Abstract
Neuroinflammation plays a crucial role in the development of various neurological diseases, including neurodegenerative disorders, leading to significant neuronal dysfunction. Current treatments involve the use of non-steroidal anti-inflammatory drugs and steroids; however, they are associated with serious adverse effects, limiting their efficacy. Exploring natural products with anti-inflammatory properties appears promising, with resveratrol, a polyphenol found in various plants, standing out for its potential benefits. Studies on resveratrol and its anti-inflammatory properties have been increasing in recent years, and analyzing the profile of this knowledge area can bring benefits to the scientific community. Therefore, this study conducted bibliometric analyses, using “resveratrol AND neuroinflammation” as search terms in the Web of Science Core Collection database. The analysis, performed with VOSviewer software version 1.6.18, encompasses 323 publications. Key terms in the studies include “resveratrol”, “neuroinflammation”, and “oxidative stress”, with China leading in the number of publications. The Federal University of Rio Grande do Sul in Brazil emerges as the institution with the highest contribution, and a phase 2 clinical study on resveratrol was the most cited. These results provide an overview of the global research landscape related to resveratrol and neuroinflammation, aiding decision making for future publications and advancing scientific understanding in this field.
1. Introduction
Numerous studies have aimed to comprehend the inflammatory processes occurring in the central nervous system (CNS) and their roles in brain pathologies. This phenomenon is termed neuroinflammation and represents a response of the innate immune system to aseptic or non-aseptic injuries [1]. Neuroinflammation is a complex process involving various cell types in the CNS, including glial cells, such as microglia and astrocytes [2], peripheral immune cells such as macrophages and mast cells, as well as oligodendrocytes and neurons [3,4,5].
Although inflammation’s initial role is protective, its chronic effects can lead to neural damage [6]. Neuroinflammation is closely linked to the onset and progression of neurodegenerative diseases [7]. There is substantial evidence demonstrating that the activation of microglia and astrocytes increases the progression of neurodegenerative diseases, suggesting that neuroinflammation contributes to neuronal dysfunction and death [8]. It is present in diseases such as Parkinson’s disease (PD), Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), and Huntington’s disease (HD), among others [9].
Currently, therapeutic approaches for inflammatory conditions comprise the use of non-steroidal anti-inflammatory drugs (NSAIDs) and steroidal drugs [2]. The most recognized effect of NSAIDs is the inhibition of cyclooxygenase (COX), which leads to a reduction in the levels of prostaglandins, prostacyclin, and thromboxane. COX comprises two functionally relevant isozymes, namely COX-1 and COX-2. Regarding the CNS, several studies have demonstrated that NSAIDs exhibit anti-inflammatory and anti-amyloidogenic effects both in vitro and in vivo, thereby enhancing cognitive function in preclinical models of AD [10,11]. However, clinical studies with NSAIDs in individuals predisposed to Alzheimer’s have given unimpressive results. Researchers assessed the effects of two NSAIDs, celecoxib and naproxen, in 2.528 patients aged over 70 with a family history of AD. The outcomes revealed that neither naproxen nor celecoxib improved cognitive function, leading to treatment discontinuation due to an observed increased cardiovascular risk with celecoxib in another prevention trial [12]. In addition to the lack of efficacy, these approaches have demonstrated adverse effects, including intestinal toxicity, ulcers, and cardiovascular problems [13]. The prolonged use of anti-inflammatory drugs can lead to resistance or tolerance, reducing their effectiveness over time [14]. This may require higher doses or frequent drug changes, increasing the risk of side effects. Despite clinical studies not being favorable, the importance of neuroinflammation in neurodegenerative diseases is well-documented, and the exploration of other molecules with inflammatory properties, such as those of natural origin, continues to be a promising strategy.
Several studies suggest the use of molecules derived from natural products as potential anti-inflammatory agents [15,16,17,18]. Numerous natural products are capable of exerting anti-inflammatory activity through multiple mechanisms of action and various signaling pathways. These natural products have demonstrated mechanisms that involve inhibiting microglia activation, reducing the release of pro-inflammatory cytokines, inhibiting Nuclear Factor Kappa-B (NF-κB) activation, and other pathways such as p38 MAPK [19]. Moreover, many of these natural molecules can activate Nrf2, a mechanism that has been shown to contribute, at least in part, to their anti-neuroinflammatory activity. It is due to these multifaceted actions of natural compounds that they have been proposed for the treatment of neurodegenerative diseases.
In particular, resveratrol, a polyphenol found in a wide variety of plants, including red grapes and walnuts, presents effectiveness as an anti-neuroinflammatory agent [20,21]. Among its molecular mechanisms in neuroinflammation, notable aspects include the inhibition of COX-2 activity [22], modulation of NF-κB signaling [23], reduction in the production of pro-inflammatory cytokines and chemokines [24], and attenuation of microglia and astrocyte activation [25], in addition to playing a significant role in inhibiting Aβ aggregation through binding to Aβ species. It has been demonstrated that resveratrol not only prevents the stacking of lower-molecular-weight oligomers into higher-molecular-weight oligomers [26], but also disrupts pre-formed Aβ aggregates [27]. Therefore, given the increasing number of studies conducted in this area, it is necessary to objectively document the growth of research on the topic.
Despite its numerous beneficial effects, resveratrol has low water solubility and rapid metabolization, which limits its absorption and distribution in the body. However, there are several approaches to increase its bioavailability, such as nanoformulations [28] coadministration with other molecules [29,30], slow-release formulations [31] and structural modifications [32].
Bibliometrics is a statistical method used to analyze publications within an important scientific subject that has been utilized for measuring the output of individuals, institutions, and countries over the years [33]. The impact of research can be explored, along with trends over time within the topic, as well as the main contributions to topics in a particular field of study through the analysis of keywords and citations [34,35].
Therefore, the aim of this study was to conduct a bibliometric review of the published literature on resveratrol and neuroinflammation in order to gain a quantitative and statistical understanding of this research area. This information is valuable for offering a quick overview of production in the field, identifying trends and assessing the impact of the most influential research and researchers, as well as recognizing the most productive institutions and the impact of the journals and countries that publish the most on the subject.
2. Materials and Methods
2.1. Data Source and Search Strategy
This bibliometric study analyzed articles on resveratrol and neuroinflammation from the first publication (2007) on the subject until September 2023. The multidisciplinary database Web of Science (WoS) was used with the following search terms (ALL = (resveratrol)) AND (ALL = (neuroinflammation)) to find publications containing these terms in the title, abstract and keywords. No filters, such as language, type of document and year, were applied in the search.
The publications identified from the search were evaluated for (1) publication year; (2) publication type (WoS category); (3) keywords; (3) most-cited bibliography; (4) the main journals; (5) countries and (6) organization. The full records and cited references of these identified publications were extracted and analyzed by the VOSviewer software for bibliometrics [36]. The VOSviewer software was also applied to analyze the semantic contents of titles, abstracts, and keywords of publications in order to relate them to the citation data count and synthesize a bubble map to visualize the results. VOSviewer is a free tool for bibliometric analysis work, as it enables advanced and interactive visualization of bibliometric networks, such as co-authorship networks, citation networks, and co-occurrence networks of key terms within the topic. This facilitates the understanding of patterns and trends within large sets of bibliographic data, as well as identifying emerging themes and areas of research related to the topic at hand [37].
2.2. Data Analysis and Presentation
The data found were imported into VOSviewer version 1.6.18 for bibliometric analysis. The following program commands were selected to create the bubble map of keywords: “Create a map based on bibliographic data”, “read data from bibliographic database files”, (1) type of analysis: “co-occurrence”, “unit of analysis: all keywords” using the “counting method: full counting”. The full count shows that a term with numerous occurrences in a single document is considered as one; each bubble represents a word, and its size reflects the frequency at which these words appear. The distance between the terms is determined by the frequency of their co-occurrence in the documents. In addition, a limit of at least 5 times for these words to appear in publications was established. To build the tables, the following commands were used: (2) type of analysis: “citation”, “unit of analysis: documents, sources, countries, organization”, and the data were exported for analysis in Excel 2019. A choropleth map of the countries that have published the most on the subject was built using Excel 2019. This thematic map is used to represent statistical data using the symbology technique of color mapping.
3. Results
3.1. Annual Publication Profile
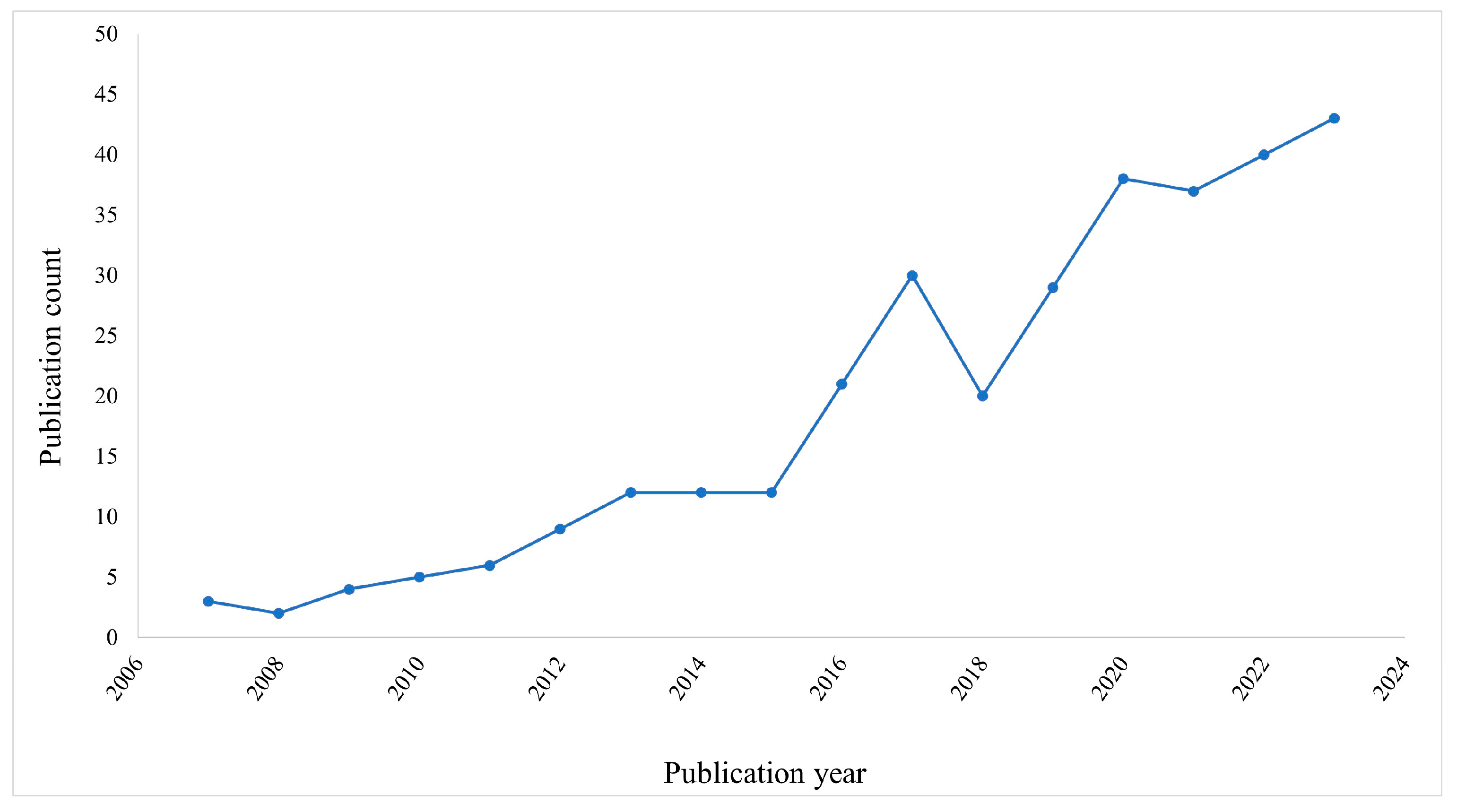
The search of published literature found 323 publications, all in English, with the first article published in 2007. There was an increase in publications from 2008. It stabilized for 3 years from 2013 to 2015. Finally, the number of publications increased considerably in the following years (except in 2018). Figure 1 shows the annual publication profile of articles dealing with resveratrol and neuroinflammation.
Figure 1.
Number of publications on resveratrol and neuroinflammation per year.
In this study, all types of documents were included in the analysis. The vast majority of the publications belong to the document types “original articles” (n = 242, 75%), “review” (n = 68, 21%), and “others” (n = 13, 4%), such as conference abstracts and retraction publications.
3.2. Keyword Analysis
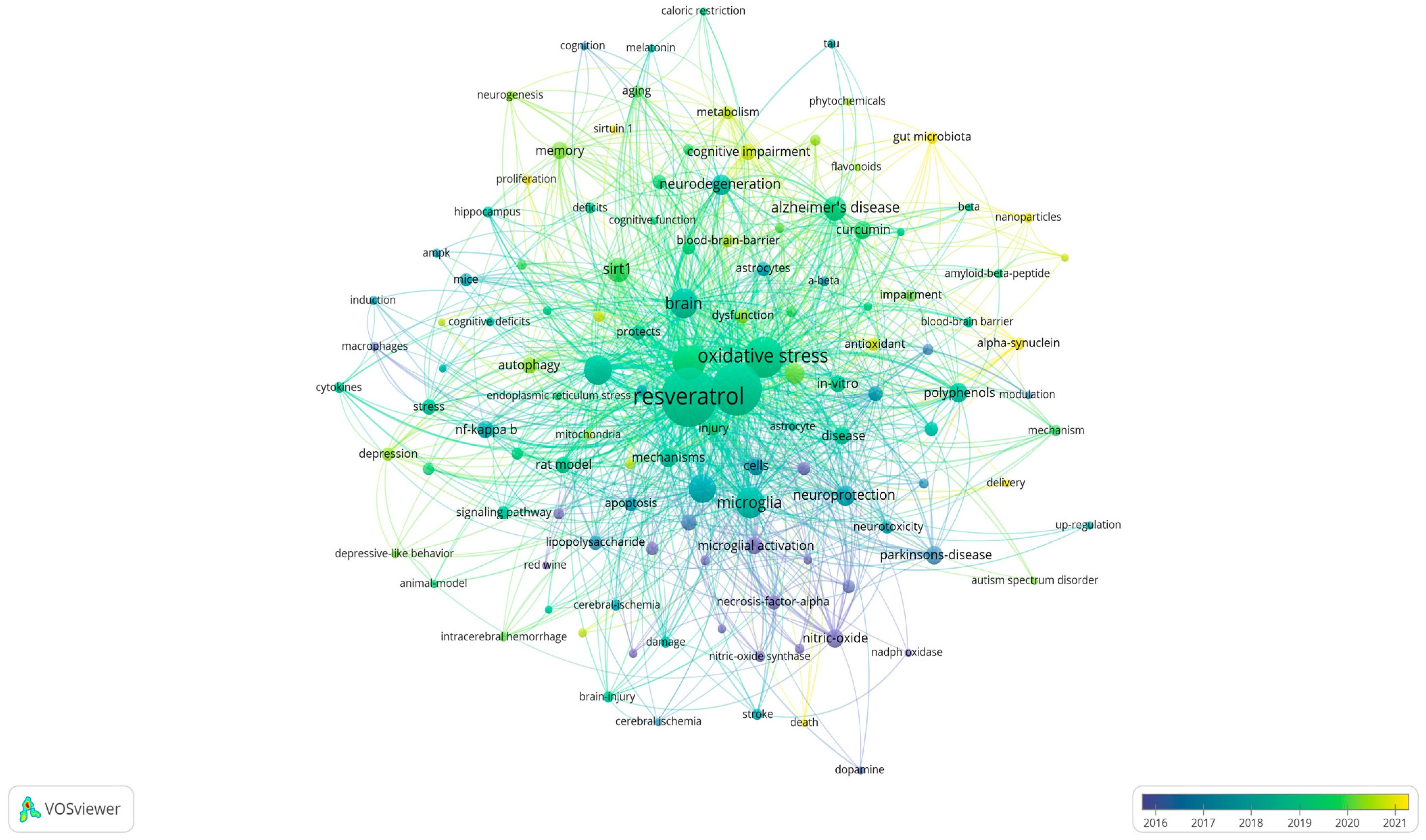
The VOSviewer software was used to analyze and visualize the recurring terms in the titles and abstracts of the 323 publications on resveratrol and neuroinflammation. Only words that appeared at least 5 times in the publications were visualized and analyzed. In total, 123 terms appeared at least five times in the evaluated publications.
Figure 2 illustrates the frequency of appearance of the keywords (multiple appearances in a single manuscript count as one). The larger the word, the more frequently it appears”. Two words that are close and connected to each other indicate more frequent co-occurrence in the evaluated publications.
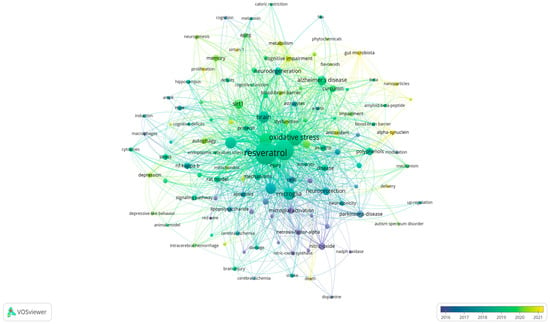
Figure 2.
Bubble map showing words from the titles and abstracts of the 323 publications on resveratrol and neuroinflammation (VOSviewer software). The colors of the bubbles represent the time scale, and the size of the word indicates its frequency of appearance.
The colors of the bubbles represent the timeline of occurrence, blue bubbles represent older articles, from 2016 onwards, and yellow bubbles represent the most recent articles. Complementing Figure 2, Table 1 presents the number of occurrences of the terms, with the three most frequently cited terms being “resveratrol”, “neuroinflammation”, and “oxidative stress”. These are followed by other processes related to inflammation and signaling pathways, such as “NF-kappa-B” and “Sirt-1” (sirtuin 1). Among the cell types, “microglia” was the most prominently studied, and “Alzheimer’s disease” was the most extensively investigated pathology in the articles.

Table 1.
The top 10 recurring terms from titles and abstracts.
3.3. Most Cited Documents
Table 2 presents data on the top 10 most cited documents among the 323 articles included in the analysis. The article from Moussa et al. [38] had the highest number of citations; it is a phase 2 clinical study that assessed the effect of resveratrol on individuals with mild to moderate AD. The second most cited document was a review by Gonzalez-Reyes et al. [39], also from 2017, focusing on the role of astrocytes in AD and neuroinflammation. The third most cited was another review by Rahimifard Mahban et al. [40], with a focus on the TLR4 signaling pathway as a therapeutic target for polyphenols in neuroinflammation. In general, among the most cited documents, there are five literature reviews, four original articles, and one phase 2 clinical study, with four studies from the year 2017. In addition, the articles were published in different journals.

Table 2.
Ten most-cited research documents.
3.4. Journals, Organizations and Countries with the Most Publications in the Field
Considering the number of publications, Table 3 presents the top 10 countries, organizations, and journals. The organizations with the highest number of publications were the Federal University of Rio Grande do Sul, from Brazil, leading with 10 publications, followed by University Jiao Tong of Shanghai, from China, and a university in the United States, Case Western Reserve University. Next, we have an Iranian university, the Tehran University of Medical Sciences, followed by Nanjing Medical University (China) and the University of Bari (Italy). These numbers not only reflect the quantity but also the diversity of the institutions involved, indicating a broad knowledge base about the impacts of resveratrol on the inflammatory response of the CNS.

Table 3.
The top ten countries, organizations and journals contributing to the 323 manuscripts.
The journals with the highest number of publications were the Journal of Neuroinflammation, an open access journal with an impact factor of 9.3 (JCR 2022), followed by molecular biology journals such as the International Journal of Molecular Sciences, an open access journal with an impact factor of 5.6. Next in line was Molecular Neurobiology, with a hybrid publication model and an impact factor of 5.1 (JCR 2022), and Cellular and Molecular Neurobiology, also with a hybrid publication model until January 2024 and an impact factor of 4.0 (JCR 2022). Other journals in the field of biochemistry included the Journal of Nutritional Biochemistry and Neurochemistry International, the latter having a high number of citations per manuscript (58.5), as well as Oxidative Medicine and Cellular Longevity. In the area of immunology, the International Immunopharmacology Journal, which accepts hybrid publications and has an impact factor of 5.6 (JCR 2022), had a high number of citations per manuscript (39.6), but journals in other areas such as Nutrients and Antioxidants had the lowest number of citations per manuscript (7.6 and 1.5, respectively), and were the least sought after in this area.
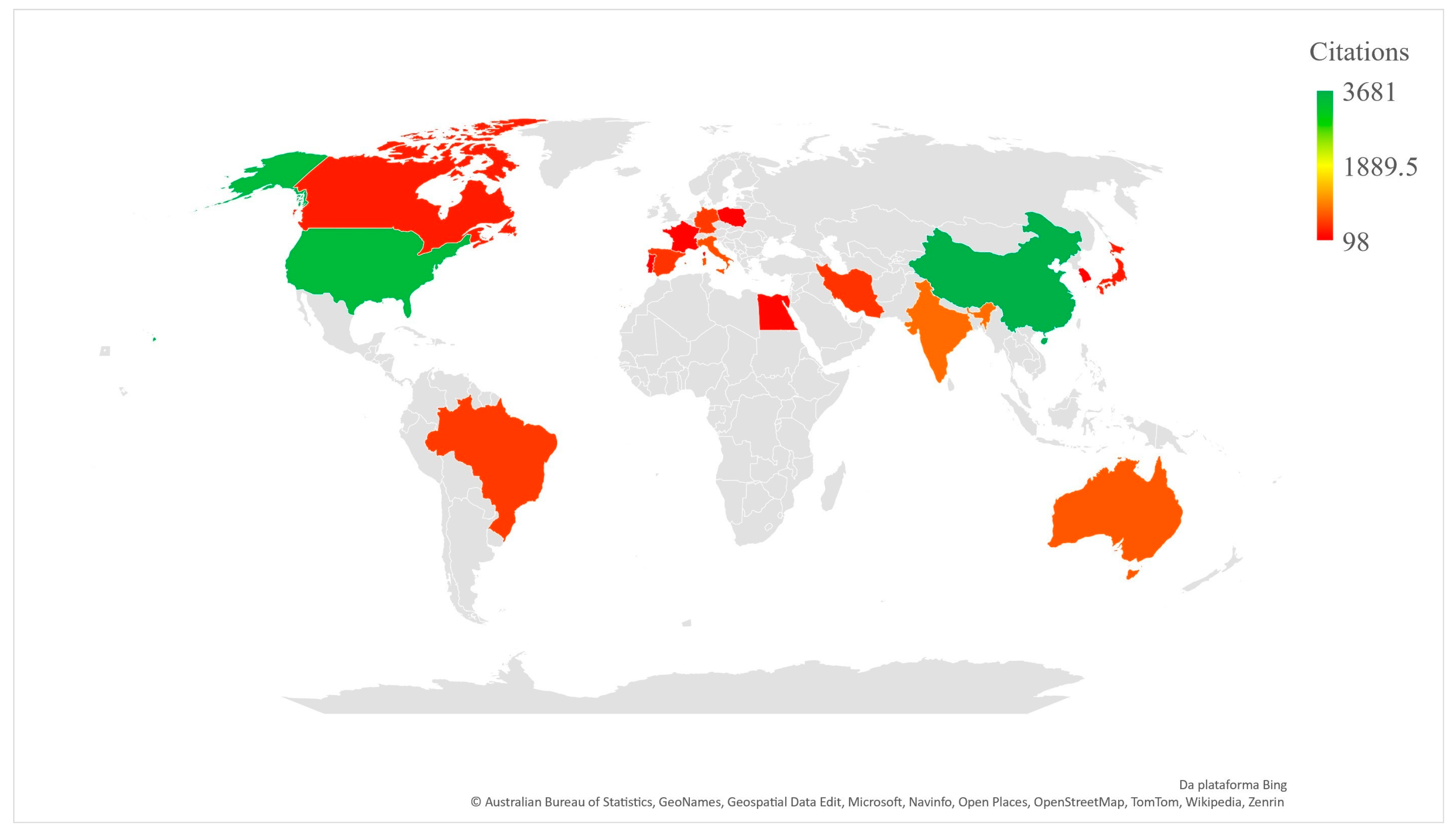
The bibliometric analysis showed that China is the country with the highest number of publications on resveratrol and neuroinflammation (Figure 3), which complements what was observed for the main authors, since most of them were Chinese. In second position is the United States, but with a higher number of citations per manuscript than China, followed by India, Brazil and Italy. The countries mentioned, along with others, can be found in Table 3, with a minimum of nine publications on the subject, as well as the number of citations per manuscript, conducted using information on the total number of citations, divided by the number of manuscripts published.
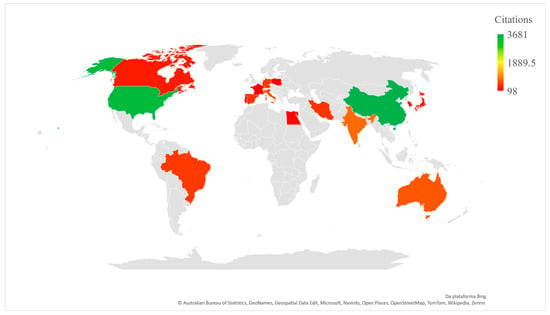
Figure 3.
Articles published by country/region according to number of total citations.
4. Discussion
This bibliometric analysis provides a quantitative analysis of scientific production on resveratrol and neuroinflammation over the years in order to identify patterns, trends and gaps in knowledge. For this analysis, the Web of Science database was used, and 323 publications were found. It was possible to observe that there has been a significant increase in the number of publications since 2015, associated with an increase in the number of countries and institutions involved in the publications, as well as a greater diversity of topics found in the studies.
The increase in the participation of Asian countries in scientific publications, consistently observed in others bibliometric reviews [48,49], highlights China as a leader in this area. This growth can be partly attributed to a combination of factors such as population size, robust investments in science, abundant access to natural resources, the establishment of international collaborations and a continued emphasis on traditional medicine. In many Asian countries, ancient medicinal traditions incorporate the use of natural products to treat diseases [50]. This cultural heritage encourages scientific research to validate and understand the therapeutic effects of natural substances, as these are elements that converge to drive the ethnopharmacological science of natural products, solidifying the position of Asian countries as significant contributors to this field of study [51].
According to a previous bibliometric study, articles on resveratrol in the research fields of nutraceuticals, such as the role of the Mediterranean diet with polyphenols as preventive agents for neurodegenerative diseases, are more extensive in Asian countries [49]. Furthermore, this pattern of contributions from Asian countries within the subject was also observed by Chinese, Japanese, and Korean institutions, represented in Table 2. It is essential to emphasize that these Asian contributions started to emerge from 2010 onward.
In addition, there are significant contributions from the United States, including a higher number of citations per manuscript compared to China, followed by India and then Brazil (Table 3). This high number of citations can be attributed to the fact that 75% of articles in the United States are open access.
Taken as a whole, the articles about resveratrol and neuroinflammation focus predominantly on molecular mechanisms, especially in the most recent publications. In addition, there have been numerous studies with a biochemical and pharmacological focus. The initial publications concentrated on neurology, exploring the polyphenol-rich Mediterranean diet and its applications in various pathologies, primarily focusing on PD, with the current shift towards AD. Through this bibliometric analysis, it is clear that the majority of studies, both original articles and reviews, address microglial activation, a fundamental feature of neuroinflammation. This information highlights that inhibiting microglial activation seems to be a promising therapeutic approach for neurological diseases.
The first review on the effects of resveratrol focusing on the brain was published in 2008 by Sun, Albert Y et al. [52]. Two years later, the second review was published by Zhang Feng et al. (2010) [44], providing a detailed exploration of the anti-inflammatory activities of resveratrol in the brain and its role in microglial activation. More recently, publications have shifted their focus to the mechanisms of action of resveratrol on other signaling pathways, such as Keap1/Nrf2/ARE, a biological system within cells that plays a crucial role in the antioxidant response and protection against oxidative stress [53], its modulation of pro-inflammatory cytokines [54], and its role in the enzymatic activity of SIRT1 [55]. Furthermore, additional reviews have examined dietary habits involving polyphenols like resveratrol in relation to lifestyle factors and neurodegenerative diseases, particularly AD [56] and PD [57].
Among the experimental studies, the first articles addressed the role of resveratrol in neuroinflammation and evaluated its effects in microglial cell cultures [58] and dopaminergic neurons [59]. It was observed that resveratrol could reduce the production of prostaglandin E2 and the formation of reactive oxygen species [58]. Furthermore, in the first study on resveratrol, its role in activating SIRT-1 was already mentioned. Researchers linked the neuroprotective and antioxidant effects of resveratrol with its ability to activate this protein [59].
In the subsequent years, most studies continued to examine the effect of resveratrol on microglia-mediated neuroinflammation [25,43,60,61,62,63]. Additionally, researchers explored its impact on neuroinflammation induced by the β-amyloid protein (Aβ), an experimental model of AD [64,65,66]. Recent articles have delved into its role in modulating the SIRT1 response [67,68,69,70] and its involvement in other signaling pathways, such as NF-κB [70,71,72,73,74].
In addition, numerous studies are currently focused on exploring tools aiming to improve the bioavailability of resveratrol. It is noteworthy that the most prolific institution among the evaluated publications is the Federal University of Rio Grande do Sul, where filiated scientists have been concentrating their studies on this aspect. Researchers from this university have assessed the potential of resveratrol as a therapeutic intervention for various neurological conditions using innovative delivery systems, such as lipid nanocapsules [64,75]. These systems show the potential to enhance the effectiveness of resveratrol, which seems to act in reducing neuroinflammation, protecting against neural damage, regulating glial activity, and promoting the release of anti-inflammatory cytokines.
The authorship analysis of publications on resveratrol and neuroinflammation was not conducted because the authors who publish the most on this subject are Chinese and often share the same initials, making a precise analysis challenging. For instance, according to the data analyzed, the most prolific author of publications on resveratrol and neuroinflammation was “Wang, Y”. This name, upon closer examination, could represent individuals like Wang Yan, Wang YP, Wang Yaping, or others such as Wang, XR, which might be the same as Wang Xiangru. Analyzing authorship by full names was also not possible since some records only provided the authors’ first names as initials.
The bubble map presents numerous terms indicating biochemical studies, focusing on oxidative stress, molecular aspects such as signaling pathways involved in the inflammatory process, especially NF-κB, microglial activation, and SIRT-1. The map shows evidence that resveratrol exhibits both antioxidant and anti-inflammatory effects, and that both processes are interconnected.
The overproduction of reactive oxygen species by activated microglia and subsequent oxidative damage induces an inflammatory state. Several studies have demonstrated that oxidative stress exacerbates the expression of inflammatory mediators in neurodegenerative diseases [76,77,78,79], and can activate various transcription factors, such as NF-κB, one of the key terms that appeared in the bibliometric analysis. This factor is sensitive to reactive species; through its phosphorylation, it becomes active and translocate to the cell nucleus, where it induces the expression of various proinflammatory molecules, such as tumor necrosis factor alfa (TNF-α), interleukin 1 beta (IL-1β) and IL-6, which are involved in the inflammatory processes [80,81].
Another term that showed significant occurrence was SIRT-1. Many studies attribute the biological properties of resveratrol to its ability to activate this SIRT-1 [68,82,83,84], an enzyme classified as an NAD+ dependent histone deacetylase, involved in metabolic processes, cellular stress regulation, and longevity [85,86]. In one study, the researchers demonstrated that the activation of SIRT1 by resveratrol (30 mg/kg/day for 8 weeks) reduced Tau protein phosphorylation induced by streptozotocin injection into the brain, confirming its protective role [87]. In another more recent study, it was shown that resveratrol significantly increased SIRT1 expression, inhibiting memory impairment [80]. This keyword analysis reflects the interaction between terms and what has been most studied regarding resveratrol and neuroinflammation in recent years.
The analysis of the most cited documents revealed that a phase 2 clinical study on the effects of resveratrol in patients with mild to moderate AD was the most cited among the articles analyzed [38]. In this study, researchers administered resveratrol (encapsulated) at a dose of 500 mg orally once a day, with a dose escalation every 13 weeks, culminating in 1000 mg twice a day; the total treatment duration was 52 weeks. They assessed the safety and tolerability of resveratrol, as well as its effects on AD biomarkers in plasma and cerebrospinal fluid (CSF). These included analyses of Aβ40 and Aβ42, tau and phospho-tau181. Pro-inflammatory cytokines were also evaluated, and cognitive analyses were conducted. The main results of this study indicated that, although resveratrol treatment did not affect tau protein in CSF, it significantly attenuated declines in Aβ42 and Aβ40 levels in CSF. Moreover, resveratrol was able to mitigate cognitive and functional decline in patients. Additionally, resveratrol reduced plasma levels of pro-inflammatory cytokines such as IL-1R4, IL-12P40, IL-12P70, and TNF-α.
Clinical studies, in general, tend to be highly cited due to their direct implications for clinical practice, especially when they demonstrate promising results, as seen in the study from Moussa et al., 2017. This study holds great relevance for the scientific community, considering its innovative therapeutic approaches and significant advances regarding the effects of resveratrol in AD. Another clinical study with resveratrol appeared in the results; however, this had fewer citations and focused on pathology other than AD [88]. Considering the promising results of resveratrol in preclinical and especially clinical studies, interest in investigating the potential of resveratrol as an intervention to improve brain health and prevent age-related cognitive decline has increased over the years [ClinicalTrials.gov NCT01794351, NCT01010009, NCT02336633]. There is growing interest in combining resveratrol with other therapies in order to potentiate its neuroprotective and anti-inflammatory effects [30,89,90], a future prospect that may offer additional benefits in the treatment of neuroinflammatory diseases.
Review articles are also commonly cited, and this is attributed to their ability to gather and synthesize information from a wide range of studies and sources, providing a comprehensive overview of the topic at hand. The most cited review article was the study by Gonzalez-Reyes, 2017 [39]. In this work, the authors compiled information on the most relevant aspects of the role of astrocytes in the neuroinflammatory changes observed in AD. Additionally, they discussed new neuroprotective and therapeutic measures, emphasizing the importance of astrocytes in this pathology. Although microglia have a fundamental role in neuroinflammation, this study has shown that it is crucial to start considering astrocytes as a new and valuable therapeutic and neuroprotective target for future studies related to the treatment of AD.
The third most cited work was also a literature review, Rahimifard Mahban et al. (2017) [40] explored the role of NF-κB factors in the central nervous system (CNS) through Toll-like receptor (TLR) activation. The researchers demonstrated the therapeutic aspects of polyphenolic compounds, including resveratrol for the treatment of neuroinflammation, by targeting TLR4, an important receptor protein that is essential in the immune system and stimulates various agonists of the inflammatory pathway [91].
A series of articles on the anti-inflammatory effects of resveratrol were collected in this review. It was observed that resveratrol exhibited potent anti-inflammatory effects through a mechanism involving the TLR4/NF-κB pathway and the transcription activation cascade (STAT) in vitro [92]. The resveratrol can prevent the activation of RAW 264.7 rat macrophages and microglial BV-2 cells targeted with a TLR4 ligand and lipopolysaccharide (LPS) [92].
Resveratrol also reduced IL-6, nitric oxide (NO) and TNF-α levels in RAW264.7 cells exposed to pathophysiological concentrations of LPS [93]. Similarly, treatment with resveratrol (5–20 µM) attenuated the increase in TLR4 expression, inhibited NF-κB activation and reduced TNF-α and IL-1β levels in cardiomyocytes exposed to anoxia/reoxygenation injury [94]. The report of resveratrol’s comprehensive mechanisms of action addressed in this review explains the large number of citations of articles that address the mechanisms of action of this molecule, because through these studies it is possible to identify that resveratrol can mediate anti-inflammatory effects by modulating the expression of TLR4 in different signaling pathways, especially NF-κB, which was one of the terms that appeared most in the keyword analyses.
5. Conclusions
In this study, a bibliometric analysis was conducted to identify the profile of publications on resveratrol and its relationship with neuroinflammation over the years, identifying trends, changes, and areas of significant growth. The study maps the most notable scientific contributions, highlighting pioneering studies and the focus of recent research in this domain. Overall, the analyzed manuscripts, especially the more recent ones, predominantly focused on molecular mechanisms and alternatives to enhance the bioavailability of resveratrol, such as studies involving nanoparticles. Early publications often addressed resveratrol as an essential component of the Mediterranean diet, which is rich in polyphenols, and its application in pathologies, with initial studies specifically focusing on PD and a current trend toward AD. This was also observed through the analysis of the most cited documents, being a phase 2 clinical study with mild to moderate AD patients treated with resveratrol that showed promising results from this molecule in the face of this pathology.
Keyword analysis revealed a close association between the terms resveratrol and neuroinflammation with oxidative stress. Considering the antioxidant and anti-inflammatory effects of resveratrol, both processes are interconnected and represent pivotal factors in the development and impairment of neurodegenerative diseases.
Although the term “gut microbiota” appeared in the keyword analysis, it did not rank among the top ten. However, there is a growing body of studies investigating the connection between gut microbiota, inflammation, and resveratrol [95,96,97]. While the precise details of how resveratrol interacts with the microbiota and modulates inflammation are still being clarified, it is known that the intestinal microbiota has the ability to metabolize resveratrol, generating metabolites that can have beneficial effects on the body and influence its bioavailability [97]. Additionally, resveratrol can also modulate the composition of the intestinal microbiota [95]; however, despite the relevance of the topic, the number of these studies is still growing.
The bibliometric analysis conducted in this study revealed research areas and/or gaps within the scientific literature about resveratrol and neuroinflammation. It identified emerging trends and collaborative networks among researchers and institutions that are prominent in publishing on this topic, thereby aiding in the formulation of well-informed decisions grounded in the available evidence within the scientific literature.
The main limitations found in this study are that bibliometric databases may not cover all relevant publications, especially those from non-English sources, and bibliometric analyses often treat all publications equally, regardless of their quality or impact. Bibliometric analyses provide quantitative data, and additional qualitative research is often needed to better complement the study.
Nevertheless, it is expected that the quantitative data analyzed here will contribute to current knowledge in the areas of resveratrol and neuroinflammation, which has increased significantly in recent years, and may help researchers identify promising areas for further study, as well as health policymakers looking for an overview of resveratrol and neuroinflammation research.
Author Contributions
Conceptualization, M.G.d.S. and M.A.H.; methodology and formal analysis M.G.d.S., D.B.d.L., F.B.d.M. and R.F.d.A.; writing -original draft preparation, M.G.d.S., D.B.d.L., F.B.d.M. and R.F.d.A.; writing -review and editing, M.G.d.S., A.M.S., B.D.A. and M.A.H.; supervision, M.A.H.; Funding acquisition: M.A.H. and A.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES) [Finance Code 001]. M.A.H and A.M.S receives Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) fellowship [research grants 309840/2022-8 and 310989/2021-3].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ransohoff, R.M. How Neuroinflammation Contributes to Neurodegeneration. Science 2016, 353, 777–783. [Google Scholar] [CrossRef]
- Patani, R.; Hardingham, G.E.; Liddelow, S.A. Functional Roles of Reactive Astrocytes in Neuroinflammation and Neurodegeneration. Nat. Rev. Neurol. 2023, 19, 395–409. [Google Scholar] [CrossRef]
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The Role and Consequences. Neurosci. Res. 2014, 79, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS Neurodegenerative Diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Q.; Zhou, J.W. Neuroinflammation in the Central Nervous System: Symphony of Glial Cells. Glia 2019, 67, 1017–1035. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Singh, S.; Tiwari, V.; Chaturvedi, S.; Wahajuddin, M.; Shukla, S. Dopamine Receptor Activation Mitigates Mitochondrial Dysfunction and Oxidative Stress to Enhance Dopaminergic Neurogenesis in 6-OHDA Lesioned Rats: A Role of Wnt Signalling. Neurochem. Int. 2019, 129, 104463. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef] [PubMed]
- de Araújo Boleti, A.P.; de Oliveira Flores, T.M.; Moreno, S.E.; dos Anjos, L.; Mortari, M.R.; Migliolo, L. Neuroinflammation: An Overview of Neurodegenerative and Metabolic Diseases and of Biotechnological Studies. Neurochem. Int. 2020, 136, 104714. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Bandopadhyay, R.; Singh, P.K.; Mishra, P.S.; Sharma, N.; Khurana, N. Neuroinflammation in Neurological Disorders: Pharmacotherapeutic Targets from Bench to Bedside. Metab. Brain Dis. 2021, 36, 1591–1626. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Sastre, M.; Dumitrescu-Ozimek, L.; Hanke, A.; Dewachter, I.; Kuiperi, C.; O’Banion, K.; Klockgether, T.; Van Leuven, F.; Landreth, G.E. Acute Treatment with the PPARγ Agonist Pioglitazone and Ibuprofen Reduces Glial Inflammation and Aβ1–42 Levels in APPV717I Transgenic Mice. Brain 2005, 128, 1442–1453. [Google Scholar] [CrossRef]
- Hirohata, M.; Ono, K.; Naiki, H.; Yamada, M. P4-243: Non-Steroidal Anti-Inflammatory Drugs Have Anti-Amyloidogenic Effects for Alzheimer’s Beta-Amyloid Fibrils In Vitro. Alzheimer’s Dement. 2006, 2, S588. [Google Scholar] [CrossRef]
- Soininen, H.; West, C.; Robbins, J.; Niculescu, L. Long-Term Efficacy and Safety of Celecoxib in Alzheimer’s Disease. Dement. Geriatr. Cogn. Disord. 2007, 23, 8–21. [Google Scholar] [CrossRef]
- Hijos-Mallada, G.; Sostres, C.; Gomollón, F. AINE, Toxicidad Gastrointestinal y Enfermedad Inflamatoria Intestinal. Gastroenterol. Hepatol. 2022, 45, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Sohail, R.; Mathew, M.; Patel, K.K.; Reddy, S.A.; Haider, Z.; Naria, M.; Habib, A.; Abdin, Z.U.; Razzaq Chaudhry, W.; Akbar, A. Effects of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and Gastroprotective NSAIDs on the Gastrointestinal Tract: A Narrative Review. Cureus 2023, 15, e37080. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Anti-Inflammatory Agents: Present and Future. Cell 2010, 140, 935–950. [Google Scholar] [CrossRef] [PubMed]
- Guilhon, C.C.; Minho, A.S.; Pouliot, M.; Boylan, F.; Fernandes, P.D. Tibouchina Granulosa Leaves Present Anti-Inflammatory Effect. Pharmaceuticals 2022, 15, 1458. [Google Scholar] [CrossRef]
- Tasneem, S.; Liu, B.; Li, B.; Choudhary, M.I.; Wang, W. Molecular Pharmacology of Inflammation: Medicinal Plants as Anti-Inflammatory Agents. Pharmacol. Res. 2019, 139, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Peng, F.; Xing, Z.; Chen, J.; Peng, C.; Li, D. Beneficial Effects of Natural Flavonoids on Neuroinflammation. Front. Immunol. 2022, 13, 1006434. [Google Scholar] [CrossRef] [PubMed]
- Mandel, S.A.; Amit, T.; Weinreb, O.; Youdim, M.B.H. Understanding the Broad-Spectrum Neuroprotective Action Profile of Green Tea Polyphenols in Aging and Neurodegenerative Diseases. J. Alzheimer’s Dis. 2011, 25, 187–208. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- dos Santos, M.G.; Schimith, L.E.; André-Miral, C.; Muccillo-Baisch, A.L.; Arbo, B.D.; Hort, M.A. Neuroprotective Effects of Resveratrol in In Vivo and In Vitro Experimental Models of Parkinson’s Disease: A Systematic Review. Neurotox. Res. 2022, 40, 319–345. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-M.; Chen, Y.-W.; Chi, P.-L.; Lin, C.-C.; Hsiao, L.-D. Resveratrol Inhibits BK-Induced COX-2 Transcription by Suppressing Acetylation of AP-1 and NF-ΚB in Human Rheumatoid Arthritis Synovial Fibroblasts. Biochem. Pharmacol. 2017, 132, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Bagul, P.K.; Deepthi, N.; Sultana, R.; Banerjee, S.K. Resveratrol Ameliorates Cardiac Oxidative Stress in Diabetes through Deacetylation of NFkB-P65 and Histone 3. J. Nutr. Biochem. 2015, 26, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Bellot, G.L.; Iskandar, K.; Chong, T.W.; Goh, F.Y.; Tai, J.J.; Schwarz, H.; Wong, S.C.; Pervaiz, S. Resveratrol Attenuates TLR-4 Mediated Inflammation and Elicits Therapeutic Potential in Models of Sepsis. Sci. Rep. 2020, 10, 18837. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Fan, L.; Li, J.; Zhang, B.; Yan, Z. Resveratrol Promoted the M2 Polarization of Microglia and Reduced Neuroinflammation after Cerebral Ischemia by Inhibiting MiR-155. Int. J. Neurosci. 2020, 130, 817–825. [Google Scholar] [CrossRef]
- Fu, Z.; Aucoin, D.; Ahmed, M.; Ziliox, M.; Van Nostrand, W.E.; Smith, S.O. Capping of Aβ42 Oligomers by Small Molecule Inhibitors. Biochemistry 2014, 53, 7893–7903. [Google Scholar] [CrossRef]
- Ghobeh, M.; Ahmadian, S.; Meratan, A.A.; Ebrahim-Habibi, A.; Ghasemi, A.; Shafizadeh, M.; Nemat-Gorgani, M. Interaction of Aβ(25–35) Fibrillation Products with Mitochondria: Effect of Small-molecule Natural Products. Pept. Sci. 2014, 102, 473–486. [Google Scholar] [CrossRef]
- Sessa, M.; Balestrieri, M.L.; Ferrari, G.; Servillo, L.; Castaldo, D.; D’Onofrio, N.; Donsì, F.; Tsao, R. Bioavailability of Encapsulated Resveratrol into Nanoemulsion-Based Delivery Systems. Food Chem. 2014, 147, 42–50. [Google Scholar] [CrossRef]
- Matencio, A.; Hernández-García, S.; García-Carmona, F.; López-Nicolás, J.M. An Integral Study of Cyclodextrins as Solubility Enhancers of α-Methylstilbene, a Resveratrol Analogue. Food Funct. 2017, 8, 270–277. [Google Scholar] [CrossRef]
- Bailey, H.H.; Johnson, J.J.; Lozar, T.; Scarlett, C.O.; Wollmer, B.W.; Kim, K.M.; Havinghurst, T.; Ahmad, N. A Randomized, Double-Blind, Dose-Ranging, Pilot Trial of Piperine with Resveratrol on the Effects on Serum Levels of Resveratrol. Eur. J. Cancer Prev. 2021, 30, 285. [Google Scholar] [CrossRef]
- Singh, G.; Pai, R.S. Trans-Resveratrol Self-Nano-Emulsifying Drug Delivery System (SNEDDS) with Enhanced Bioavailability Potential: Optimization, Pharmacokinetics and In Situ Single Pass Intestinal Perfusion (SPIP) Studies. Drug Deliv. 2015, 22, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.; Lee, S.; Park, H.; Cha, J. Enzymatic Synthesis of Resveratrol α-Glucoside by Amylosucrase of Deinococcus Geothermalis. J. Microbiol. Biotechnol. 2021, 31, 1692–1700. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.S.; Santos, I.C.; Oliveira, K.D.S.; Leão, N.C.A. Meta-Analysis as a Research Tool: A Systematic Review of Bibliometric Studies in Administration. RAM Rev. Adm. Mackenzie 2019, 20. [Google Scholar] [CrossRef]
- Wallin, J.A. Bibliometric Methods: Pitfalls and Possibilities. Basic Clin. Pharmacol. Toxicol. 2005, 97, 261–275. [Google Scholar] [CrossRef]
- Ellegaard, O.; Wallin, J.A. The Bibliometric Analysis of Scholarly Production: How Great Is the Impact? Scientometrics 2015, 105, 1809–1831. [Google Scholar] [CrossRef] [PubMed]
- van Eck, N.J.; Waltman, L. Software Survey: VOSviewer, a Computer Program for Bibliometric Mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Donthu, N.; Kumar, S.; Mukherjee, D.; Pandey, N.; Lim, W.M. How to Conduct a Bibliometric Analysis: An Overview and Guidelines. J. Bus. Res. 2021, 133, 285–296. [Google Scholar] [CrossRef]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflammation 2017, 14, 1. [Google Scholar] [CrossRef]
- González-Reyes, R.E.; Nava-Mesa, M.O.; Vargas-Sánchez, K.; Ariza-Salamanca, D.; Mora-Muñoz, L. Involvement of Astrocytes in Alzheimer’s Disease from a Neuroinflammatory and Oxidative Stress Perspective. Front. Mol. Neurosci. 2017, 10, 427. [Google Scholar] [CrossRef]
- Rahimifard, M.; Maqbool, F.; Moeini-Nodeh, S.; Niaz, K.; Abdollahi, M.; Braidy, N.; Nabavi, S.M.; Nabavi, S.F. Targeting the TLR4 Signaling Pathway by Polyphenols: A Novel Therapeutic Strategy for Neuroinflammation. Ageing Res. Rev. 2017, 36, 11–19. [Google Scholar] [CrossRef]
- Negi, G.; Kumar, A.; Sharma, S.S. Melatonin modulates neuroinflammation and oxidative stress in experimental diabetic neuropathy: Effects on NF-κB and Nrf2 cascades. J. Pineal Res. 2011, 50, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Bureau, G.; Longpré, F.; Martinoli, M.-G. Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. J. Neurosci. Res. 2008, 86, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xu, S.; Qian, Y.; Xiao, Q. Resveratrol regulates microglia M1/M2 polarization via PGC-1α in conditions of neuroinflammatory injury. Brain. Behav. Immun. 2017, 64, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, J.; Shi, J.-S. Anti-Inflammatory Activities of Resveratrol in the Brain: Role of Resveratrol in Microglial Activation. Eur. J. Pharmacol. 2010, 636, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-C. Deubiquitylation and regulation of the immune response. Nat. Rev. Immunol. 2008, 8, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Hurley, L.L.; Tizabi, Y. Neuroinflammation, Neurodegeneration, and Depression. Neurotox. Res. 2013, 23, 131–144. [Google Scholar] [CrossRef]
- Mo, X.; Wang, X.; Ge, Q.; Bian, F. The effects of SIRT1/FoxO1 on LPS induced INS-1 cells dysfunction. Stress 2019, 22. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Aggarwal, B.B.; Orhan, I.E.; Horbańczuk, O.K.; Barreca, D.; Battino, M.; Belwal, T.; Bishayee, A.; Daglia, M.; Devkota, H.P.; et al. Resveratrol, a Popular Dietary Supplement for Human and Animal Health: Quantitative Research Literature Analysis—A Review. Anim. Sci. Pap. Rep. 2019, 37, 103–118. [Google Scholar]
- Chan, E.; Tan, M.; Xin, J.; Sudarsanam, S.; Johnson, D.E. Interactions between Traditional Chinese Medicines and Western Therapeutics. Curr. Opin. Drug Discov. Dev. 2010, 13, 50–65. [Google Scholar]
- Akarasereenont, P.; Datiles, M.J.R.; Lumlerdkij, N.; Yaakob, H.; Prieto, J.M.; Heinrich, M. A South-East Asian Perspective on Ethnopharmacology. In Ethnopharmacology; Wiley: Hoboken, NJ, USA, 2015; pp. 317–332. [Google Scholar]
- Sun, A.Y.; Wang, Q.; Simonyi, A.; Sun, G.Y. Botanical Phenolics and Brain Health. Neuromol. Med. 2008, 10, 259–274. [Google Scholar] [CrossRef]
- Michalicková, D.; Hrncír, T.; Canová, N.K.; Slanar, O. Targeting Keap1/Nrf2/ARE Signaling Pathway in Multiple Sclerosis. Eur. J. Pharmacol. 2020, 873, 172973. [Google Scholar] [CrossRef]
- Vishwakarma, S.; Singh, S.; Singh, T.G. Pharmacological Modulation of Cytokines Correlating Neuroinflammatory Cascades in Epileptogenesis. Mol. Biol. Rep. 2022, 49, 1437–1452. [Google Scholar] [CrossRef]
- Li, X.; Feng, Y.; Wang, X.X.; Truong, D.; Wu, Y.C. The Critical Role of SIRT1 in Parkinson’s Disease: Mechanism and Therapeutic Considerations. Aging Dis. 2020, 11, 1608–1622. [Google Scholar] [CrossRef] [PubMed]
- El Gaamouch, F.; Chen, F.A.; Ho, L.P.; Lin, H.Y.; Yuan, C.Z.; Wong, J.; Wang, J. Benefits of Dietary Polyphenols in Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 1019942. [Google Scholar] [CrossRef] [PubMed]
- Bisaglia, M. Mediterranean Diet and Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 42. [Google Scholar] [CrossRef] [PubMed]
- Candelario-Jalil, E.; de Oliveira, A.C.P.; Gräf, S.; Bhatia, H.S.; Hüll, M.; Muñoz, E.; Fiebich, B.L. Resveratrol Potently Reduces Prostaglandin E2production and Free Radical Formation in Lipopolysaccharide-Activated Primary Rat Microglia. J. Neuroinflamm. 2007, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Okawara, M.; Katsuki, H.; Kurimoto, E.; Shibata, H.; Kume, T.; Akaike, A. Resveratrol Protects Dopaminergic Neurons in Midbrain Slice Culture from Multiple Insults. Biochem. Pharmacol. 2007, 73, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, H.; Wu, Q.; Lu, Y.; Nie, J.; Xie, X.; Shi, J. Resveratrol Protects Cortical Neurons against Microglia-Mediated Neuroinflammation. Phyther. Res. 2013, 27, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Porro, C.; Cianciulli, A.; Calvello, R.; Panaro, M. Reviewing the Role of Resveratrol as a Natural Modulator of Microglial Activities. Curr. Pharm. Des. 2015, 21, 5277–5291. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xue, J.; Zou, J.; Zhao, X.; Li, L.; Jia, R.; Zou, Y.; Wan, H.; Chen, Y.; Zhou, X.; et al. Resveratrol Alleviated Neuroinflammation Induced by Pseudorabies Virus Infection through Regulating Microglial M1/M2 Polarization. Biomed. Pharmacother. 2023, 160, 114271. [Google Scholar] [CrossRef]
- Schlotterose, L.; Pravdivtseva, M.S.; Ellermann, F.; Jansen, O.; Hövener, J.-B.; Sönnichsen, F.D.; Cossais, F.; Lucius, R.; Hattermann, K. Resveratrol Mitigates Metabolism in Human Microglia Cells. Antioxidants 2023, 12, 1248. [Google Scholar] [CrossRef]
- Frozza, R.L.; Bernardi, A.; Hoppe, J.B.; Meneghetti, A.B.; Battastini, A.M.O.; Pohlmann, A.R.; Guterres, S.S.; Salbego, C. Lipid-Core Nanocapsules Improve the Effects of Resveratrol against Abeta-Induced Neuroinflammation. J. Biomed. Nanotechnol. 2013, 9, 2086–2104. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, L. Resveratrol Suppresses Aβ-Induced Microglial Activation Through the TXNIP/TRX/NLRP3 Signaling Pathway. DNA Cell Biol. 2019, 38, 874–879. [Google Scholar] [CrossRef]
- Yao, Y.; Li, J.; Niu, Y.; Yu, J.-Q.; Yan, L.; Miao, Z.-H.; Zhao, X.-X.; Li, Y.-J.; Yao, W.-X.; Zheng, P.; et al. Resveratrol Inhibits Oligomeric Aβ-Induced Microglial Activation via NADPH Oxidase. Mol. Med. Rep. 2015, 12, 6133–6139. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, A.D.; Wątroba, M.; Witkowska, J.; Mikulska, A.; Sepúlveda, N.; Szukiewicz, D. Interplay between Systemic Glycemia and Neuroprotective Activity of Resveratrol in Modulating Astrocyte SIRT1 Response to Neuroinflammation. Int. J. Mol. Sci. 2023, 24, 11640. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Lei, M.-Y.; Liu, Z.-Q.; Liu, Z.-F.; Ma, Z.; Liu, K.; Li, J.; Deng, Y.; Liu, W.; Xu, B. Resveratrol Attenuates Manganese-Induced Oxidative Stress and Neuroinflammation through SIRT1 Signaling in Mice. Food Chem. Toxicol. 2021, 153, 112283. [Google Scholar] [CrossRef] [PubMed]
- Abozaid, O.A.R.; Sallam, M.W.; El-Sonbaty, S.; Aziza, S.; Emad, B.; Ahmed, E.S.A. Resveratrol-Selenium Nanoparticles Alleviate Neuroinflammation and Neurotoxicity in a Rat Model of Alzheimer’s Disease by Regulating Sirt1/MiRNA-134/GSK3β Expression. Biol. Trace Elem. Res. 2022, 200, 5104–5114. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.-M.; Zhang, Y.-M.; Feng, Y.-Z.; Zhang, K.-X.; Zhang, J.-Y.; Chen, J.; Luo, B.-L.; Li, X.-Y.; Chen, G.-H. Resveratrol Ameliorates Maternal Separation-Induced Anxiety- and Depression-like Behaviors and Reduces Sirt1-NF-KB Signaling-Mediated Neuroinflammation. Front. Behav. Neurosci. 2023, 17, 1172091. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Lin, H.; Chuang, C.; Wang, P.; Wan, H.; Lee, M.; Kao, M. Resveratrol Alleviates Nuclear Factor-κB-mediated Neuroinflammation in Vasculitic Peripheral Neuropathy Induced by Ischaemia–Reperfusion via Suppressing Endoplasmic Reticulum Stress. Clin. Exp. Pharmacol. Physiol. 2019, 46, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Shi, C.; Meng, J.; Xu, S.; Liu, J. Resveratrol Alleviates Ethanol-Induced Neuroinflammation in Vivo and in Vitro: Involvement of TLR2-MyD88-NF-ΚB Pathway. Int. J. Biochem. Cell Biol. 2018, 103, 56–64. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, L.; Xia, Y.; Jiang, C.; Miao, C.; Yang, C.; Yuan, M.; Wang, L. Resveratrol Suppresses Glial Activation and Alleviates Trigeminal Neuralgia via Activation of AMPK. J. Neuroinflamm. 2016, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Baek, S.-H.; Kim, S.Y. Genetically Engineered Resveratrol-Enriched Rice Inhibits Neuroinflammation in Lipopolysaccharide-Activated BV2 Microglia via Downregulating Mitogen-Activated Protein Kinase-Nuclear Factor Kappa B Signaling Pathway. Oxid. Med. Cell. Longev. 2018, 2018, 8092713. [Google Scholar] [CrossRef] [PubMed]
- Bobermin, L.D.; Roppa, R.H.A.; Quincozes-Santos, A. Adenosine Receptors as a New Target for Resveratrol-Mediated Glioprotection. Biochim. Biophys. Acta—Mol. Basis Dis. 2019, 1865, 634–647. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.N.; Shaughness, M.; Collier, S.; Hopkins, D.; Byrnes, K.R. Therapeutic Targeting of Microglia Mediated Oxidative Stress after Neurotrauma. Front. Med. 2022, 9, 1034692. [Google Scholar] [CrossRef] [PubMed]
- Ghysen, A.; Dambly-Chaudière, C. The Three-sided Romance of the Lateral Line: Glia Love Axons Love Precursors Love Glia. BioEssays 2005, 27, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Li, G.; Qian, X.; Liu, Y.; Wu, X.; Liu, B.; Hong, J.; Block, M.L. Interactive Role of the Toll-like Receptor 4 and Reactive Oxygen Species in LPS-induced Microglia Activation. Glia 2005, 52, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Sabirzhanov, B.; Li, Y.; Coll-Miro, M.; Matyas, J.J.; He, J.; Kumar, A.; Ward, N.; Yu, J.; Faden, A.I.; Wu, J. Inhibition of NOX2 Signaling Limits Pain-Related Behavior and Improves Motor Function in Male Mice after Spinal Cord Injury: Participation of IL-10/MiR-155 Pathways. Brain. Behav. Immun. 2019, 80, 73–87. [Google Scholar] [CrossRef]
- Wright, J.G.; Christman, J.W. The Role of Nuclear Factor Kappa B in the Pathogenesis of Pulmonary Diseases: Implications for Therapy. Am. J. Respir. Med. 2003, 2, 211–219. [Google Scholar] [CrossRef]
- Shi, X.-Z.; Lindholm, P.F.; Sarna, S.K. NF-ΚB Activation by Oxidative Stress and Inflammation Suppresses Contractility in Colonic Circular Smooth Muscle Cells. Gastroenterology 2003, 124, 1369–1380. [Google Scholar] [CrossRef]
- Ciccone, L.; Piragine, E.; Brogi, S.; Camodeca, C.; Fucci, R.; Calderone, V.; Nencetti, S.; Martelli, A.; Orlandini, E. Resveratrol-like Compounds as SIRT1 Activators. Int. J. Mol. Sci. 2022, 23, 15105. [Google Scholar] [CrossRef]
- Gomes, B.A.Q.; Silva, J.P.B.; Romeiro, C.F.R.; dos Santos, S.M.; Rodrigues, C.A.; Gonçalves, P.R.; Sakai, J.T.; Mendes, P.F.S.; Varela, E.L.P.; Monteiro, M.C. Neuroprotective Mechanisms of Resveratrol in Alzheimer’s Disease: Role of SIRT1. Oxid. Med. Cell. Longev. 2018, 2018, 8152373. [Google Scholar] [CrossRef]
- Sarubbo, F.; Esteban, S.; Miralles, A.; Moranta, D. Effects of Resveratrol and Other Polyphenols on Sirt1: Relevance to Brain Function During Aging. Curr. Neuropharmacol. 2018, 16, 126–136. [Google Scholar] [CrossRef]
- Dusabimana, T.; Kim, S.R.; Kim, H.J.; Park, S.W.; Kim, H. Nobiletin Ameliorates Hepatic Ischemia and Reperfusion Injury through the Activation of SIRT-1/FOXO3a-Mediated Autophagy and Mitochondrial Biogenesis. Exp. Mol. Med. 2019, 51, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ting, W.-J.; Yang, J.-J.; Kuo, C.-H.; Xiao, Z.-J.; Lu, X.-Z.; Yeh, Y.-L.; Day, C.-H.; Wen, S.-Y.; PadmaViswanadha, V.; Jiang, C.-H.; et al. Environmental Tobacco Smoke Increases Autophagic Effects but Decreases Longevity Associated with Sirt-1 Protein Expression in Young C57BL Mice Hearts. Oncotarget 2016, 7, 39017–39025. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Du, L.-L.; Xie, J.-Z.; Cheng, X.-S.; Li, X.-H.; Kong, F.-L.; Jiang, X.; Ma, Z.-W.; Wang, J.-Z.; Chen, C.; Zhou, X.-W. Activation of Sirtuin 1 Attenuates Cerebral Ventricular Streptozotocin-Induced Tau Hyperphosphorylation and Cognitive Injuries in Rat Hippocampi. Age 2014, 36, 613–623. [Google Scholar] [CrossRef]
- Hodgin, K.S.; Donovan, E.K.; Kekes-Szabo, S.; Lin, J.C.; Feick, J.; Massey, R.L.; Ness, T.J.; Younger, J.W. A Placebo-Controlled, Pseudo-Randomized, Crossover Trial of Botanical Agents for Gulf War Illness: Resveratrol (Polygonum cuspidatum), Luteolin, and Fisetin (Rhus succedanea). Int. J. Environ. Res. Public Health 2021, 18, 2483. [Google Scholar] [CrossRef]
- Wang, N.; Xu, C.; Li, N.; Wang, F.; Wang, F.; Li, Z.; Yu, Q.; Zhang, G. Synergistic Anti-Inflammatory Effects of Resveratrol and Vitamin E in Lipopolysaccharide-Induced RAW264.7 Cells. Food Sci. Technol. 2022, 42, e24122. [Google Scholar] [CrossRef]
- Chen, L.; Liu, T.; Wang, Q.; Liu, J. Anti-Inflammatory Effect of Combined Tetramethylpyrazine, Resveratrol and Curcumin in Vivo. BMC Complement. Altern. Med. 2017, 17, 233. [Google Scholar] [CrossRef]
- Coutinho-Wolino, K.S.; Almeida, P.P.; Mafra, D.; Stockler-Pinto, M.B. Bioactive Compounds Modulating Toll-like 4 Receptor (TLR4)-Mediated Inflammation: Pathways Involved and Future Perspectives. Nutr. Res. 2022, 107, 96–116. [Google Scholar] [CrossRef]
- Capiralla, H.; Vingtdeux, V.; Zhao, H.; Sankowski, R.; Al-Abed, Y.; Davies, P.; Marambaud, P. Resveratrol Mitigates Lipopolysaccharide- and Aβ-mediated Microglial Inflammation by Inhibiting the TLR4/NF-κB/STAT Signaling Cascade. J. Neurochem. 2012, 120, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, S.; Yang, Q.; Shi, Y.; Zheng, M.; Liu, Y.; Chen, F.; Song, G.; Xu, H.; Wan, T.; et al. Resveratrol Reduces the Proinflammatory Effects and Lipopolysaccharide- Induced Expression of HMGB1 and TLR4 in RAW264.7 Cells. Cell. Physiol. Biochem. 2014, 33, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lin, G.; Wan, W.; Li, X.; Zeng, B.; Yang, B.; Huang, C. Resveratrol, a Polyphenol Phytoalexin, Protects Cardiomyocytes against Anoxia/Reoxygenation Injury via the TLR4/NF-ΚB Signaling Pathway. Int. J. Mol. Med. 2012, 29, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.-T.; Ye, X.-L.; Li, R.-R.; Chen, H.; Wang, Y.-Y.; Yong, H.-J.; Pan, M.-L.; Lu, W.; Tang, Y.; Miao, H.; et al. Resveratrol Modulates the Gut Microbiota and Inflammation to Protect Against Diabetic Nephropathy in Mice. Front. Pharmacol. 2020, 11, 1249. [Google Scholar] [CrossRef]
- Wang, Y.; Hong, C.; Wu, Z.; Li, S.; Xia, Y.; Liang, Y.; He, X.; Xiao, X.; Tang, W. Resveratrol in Intestinal Health and Disease: Focusing on Intestinal Barrier. Front. Nutr. 2022, 9, 848400. [Google Scholar] [CrossRef]
- Li, F.; Han, Y.; Wu, X.; Cao, X.; Gao, Z.; Sun, Y.; Wang, M.; Xiao, H. Gut Microbiota-Derived Resveratrol Metabolites, Dihydroresveratrol and Lunularin, Significantly Contribute to the Biological Activities of Resveratrol. Front. Nutr. 2022, 9, 912591. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).