Abstract
γ-Tocopherol (γT) is the major form of vitamin E contained in plants and seed oils. Although it is readily metabolized in the liver, the function of the metabolites is not fully understood. This study investigated the antioxidant activities of the γT metabolite 2,7,8-trimethyl-2-(2′-carboxyethyl)-6-hydroxychroman (γCEHC) in comparison to its parent compound. The pretreatment of mouse hepatoma Hepa1c1c7 cells with γCEHC showed a cytoprotective effect on the hydrogen peroxide-induced cytotoxicity to a lesser extent than that of γT. A mechanistic investigation revealed that both γ-CEHC and γT significantly up-regulated the gene and protein expressions of heme oxygenase-1 (HO-1) via the promotion of the nuclear translocation of nuclear factor erythroid 2-related factor 2 (Nrf2). Furthermore, the combination of γCEHC and γT significantly increased the gene and protein levels of HO-1 and the nuclear translocation of Nrf2, suggesting that it was an additive effect. Tin protoporphyrin IX (SnPP), a representative HO-1 inhibitor, significantly impaired the cytoprotection of γCEHC and γT against the hydrogen peroxide-induced cytotoxicity. These results suggested that not only γT but also its metabolite, γCEHC, are a promising cytoprotective factor against oxidative stress-induced cytotoxicity and that the cytoprotective effect is attributable to the cooperation of both compounds.
1. Introduction
Vitamin E, widely distributed in fruits and vegetables, plays an important role in reproduction and in maintaining the structural integrity of the cells with antioxidant properties [1,2,3]. In nature, vitamin E consists of eight substances, namely α-, β-, γ-, and δ-tocopherol and α-, β-, γ-, and δ-tocotrienol. The definition of α, β, γ, and δ depends on the number and position of the methyl groups in the chroman ring of vitamin E. The difference between tocopherols and tocotrienols is that tocotrienols have three double bonds at positions 3, 7, and 11 in the phytyl chain, whereas tocopherols do not. Orally ingested vitamin E is absorbed in the small intestine, incorporated into chylomicrons, released into the lymph vessels, and finally enters blood circulation. During blood circulation, the chylomicrons containing vitamin E lose their triacylglycerols by lipoprotein lipase in the peripheral tissues to form chylomicron remnants, which are incorporated into the liver via the hepatic remnant receptors. In addition, vitamin E is transported into cells by Niemann–Pick C1-like 1 (NPC1L1), a well-known cholesterol transporter [4].
α-Tocopherol (αT) is the dominant vitamin E isoform found in humans and animals. On the other hand, γ-tocopherol (γT) is at a lower concentration than αT in our body despite the relatively high intake of plant and seed oils [5]. This is due to the low affinity of γT to the hepatic α-tocopherol transfer protein (αTTP), as shown by the relative affinity of γT being only 9% of that of the control (RRR-αT) [6]. Among the vitamin Es, αT is preferentially transferred to a very low-density lipoprotein via binding to αTTP and transportation into the extrahepatic tissues [7]. On the other hand, excessive vitamin E is metabolized to carboxyethyl hydroxychroman (CEHC) in the liver. CEHC is produced by an initial hydroxylation of the phytyl side chain catalyzed by CYP4F2, followed by consecutive β-oxidation steps [8,9]. γCEHC (also known as LLU-α) was first isolated from human urine while searching for a natriuretic hormone [10]. In addition, an in vitro study showed that γCEHC inhibited cyclooxygenase activity in macrophages and epithelial cells [11]. Since the longer-chain metabolite 13′-COOH has been shown to have anti-inflammatory and anti-proliferative activities [12], the short-chain metabolite CEHC, which retains the intact chromanol ring structure, may also contribute to health promotion. Although the in vitro antioxidant properties of αCEHC and γCEHC were reported [13,14], the intracellular antioxidative activities via up-regulation of the antioxidant enzymes have not been confirmed.
Heme oxygenase-1 (HO-1) catalyzes the degradation of heme to produce free iron, carbon monoxide (CO), and biliverdin. Biliverdin is then converted to bilirubin by biliverdin reductase. These metabolites have conferred cellular and tissue protection in multiple models of oxidative and inflammatory injury and disease [15]. The expression of the HO-1 gene is regulated by different transcription factors such as nuclear factor erythroid 2-related factor 2 (Nrf2), activator protein-1 (AP-1), and nuclear factor-kappa B (NF-κB) [16]. Nrf2 especially seems to be an important factor in the induction of the HO-1 gene [17]. Recently, food-derived phytochemicals, such as flavonoids and isothiocyanates, have been proposed to target the Nrf2-mediated signaling pathways to exert cytoprotective potentials [18]. However, it is unclear whether γCEHC exerts cytoprotective effects against oxidative stress by regulating the antioxidant enzyme. Therefore, the present study aimed to investigate the intracellular antioxidant activities of γCEHC in comparison to γT by modulating the antioxidative gene and protein. This study revealed that γCEHC, as well as γT, exerted the cytoprotective effect on hydrogen peroxide-induced cytotoxicity through the modulation of HO-1 expression.
2. Materials and Methods
2.1. Chemicals
D-γT was obtained from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). γCEHC was purchased from Cayman Chemical (Ann Arbor, MI, USA). The anti-HO-1 and anti-Nrf2 antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA). Tin Protoporphyrin IX (SnPP) was obtained from R&D Systems Inc. (Minneapolis, MN, USA). All other chemicals were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan) or Nacalai Tesque Inc. (Kyoto, Japan).
2.2. Cell Culture
A mouse hepatoma cell line Hepa1c1c7 was obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in a minimum essential media (α-MEM, Gibco, Thermo Fisher Scientific Inc., Grand Island, NY, USA) containing 10% fetal bovine serum, 100 U/mL of penicillin, and 100 μg/mL of streptomycin at 37 °C in an atmosphere of 95% O2 and 5% CO2.
2.3. MTT Assay
Hepa1c1c7 cells were seeded in a 96-well plate at 1 × 104 cells per well. After the 24 h preculture, the cells were pretreated with the chemicals γCEHC and γT or ethanol as a vehicle (final concentration, 0.1%) for 24 h. For the oxidative challenge, the cells were washed with PBS after treatment of the chemicals, followed by exposure to hydrogen peroxide at 100 μM for 6 h. For the HO-1 inhibition challenge, the cells were pretreated with SnPP for 1 h, then incubated with γCEHC and/or γT for 24 h, followed by exposure to hydrogen peroxide for 6 h. The cell viability was checked as previously reported [19].
2.4. RT-PCR
Hepa1c1c7 cells were treated with γCEHC and/or γT at the indicated concentrations for 24 h. The cells were also treated with ethanol as the control. The RT-PCR experiment was performed as previously described [20].
2.5. Western Blot Analysis
The protein expression of HO-1 and nuclear translocation of Nrf2 were determined using western blotting as previously reported with some modifications [19,21]. Hepa1c1c7 cells were exposed to γCEHC and/or γT for 24 h. The membrane was incubated with an anti-HO-1 or anti-Nrf2 antibody at room temperature for 2 h. The images were captured using ImageQuant LAS 500 (Cytiva, Marlborough, MA, USA).
2.6. Statistical Analyses
All values are presented as means ± the standard deviation (S.D.) (n > 3). Statistical significance was determined by Student’s t-test or a one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. The data were considered significant at p < 0.05.
3. Results
3.1. Effects of γCEHC and Its Parent γT on the Hydrogen Peroxide-Induced Cytotoxicity
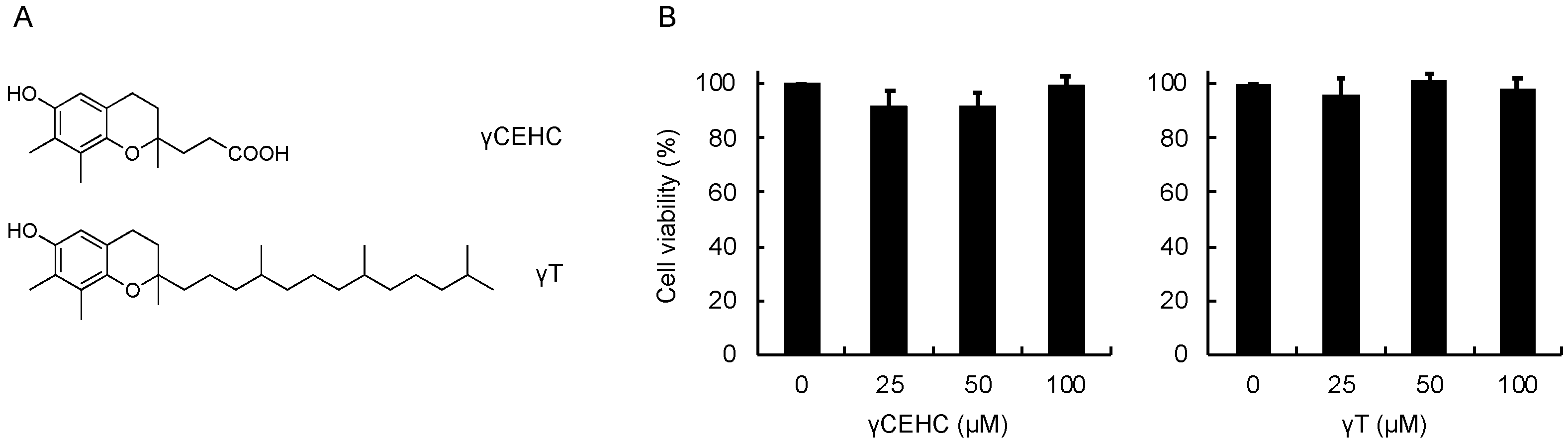
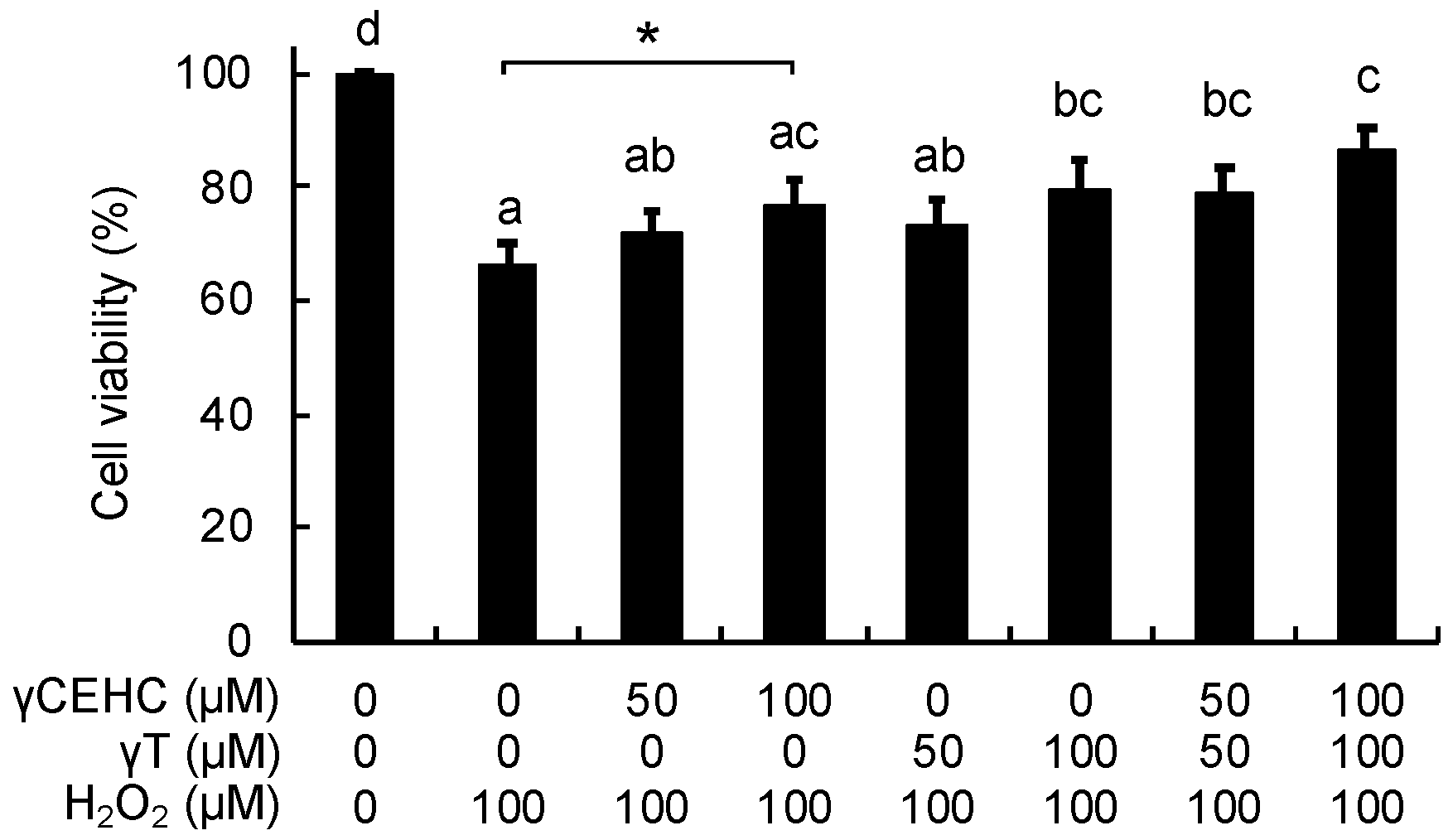
To examine the cytoprotective effect of γCEHC and γT, we first investigated the cytotoxicity of these chemicals on mouse hepatoma Hepa1c1c7 cells, the frequently used cultured hepatocyte model, using an MTT assay. As shown in Figure 1, the 24 h treatment of each compound did not show any cytotoxicity up to 100 µM. Next, their cytoprotective effects against hydrogen peroxide were examined. The pretreatment of the cells with γCEHC showed a cytoprotective effect on the hydrogen peroxide-induced cytotoxicity, which was weaker than that of γT (Figure 2). In addition, the combination of γCEHC and γT additively increased the cytoprotective effect.
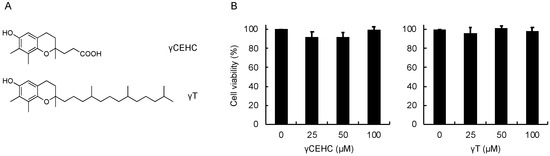
Figure 1.
Chemical structures of γCEHC and γT (A) and their cytotoxicity on mouse hepatoma Hepa1c1c7 cells (B). The cells were pretreated with γCEHC or γT for 24 h, then incubated with an MTT solution (0.5 mg/mL) for 2 h. The cell viability values were expressed as the percentages of the corresponding control. Values are the means ± S.D. (n = 3).
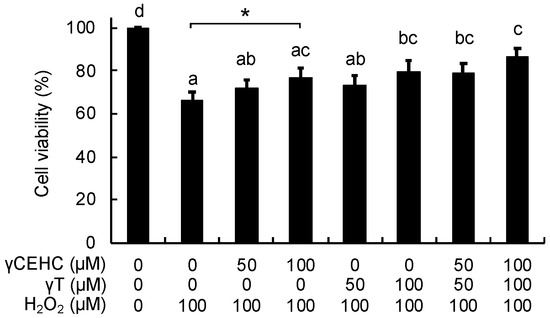
Figure 2.
Cytoprotective effects of γCEHC and γT on the hydrogen peroxide-induced cytotoxicity of Hepa1c1c7 cells. The cells were pretreated with γCEHC and/or γT for 24 h, followed by exposure to hydrogen peroxide at 100 μM for 6 h. The cells were incubated with an MTT solution (0.5 mg/mL) for 2 h. The cell viability values are expressed as the percentages of the corresponding control. Values are the means ± S.D. (n = 4). * p < 0.05 vs. control. Different letters above the bars indicate significant differences among the treatments (p < 0.05).
3.2. Modulating Effects of γCEHC and Its Parent on the Antioxidative Gene and Proteins in the Cultured Cell Model
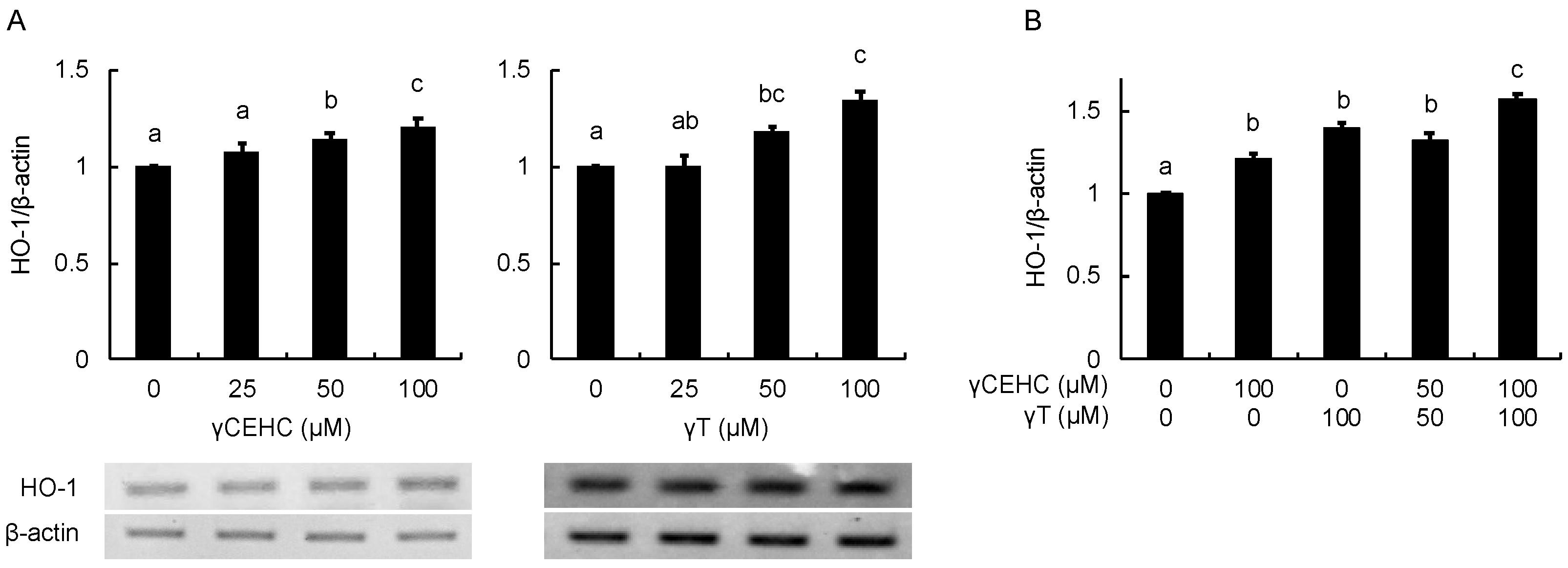
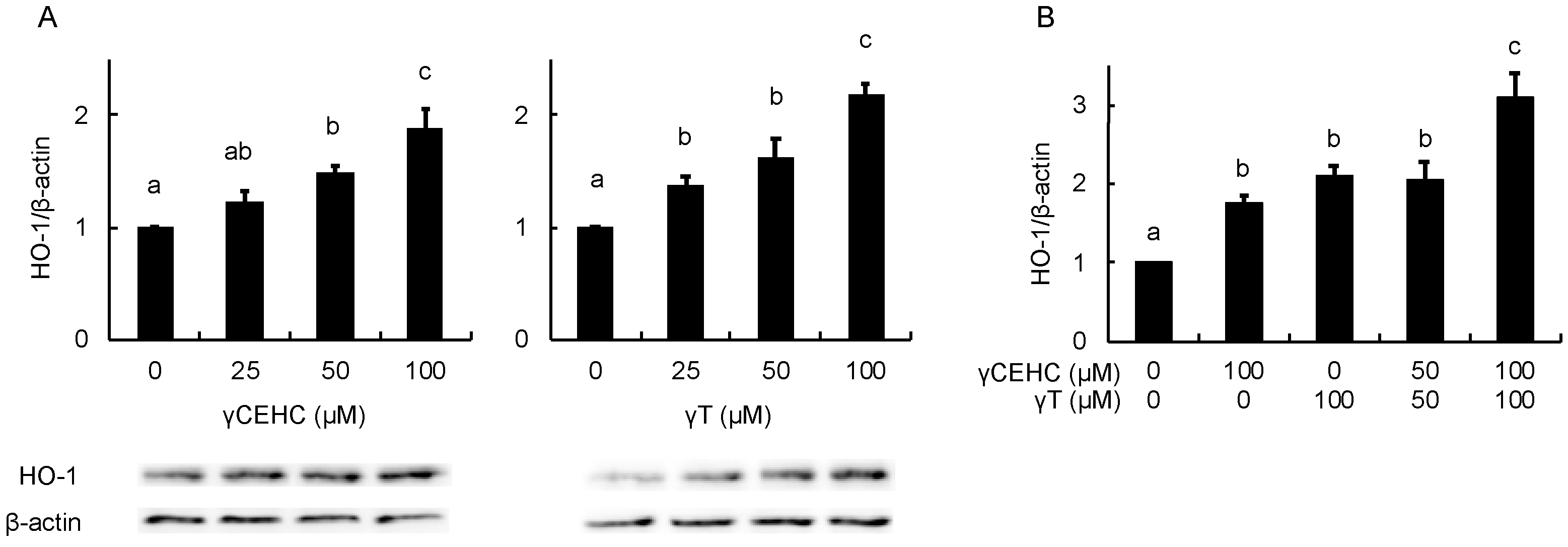
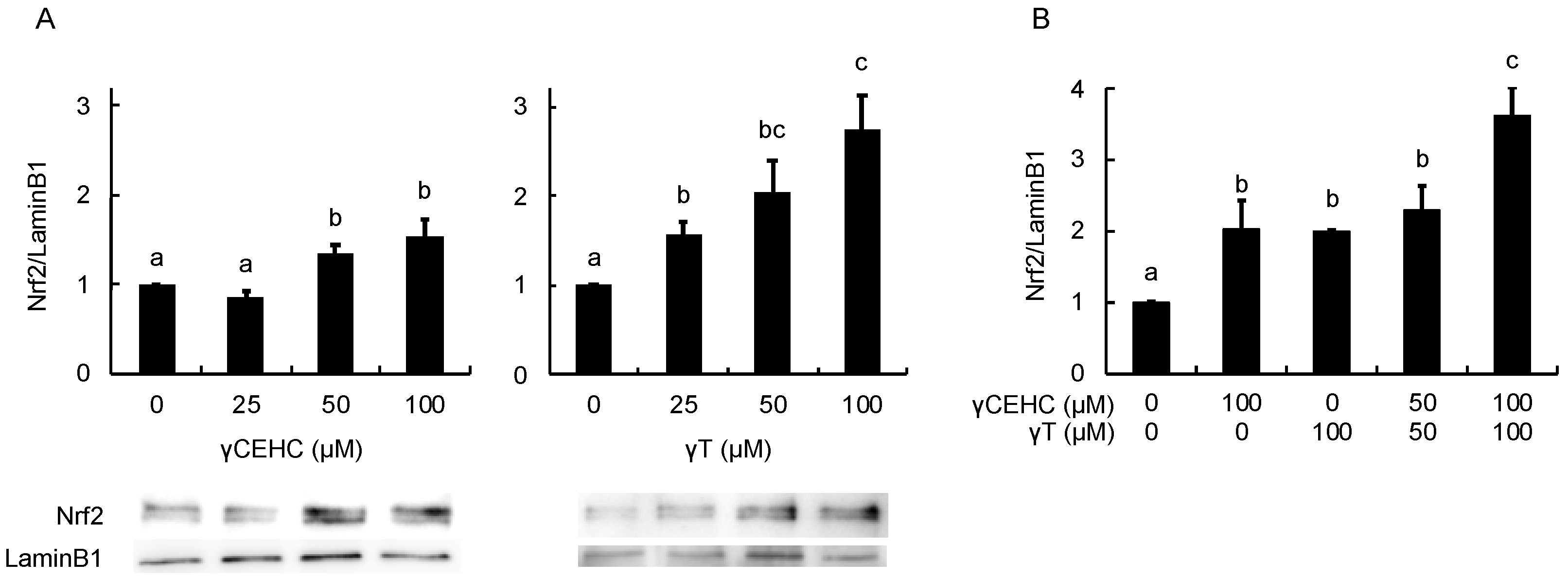
In order to estimate the intracellular antioxidant activity of the chemicals, the gene expression of HO-1 was evaluated using Hepa1c1c7 cells. The treatment with 50 µM γCEHC and 25 µM γT resulted in the significant up-regulation of HO-1 gene expression (Figure 3A). In addition, the co-treatment with γCEHC and γT enhanced HO-1 gene expression compared to that of each compound alone (Figure 3B). The protein expression of HO-1 was also enhanced by both chemicals in a dose-dependent manner (Figure 4A,B). Since Nrf2 nuclear translocation plays an important role in the induction of various cytoprotective proteins, including HO-1 [18], we checked the modulating effect of the chemicals on the nuclear Nrf2 levels. γ-CEHC and γT significantly promoted Nrf2 nuclear translocation (Figure 5A), consistent with the enhanced HO-1 expression levels. Furthermore, the combination of γCEHC and γT significantly increased the nuclear translocation of Nrf2 compared to each compound alone (Figure 5B). These data suggested that γCEHC exerted the HO-1-dependent antioxidant activity through the same pathway as γT.
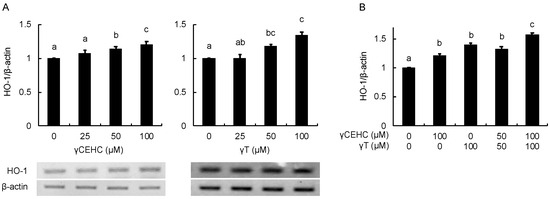
Figure 3.
The modulating effects of γCEHC and γT on HO-1 gene expression by each compound treatment (A) and the combination treatment (B). The confluent Hepa1c1c7 cells were treated with γCEHC and/or γT at the indicated concentrations for 24 h. The gene expression levels of HO-1 were determined using RT-PCR. The values are expressed as relative to the corresponding control. Values are the means ± S.D. (n = 3). Different letters above the bars indicate significant differences among the treatments (p < 0.05).
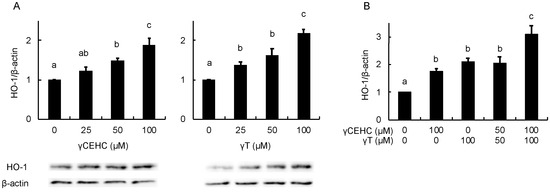
Figure 4.
The modulating effects of γCEHC and γT on the HO-1 protein levels by each compound treatment (A) and the combination treatment (B). The confluent Hepa1c1c7 cells were treated with γCEHC and/or γT at the indicated concentrations for 24 h. The protein expression levels of HO-1 were determined by western blotting. The values are expressed as relative to the corresponding control. Values are the means ± S.D. (n = 3). Different letters above the bars indicate significant differences among the treatments (p < 0.05).
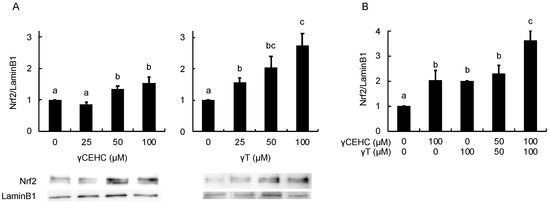
Figure 5.
The modulating effects of γCEHC and γT on the nuclear translocation of the Nrf2 protein levels by each compound treatment (A) and the combination treatment (B). The confluent Hepa1c1c7 cells were treated with γCEHC and/or γT at the indicated concentrations for 24 h. The nuclear translocation of the Nrf2 protein was determined by western blotting. The values are expressed as relative to the corresponding control. Values are the means ± S.D. (n = 3). Different letters above the bars indicate significant differences among the treatments (p < 0.05).
3.3. Influence of an HO-1 Inhibitor on the Cytoprotection of γCEHC and Its Parent against Hydrogen Peroxide-Induced Cytotoxicity
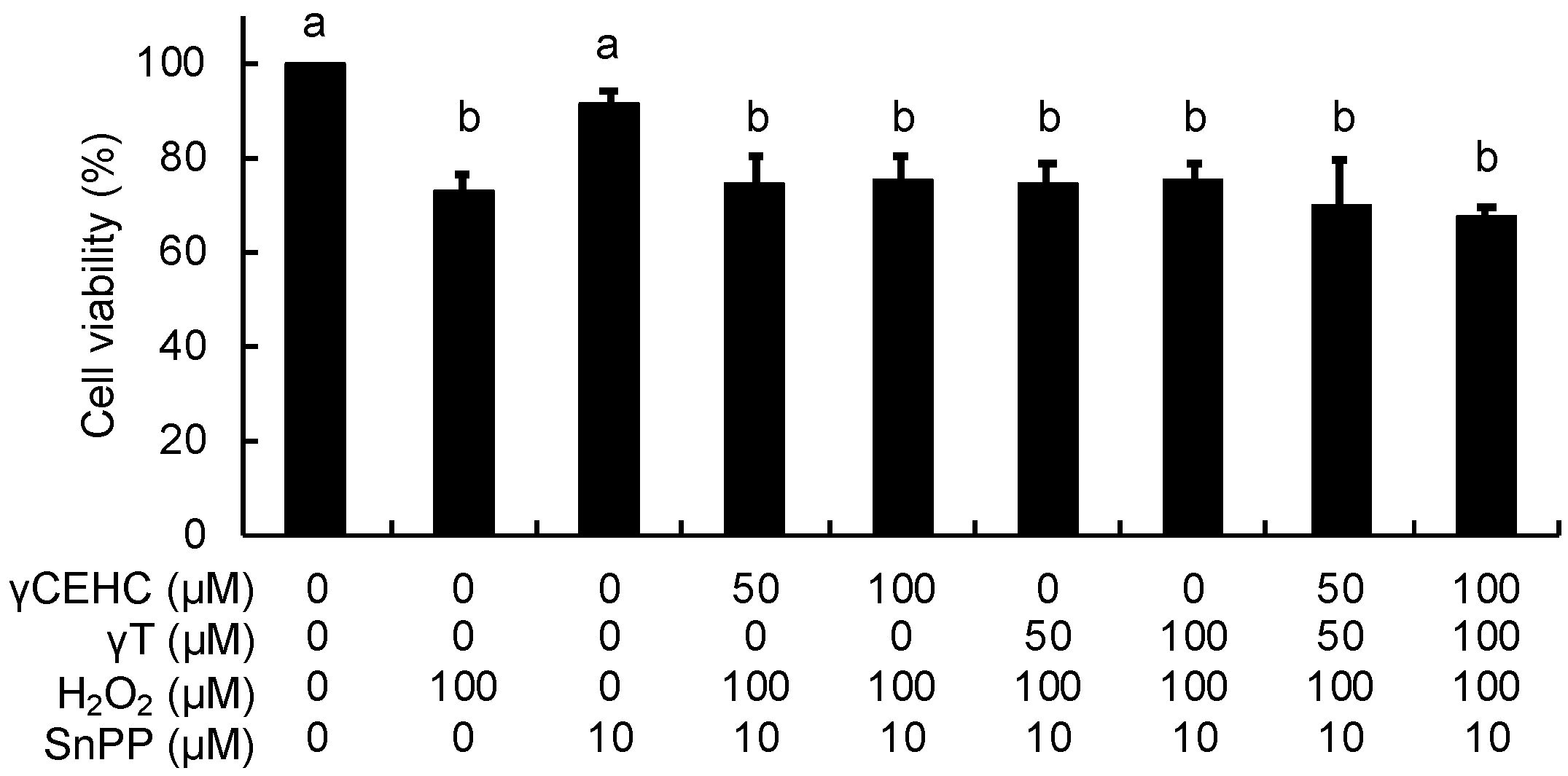
To obtain further evidence about the involvement of HO-1 in the cytoprotective mechanism, the effect of SnPP, a representative inhibitor of HO-1 activity, on the antioxidant activity was investigated. In contrast to Figure 2, γCEHC and γT did not protect the cells from the hydrogen peroxide-induced cytotoxicity under all the co-existing SnPP conditions (Figure 6). This result strongly supported the idea that HO-1-dependent metabolic products, such as CO and biliverdin/bilirubin, might be involved in the cytoprotective mechanism of γCEHC and γT.
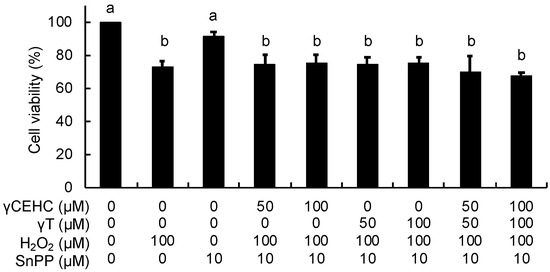
Figure 6.
The effect of a HO-1 inhibitor on the cytoprotection of γCEHC and γT to hydrogen peroxide-induced cytotoxicity. The cells were pretreated with SnPP for 1 h, then incubated with γCEHC and/or γT for 24 h, followed by exposure to hydrogen peroxide for 6 h. The cells were incubated with 0.5 mg/mL of an MTT solution for 2 h. The cell viability values are expressed as the percentages of the corresponding control. Values are the means ± S.D. (n = 3). Different letters above the bars indicate significant differences among the treatments (p < 0.05).
4. Discussion
In the present study, we demonstrated the cytoprotective effect of γCEHC and γT on hydrogen peroxide-induced cytotoxicity. Previous studies have demonstrated that vitamin E has strong antioxidative effects [3]. We have also reported that vitamin Es have cytoprotective effects on hydrogen peroxide-induced cytotoxicity [19,22]. However, there are only a few reports on the biological activities of the vitamin E metabolites. This is, to the best of our knowledge, the first report to reveal the protective effect of γCEHC on oxidative stress via the induction of the antioxidant enzyme. The effects of γCEHC were similar to those of the parent compound, indicating that the antioxidant activity of γT is maintained after metabolism.
γT is consumed in relatively large amounts on a daily basis because it is highly contained in plant and seed oils, such as corn oil and sesame oil [5]. The human plasma reference values for vitamin E were reported as 1.4–4.3 µM for γT and 161 nM for γCEHC [23]. Another report also indicated that γT and γCEHC were detected in human plasma at 2.2–2.6 μM and 123.7 nM, respectively [24]. Although CEHCs have been reported to have potential health benefits [11,23], as well as antioxidant functions [13,14], the extremely low concentrations of CEHCs in human plasma make their antioxidant functions questionable. On the other hand, the plasma concentrations of γT and γCEHC have been reported to be markedly increased by the continuous intake of γT [25,26]. Another study also indicated that γCEHC can reach up to 15 μM in plasma, and this concentration can be maintained by continuous supplementation with γT [27]. Regarding the form of γCEHC in the plasma, studies in rats have reported that most of the γCEHC in the plasma is in the conjugated form [12,28]. In contrast, several studies have shown that γCEHC is unconjugated in human plasma [27,29]. It remains unclear whether the unconjugated CEHC is the predominant metabolite in human plasma. Future studies are necessary to evaluate the plasma concentrations of the unconjugated and conjugated CEHC after vitamin E supplementation in humans.
In the present study, the cytoprotective mechanism of γCEHC and γT is suggested to involve the induction of HO-1 via the Nrf2/ARE-dependent pathway (Figure 3, Figure 4 and Figure 5). On the other hand, the induction of HO-1 by αT in rat renal cells might be mediated by the extracellular signal-regulated kinase or protein kinase A/cAMP-response element-dependent pathway but not by Nrf2/ARE [30]. Although the possibility that γCEHC and γT enhance HO-1 expression via the Nrf2-independent pathway could not be excluded, the Nrf2-mediated pathway might, at least in part, play an important role in the cytoprotective mechanism.
γCEHC and γT significantly increased the mRNA and protein levels of HO-1 (Figure 3 and Figure 4) at concentrations comparable to those required for cytoprotective effects in resistance to oxidative damage (Figure 2). Moreover, their protective effects on hydrogen peroxide-induced cytotoxicity were canceled in the presence of the HO-1 inhibitor (Figure 6). The HO-1-dependent antioxidant actions mainly result from the effects of its enzymatic reaction products, namely CO, biliverdin, and bilirubin. Although CO itself is not an antioxidant, it mediates a significant portion of the cytoprotective effects of HO-1, such as its interactions with metalloproteins [31,32,33]. In addition, the redox cycle of bilirubin by biliverdin reductase has been reported to ameliorate oxidative injury caused by hydrogen peroxide [34]. Taken together, the present findings suggested that the catalytic product of HO-1 may play an important role in the cytoprotective mechanism of γCEHC and γT. However, this study has some limitations, such as the fact that the cultured mouse hepatocyte model does not reflect the characteristics of human hepatocytes. Additionally, Hepa1c1c7 cells do not reflect the characteristics of intact hepatocytes because they are a hepatoma-derived cell line. In addition, the effective concentrations of γCEHC and γT required for antioxidant activity might be supraphysiological, except during continuous high intake. Future efforts will be required to clarify the exact effects on humans.
5. Conclusions
γCEHC, as well as γT, exerted a cytoprotective effect on oxidative stress-induced cytotoxicity by inducing HO-1 expression. Furthermore, the intracellular antioxidant activities by γCEHC may be mediated through the same pathway and additively enhanced in cooperation with γT. These results suggested that γT is a potent cytoprotective factor because it can protect against oxidative stress even when metabolized.
Author Contributions
Conceptualization, S.A. and T.N. (Toshiyuki Nakamura); Methodology, S.A., T.N. (Tomoka Nishio), D.M. and T.N. (Toshiyuki Nakamura); Formal analysis, S.A., T.N. (Tomoka Nishio), D.M. and T.N. (Toshiyuki Nakamura); Investigation, S.A., T.N. (Tomoka Nishio), D.M. and T.N. (Toshiyuki Nakamura); Data Curation, S.A., S.M., Y.M., Y.N. and T.N. (Toshiyuki Nakamura); Validation, S.M., Y.M., Y.N. and T.N. (Toshiyuki Nakamura); Supervision, Y.N. and T.N. (Toshiyuki Nakamura); Writing—Original Draft, S.A. and T.N. (Toshiyuki Nakamura); Writing—Review & Editing, S.A., Y.N. and T.N. (Toshiyuki Nakamura). All authors have read and agreed to the published version of the manuscript.
Funding
This study was partly supported by MEXT KAKENHI Grant Numbers 17H04725, 21K11676, and 24K14724 (TN), and 20H02933, 23H02161, and 23K26854 (YN).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Evans, H.M.; Bishop, K.S. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science 1922, 56, 650–651. [Google Scholar] [CrossRef] [PubMed]
- Packer, L. Protective role of vitamin E in biological systems. Am. J. Clin. Nutr. 1991, 53, 1050S–1055S. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: In vitro and in vivo evidence. Free Radic. Biol. Med. 2014, 66, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Narushima, K.; Takada, T.; Yamanashi, Y.; Suzuki, H. Niemann-pick C1-like 1 mediates alpha-tocopherol transport. Mol. Pharmacol. 2008, 74, 42–49. [Google Scholar] [CrossRef]
- Aksoz, E.; Korkut, O.; Aksit, D.; Gokbulut, C. Vitamin E (α-, β + γ- and δ-tocopherol) levels in plant oils. Flavour Fragr. J. 2020, 5, 504–510. [Google Scholar] [CrossRef]
- Hosomi, A.; Arita, M.; Sato, Y.; Kiyose, C.; Ueda, T.; Igarashi, O.; Arai, H.; Inoue, K. Affinity for alpha-tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs. FEBS Lett. 1997, 409, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Schuelke, M.; Elsner, A.; Finckh, B.; Kohlschütter, A.; Hübner, C.; Brigelius-Flohé, R. Urinary alpha-tocopherol metabolites in alpha-tocopherol transfer protein-deficient patients. J. Lipid Res. 2000, 41, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Sontag, T.J.; Parker, R.S. Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status. J. Biol. Chem. 2002, 277, 25290–25296. [Google Scholar] [CrossRef] [PubMed]
- Birringer, M.; Drogan, D.; Brigelius-Flohe, R. Tocopherols are metabolized in HepG2 cells by side chain omega-oxidation and consecutive beta-oxidation. Free Radic. Biol. Med. 2001, 31, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Wechter, W.J.; Kantoci, D.; Murray, E.D.J.; D’Amico, D.C.; Jung, M.E.; Wang, W.H. A new endogenous natriuretic factor: LLU-alpha. Proc. Natl. Acad. Sci. USA 1996, 93, 6002–6007. [Google Scholar] [CrossRef]
- Jiang, Q.; Elson-Schwab, I.; Courtemanche, C.; Ames, B.M. γ-Tocopherol and its major metabolite, in contrast to α-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc. Natl. Acad. Sci. USA 2000, 97, 11494–11499. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q. Metabolism of natural forms of vitamin E and biological actions of vitamin E metabolites. Free Radic. Biol. Med. 2022, 179, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Betancor-Fernandez, A.; Sies, H.; Stahl, W.; Polidori, M.C. In vitro antioxidant activity of 2,5,7,8-tetramethyl-2-(2-carboxyethyl)-6-hydroxychroman (alpha-CEHC), a vitamin E metabolite. Free Radic. Res. 2002, 36, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Niki, E. Antioxidant effects of alpha- and gamma-carboxyethyl-6-hydroxychromans. Biofactors 2002, 16, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.K.; Fitzgerald, H.K.; Dunne, A. Regulation of inflammation by the antioxidant haem oxygenase 1. Nat. Rev. Immunol. 2021, 21, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.V.; Sapochnik, D.; Garcia Solá, M.; Coso, O. Regulation of the Expression of Heme Oxygenase-1: Signal Transduction, Gene Promoter Activation, and Beyond. Antioxid. Redox Signal. 2020, 32, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.; Stewart, D.; Touchard, C.; Boinapally, S.; Choi, A.M.; Cook, J.L. Nrf2, a Cap’n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 1999, 274, 26071–26078. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Chen, Z.; Wen, Y.; Yi, Y.; Lv, C.; Zeng, C.; Chen, L.; Shi, M. Phytochemical activators of Nrf2: A review of therapeutic strategies in diabetes. Acta Biochim. Biophys. Sin. 2022, 55, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Nakamura, T.; Guo, Y.; Matsumoto, R.; Munemasa, S.; Murata, Y.; Nakamura, Y. Cycloartenyl ferulate is the predominant compound in brown rice conferring cytoprotective potential against oxidative stress-induced cytotoxicity. Int. J. Mol. Sci. 2023, 24, 822. [Google Scholar] [CrossRef]
- Mitsuzane, R.; Okubo, R.; Nishikawa, M.; Ikushiro, S.; Munemasa, S.; Murata, Y.; Nakamura, Y.; Nakamura, T. Enhancing effect of the coexisting alpha-tocopherol on quercetin absorption and metabolism. Free Radic. Res. 2024, 58, 88–97. [Google Scholar] [CrossRef]
- Nakamura, T.; Tsutsui, C.; Okuda, Y.; Abe-Kanoh, N.; Okazawa, S.; Munemasa, S.; Murata, Y.; Kato, Y.; Nakamura, Y. Benzyl isothiocyanate and its metabolites inhibit cell proliferation through protein modification in mouse preosteoclast RAW264.7 cells. J. Biochem. Mol. Toxicol. 2022, 36, e23184. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Nakamura, T.; Guo, Y.; Hirooka, M.; Zhang, G.; Munemasa, S.; Murata, Y.; Fujita, A.; Nakamura, Y. White rice ethanol extract is qualitatively, but not quantitatively, equivalent to that of brown rice as an antioxidant source. Biosci. Biotechnol. Biochem. 2021, 85, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Hensley, K.; Benaksas, E.J.; Bolli, R.; Comp, P.; Grammas, P.; Hamdheydari, L.; Mou, S.; Pye, Q.N.; Stoddard, M.F.; Wallis, G.; et al. New perspectives on vitamin E: γ-tocopherol and carboxyelthylhydroxychroman metabolites in biology and medicine. Free Radic. Biol. Med. 2004, 36, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Radosavac, D.; Graf, P.; Polidori, M.C.; Sies, H.; Stahl, W. Tocopherol metabolites 2, 5, 7, 8-tetramethyl-2-(2′-carboxyethyl)-6-hydroxychroman (alpha-CEHC) and 2, 7, 8-trimethyl-2-(2′-carboxyethyl)-6-hydroxychroman (gamma-CEHC) in human serum after a single dose of natural vitamin E. Eur. J. Nutr. 2002, 41, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, S.; Morinobu, T.; Hamamura, K.; Hirahara, F.; Iwamoto, T.; Tamai, H. The effect of γ-tocopherol administration on α-tocopherol levels and metabolism in humans. Eur. J. Clin. Nutr. 2005, 59, 900–905. [Google Scholar] [CrossRef]
- Wiser, J.; Alexis, N.E.; Jiang, Q.; Wu, W.; Robinette, C.; Roubey, R.; Peden, D.B. In vivo gamma-tocopherol supplementation decreases systemic oxidative stress and cytokine responses of human monocytes in normal and asthmatic subjects. Free Radic. Biol. Med. 2008, 45, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Burbank, A.J.; Duran, C.G.; Pan, Y.; Burns, P.; Jones, S.; Jiang, Q.; Yang, C.; Jenkins, S.; Wells, H.; Alexis, N.; et al. Gamma tocopherol-enriched supplement reduces sputum eosinophilia and endotoxin-induced sputum neutrophilia in volunteers with asthma. J. Allergy Clin. Immunol. 2018, 141, 1231–1238.e1. [Google Scholar] [CrossRef] [PubMed]
- Freiser, H.; Jiang, Q. Gamma-tocotrienol and gamma-tocopherol are primarily metabolized to conjugated 2-(beta-carboxyethyl)-6-hydroxy-2,7,8-trimethylchroman and sulfated long-chain carboxychromanols in rats. J. Nutr. 2009, 139, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Burbank, A.J.; Duran, C.G.; Almond, M.; Wells, H.; Jenkins, S.; Jiang, Q.; Yang, C.; Wang, T.; Zhou, H.; Hernandez, M.L.; et al. A short course of gamma-tocopherol mitigates LPS-induced inflammatory responses in humans ex vivo. J. Allergy Clin. Immunol. 2017, 140, 1179–1181.e4. [Google Scholar] [CrossRef]
- Reed, D.K.; Hall, S.; Arany, I. alpha-Tocopherol protects renal cells from nicotine- or oleic acid-provoked oxidative stress via inducing heme oxygenase-1. J. Physiol. Biochem. 2015, 71, 1–7. [Google Scholar] [CrossRef]
- Gozzelino, R.; Jeney, V.; Soares, M.P. Mechanisms of cell protection by heme oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 323–354. [Google Scholar] [CrossRef] [PubMed]
- Motterlini, R.; Otterbein, L.E. The therapeutic potential of carbon monoxide. Nat. Rev. Drug Discov. 2010, 9, 728–743. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.P.; Bach, F.H. Heme oxygenase-1: From biology to therapeutic potential. Trends Mol. Med. 2009, 15, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Baranano, D.E.; Rao, M.; Ferris, C.D.; Snyder, S.H. Biliverdin reductase: A major physiologic cytoprotectant. Proc. Natl. Acad. Sci. USA 2002, 99, 16093–16098. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).