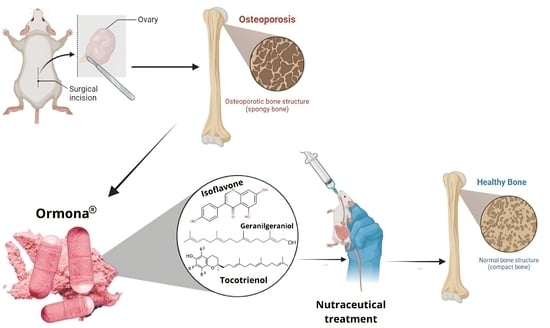
Osteoprotective Effect of the Phytonutraceutical Ormona® on Ovariectomy-Induced Osteoporosis in Wistar Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Material
2.2. Animals and Ethical Aspects
2.3. Experimental Design and Ovariectomy
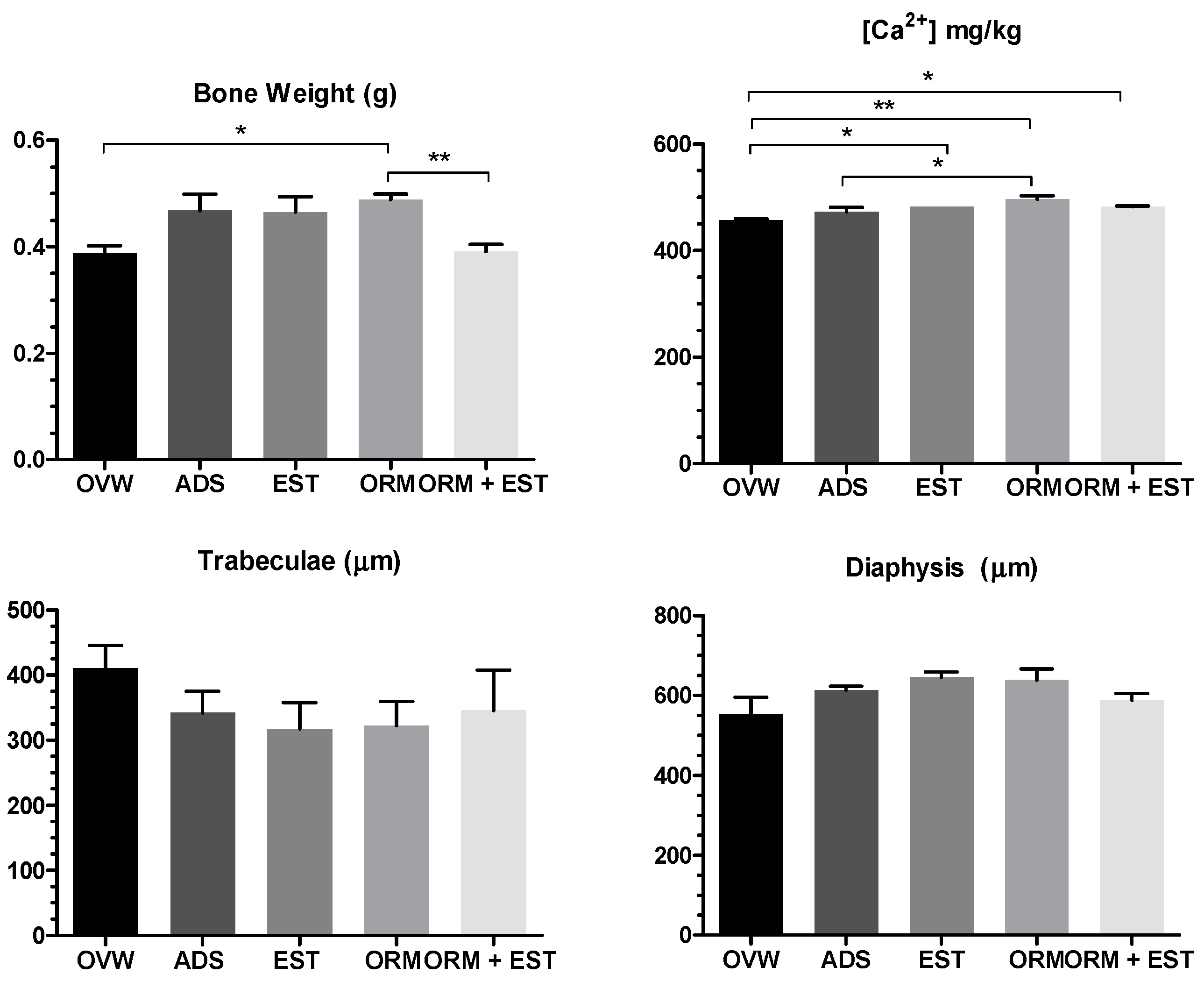
- Negative control group (OVW): ovariectomized rats treated with distilled water (1 mL/kg, p.o);
- Alendronate sodium group (ADS): ovariectomized rats treated with alendronate sodium (4 mg/kg, p.o);
- Estrogen group (EST): ovariectomized rats treated with estrogen (2 µg/kg, p.o);
- Ormona® group (ORM): ovariectomized rats treated with Ormona® (20 mg/kg, p.o);
- Experimental group (ORM + EST): ovariectomized rats treated with Ormona® (20 mg/kg, p.o) + estrogen (2 µg/kg, p.o).
2.4. Hormonal and Biochemical Analysis
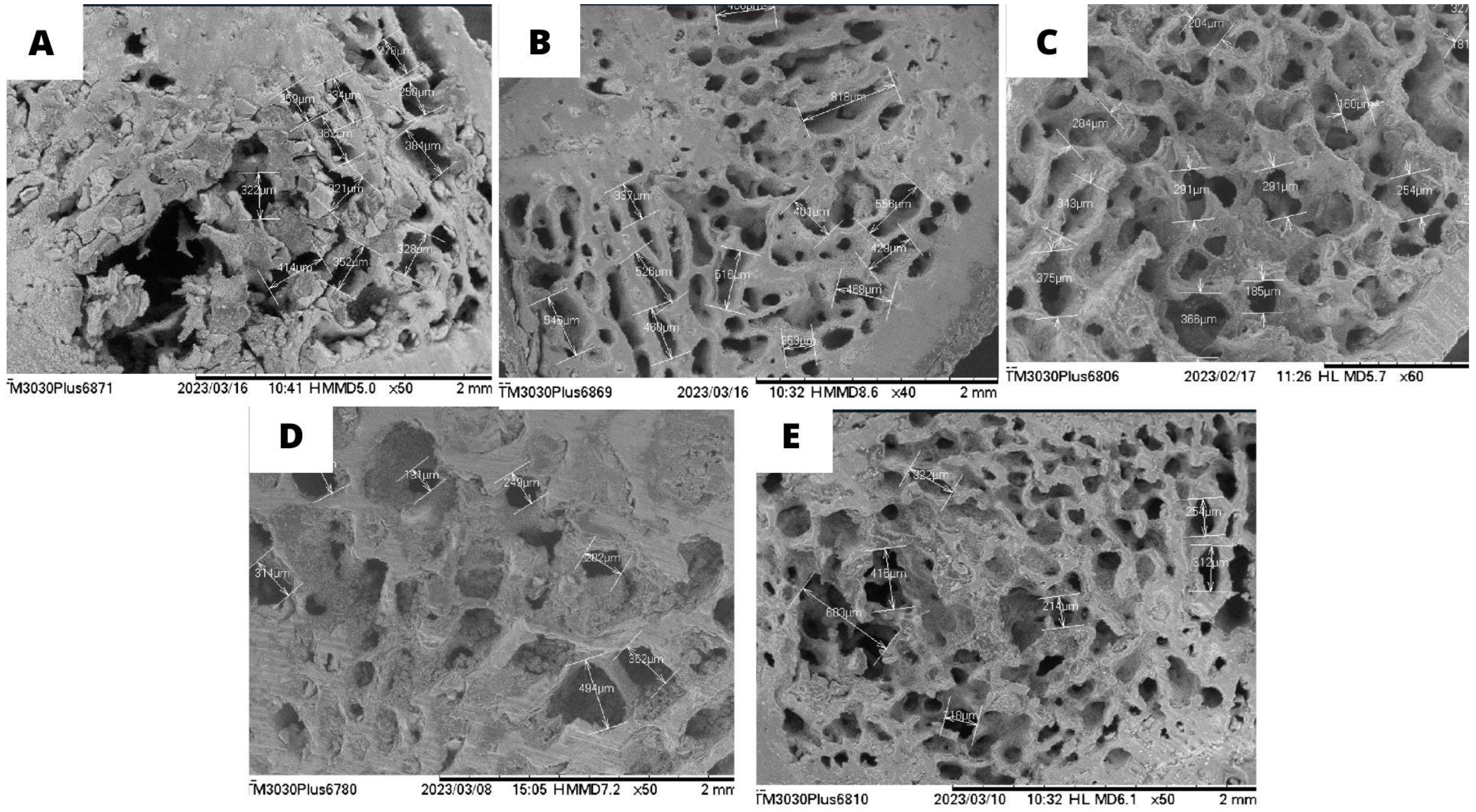
2.5. Scanning Electron Microscopy (SEM) of the Femur
2.6. Quantification of Calcium in Bone Matrix by Atomic Absorption Spectrophotometry
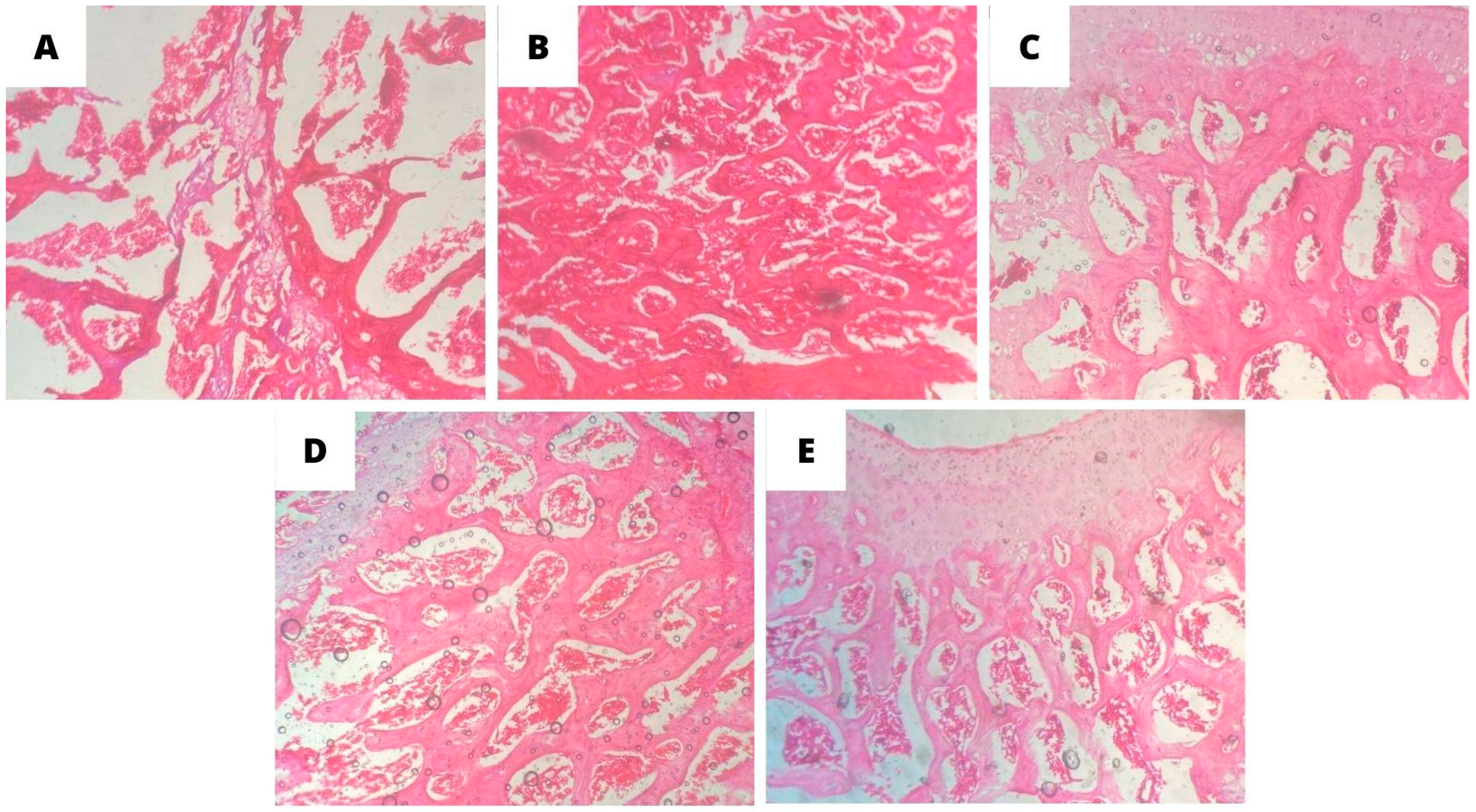
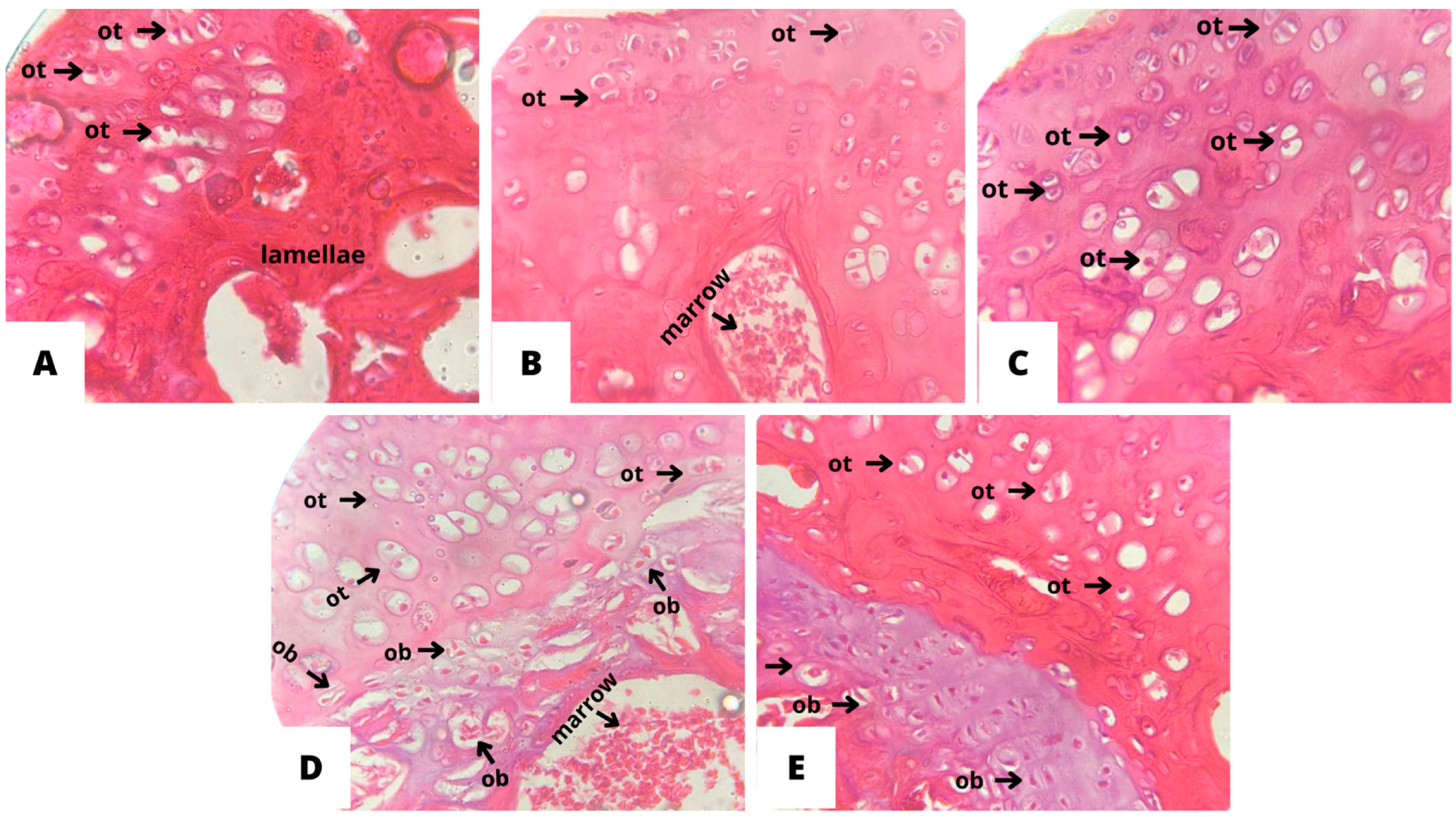
2.7. Histopathological Analysis of Bone Tissue
2.8. Statistical Analysis
3. Results
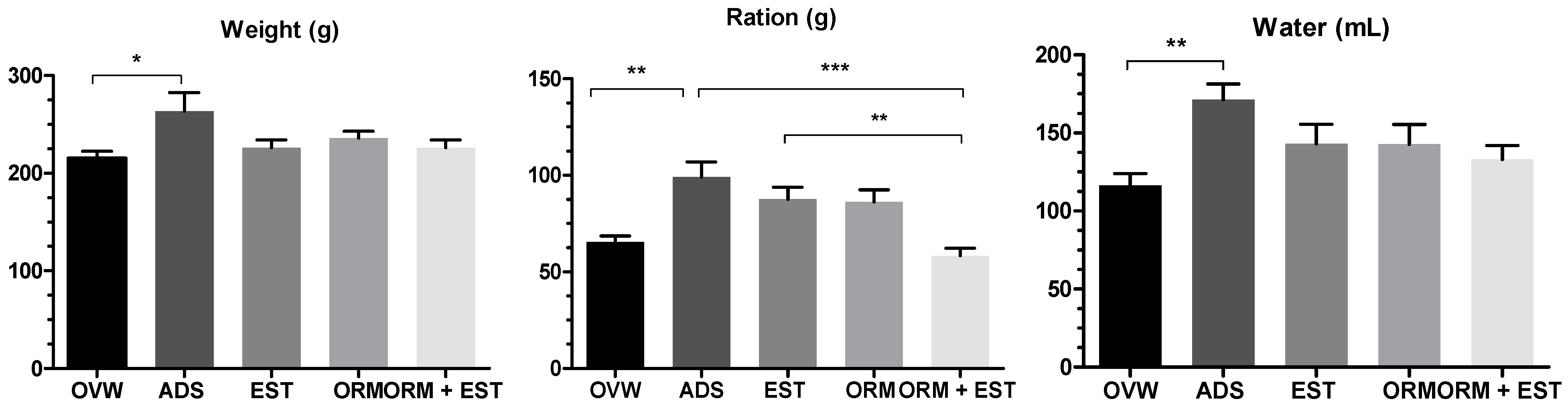
3.1. Ponderal Development
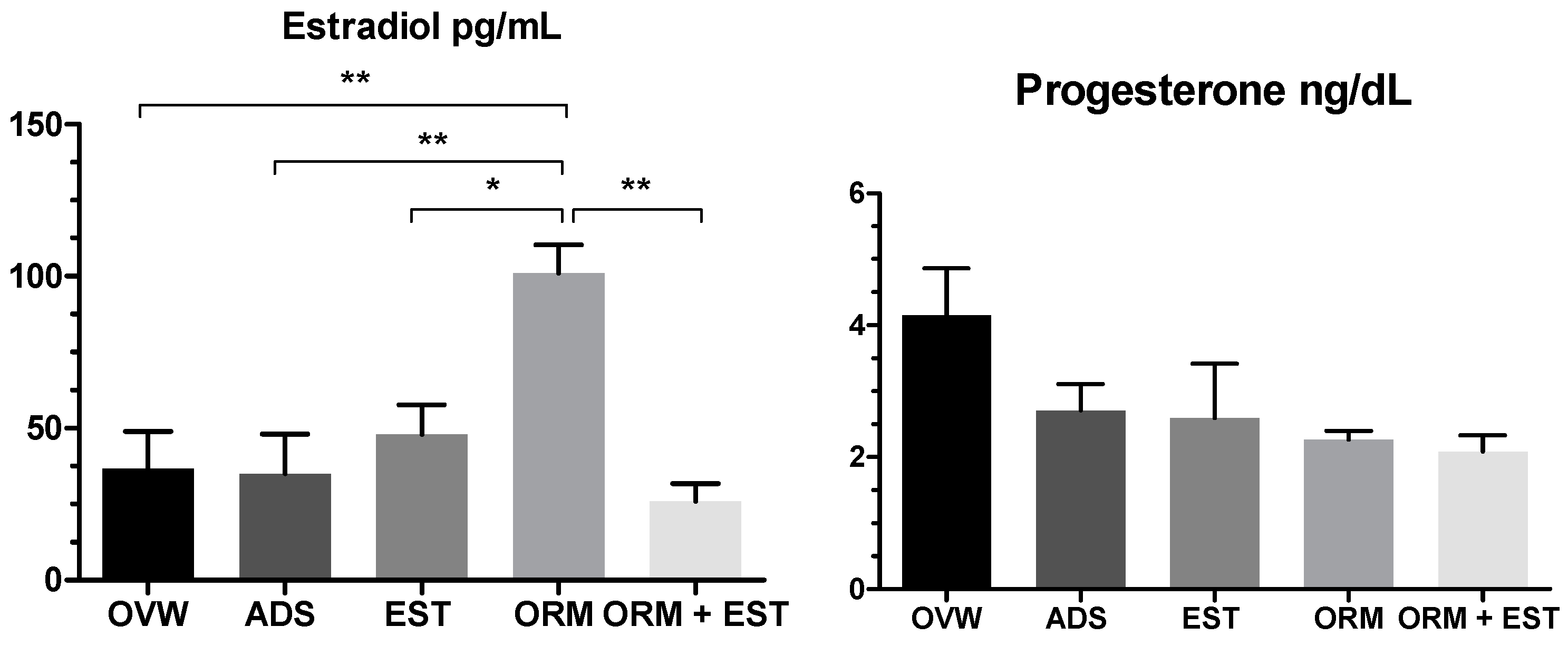
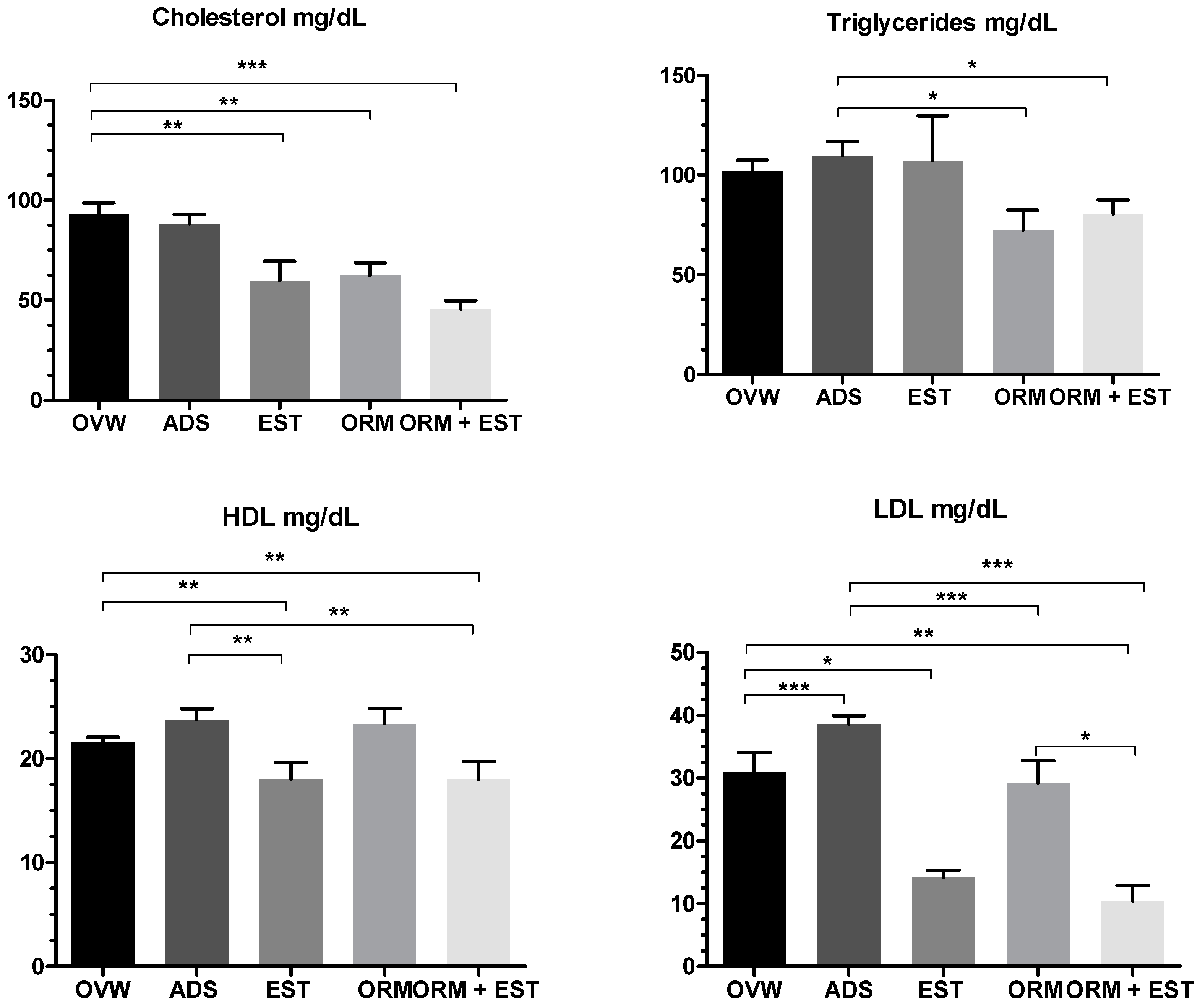
3.2. Hormonal and Biochemical Analysis
3.3. Scanning Electron Microscopy (SEM) of the Femur and Calcium Quantification
3.4. Histopathological Analysis of Bone Tissue
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, A.C.V.; da Rosa, M.I.; Fernandes, B.; Lumertz, S.; Diniz, R.M.; dos Reis, D.M.E.F. Fatores associados à osteopenia e osteoporose em mulheres submetidas à densitometria óssea. Rev. Bras. Reumatol. 2015, 55, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Radominski, S.C.; Bernardo, W.; Paula, A.P.; Albergaria, B.H.; Moreira, C.; Fernandes, C.E.; Castro, C.H.M.; Zerbini, C.A.F.; Domiciano, D.S.; Mendonça, L.; et al. Brazilian guidelines for the diagnosis and treatment of postmenopausal osteoporosis. Rev. Bras. Reumatol. 2017, 57, 452–466. [Google Scholar] [CrossRef]
- da Costa, J.R.G.; de Freitas Tavares, A.L.; Bertolini, G.R.F.; Costa, R.M.; Ribeiro, L.D.F.C. Efeito da reposição fitoterápica com isoflavonas na histomorfometria do tecido ósseo de ratas Wistar ooforectomizadas. Biosaúde 2021, 23, 1–8. [Google Scholar]
- Gosset, A.; Pouillès, J.-M.; Trémollieres, F. Menopausal hormone therapy for the management of osteoporosis. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101551. [Google Scholar] [CrossRef]
- Deli, T.; Orosz, M.; Jakab, A. Hormone Replacement Therapy in Cancer Survivors—Review of the Literature. Pathol. Oncol. Res. 2019, 26, 63–78. [Google Scholar] [CrossRef]
- Lacey, J.V., Jr.; Mink, P.J.; Lubin, J.H.; Sherman, M.E.; Troisi, R.; Hartge, P.; Schatzkin, A.; Schairer, C. Menopausal Hormone Replacement Therapy and the Risk of Ovarian Cancer: A Meta-Analysis. Front. Endocrinol. 2019, 10, 486180. [Google Scholar]
- Frigo, M.; Barros, E.D.; Santos, P.C.D.B.D.; Koehnlein, E.A. Isoflavonas como tratamento alternativo na sintomatologia climatérica: Uma revisão sistemática. Rev. Inst. Adolfo Lutz. 2021, 80, e37249. [Google Scholar] [CrossRef]
- Viggiani, M.T.; Polimeno, L.; Di Leo, A.; Barone, M. Phytoestrogens: Dietary Intake, Bioavailability, and Protective Mechanisms against Colorectal Neoproliferative Lesions. Nutrients 2019, 11, 1709. [Google Scholar] [CrossRef]
- Aronson, J.K. Defining ‘nutraceuticals’: Neither nutritious nor pharmaceutical. Br. J. Clin. Pharmacol. 2017, 83, 8–19. [Google Scholar] [CrossRef]
- da Rocha, C.F.; Flexa, C.D.N.N.; de Souza, G.C.; Pereira, A.C.M.; Carvalho, H.D.O.; do Nascimento, A.L.; de Jesus Vasconcelos, N.J.P.; da Silva, H.R.; Carvalho, J.C.T. Acute and Re-productive Toxicity Evaluation of Ormona® SI and Ormona® RC-Two New Nutraceuticals with Geranylgeraniol, Tocotri-enols, Anthocyanins, and Isoflavones-In Adult Zebrafish. Pharmaceuticals 2022, 15, 1434. [Google Scholar] [CrossRef]
- Rodrigues, A.P.S.; da Silva Barbosa, R.; Pereira, A.C.M.; Batista, M.A.; Sales, P.F.; Ferreira, A.M.; Colares, N.N.D.; da Silva, H.R.; Soares, M.O.D.S.; da Silva Hage-Melim, L.I.; et al. Ormona® SI and Ormona® RC—New Nutraceuticals with Geranylgeraniol, Tocotrienols, Anthocyanins, and Isoflavones—Decrease High-Fat Diet-Induced Dyslipidemia in Wistar Rats. Nutraceuticals 2022, 2, 311–322. [Google Scholar] [CrossRef]
- Tsuang, Y.H.; Chen, L.T.; Chiang, C.J.; Wu, L.C.; Chiang, Y.F.; Chen, P.Y.; Sun, J.S.; Wang, C.C. Isoflavones prevent bone loss following ovariectomy in young adult rats. J. Orthop. Surg. Res. 2008, 3, 1–9. [Google Scholar] [CrossRef]
- Yousefzadeh, N.; Kashfi, K.; Jeddi, S.; Ghasemi, A. Ovariectomized rat model of osteoporosis: A Practical Guide. EXCLI J. 2020, 19, 89–107. [Google Scholar]
- Carvalho, H.D.O.; Santos, I.V.F.D.; Resque, R.L.; Keita, H.; Fernandes, C.P.; Carvalho, J.C.T. Hypoglycemic effect of formulation containing hydroethanolic extract of Calophyllum brasiliense in diabetic rats induced by streptozotocin. Rev. Bras. Farmacogn. 2016, 26, 634–639. [Google Scholar] [CrossRef]
- Palma, M.N.N.; Rocha, G.C.; Filho, S.C.V.; Detmann, E. Evaluation of Acid Digestion Procedures to Estimate Mineral Contents in Materials from Animal Trials. Asian-Australas J. Anim. Sci. 2015, 28, 1624–1628. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.C.M.; de Oliveira Carvalho, H.; Gonçalves, D.E.S.; Picanço, K.R.T.; de Lima Teixeira dos Santos, A.V.T.; da Silva, H.R.; Braga, F.S.; Bezerra, R.M.; de Sousa Nunes, A.; Nazima, M.T.S.T.; et al. Co-Treatment of Purified Annatto Oil (Bixa orellana L.) and Its Granules (Chronic®) Improves the Blood Lipid Profile and Bone Protective Effects of Testosterone in the Orchiectomy-Induced Osteoporosis in Wistar Rats. Molecules 2021, 26, 4720. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Zhang, L.; Yang, X. Ferulic acid, a natural polyphenol, protects against osteoporosis by activating SIRT1 and NF-κB in neonatal rats with glucocorticoid-induced osteoporosis. Biomed. Pharmacother. 2019, 120, 109205. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.A. Animal models of osteoporosis—necessity and limitations. Eur. Cell Mater. 2001, 1, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, T.; Otsuka, E.; Hagiwara, H. Reciprocal Control of Expression of mRNAs for Osteoclast Differentiation Factor and OPG in Osteogenic Stromal Cells by Genistein: Evidence for the Involvement of Topoisomerase II in Osteoclastogenesis. Endocrinology 2001, 142, 3632–3637. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.D.; Simmons, H.A.; Pirie, C.M.; Ke, H.Z. FDA guidelines and animal models for osteoporosis. Bone 1995, 17 (Suppl. S4), S125–S133. [Google Scholar] [CrossRef] [PubMed]
- Silveira, V. Efeitos Iniciais da Ovariectomia e do Tratamento com Estrógeno e Isoflavonas da Soja, Isolados e Associados, na Reparação Óssea Alveolar e no Útero de Ratas. Ph.D. Thesis, Universidade Estadual Paulista, São Paulo, Brazil, 2007. [Google Scholar]
- Shaffie, N.M.; Sharaf, H.; Morsy, F.; Badawi, M.; Abbas, N.F. Role of some phytoestrogens in recovering bone loss: Histological results from experimental ovariectomized rat models. J. Arab. Soc. Med. Res. 2015, 10, 65. [Google Scholar] [CrossRef]
- Kalleny, N.K. Histological and morphometric studies on the effect of alpha-lipoic acid on postovariectomy osteoporosis induced in adult female albino rats. Egypt. J. Histol. 2011, 34, 139–155. [Google Scholar] [CrossRef]
- Park, J.A.; Ha, S.K.; Kang, T.H.; Oh, M.S.; Cho, M.H.; Lee, S.Y.; Park, J.H.; Kim, S.Y. Protective effect of apigenin on ovariectomy-induced bone loss in rats. Life Sci. 2008, 82, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.; Kaschig, C.; Erben, R.G. 1α-Hydroxyvitamin D2 and 1α-hydroxyvitamin D3 have anabolic effects on cortical bone, but induce intracortical remodeling at toxic doses in ovariectomized rats. Bone 2004, 35, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Barlet, J.-P.; Picherit, C.; Coxam, V.; Bennetau-Pelissero, C.; Lebecque, P.; Kati-Coulibaly, S.; Davicco, M.-J. Daidzein Is More Efficient than Genistein in Preventing Ovariectomy-Induced Bone Loss in Rats. J. Nutr. 2000, 130, 1675–1681. [Google Scholar] [CrossRef] [PubMed]
- Fanti, P.; Monier-Faugere, M.C.; Geng, Z.; Schmidt, J.; Morris, P.E.; Cohen, D.; Malluche, H.H. The Phytoestrogen Genistein Reduces Bone Loss in Short-Term Ovariectomized Rats. Osteoporos. Int. 1998, 8, 274–281. [Google Scholar] [CrossRef] [PubMed]
- López, M.; Tena-Sempere, M. Estrogens and the control of energy homeostasis: A brain perspective. Trends Endocrinol. Metab. 2015, 26, 411–421. [Google Scholar] [CrossRef] [PubMed]
- de Morentin, P.B.M.; González-García, I.; Martins, L.; Lage, R.; Fernández-Mallo, D.; Martínez-Sánchez, N.; Ruíz-Pino, F.; Liu, J.; Morgan, D.A.; Pinilla, L.; et al. Estradiol Regulates Brown Adipose Tissue Thermogenesis via Hypothalamic AMPK. Cell Metab. 2014, 20, 41–53. [Google Scholar]
- Xu, Y.; López, M. Central regulation of energy metabolism by estrogens. Mol. Metab. 2018, 15, 104–115. [Google Scholar] [CrossRef]
- Hirschberg, A.L. Sex hormones, appetite and eating behaviour in women. Maturitas 2012, 71, 248–256. [Google Scholar] [CrossRef]
- Liu, Z.P.; Li, W.X.; Yu, B.; Huang, J.; Sun, J.; Huo, J.S.; Liu, C.X. Effects of trans-Resveratrol from Polygonum cuspidatum on Bone Loss Using the Ovariectomized Rat Model. J. Med. Food. 2005, 8, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; He, Y.; Hyseni, I.; Pei, Z.; Yang, Y.; Xu, P.; Cai, X.; Liu, H.; Qu, N.; Liu, H.; et al. 17β-estradiol promotes acute refeeding in hungry mice via mem-brane-initiated ERα signaling. Mol. Metab. 2020, 42, 101053. [Google Scholar] [CrossRef]
- Li, L.L.; Yang, Y.; Ma, C.M.; Li, X.M.; Bian, X.; Fu, Y.; Ren, L.K.; Wang, R.M.; Shi, Y.G.; Zhang, N. Effects of soybean isoflavone aglycone on osteoporosis in ovariectomized rats. Front. Nutr. 2023, 10, 1122045. [Google Scholar] [CrossRef]
- Xu, H.; Liu, T.; Hu, L.; Li, J.; Gan, C.; Xu, J.; Chen, F.; Xiang, Z.; Wang, X.; Sheng, J. Effect of caffeine on ovariectomy-induced osteoporosis in rats. Biomed. Pharmacother. 2019, 112, 108650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Song, X.; Chen, X.; Jiang, R.; Peng, K.; Tang, X.; Liu, Z. Antiosteoporotic effect of hesperidin against ovariectomy-induced osteoporosis in rats via reduction of oxidative stress and inflammation. J. Biochem. Mol. Toxicol. 2021, 35, e22832. [Google Scholar] [CrossRef]
- Khattab, H.A.H.; Ardawi, M.S.; Ateeq, R.A.M.; Hala, K.A.H. Ateeq Effect RAM. Effect of Phytoestrogens Derived from Red Clover (Trifolium Pratense L.) in Ovariectomized Rats. Life Sci. J. 2013, 20, 1096–1099. [Google Scholar]
- Chen, Y.M.; Wang, I.L.; Zhu, X.Y.; Chiu, W.C.; Chiu, Y.S. Red Clover Isoflavones Influence Estradiol Concentration, Exercise Performance, and Gut Microbiota in Female Mice. Front. Nutr. 2021, 8, 623698. [Google Scholar] [CrossRef]
- Hooper, L.; Ryder, J.; Kurzer, M.; Lampe, J.; Messina, M.; Phipps, W.; Cassidy, A. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: A systematic review and meta-analysis. Hum. Reprod. Updat. 2009, 15, 423–440. [Google Scholar] [CrossRef]
- Lacey, M.; Bohday, J.; Fonseka, S.M.; Ullah, A.I.; Whitehead, S.A. Dose–response effects of phytoestrogens on the activity and expression of 3β-hydroxysteroid dehydrogenase and aromatase in human granulosa-luteal cells. J. Steroid Biochem. Mol. Biol. 2005, 96, 279–286. [Google Scholar] [CrossRef]
- Liu, J.; Burdette, J.E.; Xu, H.; Gu, C.; Van Breemen, R.B.; Bhat, K.P.; Booth, N.; Constantinou, A.I.; Pezzuto, J.M.; Fong, H.H.; et al. Evaluation of Estrogenic Activity of Plant Extracts for the Potential Treatment of Menopausal Symptoms. J. Agric. Food Chem. 2001, 49, 2472–2479. [Google Scholar] [CrossRef]
- Burdette, J.E.; Liu, J.; Lantvit, D.; Lim, E.; Booth, N.; Bhat, K.P.; Hedayat, S.; Van Breemen, R.B.; Constantinou, A.I.; Pezzuto, J.M.; et al. Trifolium pratense (Red Clover) Exhibits Estrogenic Effects In Vivo in Ovariectomized Sprague-Dawley Rats. J. Nutr. 2002, 132, 27–30. [Google Scholar] [CrossRef]
- Greenwood, S.; Barnes, S.; Clarkson, T.B.; Eden, J.; Helferich, W.G.; Hughes, C.; Messina, M.; Setchell, K.D.R. The role of isoflavones in menopausal health: Consensus opinion of the North American Menopause Society. Menopause 2000, 7, 215–229. [Google Scholar]
- Tripathi, A.; Singh, S.P.; Raju, K.S.R.; Wahajuddin; Gayen, J.R. Effect of Red Clover on CYP Expression: An Investigation of Herb-Drug Interaction at Molecular Level. Indian J. Pharm. Sci. 2014, 76, 261–266. [Google Scholar]
- Hwang, C.S.; Kwak, H.S.; Lim, H.J.; Lee, S.H.; Kang, Y.S.; Choe, T.B.; Hur, H.G.; Han, K.O. Isoflavone metabolites and their in vitro dual functions: They can act as an estrogenic agonist or antagonist depending on the estrogen concentration. J. Steroid Biochem. Mol. Biol. 2006, 101, 246–253. [Google Scholar] [CrossRef]
- Stubert, J.; Gerber, B. Isoflavones—Mechanism of Action and Impact on Breast Cancer Risk. Breast Care 2009, 4, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic activity, biological effect and bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 15–25. [Google Scholar] [CrossRef]
- Medina-Contreras, J.; Villalobos-Molina, R.; Zarain-Herzberg, A.; Balderas-Villalobos, J. Ovariectomized rodents as a meno-pausal metabolic syndrome model. A minireview. Mol. Cell Biochem. 2020, 475, 261–276. [Google Scholar] [CrossRef]
- Nigro, M.; Santos, A.T.; Barthem, C.S.; Louzada, R.A.N.; Fortunato, R.S.; Ketzer, L.A.; Carvalho, D.P.; de Meis, L. A Change in Liver Metabolism but Not in Brown Adipose Tissue Thermogenesis Is an Early Event in Ovariectomy-Induced Obesity in Rats. Endocrinology 2014, 155, 2881–2891. [Google Scholar] [CrossRef] [PubMed]
- Heine, P.A.; Taylor, J.A.; Iwamoto, G.A.; Lubahn, D.B.; Cooke, P.S. Increased adipose tissue in male and female estrogen re-ceptor-α knockout mice. Proc. Natl. Acad. Sci. USA 2000, 97, 12729–12734. [Google Scholar] [CrossRef]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex Differ. 2017, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, B.T.; Zhu, L.; Stafford, J.M. Role of Estrogens in the Regulation of Liver Lipid Metabolism. Adv. Exp. Med. Biol. 2017, 1043, 227–256. [Google Scholar]
- Berman, D.M.; Nicklas, B.J.; Ryan, A.S.; Rogus, E.M.; Dennis, K.E.; Goldberg, A.P. Regulation of Lipolysis and Lipoprotein Lipase after Weight Loss in Obese, Postmenopausal Women. Obes Res. 2004, 12, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Faulds, M.H.; Zhao, C.; Dahlman-Wright, K.; Gustafsson, J. The diversity of sex steroid action: Regulation of metabolism by estrogen signaling. J. Endocrinol. 2012, 212, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Luís, Â.; Domingues, F.; Pereira, L. Effects of red clover on perimenopausal and postmenopausal women’s blood lipid profile: A meta-analysis. Climacteric 2018, 21, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Chiu, H.M.; Chen, C.L.; Yang, W.S.; Yang, Y.S.; Ho, H.N. Hyperandrogenemia Is Independently Associated with Elevated Alanine Aminotransferase Activity in Young Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2010, 95, 3332–3341. [Google Scholar] [CrossRef][Green Version]
- Kobayashi, A.; Suzuki, Y.; Sugai, S. Specificity of transaminase activities in the prediction of drug-induced hepatotoxicity. J. Toxicol. Sci. 2020, 45, 515–537. [Google Scholar] [CrossRef]
- Grigoryan, A.V.; Dimitrova, A.A.; Kostov, K.G.; Russeva, A.L.; Atanasova, M.A.; Blagev, A.B.; Betova, T.M.; Trifonov, R.G. Changes of Serum Concen-trations of Alkaline Phosphatase and Metalloproteinase-9 in an Ovariectomized Wistar Rat Model of Osteoporosis. J. Biomed. Clin. Res. 2017, 10, 32–36. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, M.H.; Rhee, M.H. Studies on the Effects of Biomedicinal Agents on Serum Concentration of Ca2+, P and ALP Activity in Osteoporosis-Induced Rats. J. Vet. Sci. 2003, 4, 151. [Google Scholar] [CrossRef]
- Devareddy, L.; Hooshmand, S.; Collins, J.K.; Lucas, E.A.; Chai, S.C.; Arjmandi, B.H. Blueberry prevents bone loss in ovariecto-mized rat model of postmenopausal osteoporosis. J. Nutr. Biochem. 2008, 19, 694–699. [Google Scholar] [CrossRef]
- Zhang, R.; Hu, S.J.; Li, C.; Zhang, F.; Gan, H.Q.; Mei, Q.B. Achyranthes bidentata root extract prevent OVX-induced osteoporosis in rats. J. Ethnopharmacol. 2012, 139, 12–18. [Google Scholar] [CrossRef]
- Chang, K.-L.; Hu, Y.-C.; Hsieh, B.-S.; Cheng, H.-L.; Hsu, H.-W.; Huang, L.-W.; Su, S.-J. Combined effect of soy isoflavones and vitamin D3 on bone loss in ovariectomized rats. Nutrition 2013, 29, 250–257. [Google Scholar] [CrossRef]
- Hooshmand, S.; Juma, S.; Arjmandi, B.H. Combination of Genistin and Fructooligosaccharides Prevents Bone Loss in Ovarian Hormone Deficiency. J. Med. Food 2010, 13, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.H.; Yamaguchi, M. Suppressive effect of genistein on rat bone osteoclasts: Involvement of protein kinase inhibition and protein tyrosine phosphatase activation. Int. J. Mol. Med. 2000, 5, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Orsatti, F.L.; Nahas, E.P.; Nahas-Neto, J.; Orsatti, C.L.; Teixeira, A.S. Efeito do treinamento contrarresistência e isoflavona na densidade mineral óssea em. Rev. Bras. Cineantropometria E Desempenho Hum. 2013, 15, 726–736. [Google Scholar] [CrossRef]
- Cegieła, U.; Folwarczna, J.; Pytlik, M.; Zgórka, G. Effects of Extracts from Trifolium medium L. and Trifolium pratense L. on Development of Estrogen Deficiency-Induced Osteoporosis in Rats. Evidence-Based Complement. Altern. Med. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Al-Ghaban, N.M.; Jasem, G.H. Histomorphometric evaluation of the effects of local application of red cloveroil (Trifolium pratense) on bone healing in rats. J. Baghdad Coll. Dent. 2020, 32, 26–31. [Google Scholar] [CrossRef]
- Occhiuto, F.; De Pasquale, R.; Guglielmo, G.; Palumbo, D.; Zangla, G.; Samperi, S.; Renzo, A.; Circosta, C. Effects of phytoestrogenic isoflavones from red clover (Trifolium pratense L.) on experimental osteoporosis. Phytother. Res. 2007, 21, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Hiruma, Y.; Nakahama, K.-I.; Fujita, H.; Morita, I. Vitamin K2 and geranylgeraniol, its side chain component, inhibited osteoclast formation in a different manner. Biochem. Biophys. Res. Commun. 2004, 314, 24–30. [Google Scholar] [CrossRef]
- Andres, S.; Hansen, U.; Niemann, B.; Palavinskas, R.; Lampen, A. Determination of the isoflavone composition and estrogenic activity of commercial dietary supplements based on soy or red clover. Food Funct. 2015, 6, 2017–2025. [Google Scholar] [CrossRef]
- Pfitscher, A.; Reiter, E.; Jungbauer, A. Receptor binding and transactivation activities of red clover isoflavones and their me-tabolites. J. Steroid. Biochem. Mol. Biol. 2008, 112, 87–94. [Google Scholar] [CrossRef]
- Mathey, J.; Mardon, J.; Fokialakis, N.; Puel, C.; Kati-Coulibaly, S.; Mitakou, S.; Bennetau-Pelissero, C.; Lamothe, V.; Davicco, M.J.; Lebecque, P.; et al. Modulation of soy isoflavones bioavailability and subsequent effects on bone health in ovariectomized rats: The case for equol. Osteoporos. Int. 2007, 18, 671–679. [Google Scholar] [CrossRef]
- Somjen, D.; Katzburg, S.; Kohen, F.; Gayer, B.; Livne, E. Daidzein but not other phytoestrogens preserves bone architecture in ovariectomized female rats in vivo. J. Cell Biochem. 2008, 103, 1826–1832. [Google Scholar] [CrossRef]
- Sehmisch, S.; Uffenorde, J.; Maehlmeyer, S.; Tezval, M.; Jarry, H.; Stuermer, K.; Stuermer, E. Evaluation of bone quality and quantity in osteoporotic mice—The effects of genistein and equol. Phytomedicine 2010, 17, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.J.B.; Ambrose, W.W.; Garner, S.C. Biphasic Effects of Genistein on Bone Tissue in the Ovariectomized, Lactating Rat Model. Exp. Biol. Med. 1998, 217, 345–350. [Google Scholar] [CrossRef]
- Gautam, A.K.; Bhargavan, B.; Tyagi, A.M.; Srivastava, K.; Yadav, D.K.; Kumar, M.; Singh, A.; Mishra, J.S.; Singh, A.B.; Sanyal, S.; et al. Differential effects of formononetin and cladrin on osteoblast function, peak bone mass achievement and bioavailability in rats. J. Nutr. Biochem. 2011, 22, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; Lee, H.Y.; Lee, J.H.; Jung, D.; Choi, J.; Song, K.Y.; Jung, H.J.; Choi, J.S.; Chang, S.I.; Kim, C. Formononetin prevents ovariectomy-induced bone loss in rats. Arch. Pharm. Res. 2010, 33, 625–632. [Google Scholar] [CrossRef]
- Harahap, I.A.; Suliburska, J. An overview of dietary isoflavones on bone health: The association between calcium bioavaila-bility and gut microbiota modulation. Mater. Today Proc. 2022, 63, S368–S372. [Google Scholar] [CrossRef]
- de Souza, A.P.; Gonsalves, I.F.; Schneider, M.J.F.; Gianini, N.M.; Kuroiwa, V.Y.; Quinones, E.M.; Coimbra, C.N.; Diniz, R.; Maccagnan, P. Uso de isoflavonas em casos de osteoporose nas mulheres: Uma revisão bibliográfica. Rev. Higei 2022, 4, 1–10. [Google Scholar]
- De Franciscis, P.; Colacurci, N.; Riemma, G.; Conte, A.; Pittana, E.; Guida, M.; Schiattarella, A. A Nutraceutical Approach to Menopausal Complaints. Medicina 2019, 55, 544. [Google Scholar] [CrossRef]
- Lambert, M.N.T.; Jeppesen, P.B. Isoflavones and bone health in perimenopausal and postmenopausal women. Curr. Opin. Clin. Nutr. Metab. Care. 2018, 21, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.L.; Cao, J.; Sun, M.; Yuen, T.; Zhou, R.; Li, J.; Peng, Y.; Moonga, S.S.; Guo, L.; Mechanick, J.I.; et al. Vitamin C Prevents Hypogonadal Bone Loss. PLoS ONE 2012, 7, e47058. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Jiang, J.; Hong, R.; Xu, F.; Dai, S. Circulating IGFBP-3 and Interleukin 6 as Predictors of Osteoporosis in Postmenopausal Women: A Cross-Sectional Study. Mediat. Inflamm. 2023, 2023, 2613766. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.-Y.; Yoshida, H.; Sarosi, I.; Tan, H.-L.; Timms, E.; Capparelli, C.; Morony, S.; Oliveira-Dos-Santos, A.J.; Van, G.; Itie, A.; et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 1999, 397, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.W.; Garner, S.C.; Anderson, J.J.B. Isoflavones regulate interleukin-6 and osteoprotegerin synthesis during osteoblast cell differentiation via an estrogen-receptor-dependent pathway. Biochem. Biophys. Res. Commun. 2002, 295, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Chen, X.; Anderson, J.J.B. Physiological concentrations of genistein stimulate the proliferation and protect against free radical-induced oxidative damage of MC3T3-E1 osteoblast-like cells. Nutr. Res. 2001, 21, 1287–1298. [Google Scholar] [CrossRef]
- Choi, E.M.; Suh, K.S.; Kim, Y.S.; Choue, R.W.; Koo, S.J. Soybean ethanol extract increases the function of osteoblastic MC3T3-E1 cells. Phytochemistry 2001, 56, 733–739. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Gao, Y.H. Anabolic effect of genistein on bone metabolism in the femoral-metaphyseal tissues of elderly rats is inhibited by the anti-estrogen tamoxifen. Res. Exp. Med. 1997, 197, 101–107. [Google Scholar] [CrossRef]
| Parameters | Urea (mg/dL) | Creatinine | AST (U/dL) | ALT (U/dL) | Alkaline Phosphatase (U/L) |
|---|---|---|---|---|---|
| OVW | 29.40 ± 4.10 | 0.42 ± 0.16 | 144.40 ± 24.48 | 20.40 ± 2.07 | 55.00 ± 12.12 |
| ADS | 22.20 ± 6.42 | 0.38 ± 0.08 | 156.60 ± 33.95 | 37.60 ± 2.61 *** | 50.80 ± 15.47 |
| EST | 31.40 ± 2.88 | 0.46 ± 0.15 | 276.60 ± 102.10 *,# | 33.80 ± 7.22 ** | 44.60 ± 8.53 |
| ORM | 25.40 ± 5.40 | 0.38 ± 0.04 | 201.30 ± 49.27 | 33.00 ± 3.74 ** | 51.40 ± 19.26 |
| ORM + EST | 26.40 ± 7.27 | 0.48± 0.05 | 156.80 ± 48.02 | 28.80 ± 7.56 | 51.60 ± 4.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
do Nascimento, A.L.; Furtado, G.d.C.; Vilhena, V.M.; Carvalho, H.d.O.; Sales, P.F.; Barcellos, A.O.N.; de Maria, K.C.; Braga, F.S.; da Silva, H.R.; Bezerra, R.M.; et al. Osteoprotective Effect of the Phytonutraceutical Ormona® on Ovariectomy-Induced Osteoporosis in Wistar Rats. Nutraceuticals 2024, 4, 147-164. https://doi.org/10.3390/nutraceuticals4020010
do Nascimento AL, Furtado GdC, Vilhena VM, Carvalho HdO, Sales PF, Barcellos AON, de Maria KC, Braga FS, da Silva HR, Bezerra RM, et al. Osteoprotective Effect of the Phytonutraceutical Ormona® on Ovariectomy-Induced Osteoporosis in Wistar Rats. Nutraceuticals. 2024; 4(2):147-164. https://doi.org/10.3390/nutraceuticals4020010
Chicago/Turabian Styledo Nascimento, Aline Lopes, Gabriel da Costa Furtado, Vinicius Maciel Vilhena, Helison de Oliveira Carvalho, Priscila Faimann Sales, Alessandra Ohana Nery Barcellos, Kaio Coutinho de Maria, Francinaldo Sarges Braga, Heitor Ribeiro da Silva, Roberto Messias Bezerra, and et al. 2024. "Osteoprotective Effect of the Phytonutraceutical Ormona® on Ovariectomy-Induced Osteoporosis in Wistar Rats" Nutraceuticals 4, no. 2: 147-164. https://doi.org/10.3390/nutraceuticals4020010
APA Styledo Nascimento, A. L., Furtado, G. d. C., Vilhena, V. M., Carvalho, H. d. O., Sales, P. F., Barcellos, A. O. N., de Maria, K. C., Braga, F. S., da Silva, H. R., Bezerra, R. M., & Carvalho, J. C. T. (2024). Osteoprotective Effect of the Phytonutraceutical Ormona® on Ovariectomy-Induced Osteoporosis in Wistar Rats. Nutraceuticals, 4(2), 147-164. https://doi.org/10.3390/nutraceuticals4020010