The Marine Alga Sargassum horneri Is a Functional Food with High Bioactivity
Abstract
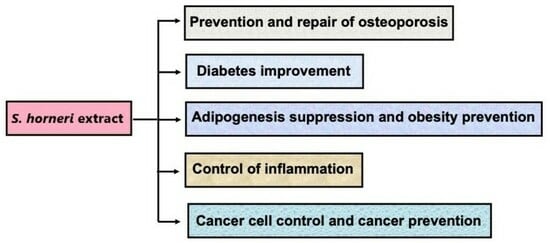
1. Introduction
2. S. horneri Component May Prevent Osteoporosis
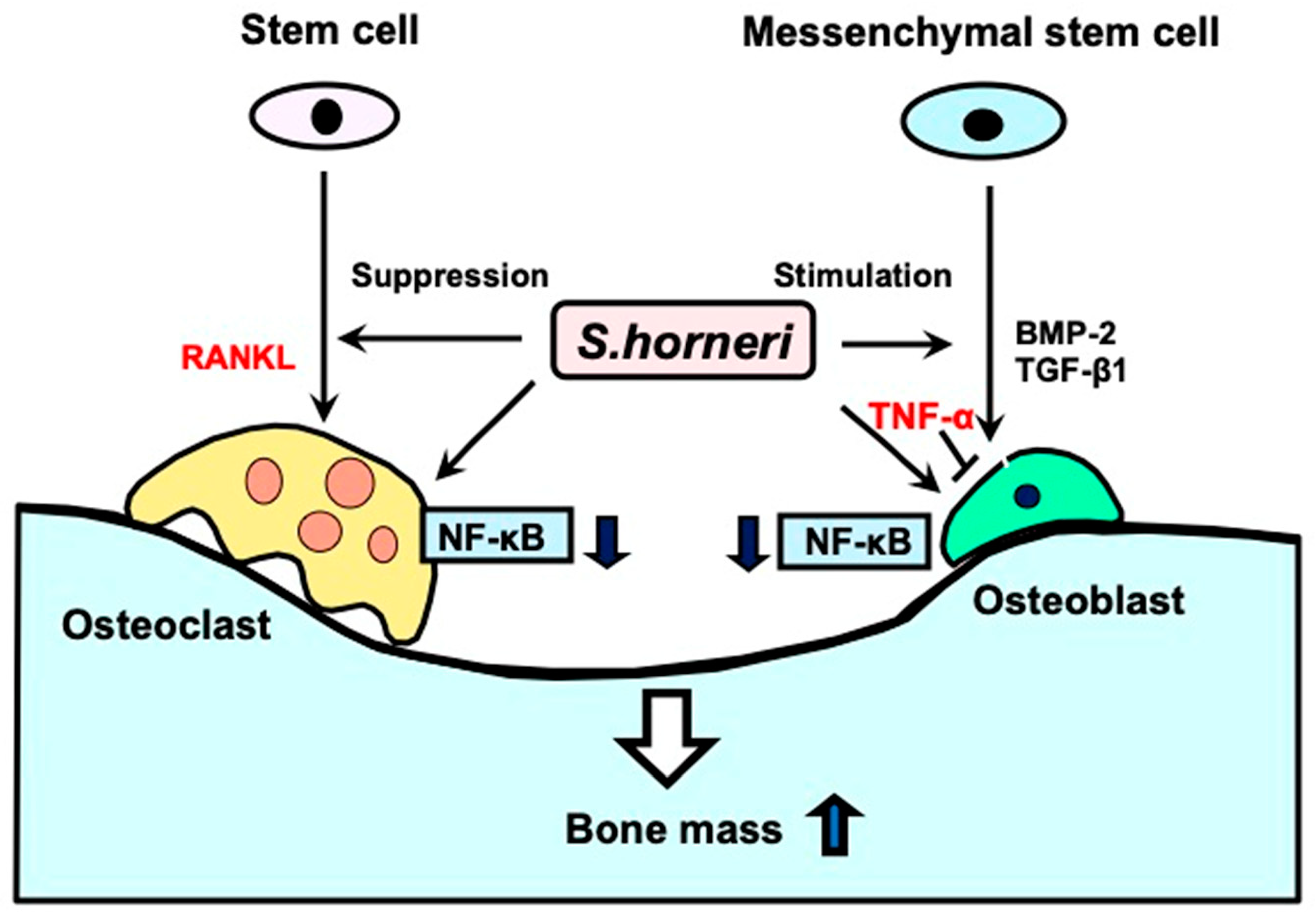
2.1. Unique Anabolic Effect of S. horneri Component in Bone Tissue
2.2. Characterisation of the Active Ingredient in S. horneri Aqueous Extract
2.3. S. horneri Component Has an Inhibitory Effect on the Activation of the NF-κB Signalling Pathway
2.4. Involvement of S. horneri Water Extract Component in Osteoporosis Prevention
2.5. Complementary Effects of S. horneri Water Extract Component in Humans
3. S. horneri Water Extract Component Has a Preventive Effect on Diabetes
4. S. horneri Extract Component Suppresses Adipogenesis in Bone Marrow Cells
5. Suppressive Effects of the S. horneri Component on the Inflammatory State Associated with TNF-α in Various Cell Types
6. Suppressive Effects of the S. horneri Component on Cancer Cells
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamaguchi, M. Regulatory mechanism of food factors in bone metabolism and prevention of osteoporosis. Yakugaku Zasshi 2006, 126, 1117–1137. [Google Scholar] [CrossRef]
- Yamaguchi, M. Nutritional Factors and Osteoporosis Prevention; Nova Science Publishers, Inc.: New York, NY, USA, 2010. [Google Scholar]
- Yamaguchi, M. Biomedical Osteoporosis Treatment; Nova Science Publishers, Inc.: New York, NY, USA, 2013. [Google Scholar]
- Raggatt, L.J.; Partridge, N.C. Cellular and Molecular Mechanisms of Bone Remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef]
- Xu, J.; Yu, L.; Liu, F.; Wan, L.; Deng, Z. The effect of cytokines on osteoblasts and osteoclasts in bone remodeling in osteoporosis: A review. Front. Immunol. 2023, 14, 1222129. [Google Scholar] [CrossRef]
- Chin, K.-Y.; Ng, B.N.; Rostam, M.K.I.; Fadzil, N.F.D.M.; Raman, V.; Yunus, F.M.; Hashim, S.A.S.; Ekeuku, S.O. A Mini Review on Osteoporosis: From biology to pharmacological management of bone loss. J. Clin. Med. 2022, 11, 6434. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Hachiya, S.; Hiratuka, S.; Suzuki, T. Effect of marine algae extract on bone calcification in the femoral-metaphyseal tissues of rats: Anabolic effect of Sargassum horneri. J. Health Sci. 2001, 47, 533–538. [Google Scholar] [CrossRef][Green Version]
- Park, M.H.; Kim, S.; Cheon, J.; Lee, J.; Kim, B.K.; Lee, S.H.; Kong, C.; Kim, Y.Y.; Kim, M. Effects of Scytosiphon lomentaria on osteoblastic proliferation and differentiation of MC3T3- E1 cells. Nutr. Res. Pract. 2016, 10, 148–153. [Google Scholar] [CrossRef]
- Koshi, R.; Nakai, K.; Tanaka, H.; Kato, K.; Charlestoncoad, T.; Matsuike, R.; Nakasugi, T. Original an extract of Eisenia bicyclis stimulates mineralized nodule formation by osteoblasts. J. Hard Tissue Biol. 2019, 28, 359–364. [Google Scholar] [CrossRef]
- Kim, M.; Kang, J.H.; Oh, G.H.; Park, M.H. Ishige sinicola extract stimulates osteoblast proliferation and differentiation via the bone morphogenetic protein 2/runt-related gene 2 signalling pathway. Z. Für Naturforschung C 2019, 74, 167–174. [Google Scholar] [CrossRef]
- Minetti, M.; Bernardini, G.; Biazzo, M.; Gutierrez, G.; Geminiani, M.; Petrucci, T.; Santucci, A. Padina pavonica extract promotes in vitro differentiation and functionality of human primary osteoblasts. Mar. Drugs 2019, 17, 473. [Google Scholar] [CrossRef]
- Walsh, P.J.; McGrath, S.; McKelvey, S.; Ford, L.; Sheldrake, G.; Clarke, S.A. The osteogenic potential of brown seaweed extracts. Mar. Drugs 2019, 17, 141. [Google Scholar] [CrossRef]
- Gyu, D. Floridoside from Laurencia undulata promotes osteogenic differentiation in murine bone marrow mesenchymal cells. J. Funct. Foods 2015, 19, 505–511. [Google Scholar] [CrossRef]
- Saleng, S.; Hendra, F.N.; Ruslin, M.; Forouzanfar, T.; Helder, M.N. The osteogenic potential of seaweed: A systematic review and meta-analysis. Algal Res. 2024, 79, 103445. [Google Scholar] [CrossRef]
- Uchiyama, S.; Yamaguchi, M. Preventive effect of marine alga Sargassum horneri extract on bone loss in streptozotocin-diabetic rats in vivo. J. Health Sci. 2003, 49, 149–155. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Matsumoto, T. Marine algae Sargassum horneri bioactive factor stimulates osteoblastogenesis and suppresses osteoclastogenesis in vitro. OA Biotechnol. 2012, 1, 3. [Google Scholar] [CrossRef]
- Uchiyama, S.; Yamaguchi, M. Stimulatory effect of Sargassum horneri extract on bone formation in rat femoral-diaphyseal and -metaphyseal tissues in vitro. J. Health Sci. 2002, 48, 148–153. [Google Scholar] [CrossRef][Green Version]
- Uchiyama, S.; Yamaguchi, M. Inhibitory effect of marine alga Sargassum horneri extract on bone resorption in tissue culture in vitro. J. Health Sci. 2002, 48, 154–160. [Google Scholar] [CrossRef][Green Version]
- Uchiyama, S.; Hashizume, M.; Hokari, Y.; Nakagawa, T.; Igarashi, A.; Yamaguchi, M. Characterization of active component in marine alga Sargassum horneri extract in stimulating bone calcification in vitro. J. Health Sci. 2004, 50, 634–639. [Google Scholar] [CrossRef]
- Nanes, M.S. Tumor necrosis factor-α: Molecular and cellular mechanisms in skeletal pathology. Gene 2003, 321, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, A.; Strait, K.; Zhang, H.; Nanes, M.S.; Weitzmann, M.N. Endogenous TNFα lowers maximum peak bone mass and inhibits osteoblastic smad activation through NF-κB. J. Bone Miner. Res. 2007, 22, 646–655. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Weitzmann, M.N. Vitamin K2 stimulates osteoblastogenesis and suppresses osteoclastogenesis by suppressing NF-κB activation. Int. J. Mol. Med. 2011, 27, 3–14. [Google Scholar] [CrossRef]
- Uchiyama, S.; Yamaguchi, M. Anabolic Effect of Marine Alga Sargassum horneri Extract on Bone Components in the Femoral-diaphyseal and -metaphyseal Tissues of Young and Aged Rats in Vivo. J. Health Sci. 2002, 48, 325–330. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, W.; Lin, X.; Li, F. AI algorithms for accurate prediction of osteoporotic fractures in patients with diabetes: An up-to-date review. J. Orthop. Surg. Res. 2023, 18, 956. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, G.C.; Coelho, J.B.C.; Silva, J.G.O.; de Sant’ana, A.C.C.; de Sá, C.A.C.; Moreno, J.M.; Reis, L.M.; Guimarães, C.S.d.O. Obesity, diabetes and risk of bone fragility: How BMAT behavior is affected by metabolic disturbances and its influence on bone health. Osteoporos. Int. 2023, 35, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Jeddi, S. Streptozotocin as a tool for induction of rat models of diabetes: A practical guide. EXCLI J. 2023, 22, 274–294. [Google Scholar] [CrossRef]
- Matsumoto, T.; Hokari, Y.; Hashizume, M.; Yamaguchi, M. Effect of Sargassum horneri extract on circulating bone metabolic markers: Supplemental intake has an effect in healthy humans. J. Health Sci. 2008, 54, 50–55. [Google Scholar] [CrossRef]
- Gomez, B.; Ardakani, S.; Ju, J.; Jenkins, D.; Cerelli, M.J.; Daniloff, G.Y.; Kung, V.T. Monoclonal antibody assay for measuring bone-specific alkaline phosphatase activity in serum. Clin. Chem. 1995, 41, 1560–1566. [Google Scholar] [CrossRef] [PubMed]
- Koyama, N.; Ohara, K.; Yokota, H.; Kurome, T.; Katayama, M.; Hino, F.; Kato, I.; Akai, T. A one step sandwich enzyme immunoassay for γ-carboxylated osteocalcin using monoclonal antibodies. J. Immunol. Methods 1991, 139, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Halleen, J.M.; Alatalo, S.L.; Suominen, H.; Cheng, S.; Janckila, A.J.; Väänänen, H.K. Tartrate-resistant acid phosphatase 5b: A novel serum marker of bone resorption. J. Bone Miner. Res. 2000, 15, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.D.; Herrich, M.V.; Singer, F.R.; Eyre, D. Evidence that serum NTx (collagen-type I N-telopeptides) can act as an immunochemical marker of bone resorption. Clin. Chem. 1997, 43, 2058–2063. [Google Scholar] [CrossRef]
- Minguell, J.J.; Erices, A.; Conget, P. Mesenchymal stem cells. Exp. Biol. Med. 2001, 226, 507–520. [Google Scholar] [CrossRef]
- Muruganandan, S.; Roman, A.A.; Sinal, C.J. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: Cross talk with the osteoblastogenic program. Cell. Mol. Life Sci. 2009, 66, 236–253. [Google Scholar] [CrossRef] [PubMed]
- Gharibi, B.; Abraham, A.A.; Ham, J.; Evans, B.A.J. Adenosine receptor subtype expression and activation influence the differentiation of mesenchymal stem cells to osteoblasts and adipocytes. J. Bone Miner. Res. 2011, 26, 2112–2124. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Matsumoto, T. Marine algae Sargassum horneri bioactive factor suppresses adipogenesis in mouse bone marrow culture in vitro. OA Biotechnol 2014, 3, 7. [Google Scholar]
- Lee, H.-G.; Kim, H.-S.; Je, J.-G.; Hwang, J.; Sanjeewa, K.K.A.; Lee, D.-S.; Song, K.-M.; Choi, Y.-S.; Kang, M.-C.; Jeon, Y.-J. Lipid inhibitory effect of (−)-loliolide isolated from Sargassum horneri in 3T3-L1 adipocytes: Inhibitory mechanism of adipose-specific proteins. Mar. Drugs 2021, 19, 96. [Google Scholar] [CrossRef] [PubMed]
- Proietto, J. Obesity and Bone. F1000Research 2020, 9, 1111. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Curtis, M.E.; Fears, L.S.; Nahashon, S.N.; Fentress, H.M. Molecular mechanisms of obesity-induced osteoporosis and muscle atrophy. Front. Physiol. 2016, 7, 439. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Jo, H.G.; Ramakrishna, C.; Lee, S.-J.; Lee, D.-S.; Cheong, S.H. Anti-inflammatory and antioxidant activities of Sargassum horneri extract in RAW264.7 macrophages. Phys. Act. Nutr. 2021, 25, 45–53. [Google Scholar] [CrossRef]
- Nagahawatta, D.P.; Kim, H.-S.; Jee, Y.-H.; Jayawardena, T.U.; Ahn, G.; Namgung, J.; Yeo, I.-K.; Sanjeewa, K.K.A.; Jeon, Y.-J. Sargachromenol isolated from Sargassum horneri inhibits particulate matter-induced inflammation in macrophages through toll-like receptor-mediated cell signaling pathways. Mar. Drugs 2021, 20, 28. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-N.; Nabende, W.Y.; Jeong, H.; Hahn, D.; Jeong, G.-S. The marine-derived natural product epiloliolide isolated from Sargassum horneri regulates NLRP3 via PKA/CREB, promoting proliferation and anti-inflammatory effects of human periodontal ligament cells. Mar. Drugs 2021, 19, 388. [Google Scholar] [CrossRef]
- Sanjeewa, K.A.; Jayawardena, T.U.; Kim, S.-Y.; Lee, H.G.; Je, J.-G.; Jee, Y.; Jeon, Y.-J. Sargassum horneri (Turner) inhibit urban particulate matter-induced inflammation in MH-S lung macrophages via blocking TLRs mediated NF-κB and MAPK activation. J. Ethnopharmacol. 2020, 249, 112363. [Google Scholar] [CrossRef]
- Herath, K.H.I.N.M.; Cho, J.; Kim, H.J.; Dinh, D.T.T.; Kim, H.-S.; Ahn, G.; Jeon, Y.-J.; Jee, Y. Polyphenol containing Sargassum horneri attenuated Th2 differentiation in splenocytes of ovalbumin-sensitised mice: Involvement of the transcription factors GATA3/STAT5/NLRP3 in Th2 polarization. Pharm. Biol. 2021, 59, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Park, J.-S.; Umanzor, S.; Yarish, C.; Kim, J.K. Effects of extraction methods for a new source of biostimulant from Sargassum horneri on the growth of economically important red algae, Neopyropia yezoensis. Sci. Rep. 2022, 12, 11878. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Hyun, S.H.; Kim, H.; Park, S.; Lee, J.; Lee, I.; Bae, J. The effects of fucoidan-rich polysaccharides extracted from Sargassum horneri on enhancing collagen-related skin barrier function as a potential cosmetic product. J. Cosmet. Dermatol. 2024, 23, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, H.-S.; Jeon, Y.-J.; Jee, Y.; Kim, K.-N.; Lee, K.; Fernando, I.P.S.; Ahn, G. Sargassum horneri (Turner) C. Agardh ethanol extract attenuates fine dust-induced inflammatory responses and impaired skin barrier functions in HaCaT keratinocytes. J. Ethnopharmacol. 2021, 273, 114003. [Google Scholar] [CrossRef]
- Han, E.J.; Kim, H.-S.; Sanjeewa, K.K.A.; Jung, K.; Jee, Y.; Jeon, Y.-J.; Fernando, I.P.S.; Ahn, G. Sargassum horneri as a functional food ameliorated ige/bsa-induced mast cell activation and passive cutaneous anaphylaxis in mice. Mar. Drugs 2020, 18, 594. [Google Scholar] [CrossRef] [PubMed]
- Han, E.J.; Fernando, I.P.S.; Kim, H.-S.; Jeon, Y.-J.; Madusanka, D.M.D.; Dias, M.K.H.M.; Jee, Y.; Ahn, G. Oral administration of Sargassum horneri improves the HDM/DNCB-Induced atopic dermatitis in NC/Nga mice. Nutrients 2020, 12, 2482. [Google Scholar] [CrossRef]
- Herath, K.H.I.N.M.; Kim, H.J.; Jang, J.-H.; Kim, H.-S.; Kim, H.J.; Jeon, Y.-J.; Jee, Y. Mojabanchromanol isolated from Sargassum horneri attenuates particulate matter induced inflammatory responses via suppressing TLR2/4/7-MAPK signaling in MLE-12 cells. Mar. Drugs 2020, 18, 355. [Google Scholar] [CrossRef]
- Wen, Z.-S.; Liu, L.-J.; OuYang, X.-K.; Qu, Y.-L.; Chen, Y.; Ding, G.-F. Protective effect of polysaccharides from Sargassum horneri against oxidative stress in RAW264.7 cells. Int. J. Biol. Macromol. 2014, 68, 98–106. [Google Scholar] [CrossRef]
- Herath, K.H.I.N.M.; Cho, J.; Kim, A.; Kim, H.-S.; Han, E.J.; Kim, H.J.; Kim, M.S.; Ahn, G.; Jeon, Y.-J.; Jee, Y. Differential modulation of immune response and cytokine profiles of Sargassum horneri ethanol extract in murine spleen with or without Concanavalin A stimulation. Biomed. Pharmacother. 2019, 110, 930–942. [Google Scholar] [CrossRef]
- Roodman, G.D. Mechanisms of Bone Metastasis. N. Engl. J. Med. 2004, 350, 1655–1664. [Google Scholar] [CrossRef]
- Pang, L.; Gan, C.; Xu, J.; Jia, Y.; Chai, J.; Huang, R.; Li, A.; Ge, H.; Yu, S.; Cheng, H. Bone metastasis of breast cancer: Molecular mechanisms and therapeutic strategies. Cancers 2022, 14, 5727. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Chang JC: Cancer stem cells: Role in tumor growth, recurrence, metastasis, and treatment resistance. Medicine 2016, 95, S20–S25. [CrossRef] [PubMed]
- Boyce, B.F.; Yoneda, T.; Guise, T. Factors regulating the growth of metastasis cancer in bone. Endocr. Relat. Cancer 1999, 6, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Mundy, G. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2002, 2, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Yeeravalli, R.; Das, A. Molecular mediators of breast cancer metastasis. Hematol./Oncol. Stem Cell Ther. 2021, 14, 275–289. [Google Scholar]
- Yoneda, T.; Williams, P.J.; Hiraga, T.; Niewolna, M.; Nishimura, R. A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J. Bone Miner Res. 2001, 16, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Matsumoto, T. Marine algae Sargassum horneri bioactive factor suppresses proliferation and stimulates apoptotic cell death in human breast cancer MDA-MB-231 cells in vitro. Integr. Mol. Med. 2015, 2, 135–138. [Google Scholar] [CrossRef]
- Shao, P.; Liu, J.; Chen, X.; Fang, Z.; Sun, P. Structural features and antitumor activity of a purified polysaccharide extracted from Sargassum horneri. Int. J. Biol. Macromol. 2015, 73, 1240130. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Chen, X.; Suri, P. Chemical characterization, antioxidant and antitumor activity o sulfated polysaccharide from Sargassum horneri. Carbohydr. Polym. 2014, 105, 260–269. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, M. The Marine Alga Sargassum horneri Is a Functional Food with High Bioactivity. Nutraceuticals 2024, 4, 181-189. https://doi.org/10.3390/nutraceuticals4020012
Yamaguchi M. The Marine Alga Sargassum horneri Is a Functional Food with High Bioactivity. Nutraceuticals. 2024; 4(2):181-189. https://doi.org/10.3390/nutraceuticals4020012
Chicago/Turabian StyleYamaguchi, Masayoshi. 2024. "The Marine Alga Sargassum horneri Is a Functional Food with High Bioactivity" Nutraceuticals 4, no. 2: 181-189. https://doi.org/10.3390/nutraceuticals4020012
APA StyleYamaguchi, M. (2024). The Marine Alga Sargassum horneri Is a Functional Food with High Bioactivity. Nutraceuticals, 4(2), 181-189. https://doi.org/10.3390/nutraceuticals4020012