Exploring the In Vitro Protective Effects of Green-Lipped Mussel (GLM) Oil Extract against Biomarkers of Glucose Metabolism and Inflammation in Chondrocyte Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Mussel Crude Oils Treatment and Cell Viability
2.2. Cellular Proliferation Assay
2.3. Cellular ROS Assay
2.4. Glucose Uptake Assay
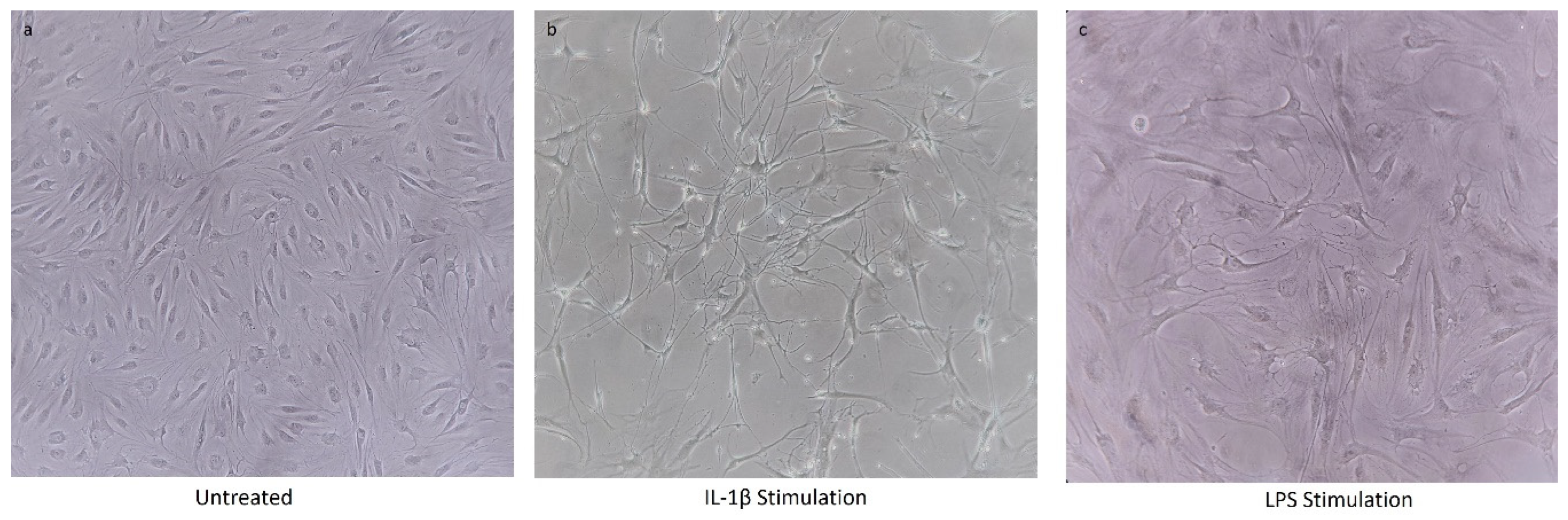
2.5. Chondrocyte Cell Line Stimulation
2.6. Enzyme-Linked Immunosorbent Assay (ELISA) of Proinflammatory Cytokines
2.7. Statistical Analysis
3. Results
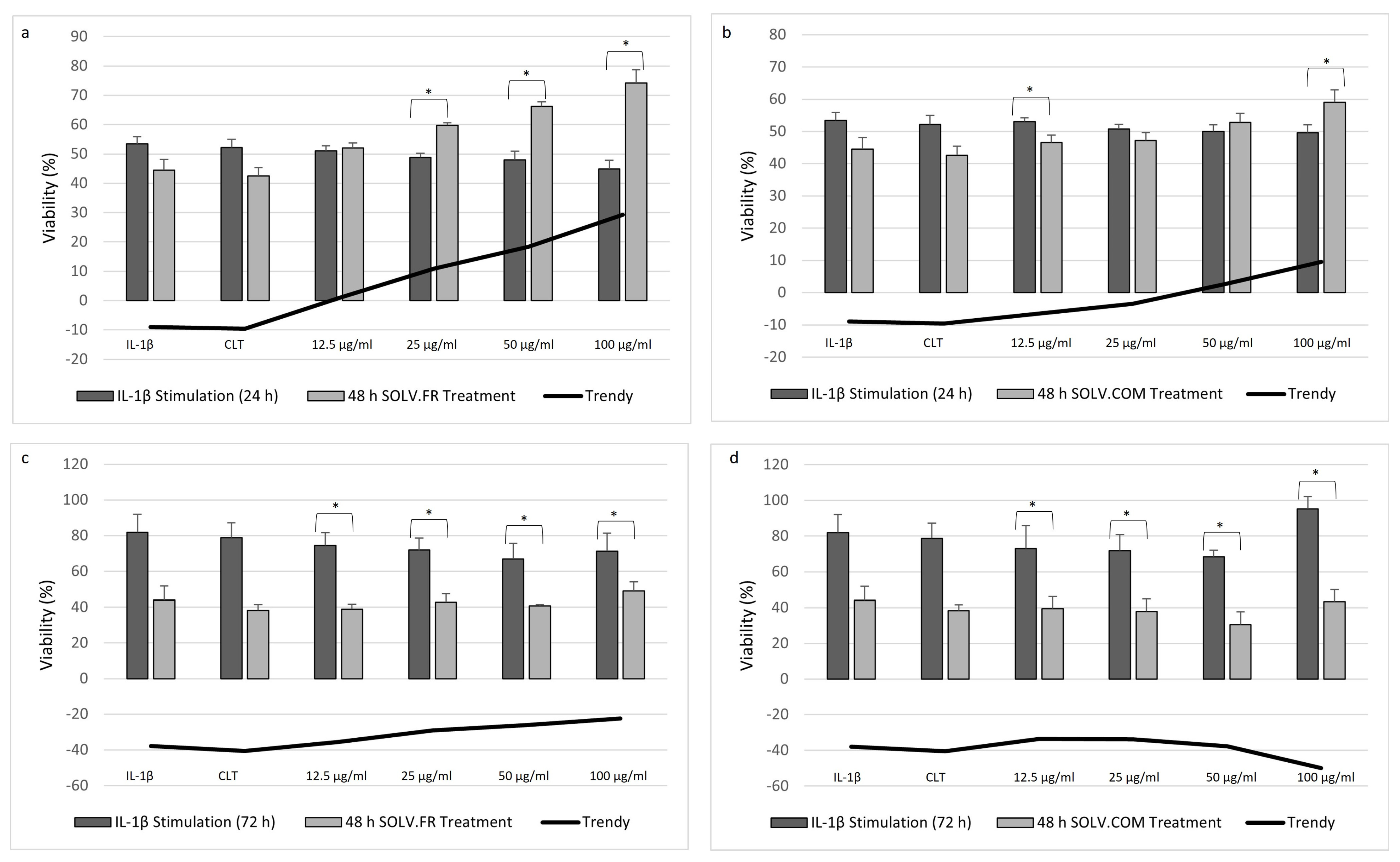
3.1. Cellular Proliferation Assay
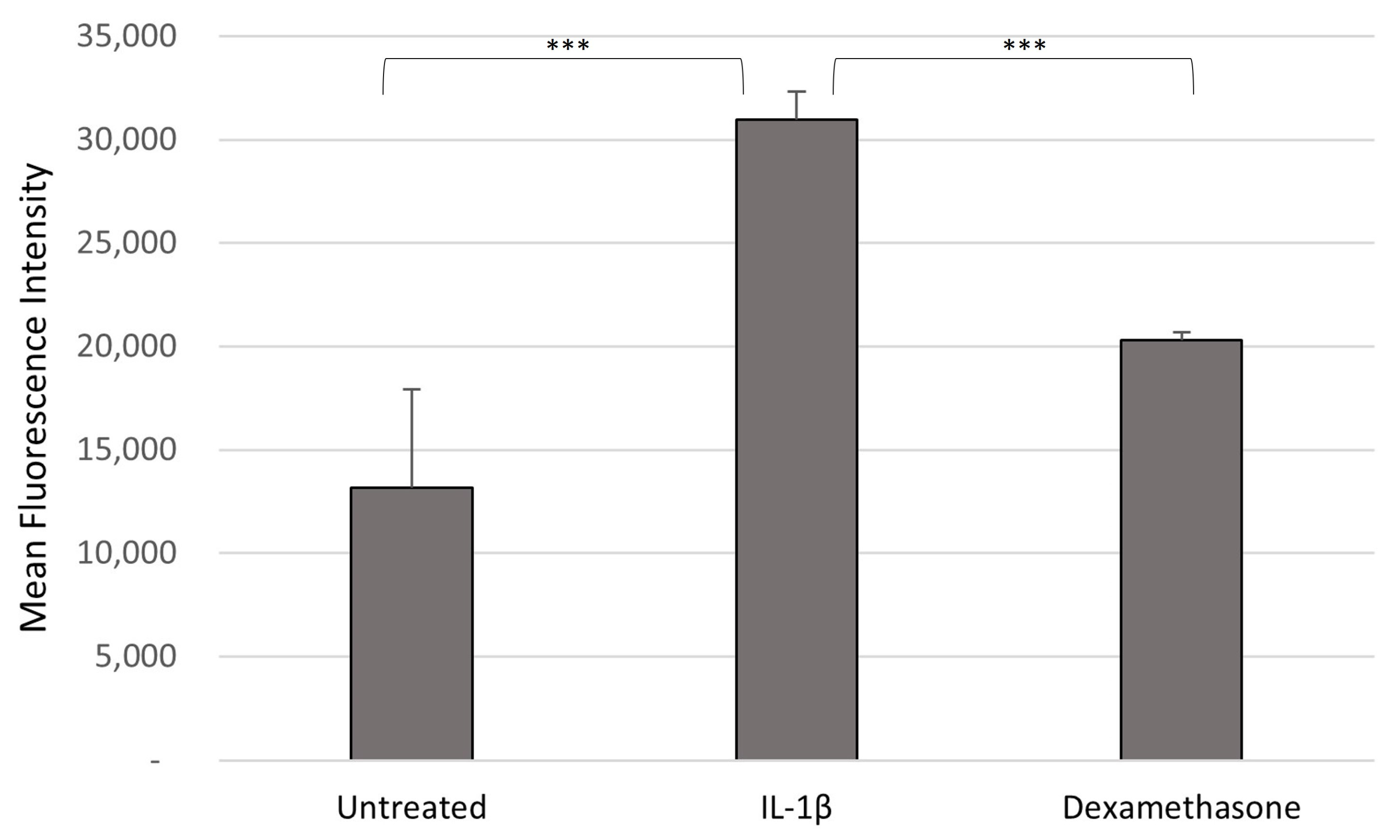
3.2. ROS Production Following Sample Treatment
3.3. Cell Glucose Levels Recovered after Sample Treatment
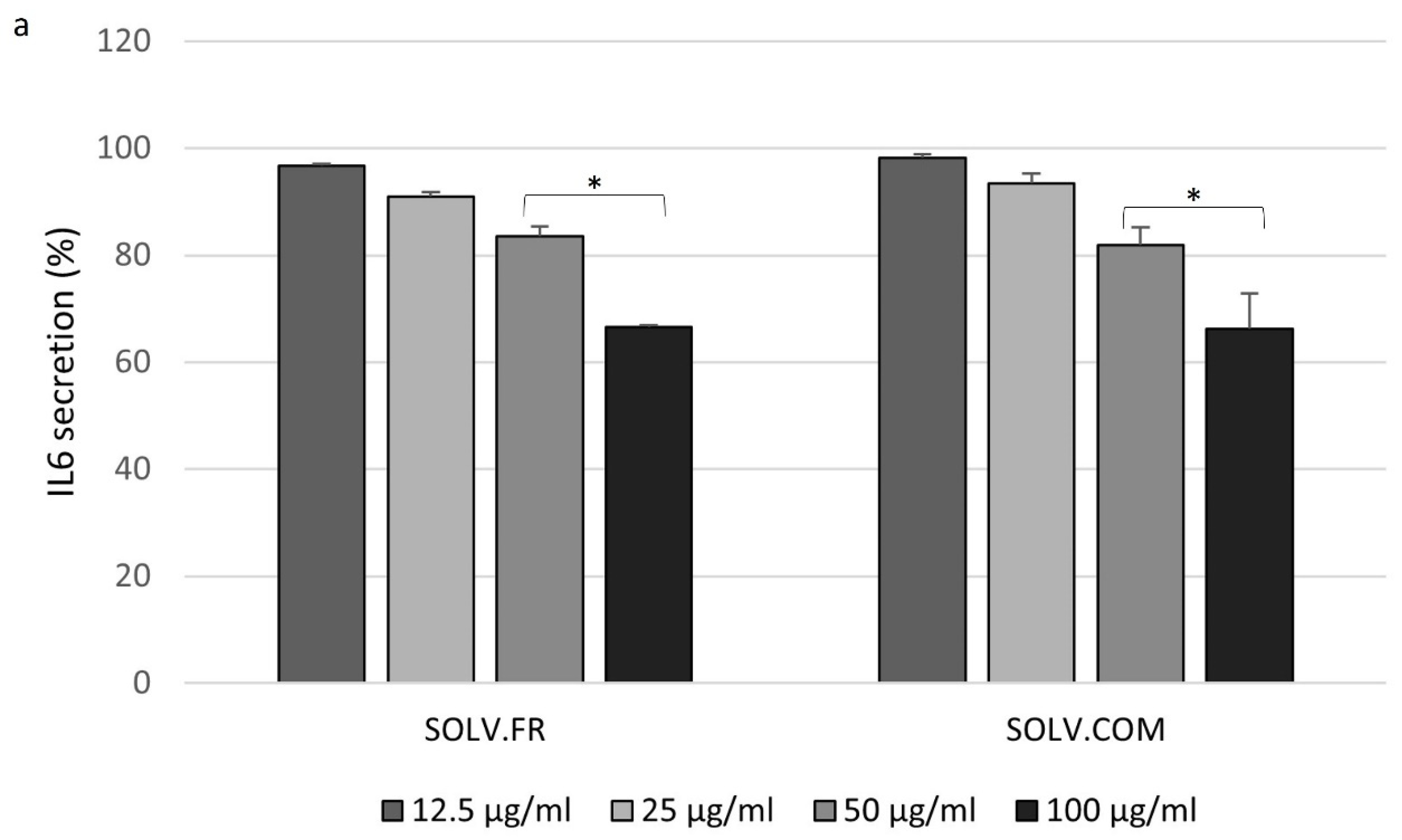
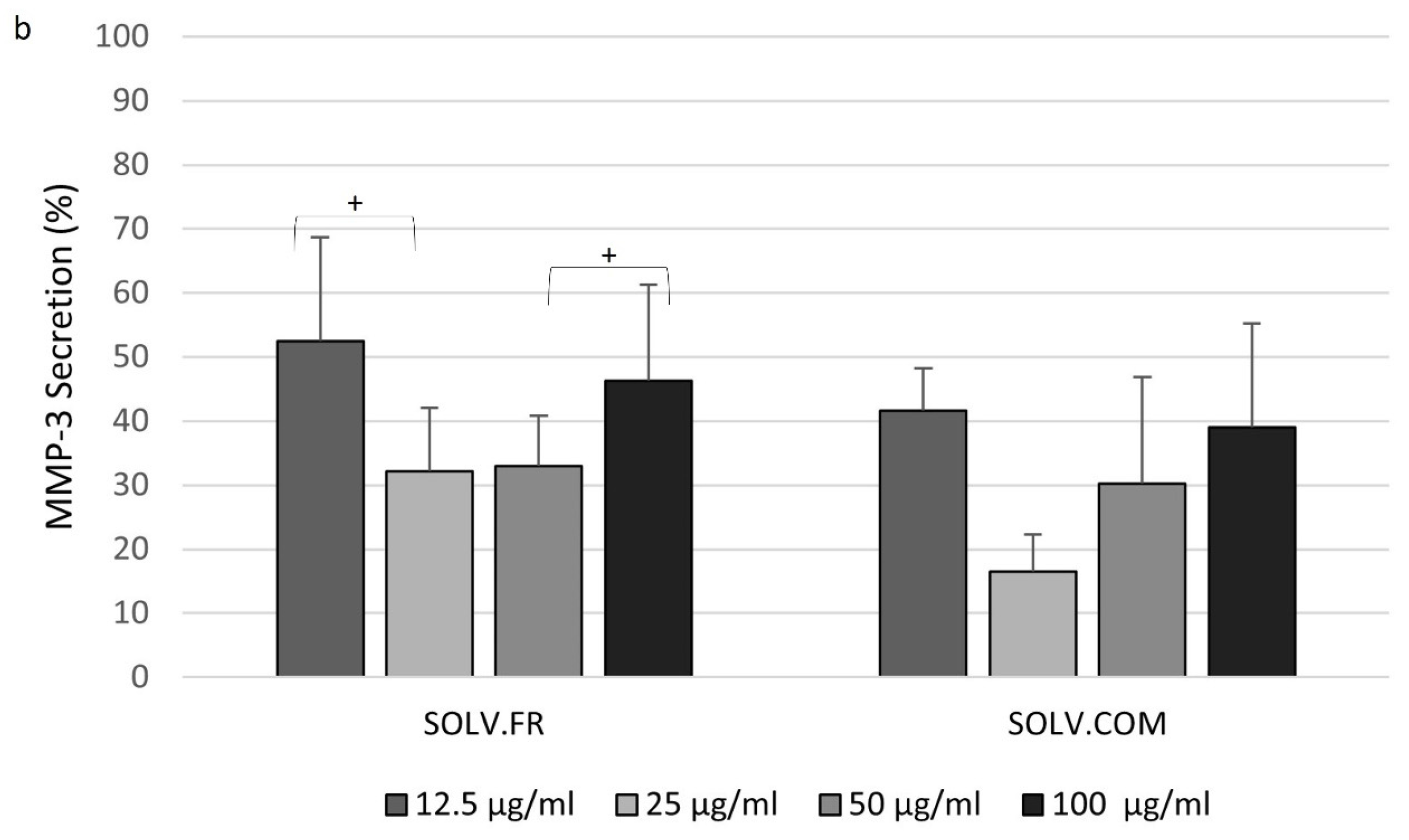
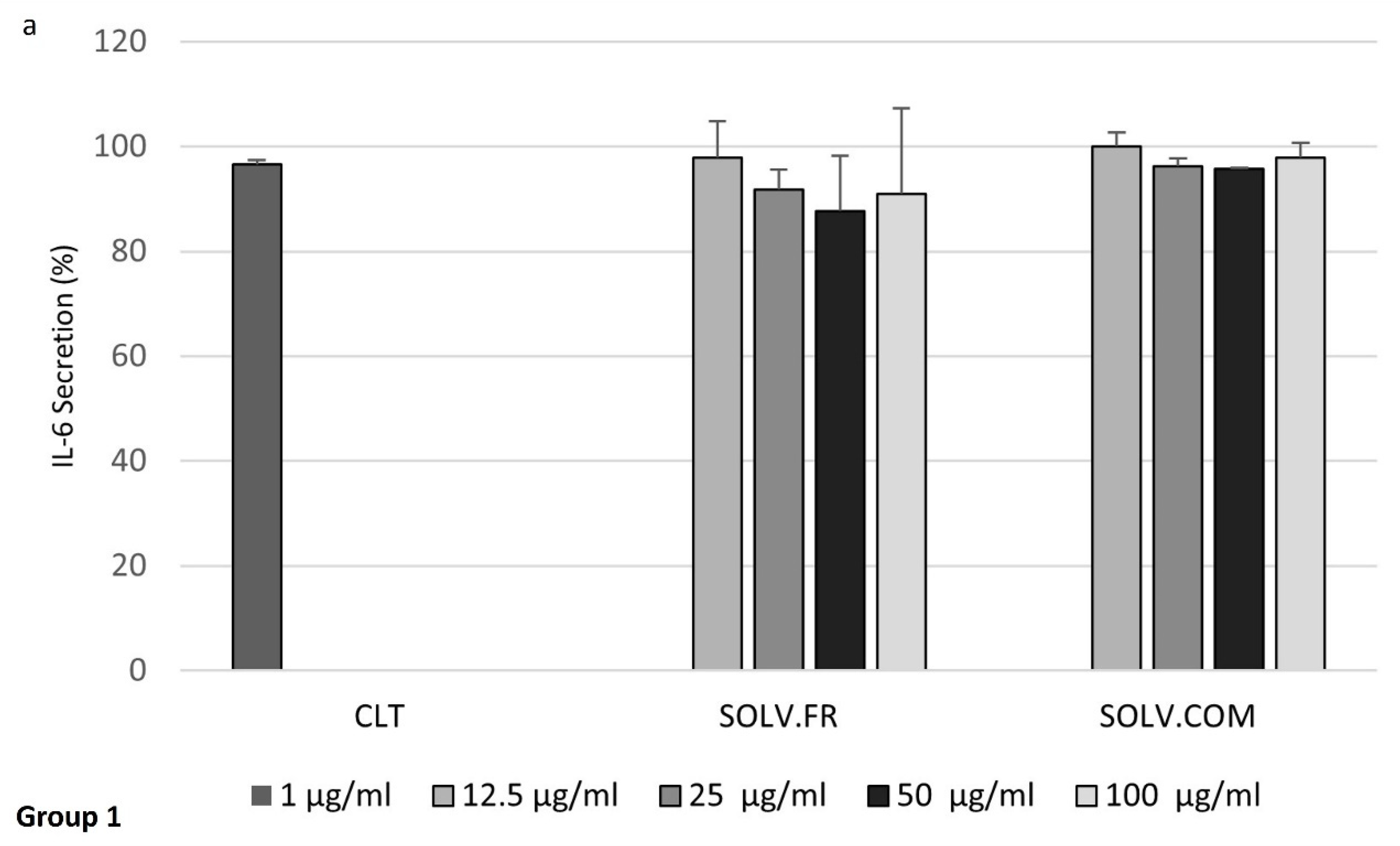
3.4. Modulation of IL-6 and MMP-3 Secretion in Chondrocyte Cells Following Treatment with Mussel Oil Extracts
4. Discussion
4.1. Chondrocytes Cell Viability after IL-1β Stimulation
4.2. Green-Lipped Mussel Extract in the Control in ROS Generation
4.3. Increased Glucose Transport in Response to Green-Lipped Mussel Extract in Chondrocytes Cells
4.4. Exploring the Interplay of Inflammatory Cytokines and Green-Lipped Mussel Oil Extracts in Stimulated Chondrocyte Cells
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2DG | 2-Deoxy-D-glucose |
| 2DG6P | 2-deoxy-D-glucose-6-phosphate |
| ADAMTS | A disintegrin and metalloproteinase with thrombospondin motifs |
| ALA | Alpha-linolenic acid |
| CO2 | Carbonate dioxide |
| COX | Cyclooxygenase |
| DCFH-DA | Diacetyldichlorofluorescein |
| DHA | Docosahexaenoic acid |
| ECM | Extracellular matrix |
| EPA | Eicosapentaenoic acid |
| GLM | Green-lipped mussel |
| IL | Interleukin |
| LPS | Lipopolysaccharides |
| MAPK | Mitogen-activated protein kinases |
| MMPs | Matrix metalloproteinases |
| MTBE: Me | Methyl tert-butyl ether: methanol |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide |
| MUFA | Monounsaturated fatty acids |
| n-3 | Omega-3 |
| NF-κB | Nuclear factor kappa B |
| OA | Osteoarthritis |
| PBS | Phosphate-buffered saline |
| PUFAs | Polyunsaturated fatty acids |
| ROS | Reactive oxygen species |
| TNF-α | Tumor necrosis factor-alpha |
References
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic signaling pathways and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Fontanella, C.G.; Belluzzi, E.; Pozzuoli, A.; Scioni, M.; Olivotto, E.; Reale, D.; Ruggieri, P.; De Caro, R.; Ramonda, R.; Carniel, E.L. Exploring Anatomo-Morphometric Characteristics of Infrapatellar, Suprapatellar Fat Pad, and Knee Ligaments in Osteoarthritis Compared to Post-Traumatic Lesions. Biomedicines 2022, 10, 1369. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, Z.; Alexander, P.G.; Ocasio-Nieves, B.D.; Yocum, L.; Lin, H.; Tuan, R.S. Pathogenesis of osteoarthritis: Risk factors, regulatory pathways in chondrocytes, and experimental models. Biology 2020, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Ege, F. Pathogenesis, Pathology and Genetics of Osteoarthritis. In Rheum. Arthritis; IntechOpen: London, UK, 2021. [Google Scholar]
- Man, G.; Mologhianu, G. Osteoarthritis pathogenesis–a complex process that involves the entire joint. J. Med. Life 2014, 7, 37. [Google Scholar] [PubMed]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.-P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Defois, A.; Bon, N.; Charpentier, A.; Georget, M.; Gaigeard, N.; Blanchard, F.; Hamel, A.; Waast, D.; Armengaud, J.; Renoult, O. Osteoarthritic chondrocytes undergo a glycolysis-related metabolic switch upon exposure to IL-1b or TNF. Cell Commun. Signal. 2023, 21, 137. [Google Scholar] [CrossRef]
- Shikhman, A.R.; Brinson, D.C.; Valbracht, J.; Lotz, M.K. Cytokine regulation of facilitated glucose transport in human articular chondrocytes. J. Immunol. 2001, 167, 7001–7008. [Google Scholar] [CrossRef]
- Wu, X.; Liyanage, C.; Plan, M.; Stark, T.; McCubbin, T.; Barrero, R.; Batra, J.; Crawford, R.; Xiao, Y.; Prasadam, I. Dysregulated energy metabolism impairs chondrocyte function in osteoarthritis. Osteoarthr. Cartil. 2023, 31, 613–626. [Google Scholar] [CrossRef]
- Zhou, J.; Fang, C.; Rong, C.; Luo, T.; Liu, J.; Zhang, K. Reactive oxygen species-sensitive materials: A promising strategy for regulating inflammation and favoring tissue regeneration. Smart Mater. Med. 2023, 4, 427–446. [Google Scholar] [CrossRef]
- Zahan, O.-M.; Serban, O.; Gherman, C.; Fodor, D. The evaluation of oxidative stress in osteoarthritis. Med. Pharm. Rep. 2020, 93, 12. [Google Scholar] [CrossRef]
- Wood, M.J.; Miller, R.E.; Malfait, A.-M. The genesis of pain in osteoarthritis: Inflammation as a mediator of osteoarthritis pain. Clin. Geriatr. Med. 2022, 38, 221–238. [Google Scholar] [CrossRef]
- Hunter, D.J.; McDougall, J.J.; Keefe, F.J. The symptoms of osteoarthritis and the genesis of pain. Rheum. Dis. Clin. N. Am. 2008, 34, 623–643. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Yang, X.; Li, J. Research progress on the mechanism of interleukin-1β on epiphyseal plate chondrocytes. Eur. J. Med. Res. 2022, 27, 313. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.; Yang, Y.; Wang, H.; Guo, X.; Wu, Z.; Jin, Q. Impact of prevention strategies on quality of life in patients with osteoarthritis: A systematic review and meta-analysis of randomized controlled trials. World Neurosurg. 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Bowden, J.L.; Hunter, D.J.; Deveza, L.A.; Duong, V.; Dziedzic, K.S.; Allen, K.D.; Chan, P.-K.; Eyles, J.P. Core and adjunctive interventions for osteoarthritis: Efficacy and models for implementation. Nat. Rev. Rheumatol. 2020, 16, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Orlowsky, E.W.; Kraus, V.B. The role of innate immunity in osteoarthritis: When our first line of defense goes on the offensive. J. Rheumatol. 2015, 42, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Innes, A.Q.; Burns, D.S.; Deniszczyc, D.; Selfe, J.; MacConville, S.; Deighton, K.; Kelly, B.M. A scalable 12-week exercise and education programme reduces symptoms and improves function and wellbeing in people with hip and knee osteoarthritis. Front. Rehabil. Sci. 2023, 4, 1147938. [Google Scholar] [CrossRef] [PubMed]
- Dantas, L.O.; de Fátima Salvini, T.; McAlindon, T.E. Knee osteoarthritis: Key treatments and implications for physical therapy. Braz. J. Phys. Ther. 2021, 25, 135–146. [Google Scholar] [CrossRef]
- Valsamidou, E.; Amerikanou, C.; Tzavara, C.; Skarpas, G.; Mariolis-Sapsakos, T.D.; Zoumpoulakis, P.; Kaliora, A.C. A standardized nutraceutical supplement contributes to pain relief, improves quality of life and regulates inflammation in knee osteoarthritis patients; A randomized clinical trial. Heliyon 2023, 9, e20143. [Google Scholar] [CrossRef]
- Gahivad, M.D.P.; Gahivad, M.R.P.; Deo, S.D.; Daphal, G.T.; Borse, M.H.D.; Ahmed, M.N.; Mahajan, M.B. The trends of herbal nutraceuticals used in chronic disease. Int. J. Prog. Res. Eng. Manag. Sci. (IJPREMS) 2023, 11, 68–80. [Google Scholar]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef]
- Lunn, J.; Theobald, H. The health effects of dietary unsaturated fatty acids. Nutr. Bull. 2006, 31, 178–224. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Kim, S.-K. Marine invertebrate natural products for anti-inflammatory and chronic diseases. Evid.-Based Complement. Altern. Med. 2013, 2013, 572859. [Google Scholar] [CrossRef] [PubMed]
- Kostoglou-Athanassiou, I.; Athanassiou, L.; Athanassiou, P. The effect of omega-3 fatty acids on rheumatoid arthritis. Mediterr. J. Rheumatol. 2020, 31, 190. [Google Scholar] [CrossRef] [PubMed]
- Jhun, J.; Na, H.S.; Cho, K.-H.; Kim, J.; Moon, Y.-M.; Lee, S.Y.; Lee, J.S.; Lee, A.R.; Kim, S.J.; Cho, M.-L. A green-lipped mussel reduces pain behavior and chondrocyte inflammation and attenuated experimental osteoarthritis progression. PLoS ONE 2021, 16, e0259130. [Google Scholar] [CrossRef] [PubMed]
- Knott, L.; Avery, N.; Hollander, A.; Tarlton, J. Regulation of osteoarthritis by omega-3 (n-3) polyunsaturated fatty acids in a naturally occurring model of disease. Osteoarthr. Cartil. 2011, 19, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Feng, X.; Zhang, H.; Wei, Y.; Yang, Y.; Tian, Y.; Bai, L. Maresin-1 suppresses IL-1β-induced MMP-13 secretion by activating the PI3K/AKT pathway and inhibiting the NF-κB pathway in synovioblasts of an osteoarthritis rat model with treadmill exercise. Connect. Tissue Res. 2021, 62, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhou, W.; Zhong, Z.; Yu, H.; Zhang, P.; Shen, H. Docosahexaenoic acid inhibits bone remodeling and vessel formation in the osteochondral unit in a rat model. Biomed. Pharmacother. 2019, 114, 108811. [Google Scholar] [CrossRef]
- Zainal, Z.; Longman, A.J.; Hurst, S.; Duggan, K.; Caterson, B.; Hughes, C.E.; Harwood, J.L. Relative efficacies of omega-3 polyunsaturated fatty acids in reducing expression of key proteins in a model system for studying osteoarthritis. Osteoarthr. Cartil. 2009, 17, 896–905. [Google Scholar] [CrossRef]
- Loef, M.; Schoones, J.W.; Kloppenburg, M.; Ioan-Facsinay, A. Fatty acids and osteoarthritis: Different types, different effects. Jt. Bone Spine 2019, 86, 451–458. [Google Scholar] [CrossRef]
- Cho, S.H.; Jung, Y.B.; Seong, S.C.; Park, H.B.; Byun, K.Y.; Lee, D.C.; Song, E.K.; Son, J.H. Clinical efficacy and safety of Lyprinol, a patented extract from New Zealand green-lipped mussel (Perna canaliculus) in patients with osteoarthritis of the hip and knee: A multicenter 2-month clinical trial. Eur. Ann. Allergy Clin. Immunol. 2003, 35, 212–216. [Google Scholar]
- Coulson, S.; Vecchio, P.; Gramotnev, H.; Vitetta, L. Green-lipped mussel (Perna canaliculus) extract efficacy in knee osteoarthritis and improvement in gastrointestinal dysfunction: A pilot study. Inflammopharmacology 2012, 20, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Coulson, S.; Butt, H.; Vecchio, P.; Gramotnev, H.; Vitetta, L. Green-lipped mussel extract (Perna canaliculus) and glucosamine sulphate in patients with knee osteoarthritis: Therapeutic efficacy and effects on gastrointestinal microbiota profiles. Inflammopharmacology 2013, 21, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Coulson, S.; Palacios, T.; Vitetta, L. Perna canaliculus (Green-Lipped Mussel): Bioactive components and therapeutic evaluation for chronic health conditions. Prog. Drug Res. 2015, 70, 91–132. [Google Scholar] [PubMed]
- Lau, C.; Chiu, P.; Chu, E.; Cheng, I.; Tang, W.; Man, R.; Halpern, G. Treatment of knee osteoarthritis with Lyprinol®, lipid extract of the green-lipped mussel—A double-blind placebo-controlled study. Prog. Nutr. 2004, 6, 17–31. [Google Scholar]
- Mickleborough, T.D.; Sinex, J.A.; Platt, D.; Chapman, R.F.; Hirt, M. The effects PCSO-524®, a patented marine oil lipid and omega-3 PUFA blend derived from the New Zealand green lipped mussel (Perna canaliculus), on indirect markers of muscle damage and inflammation after muscle damaging exercise in untrained men: A randomized, placebo controlled trial. J. Int. Soc. Sports Nutr. 2015, 12, 10. [Google Scholar]
- Stebbings, S.; Gray, A.; Schneiders, A.G.; Sansom, A. A randomized double-blind placebo-controlled trial to investigate the effectiveness and safety of a novel green-lipped mussel extract-BioLex®-for managing pain in moderate to severe osteoarthritis of the hip and knee. BMC Complement. Altern. Med. 2017, 17, 416. [Google Scholar] [CrossRef]
- Zawadzki, M.; Janosch, C.; Szechinski, J. Perna canaliculus lipid complex PCSO-524™ demonstrated pain relief for osteoarthritis patients benchmarked against fish oil, a randomized trial, without placebo control. Mar. Drugs 2013, 11, 1920–1935. [Google Scholar] [CrossRef]
- Cordingley, D.M.; Cornish, S.M. Omega-3 fatty acids for the management of osteoarthritis: A narrative review. Nutrients 2022, 14, 3362. [Google Scholar] [CrossRef]
- Salem, M.; Bernach, M.; Bajdzienko, K.; Giavalisco, P. A simple fractionated extraction method for the comprehensive analysis of metabolites, lipids, and proteins from a single sample. JoVE (J. Vis. Exp.) 2017, e55802. [Google Scholar]
- Lorenz, W.; Buhrmann, C.; Mobasheri, A.; Lueders, C.; Shakibaei, M. Bacterial lipopolysaccharides form procollagen-endotoxin complexes that trigger cartilage inflammation and degeneration: Implications for the development of rheumatoid arthritis. Arthritis Res. Ther. 2013, 15, R111. [Google Scholar] [CrossRef]
- Zhuang, J.C.; Wogan, G.N. Growth and viability of macrophages continuously stimulated to produce nitric oxide. Proc. Natl. Acad. Sci. USA 1997, 94, 11875–11880. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, A.; Haqqi, T.M. Immunopathogenesis of osteoarthritis. Clin. Immunol. 2013, 146, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Ren, Y.; Luo, X.; Mirando, A.J.; Long, J.T.; Leinroth, A.; Ji, R.-R.; Hilton, M.J. Interleukin-6 signaling mediates cartilage degradation and pain in posttraumatic osteoarthritis in a sex-specific manner. Sci. Signal. 2022, 15, eabn7082. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, A.; Ansari, M.Y.; Haqqi, T.M. Harpagoside suppresses IL-6 expression in primary human osteoarthritis chondrocytes. J. Orthop. Res. 2017, 35, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Wiegertjes, R.; van de Loo, F.A.; Blaney Davidson, E.N. A roadmap to target interleukin-6 in osteoarthritis. Rheumatology 2020, 59, 2681–2694. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, G.; Li, X.; Qiu, X.; Ouyang, J.; Dai, J.; Min, S. Matrix metalloproteinase 3: A promoting and destabilizing factor in the pathogenesis of disease and cell differentiation. Front. Physiol. 2021, 12, 663978. [Google Scholar] [CrossRef]
- Lv, M.; Zhou, Y.; Polson, S.W.; Wan, L.Q.; Wang, M.; Han, L.; Wang, L.; Lu, X.L. Identification of chondrocyte genes and signaling pathways in response to acute joint inflammation. Sci. Rep. 2019, 9, 93. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, J.; Liu, J. Baicalin protects human OA chondrocytes against IL-1β-induced apoptosis and ECM degradation by activating autophagy via MiR-766-3p/AIFM1 axis. Drug Des. Dev. Ther. 2020, 14, 2645–2655. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef]
- Sato, K.; Ishida, Y.; Ikuta, N.; Uekaji, Y.; Nakata, D.; Terao, K. Stabilization of unstable functional food ingredients by complexation with cyclodextrin. Oleo Sci. 2013, 13, 123–130. [Google Scholar]
- Chen, J.; Bao, C.; Cho, S.H.; Lee, H.J. Green lipped mussel oil complex suppresses lipopolysaccharide stimulated inflammation via regulating nuclear factor-κB and mitogen activated protein kinases signaling in RAW264. 7 murine macrophages. Food Sci. Biotechnol. 2017, 26, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Clementi, M.E.; Lazzarino, G.; Sampaolese, B.; Brancato, A.; Tringali, G. DHA protects PC12 cells against oxidative stress and apoptotic signals through the activation of the NFE2L2/HO-1 axis. Int. J. Mol. Med. 2019, 43, 2523–2531. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, L.; Recalde, S.; Fernandez-Robredo, P.; Hernandez, M.; Ispizua-Madariaga, N.; Reiter, N.; Albiasu, E.; Bezunartea, J.; Alonso, E.; Layana, A.G. Effect of EPA: DHA formulations on cell viability, proliferation and reactive oxygen species generation under inflamatory and oxidative stress conditions. Investig. Ophthalmol. Vis. Sci. 2015, 56, 16. [Google Scholar]
- Sakai, C.; Ishida, M.; Ohba, H.; Yamashita, H.; Uchida, H.; Yoshizumi, M.; Ishida, T. Fish oil omega-3 polyunsaturated fatty acids attenuate oxidative stress-induced DNA damage in vascular endothelial cells. PLoS ONE 2017, 12, e0187934. [Google Scholar] [CrossRef] [PubMed]
- Sakata, S.; Hayashi, S.; Fujishiro, T.; Kawakita, K.; Kanzaki, N.; Hashimoto, S.; Iwasa, K.; Chinzei, N.; Kihara, S.; Haneda, M. Oxidative stress-induced apoptosis and matrix loss of chondrocytes is inhibited by eicosapentaenoic acid. J. Orthop. Res. 2015, 33, 359–365. [Google Scholar] [CrossRef]
- Schönfeld, P.; Wojtczak, L. Fatty acids decrease mitochondrial generation of reactive oxygen species at the reverse electron transport but increase it at the forward transport. Biochim. Biophys. Acta (BBA)-Bioenerg. 2007, 1767, 1032–1040. [Google Scholar] [CrossRef]
- Heshmati, J.; Morvaridzadeh, M.; Maroufizadeh, S.; Akbari, A.; Yavari, M.; Amirinejad, A.; Maleki-Hajiagha, A.; Sepidarkish, M. Omega-3 fatty acids supplementation and oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019, 149, 104462. [Google Scholar] [CrossRef]
- Rotter Sopasakis, V.; Wickelgren, R.; Sukonina, V.; Brantsing, C.; Svala, E.; Hansson, E.; Enerbäck, S.; Lindahl, A.; Skiöldebrand, E. Elevated glucose levels preserve glucose uptake, hyaluronan production, and low glutamate release following interleukin-1β stimulation of differentiated chondrocytes. Cartilage 2019, 10, 491–503. [Google Scholar] [CrossRef]
- Su, Z.; Zong, Z.; Deng, J.; Huang, J.; Liu, G.; Wei, B.; Cui, L.; Li, G.; Zhong, H.; Lin, S. Lipid metabolism in cartilage development, degeneration, and regeneration. Nutrients 2022, 14, 3984. [Google Scholar] [CrossRef]
- Villalvilla, A.; Gómez, R.; Largo, R.; Herrero-Beaumont, G. Lipid transport and metabolism in healthy and osteoarthritic cartilage. Int. J. Mol. Sci. 2013, 14, 20793–20808. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Vannucci, S.; Bondy, C.; Carter, S.; Innes, J.; Arteaga, M.; Trujillo, E.; Ferraz, I.; Shakibaei, M.; Martín Vasallo, P. Glucose transport and metabolism in chondrocytes: A key to understanding chondrogenesis, skeletal development and cartilage degradation in osteoarthritis. Histol. Histopathol. 2002, 17, 1239–1267. [Google Scholar] [PubMed]
- Cruz, M.M.; Lopes, A.B.; Crisma, A.R.; de Sá, R.C.; Kuwabara, W.M.; Curi, R.; de Andrade, P.B.; Alonso-Vale, M.I. Palmitoleic acid (16: 1n7) increases oxygen consumption, fatty acid oxidation and ATP content in white adipocytes. Lipids Health Dis. 2018, 17, 55. [Google Scholar] [CrossRef] [PubMed]
- Egalini, F.; Guardamagna, O.; Gaggero, G.; Varaldo, E.; Giannone, B.; Beccuti, G.; Benso, A.; Broglio, F. The Effects of Omega 3 and Omega 6 Fatty Acids on Glucose Metabolism: An Updated Review. Nutrients 2023, 15, 2672. [Google Scholar] [CrossRef] [PubMed]
- Jeromson, S.; Mackenzie, I.; Doherty, M.K.; Whitfield, P.D.; Bell, G.; Dick, J.; Shaw, A.; Rao, F.V.; Ashcroft, S.P.; Philp, A. Lipid remodeling and an altered membrane-associated proteome may drive the differential effects of EPA and DHA treatment on skeletal muscle glucose uptake and protein accretion. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E605–E619. [Google Scholar] [CrossRef]
- Kim, N.; Kang, M.S.; Nam, M.; Kim, S.A.; Hwang, G.-S.; Kim, H.S. Eicosapentaenoic acid (EPA) modulates glucose metabolism by targeting AMP-activated protein kinase (AMPK) pathway. Int. J. Mol. Sci. 2019, 20, 4751. [Google Scholar] [CrossRef]
- Mone, P.; Varzideh, F.; Kansakar, U.; Infante, C.; Lombardi, A.; de Donato, A.; Frullone, S.; Santulli, G. Omega-3 fatty acids coordinate glucose and lipid metabolism in diabetic patients. Lipids Health Dis. 2022, 21, 31. [Google Scholar] [CrossRef]
- Pifferi, F.; Jouin, M.; Roux, F.; Perrière, N.; Alessandri, J.; Lavialle, M.; Guesnet, P. Omega-3 fatty acids and brain energy metabolism: Impact on the expression of glucose transporters and glucose transport activity in endothelial cells in culture. Oléagineux Corps Gras Lipides 2007, 14, 235. [Google Scholar] [CrossRef][Green Version]
- Pifferi, F.; Cunnane, S.C.; Guesnet, P. Evidence of the role of Omega-3 polyunsaturated fatty acids in brain glucose metabolism. Nutrients 2020, 12, 1382. [Google Scholar] [CrossRef]
- Huang, Z.; Kraus, V.B. Does lipopolysaccharide-mediated inflammation have a role in OA? Nat. Rev. Rheumatol. 2016, 12, 123–129. [Google Scholar] [CrossRef]
- Haglund, L.; Bernier, S.M.; Önnerfjord, P.; Recklies, A.D. Proteomic analysis of the LPS-induced stress response in rat chondrocytes reveals induction of innate immune response components in articular cartilage. Matrix Biol. 2008, 27, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Söder, S.; Oehler, S.; Fundel, K.; Aigner, T. Activation of interleukin-1 signaling cascades in normal and osteoarthritic articular cartilage. Am. J. Pathol. 2007, 171, 938–946. [Google Scholar] [CrossRef]
- Gao, C.; Pu, H.; Zhou, Q.; Tao, T.; Liu, H.; Sun, X.; He, X.; Xiao, J. Two reactive behaviors of chondrocytes in an IL-1β-induced inflammatory environment revealed by the single-cell RNA sequencing. Aging 2021, 13, 11646. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Topete, D.; Cidlowski, J.A. One hormone, two actions: Anti-and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation 2014, 22, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Black, R.; Grodzinsky, A.J. Dexamethasone: Chondroprotective corticosteroid or catabolic killer? Eur. Cells Mater. 2019, 38, 246. [Google Scholar] [CrossRef] [PubMed]
- Makki, M.S.; Haqqi, T.M. Histone deacetylase inhibitor vorinostat (SAHA) suppresses IL-1β–induced matrix metallopeptidase-13 expression by inhibiting IL-6 in osteoarthritis chondrocyte. Am. J. Pathol. 2016, 186, 2701–2708. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Yang, S.; Shin, Y.; Rhee, J.; Chun, C.H.; Chun, J.S. Interleukin-6 plays an essential role in hypoxia-inducible factor 2α–induced experimental osteoarthritic cartilage destruction in mice. Arthritis Rheum. 2011, 63, 2732–2743. [Google Scholar] [CrossRef]
- Sahu, N.; Viljoen, H.J.; Subramanian, A. Continuous low-intensity ultrasound attenuates IL-6 and TNFα-induced catabolic effects and repairs chondral fissures in bovine osteochondral explants. BMC Musculoskelet. Disord. 2019, 20, 193. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef]
- Ioan-Facsinay, A.; Kloppenburg, M. Bioactive lipids in osteoarthritis: Risk or benefit? Curr. Opin. Rheumatol. 2018, 30, 108–113. [Google Scholar] [CrossRef]
- Yang, S.; Min, H.K.; Park, J.-S.; Na, H.S.; Cho, M.-L.; Park, S.-H. A green-lipped mussel prevents rheumatoid arthritis via regulation of inflammatory response and osteoclastogenesis. PLoS ONE 2023, 18, e0280601. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lessa, R.C.; Ebrahimi, B.; Guan, X.; Li, Y.; Lu, J. Exploring the In Vitro Protective Effects of Green-Lipped Mussel (GLM) Oil Extract against Biomarkers of Glucose Metabolism and Inflammation in Chondrocyte Cells. Nutraceuticals 2024, 4, 1-22. https://doi.org/10.3390/nutraceuticals4010001
Lessa RC, Ebrahimi B, Guan X, Li Y, Lu J. Exploring the In Vitro Protective Effects of Green-Lipped Mussel (GLM) Oil Extract against Biomarkers of Glucose Metabolism and Inflammation in Chondrocyte Cells. Nutraceuticals. 2024; 4(1):1-22. https://doi.org/10.3390/nutraceuticals4010001
Chicago/Turabian StyleLessa, Roberta Cardim, Belgheis Ebrahimi, Xiao Guan, Yan Li, and Jun Lu. 2024. "Exploring the In Vitro Protective Effects of Green-Lipped Mussel (GLM) Oil Extract against Biomarkers of Glucose Metabolism and Inflammation in Chondrocyte Cells" Nutraceuticals 4, no. 1: 1-22. https://doi.org/10.3390/nutraceuticals4010001
APA StyleLessa, R. C., Ebrahimi, B., Guan, X., Li, Y., & Lu, J. (2024). Exploring the In Vitro Protective Effects of Green-Lipped Mussel (GLM) Oil Extract against Biomarkers of Glucose Metabolism and Inflammation in Chondrocyte Cells. Nutraceuticals, 4(1), 1-22. https://doi.org/10.3390/nutraceuticals4010001







