to inflammatory processes in the gut. This is particularly important given the complexity of the signaling mechanisms surrounding many of these important neuroactive receptors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nutraceuticals4030023/s1, Table S1: Relative Normalized Quantities (RNQ) of targets explored in the present study along with previously published QPCR data (RNQ) on same samples [44]. Table S2: Re-analysis of gene expression data (RNQ) [44] from Rattigan et al., 2020 and additional targets (CXCL8, CDH2, MMP7), exploring the effects of DSS on gene expression in the colon compared to Basal diet alone (least square means with standard errors).
Author Contributions
Conceptualization, M.T.R. and T.S.; methodology, T.S., M.T.R. and J.V.O.; formal analysis, M.T.R. and J.V.O.; resources, J.V.O. and T.S.; data curation, M.T.R.; writing—original draft preparation, M.T.R.; writing—review and editing, M.T.R., T.S. and J.V.O.; supervision, T.S. and J.V.O.; project administration, T.S.; funding acquisition, T.S. and J.V.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work is funded by the Irish Department of Agriculture, Food and the Marine (MARINE-IBD project (13/F/516) and Science Foundation Ireland (14/IA/2548).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Acknowledgments
We wish to acknowledge the contribution of RNA and normalized QPCR data for this study from (Rattigan et al., 2020).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Suganya, K.; Koo, B.-S. Gut–Brain Axis: Role of Gut Microbiota on Neurological Disorders and How Probiotics/Prebiotics Beneficially Modulate Microbial and Immune Pathways to Improve Brain Functions. Int. J. Mol. Sci. 2020, 21, 7551. [Google Scholar] [CrossRef]
- Fleming, M.A., 2nd; Ehsan, L.; Moore, S.R.; Levin, D.E. The Enteric Nervous System and Its Emerging Role as a Therapeutic Target. Gastroenterol. Res. Pract. 2020, 2020, 8024171. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Gut Bacteria and Neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef]
- Mittal, R.; Debs, L.H.; Patel, A.P.; Nguyen, D.; Patel, K.; O’Connor, G.; Grati, M.h.; Mittal, J.; Yan, D.; Eshraghi, A.A. Neurotransmitters: The critical modulators regulating gut–brain axis. J. Cell. Physiol. 2017, 232, 2359–2372. [Google Scholar] [CrossRef]
- Yılmaz, C.; Gökmen, V. Neuroactive compounds in foods: Occurrence, mechanism and potential health effects. Food Res. Int. 2020, 128, 108744. [Google Scholar] [CrossRef]
- Baj, A.; Moro, E.; Bistoletti, M.; Orlandi, V.; Crema, F.; Giaroni, C. Glutamatergic Signaling Along the Microbiota-Gut-Brain Axis. Int. J. Mol. Sci. 2019, 20, 1482. [Google Scholar] [CrossRef]
- Carretta, M.D.; Quiroga, J.; López, R.; Hidalgo, M.A.; Burgos, R.A. Participation of Short-Chain Fatty Acids and Their Receptors in Gut Inflammation and Colon Cancer. Front. Physiol. 2021, 12, 662739. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Pizzoferrato, M.; Gasbarrini, A.; Miggiano, G.A.D. Nutrition, IBD and Gut Microbiota: A Review. Nutrients 2020, 12, 944. [Google Scholar] [CrossRef]
- Magalhães, H.I.R.; Castelucci, P. Enteric nervous system and inflammatory bowel diseases: Correlated impacts and therapeutic approaches through the P2X7 receptor. World J. Gastroenterol. 2021, 27, 7909–7924. [Google Scholar] [CrossRef]
- Günther, C.; Rothhammer, V.; Karow, M.; Neurath, M.; Winner, B. The Gut-Brain Axis in Inflammatory Bowel Disease-Current and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 8870. [Google Scholar] [CrossRef]
- Oshaghi, M.; Kourosh-Arami, M.; Roozbehkia, M. Role of neurotransmitters in immune-mediated inflammatory disorders: A crosstalk between the nervous and immune systems. Neurol. Sci. 2023, 44, 99–113. [Google Scholar] [CrossRef]
- Sochal, M.; Ditmer, M.; Gabryelska, A.; Białasiewicz, P. The Role of Brain-Derived Neurotrophic Factor in Immune-Related Diseases: A Narrative Review. J. Clin. Med. 2022, 11, 6023. [Google Scholar] [CrossRef]
- Cox, M.A.; Bassi, C.; Saunders, M.E.; Nechanitzky, R.; Morgado-Palacin, I.; Zheng, C.; Mak, T.W. Beyond neurotransmission: Acetylcholine in immunity and inflammation. J. Intern. Med. 2020, 287, 120–133. [Google Scholar] [CrossRef]
- Chen, S.; Wu, X.; Xia, Y.; Wang, M.; Liao, S.; Li, F.; Yin, J.; Ren, W.; Tan, B.; Yin, Y. Effects of dietary gamma-aminobutyric acid supplementation on amino acid profile, intestinal immunity, and microbiota in ETEC-challenged piglets. Food Funct. 2020, 11, 9067–9074. [Google Scholar] [CrossRef]
- Gaskill, P.J.; Khoshbouei, H. Dopamine and norepinephrine are embracing their immune side and so should we. Curr. Opin. Neurobiol. 2022, 77, 102626. [Google Scholar] [CrossRef]
- Liu, G.; Gu, K.; Liu, X.; Jia, G.; Zhao, H.; Chen, X.; Wang, J. Dietary glutamate enhances intestinal immunity by modulating microbiota and Th17/Treg balance-related immune signaling in piglets after lipopolysaccharide challenge. Food Res. Int. 2023, 166, 112597. [Google Scholar] [CrossRef]
- Channer, B.; Matt, S.M.; Nickoloff-Bybel, E.A.; Pappa, V.; Agarwal, Y.; Wickman, J.; Gaskill, P.J. Dopamine, Immunity, and Disease. Pharmacol. Rev. 2023, 75, 62–158. [Google Scholar] [CrossRef] [PubMed]
- Tolstanova, G.; Deng, X.; Ahluwalia, A.; Paunovic, B.; Prysiazhniuk, A.; Ostapchenko, L.; Tarnawski, A.; Sandor, Z.; Szabo, S. Role of Dopamine and D2 Dopamine Receptor in the Pathogenesis of Inflammatory Bowel Disease. Dig. Dis. Sci. 2015, 60, 2963–2975. [Google Scholar] [CrossRef] [PubMed]
- Hamamah, S.; Aghazarian, A.; Nazaryan, A.; Hajnal, A.; Covasa, M. Role of Microbiota-Gut-Brain Axis in Regulating Dopaminergic Signaling. Biomedicines 2022, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Ugalde, V.; Contreras, F.; Prado, C.; Chovar, O.; Espinoza, A.; Pacheco, R. Dopaminergic signalling limits suppressive activity and gut homing of regulatory T cells upon intestinal inflammation. Mucosal Immunol. 2021, 14, 652–666. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Aneman, A.; Friberg, P.; Hooper, D.; Fåndriks, L.; Lonroth, H.; Hunyady, B.; Mezey, E. Substantial production of dopamine in the human gastrointestinal tract. J. Clin. Endocrinol. Metab. 1997, 82, 3864–3871. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Cheng, Z.; Piao, J.; Cui, R.; Li, B. Dopamine Receptors: Is It Possible to Become a Therapeutic Target for Depression? Front. Pharmacol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Niewiarowska-Sendo, A.; Polit, A.; Piwowar, M.; Tworzydło, M.; Kozik, A.; Guevara-Lora, I. Bradykinin B2 and dopamine D2 receptors form a functional dimer. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2017, 1864, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Zhang, X.-L.; Feng, X.-Y.; Liu, C.-Z.; Zhang, X.-N.; Quan, Z.-S.; Yan, J.-T.; Zhu, J.-X. Dopamine promotes colonic mucus secretion through dopamine D5 receptor in rats. Am. J. Physiol.-Cell Physiol. 2019, 316, C393–C403. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.C.; Vaz de Castro, P.A.S.; Yaqub, D.; Jose, P.A.; Armando, I. Anti-Inflammatory Effects of Peripheral Dopamine. Int. J. Mol. Sci. 2023, 24, 13816. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, R.; Contreras, F.; Zouali, M. The dopaminergic system in autoimmune diseases. Front. Immunol. 2014, 5, 117. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y.; Hiramoto, T.; Nishino, R.; Aiba, Y.; Kimura, T.; Yoshihara, K.; Koga, Y.; Sudo, N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2012, 303, G1288–G1295. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.S.; Schmauss, C.; Cuenca, A.; Ratcliffe, E.; Gershon, M.D. Physiological modulation of intestinal motility by enteric dopaminergic neurons and the D2 receptor: Analysis of dopamine receptor expression, location, development, and function in wild-type and knock-out mice. J. Neurosci. 2006, 26, 2798–2807. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Barrios, F.; Navarro, G.; Campos, J.; Ugalde, V.; Prado, C.; Raïch, I.; Contreras, F.; López, E.; Espinoza, A.; Lladser, A.; et al. The Heteromeric Complex Formed by Dopamine Receptor D5 and CCR9 Leads the Gut Homing of CD4+ T Cells Upon Inflammation. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 489–506. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, Q.; Ma, S.-R.; Zhao, Z.-X.; Pan, L.-B.; Cong, L.; Han, P.; Peng, R.; Yu, H.; Lin, Y.; et al. Oral berberine improves brain dopa/dopamine levels to ameliorate Parkinson’s disease by regulating gut microbiota. Signal Transduct. Target. Ther. 2021, 6, 77. [Google Scholar] [CrossRef]
- Olivier, B. Serotonin: A never-ending story. Eur. J. Pharmacol. 2015, 753, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Legan, T.B.; Lavoie, B.; Mawe, G.M. Direct and indirect mechanisms by which the gut microbiota influence host serotonin systems. Neurogastroenterol. Motil. 2022, 34, e14346. [Google Scholar] [CrossRef] [PubMed]
- El-Merahbi, R.; Löffler, M.; Mayer, A.; Sumara, G. The roles of peripheral serotonin in metabolic homeostasis. FEBS Lett. 2015, 589, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Kanova, M.; Kohout, P. Serotonin-Its Synthesis and Roles in the Healthy and the Critically Ill. Int. J. Mol. Sci. 2021, 22, 4837. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.A.; Sun, E.W.; Martin, A.M.; Keating, D.J. The ever-changing roles of serotonin. Int. J. Biochem. Cell Biol. 2020, 125, 105776. [Google Scholar] [CrossRef] [PubMed]
- Pergolizzi, S.; Alesci, A.; Centofanti, A.; Aragona, M.; Pallio, S.; Magaudda, L.; Cutroneo, G.; Lauriano, E.R. Role of Serotonin in the Maintenance of Inflammatory State in Crohn’s Disease. Biomedicines 2022, 10, 765. [Google Scholar] [CrossRef] [PubMed]
- Spohn, S.N.; Mawe, G.M. Non-conventional features of peripheral serotonin signalling—The gut and beyond. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Fabà, L.; de Groot, N.; Ramis, G.; Cabrera-Gómez, C.G.; Doelman, J. Serotonin receptors and their association with the immune system in the gastrointestinal tract of weaning piglets. Porc. Health Manag. 2022, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Costedio, M.M.; Coates, M.D.; Brooks, E.M.; Glass, L.M.; Ganguly, E.K.; Blaszyk, H.; Ciolino, A.L.; Wood, M.J.; Strader, D.; Hyman, N.H. Mucosal serotonin signaling is altered in chronic constipation, but not in opiate-induced constipation. Am. J. Gastroenterol. 2010, 105, 1173. [Google Scholar] [CrossRef]
- Hoffman, J.M.; Tyler, K.; MacEachern, S.J.; Balemba, O.B.; Johnson, A.C.; Brooks, E.M.; Zhao, H.; Swain, G.M.; Moses, P.L.; Galligan, J.J. Activation of colonic mucosal 5-HT4 receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology 2012, 142, 844–854.e844. [Google Scholar] [CrossRef]
- Skadhauge, E.; Grondahl, M.; Hansen, M. Pathophysiology and symptomatic treatment of secretory and osmotic diarrhoea. In Proceedings of the Digestive Physiology in Pigs: Proceedings of the VIIth International Symposium on Digestive Physiology in Pigs, St Malo, France, 26–28 May 1997. [Google Scholar]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15.25.11–15.25.14. [Google Scholar] [CrossRef]
- Kiesler, P.; Fuss, I.; Strober, W. Experimental Models of Inflammatory Bowel Diseases. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 154–170. [Google Scholar] [CrossRef]
- Rattigan, R.; O’Doherty, J.V.; Vigors, S.; Ryan, M.T.; Sebastiano, R.S.; Callanan, J.J.; Thornton, K.; Rajauria, G.; Margassery, L.M.; Dobson, A.D.W.; et al. The Effects of the Marine-Derived Polysaccharides Laminarin and Chitosan on Aspects of Colonic Health in Pigs Challenged with Dextran Sodium Sulphate. Mar. Drugs 2020, 18, 262. [Google Scholar] [CrossRef]
- Council, N.R. Nutrient Requirements of Swine: Eleventh Revised Edition; The National Academies Press: Washington, DC, USA, 2012; p. 420. [Google Scholar]
- O’Shea, C.; O’Doherty, J.; Callanan, J.; Doyle, D.; Thornton, K.; Sweeney, T. The effect of algal polysaccharides laminarin and fucoidan on colonic pathology, cytokine gene expression and Enterobacteriaceae in a dextran sodium sulfate-challenged porcine model. J. Nutr. Sci. 2016, 5, e15. [Google Scholar] [CrossRef]
- Egan, Á.M.; O’Doherty, J.V.; Vigors, S.; Sweeney, T. Prawn Shell Chitosan Exhibits Anti-Obesogenic Potential through Alterations to Appetite, Affecting Feeding Behaviour and Satiety Signals In Vivo. PLoS ONE 2016, 11, e0149820. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Simko, V. Package ‘Corrplot’: Visualization of a Correlation Matrix, Version 0.92. Available online: https://github.com/taiyun/corrplot/ (accessed on 1 November 2023).
- Schloerke, B.; Cook, D.; Larmarange, J.; Briatte, F.; Marbach, M.; Thoen, E.; Elberg, A.; Crowley, J. GGally: Extension to ‘ggplot2’. Available online: https://ggobi.github.io/ggally/ (accessed on 1 November 2023).
- Human Protein Atlas. Available online: https://www.proteinatlas.org/ (accessed on 22 March 2024).
- Coates, M.D.; Tekin, I.; Vrana, K.E.; Mawe, G.M. Review article: The many potential roles of intestinal serotonin (5-hydroxytryptamine, 5-HT) signalling in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017, 46, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Kurnik-Łucka, M.; Pasieka, P.; Łączak, P.; Wojnarski, M.; Jurczyk, M.; Gil, K. Gastrointestinal Dopamine in Inflammatory Bowel Diseases: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 12932. [Google Scholar] [CrossRef]
- Alvarado, D.M.; Ciorba, M.A. Serotonin Receptors Regulate Inflammatory Response in Experimental Colitis. J. Nutr. 2020, 150, 1678–1679. [Google Scholar] [CrossRef]
- Spohn, S.N.; Bianco, F.; Scott, R.B.; Keenan, C.M.; Linton, A.A.; O’Neill, C.H.; Bonora, E.; Dicay, M.; Lavoie, B.; Wilcox, R.L.; et al. Protective Actions of Epithelial 5-Hydroxytryptamine 4 Receptors in Normal and Inflamed Colon. Gastroenterology 2016, 151, 933–944.e933. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Cheng, L.; Wang, Q.; Zhou, L. Comparative Transcriptomic Analysis Reveals the Immunosuppressive Targets of Mesalazine in Dextran Sulfate Sodium-Induced Ulcerative Colitis. Front. Genet. 2021, 12, 698983. [Google Scholar] [CrossRef]
- Auteri, M.; Zizzo, M.G.; Serio, R. GABA and GABA receptors in the gastrointestinal tract: From motility to inflammation. Pharmacol. Res. 2015, 93, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rosas, R.; Yehia, G.; Peña, G.; Mishra, P.; del Rocio Thompson-Bonilla, M.; Moreno-Eutimio, M.A.; Arriaga-Pizano, L.A.; Isibasi, A.; Ulloa, L. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat. Med. 2014, 20, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Vidal, P.M.; Pacheco, R. Targeting the Dopaminergic System in Autoimmunity. J. Neuroimmune Pharmacol. 2020, 15, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Cunha, E.; Araujo, F.; Meireles, E.; Pereira, P.; Dinis-Ribeiro, M.; Veloso, F.T.; Medeiros, R.; Soares-da-Silva, P. Dopamine D2 receptor polymorphisms in inflammatory bowel disease and the refractory response to treatment. Dig. Dis. Sci. 2006, 51, 2039–2044. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.A.; Fu, J.; Chang, P.V. Dopamine receptor D2 confers colonization resistance via gut microbial metabolites. Nature 2024, 628, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Carli, M.; Kolachalam, S.; Aringhieri, S.; Rossi, M.; Giovannini, L.; Maggio, R.; Scarselli, M. Dopamine D2 Receptors Dimers: How can we Pharmacologically Target Them? Curr. Neuropharmacol. 2018, 16, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Shin, S.H.; Santangelo, B.; Veronese, M.; Kang, S.K.; Lee, J.S.; Cheon, G.J.; Lee, W.; Kwon, J.S.; Howes, O.D. Dopamine dysregulation in psychotic relapse after antipsychotic discontinuation: An [18F] DOPA and [11C] raclopride PET study in first-episode psychosis. Mol. Psychiatry 2021, 26, 3476–3488. [Google Scholar] [CrossRef]
- Lovisa, S.; Genovese, G.; Danese, S. Role of Epithelial-to-Mesenchymal Transition in Inflammatory Bowel Disease. J. Crohn’s Colitis 2019, 13, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Marconi, G.D.; Fonticoli, L.; Rajan, T.S.; Pierdomenico, S.D.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in Wound Healing, Tissue Regeneration and Organ Fibrosis. Cells 2021, 10, 1587. [Google Scholar] [CrossRef]
- Jiang, H.; Shen, J.; Ran, Z. Epithelial–mesenchymal transition in Crohn’s disease. Mucosal Immunol. 2018, 11, 294–303. [Google Scholar] [CrossRef]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Maître, J.L.; Heisenberg, C.P. Three functions of cadherins in cell adhesion. Curr. Biol. 2013, 23, R626–R633. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, M.; Tarutani, M.; Takeda, J.; Sano, S. Mesenchymal to Epithelial Transition Induced by Reprogramming Factors Attenuates the Malignancy of Cancer Cells. PLoS ONE 2016, 11, e0156904. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.-Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, Y.; Wang, B.; Jiang, Y.; Lin, L.; Li, X.; Yang, S. DA-DRD5 signaling controls colitis by regulating colonic M1/M2 macrophage polarization. Cell Death Dis. 2021, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- Corridoni, D.; Chapman, T.; Antanaviciute, A.; Satsangi, J.; Simmons, A. Inflammatory Bowel Disease Through the Lens of Single-cell RNA-seq Technologies. Inflamm. Bowel Dis. 2020, 26, 1658–1668. [Google Scholar] [CrossRef] [PubMed]
- Miles, P. IBD BioResource: An open-access platform of 25,000 patients to accelerate research in Crohn’s and Colitis. Gut 2019, 68, 1537. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).