Yohimbine Ingestion Mitigates Morning-Associated Decrements in High-Intensity Exercise Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Supplementation
2.4. Metabolic Biomarkers (Blood Lactate and Plasma Hypoxanthine)
2.5. Procedures
2.6. Data Analysis
3. Results
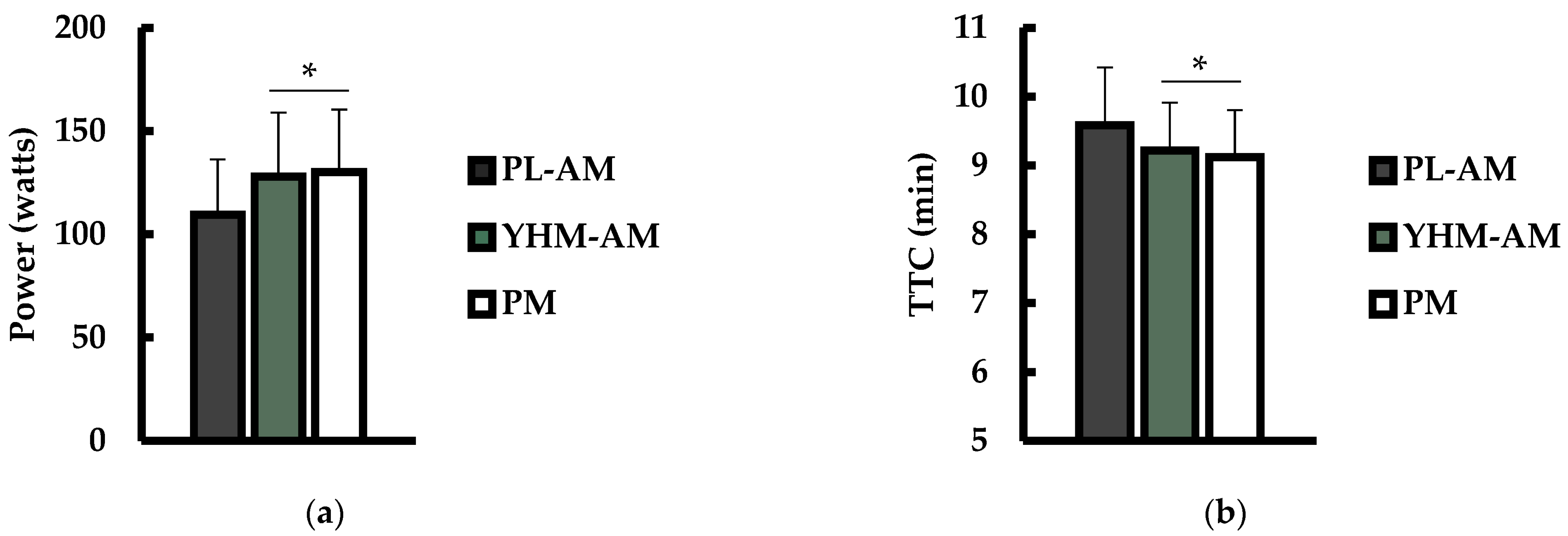
3.1. Power Output and Time to Completion (TTC)
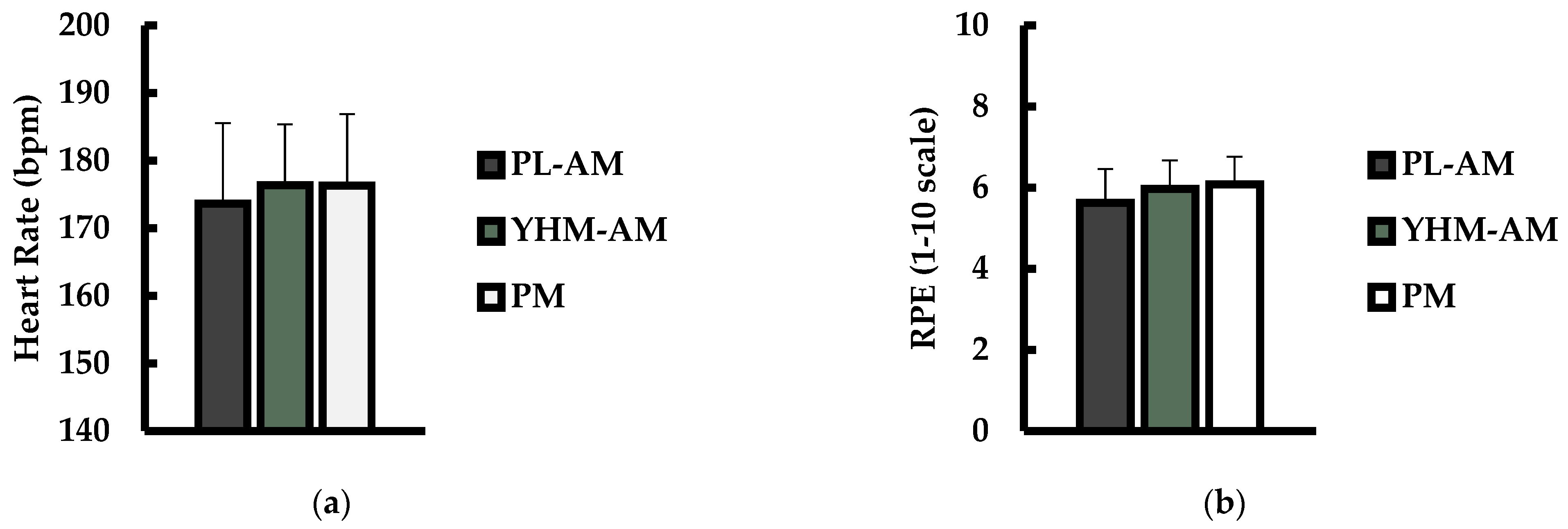
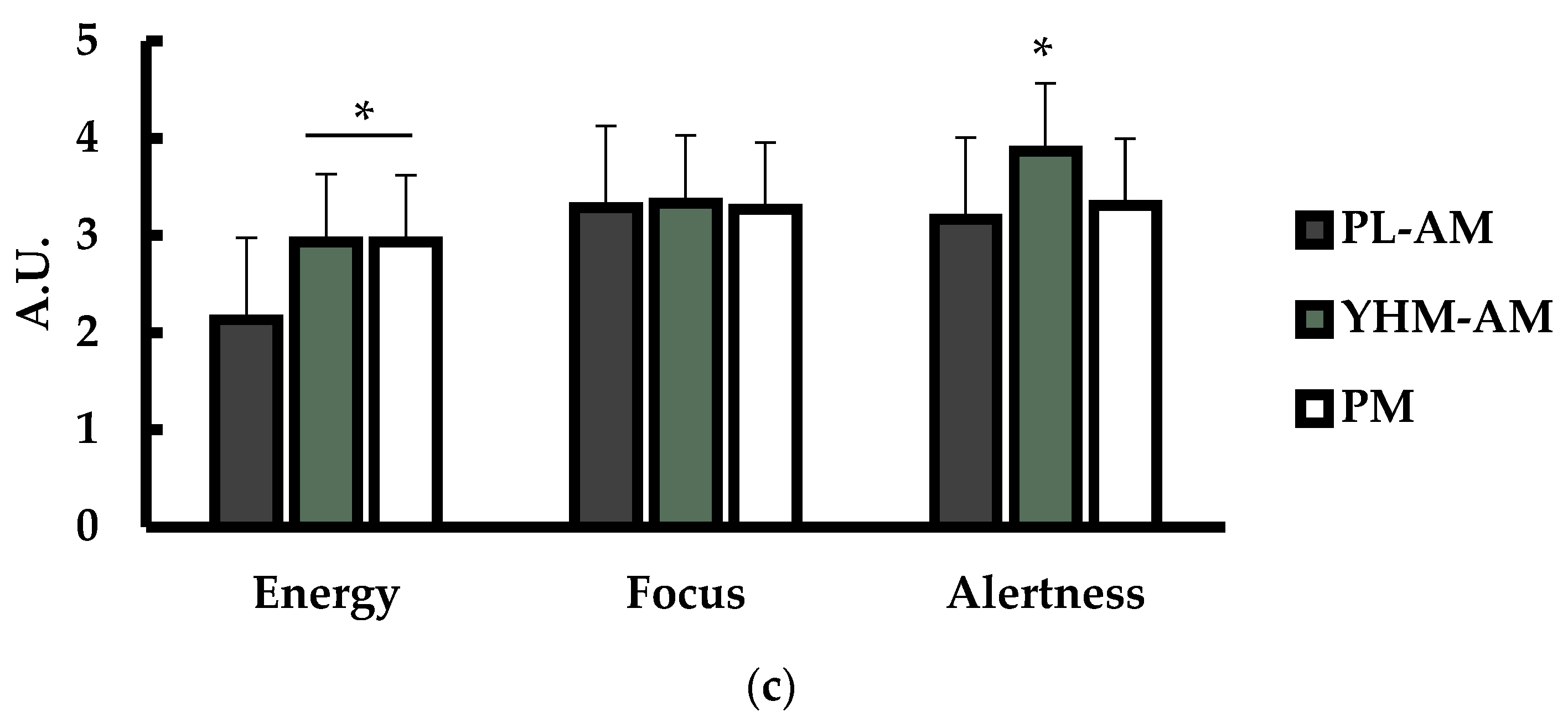
3.2. Heart Rate (HR), Rate of Perceived Exertion (RPE), and Subjective Ratings of Energy, Focus, and Alertness
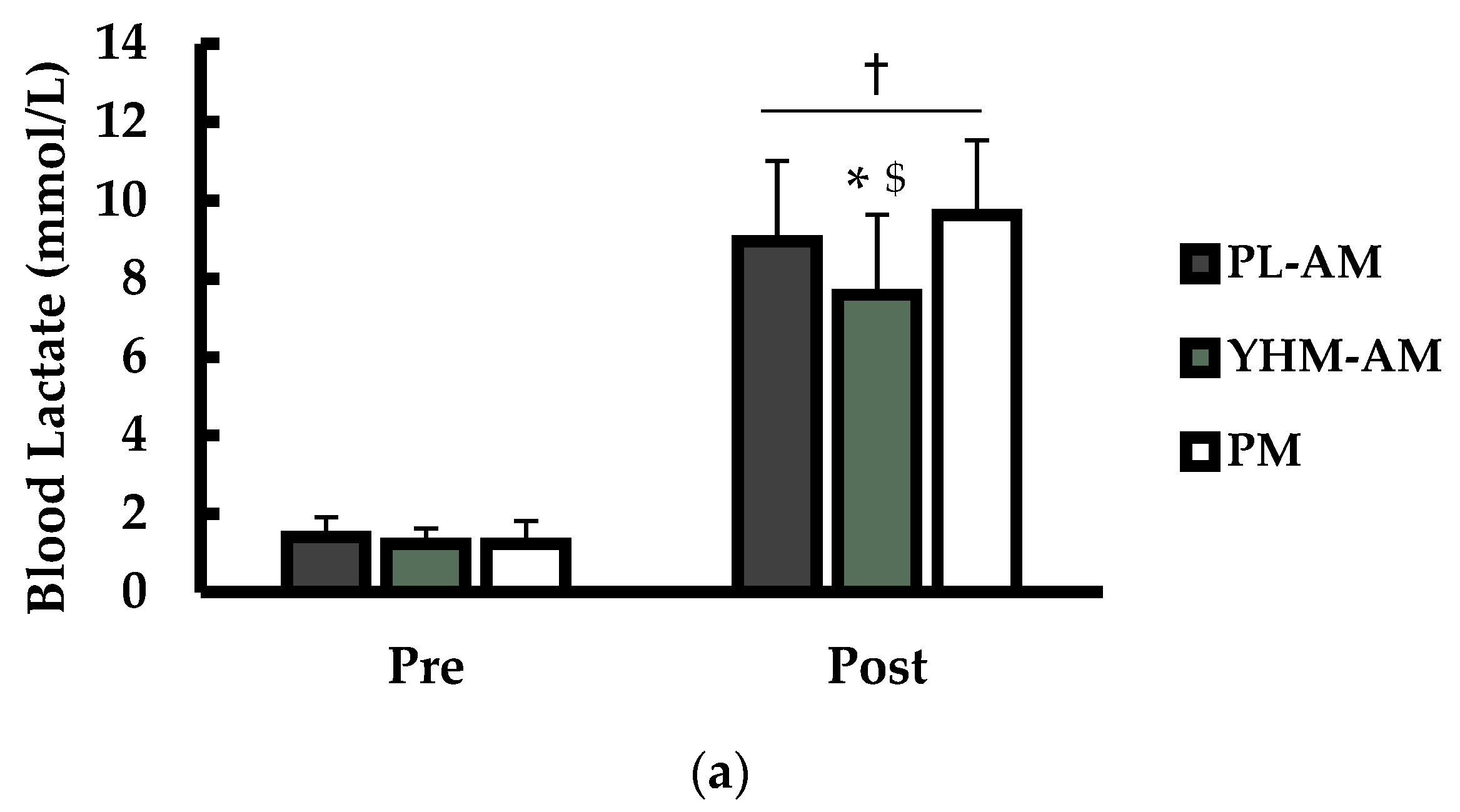
3.3. Blood Lactate (La) and Plasma Hypoxanthine (HX)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGinnis, G.R.; Young, M.E. Circadian regulation of metabolic homeostasis: Causes and consequences. Nat. Sci. Sleep. 2016, 8, 163–180. [Google Scholar] [PubMed]
- Kaneko, M.; Zechman, F.W.; Smith, R.E. Circadian variation in human peripheral blood flow levels and exercise responses. J. Appl. Physiol. 1968, 25, 109–114. [Google Scholar] [CrossRef]
- Racinais, S.; Blonc, S.; Hue, O. Effects of active warm-up and diurnal increase in temperature on muscular power. Med. Sci. Sports Exerc. 2005, 37, 2134–2139. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Carpentier, A.; Guissard, N.; Van Hoecke, J.; Duchateau, J. Effect of time of day on force variation in a human muscle. Muscle Nerve 1999, 22, 1380–1387. [Google Scholar] [CrossRef]
- Ayala, V.; Martínez-Bebia, M.; Latorre, J.A.; Gimenez-Blasi, N.; Jimenez-Casquet, M.J.; Conde-Pipo, J.; Bach-Faig, A.; Mariscal-Arcas, M. Influence of circadian rhythms on sports performance. Chronobiol. Int. 2021, 38, 1522–1536. [Google Scholar] [CrossRef] [PubMed]
- Nobari, H.; Azarian, S.; Saedmocheshi, S.; Valdés-Badilla, P.; Calvo, T.G. Narrative review: The role of circadian rhythm on sports performance, hormonal regulation, immune system function, and injury prevention in athletes. Heliyon 2023, 9, e19636. [Google Scholar] [CrossRef] [PubMed]
- Blazer, H.J.; Jordan, C.L.; Pederson, J.A.; Rogers, R.R.; Williams, T.D.; Marshall, M.R.; Ballmann, C.G. Effects of time-of-day training preference on resistance-exercise performance. Res. Q. Exerc. Sport. 2021, 92, 492–499. [Google Scholar] [CrossRef]
- Dumar, A.M.; Huntington, A.F.; Rogers, R.R.; Kopec, T.J.; Williams, T.D.; Ballmann, C.G. Acute beetroot juice supplementation attenuates morning-associated decrements in supramaximal exercise performance in trained sprinters. Int. J. Environ. Res. Public. Health 2021, 18, 412. [Google Scholar] [CrossRef]
- Shannon, M.; Neuman, M.I. Yohimbine. Pediatr. Emerg. Care 2000, 16, 49–50. [Google Scholar] [CrossRef]
- Small, J. Yohimbe bark: Its history and identification in commerce. Pharma J. 1922, 108, 264–311. [Google Scholar]
- Swann, A.C.; Birnbaum, D.; Jagar, A.A.; Dougherty, D.M.; Moeller, F.G. Acute yohimbine increases laboratory-measured impulsivity in normal subjects. Biol. Psychiatry 2005, 57, 1209–1211. [Google Scholar] [CrossRef]
- Grossman, E.; Rea, R.F.; Hoffman, A.; Goldstein, D.S. Yohimbine increases sympathetic nerve activity and norepinephrine spillover in normal volunteers. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1991, 260, R142–R147. [Google Scholar] [CrossRef]
- Williams, T.D.; Boag, L.E.; Helton, C.L.; Middleton, M.L.; Rogers, R.R.; Sternenberg, L.H.; Ballmann, C.G. Effects of Acute Yohimbine Hydrochloride Ingestion on Bench Press Performance in Resistance-Trained Males. Muscles 2022, 1, 82–91. [Google Scholar] [CrossRef]
- Roatta, S.; Farina, D. Sympathetic actions on the skeletal muscle. Exerc. Sport. Sci. Rev. 2010, 38, 31–35. [Google Scholar] [CrossRef]
- Cameron, O.G.; Zubieta, J.K.; Grunhaus, L.; Minoshima, S. Effects of yohimbine on cerebral blood flow, symptoms, and physiological functions in humans. Psychosom. Med. 2000, 62, 549–559. [Google Scholar] [CrossRef]
- Barnes, M.E.; Cowan, C.R.; Boag, L.E.; Hill, J.G.; Jones, M.L.; Nixon, K.M.; Parker, M.G.; Parker, S.K.; Raymond, M.V.; Sternenberg, L.H.; et al. Effects of Acute Yohimbine Hydrochloride Supplementation on Repeated Supramaximal Sprint Performance. Int. J. Environ. Res. Public. Health 2022, 19, 1316. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; AN Abood, H.; Al-Gareeb, I.A. Ergogenic Effects of Yohimbine: Standardized Cycling Clinical Study. Kerbala J. Med. 2014, 7, 1850–1855. [Google Scholar]
- Laakso, M.-L.; Mustanoja, S.M.; Hätönen, T.; Alila-Johansson, A. Alpha2-adrenoceptor agonist medetomidine affects the melatonin rhythm in rats. Neurosci. Lett. 1997, 238, 61–64. [Google Scholar] [CrossRef]
- Thosar, S.S.; Shea, S.A. Circadian control of human cardiovascular function. Curr. Opin. Pharmacol. 2021, 57, 89–97. [Google Scholar] [CrossRef]
- Souissi, M.; Abedelmalek, S.; Chtourou, H.; Boussita, A.; Hakim, A.; Sahnoun, Z. Effects of time-of-day and caffeine ingestion on mood states, simple reaction time, and short-term maximal performance in elite judoists. Biol. Rhythm. Res. 2013, 44, 897–907. [Google Scholar] [CrossRef]
- Mora-Rodríguez, R.; Pallarés, J.G.; López-Gullón, J.M.; López-Samanes, Á.; Fernández-Elías, V.E.; Ortega, J.F. Improvements on neuromuscular performance with caffeine ingestion depend on the time-of-day. J. Sci. Med. Sport. 2015, 18, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Riebe, D.; Ehrman, J.K.; Liguori, G.; Magal, M.; American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription; Wolters Kluwer: Amsterdam, The Netherland, 2018. [Google Scholar]
- Williams, T.D.; Langley, H.N.; Roberson, C.C.; Rogers, R.R.; Ballmann, C.G. Effects of short-term golden root extract (Rhodiola rosea) supplementation on resistance exercise performance. Int. J. Environ. Res. Public. Health 2021, 18, 6953. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Ibelles, P.; Gutiérrez-Vidal, R.; Calixto-Tlacomulco, S.; Delgado-Coello, B.; Mas-Oliva, J. Hepatic accumulation of hypoxanthine: A link between hyperuricemia and nonalcoholic fatty liver disease. Arch. Med. Res. 2021, 52, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Nixon, K.M.; Parker, M.G.; Elwell, C.C.; Pemberton, A.L.; Rogers, R.R.; Ballmann, C.G. Effects of music volume preference on endurance exercise performance. J. Funct. Morphol. Kinesiol. 2022, 7, 35. [Google Scholar] [CrossRef]
- Karow, M.C.; Rogers, R.R.; Pederson, J.A.; Williams, T.D.; Marshall, M.R.; Ballmann, C.G. Effects of preferred and nonpreferred warm-up music on exercise performance. Percept. Mot. Ski. 2020, 127, 912–924. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Kang, J.; Ratamess, N.A.; Hoffman, M.W.; Tranchina, C.P.; Faigenbaum, A.D. Examination of a pre-exercise, high energy supplement on exercise performance. J. Int. Soc. Sports Nutr. 2009, 6, 2. [Google Scholar] [CrossRef]
- Wei, J.; Carroll, R.J.; Harden, K.K.; Wu, G. Comparisons of treatment means when factors do not interact in two-factorial studies. Amino Acids 2012, 42, 2031–2035. [Google Scholar] [CrossRef]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum Associates: Hillsdale, MI, USA, 1988. [Google Scholar]
- Teo, W.; Newton, M.J.; McGuigan, M.R. Circadian rhythms in exercise performance: Implications for hormonal and muscular adaptation. J. Sports Sci. Med. 2011, 10, 600. [Google Scholar]
- Knaier, R.; Qian, J.; Roth, R.; Infanger, D.; Notter, T.; Wang, W.; Cajochen, C.; Scheer, F.A. Diurnal variation in maximum endurance and maximum strength performance: A systematic review and meta-analysis. Med. Sci. Sports Exerc. 2022, 54, 169. [Google Scholar] [CrossRef]
- Lambert, E.A.; Chatzivlastou, K.; Schlaich, M.; Lambert, G.; Head, G.A. Morning surge in blood pressure is associated with reactivity of the sympathetic nervous system. Am. J. Hypertens. 2014, 27, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Chtourou, H.; Driss, T.; Souissi, S.; Gam, A.; Chaouachi, A.; Souissi, N. The effect of strength training at the same time of the day on the diurnal fluctuations of muscular anaerobic performances. J. Strength. Cond. Res. 2012, 26, 217–225. [Google Scholar] [CrossRef]
- Hassler, C.; Burnier, M. Circadian variations in blood pressure: Implications for chronotherapeutics. Am. J. Cardiovasc. Drugs 2005, 5, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Kario, K.; Pickering, T.G.; Hoshide, S.; Eguchi, K.; Ishikawa, J.; Morinari, M.; Hoshide, Y.; Shimada, K. Morning blood pressure surge and hypertensive cerebrovascular disease: Role of the alpha adrenergic sympathetic nervous system. Am. J. Hypertens. 2004, 17, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Roatta, S.; Arendt-Nielsen, L.; Farina, D. Sympathetic-induced changes in discharge rate and spike-triggered average twitch torque of low-threshold motor units in humans. J. Physiol. 2008, 586, 5561–5574. [Google Scholar] [CrossRef] [PubMed]
- Jabir, N.R.; Firoz, C.K.; Zughaibi, T.A.; Alsaadi, M.A.; Abuzenadah, A.M.; Al-Asmari, A.I.; Alsaieedi, A.; Ahmed, B.A.; Ramu, A.K.; Tabrez, S. A literature perspective on the pharmacological applications of yohimbine. Ann. Med. 2022, 54, 2849–2863. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Green, T.A.; Theobald, D.E.; Birnbaum, S.G.; Graham, D.L.; Zeeb, F.D.; Nestler, E.J.; Winstanley, C.A. Yohimbine increases impulsivity through activation of cAMP response element binding in the orbitofrontal cortex. Biol. Psychiatry 2010, 67, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Reilly, T.; Atkinson, G.; Coldwells, A. The relevance to exercise performance of the circadian rhythms in body temperature and arousal. Biol. Sport. 1993, 10, 204. [Google Scholar]
- Delp, M.; Manning, R.; Bruckner, J.; Armstrong, R. Distribution of cardiac output during diurnal changes of activity in rats. Am. J. Physiol.-Heart Circ. Physiol. 1991, 261, H1487–H1493. [Google Scholar] [CrossRef]
- Fritzsche, R.G.; Switzer, T.W.; Hodgkinson, B.J.; Coyle, E.F. Stroke volume decline during prolonged exercise is influenced by the increase in heart rate. J. Appl. Physiol. 1999, 86, 799–805. [Google Scholar] [CrossRef]
- Spina, R.J.; Ogawa, T.; Martin, W., 3rd; Coggan, A.R.; Holloszy, J.; Ehsani, A. Exercise training prevents decline in stroke volume during exercise in young healthy subjects. J. Appl. Physiol. 1992, 72, 2458–2462. [Google Scholar] [CrossRef] [PubMed]
- Garb, S. Inotropic Action of Epinephrine, Nor-epinephrine, and N-Isopropyl-Norepinephrine on Heart Muscle. Proc. Soc. Exp. Biol. Med. 1950, 73, 134–135. [Google Scholar] [CrossRef] [PubMed]
- Zinoubi, B.; Zbidi, S.; Vandewalle, H.; Chamari, K.; Driss, T. Relationships between rating of perceived exertion, heart rate and blood lactate during continuous and alternated-intensity cycling exercises. Biol. Sport. 2018, 35, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Costello, J.T.; Bieuzen, F.; Bleakley, C.M. Where are all the female participants in sports and exercise medicine research? Eur. J. Sport. Sci. 2014, 14, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Bushman, B.; Masterson, G.; Nelsen, J. Anaerobic power performance and the menstrual cycle: Eumenorrheic and oral contraceptive users. J. Sports Med. Phys. Fit. 2006, 46, 132. [Google Scholar]
- Wiecek, M.; Szymura, J.; Maciejczyk, M.; Cempla, J.; Szygula, Z. Effect of sex and menstrual cycle in women on starting speed, anaerobic endurance and muscle power. Acta Physiol. Hung. 2016, 103, 127–132. [Google Scholar] [CrossRef]
- Ghazel, N.; Souissi, A.; Chtourou, H.; Aloui, G.; Souissi, N. The effect of music on short-term exercise performance during the different menstrual cycle phases in female handball players. Res. Sports Med. 2022, 30, 50–60. [Google Scholar] [CrossRef]
- Cowley, E.S.; Olenick, A.A.; McNulty, K.L.; Ross, E.Z. “Invisible sportswomen”: The sex data gap in sport and exercise science research. Women Sport. Phys. Act. J. 2021, 29, 146–151. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballmann, C.G.; Rogers, R.R.; Barnes, M.E.; Cowan, C.R.; Elwell, C.C.; Luiken, K.A.; Lehman, G.Y.; Kaylor, J.C.; Simpson, E.G.; Westbrooks, S.B.; et al. Yohimbine Ingestion Mitigates Morning-Associated Decrements in High-Intensity Exercise Performance. Nutraceuticals 2024, 4, 23-34. https://doi.org/10.3390/nutraceuticals4010002
Ballmann CG, Rogers RR, Barnes ME, Cowan CR, Elwell CC, Luiken KA, Lehman GY, Kaylor JC, Simpson EG, Westbrooks SB, et al. Yohimbine Ingestion Mitigates Morning-Associated Decrements in High-Intensity Exercise Performance. Nutraceuticals. 2024; 4(1):23-34. https://doi.org/10.3390/nutraceuticals4010002
Chicago/Turabian StyleBallmann, Christopher G., Rebecca R. Rogers, Megan E. Barnes, Camryn R. Cowan, Carson C. Elwell, Kailey A. Luiken, Grace Y. Lehman, Julia C. Kaylor, Ella G. Simpson, Spencer B. Westbrooks, and et al. 2024. "Yohimbine Ingestion Mitigates Morning-Associated Decrements in High-Intensity Exercise Performance" Nutraceuticals 4, no. 1: 23-34. https://doi.org/10.3390/nutraceuticals4010002
APA StyleBallmann, C. G., Rogers, R. R., Barnes, M. E., Cowan, C. R., Elwell, C. C., Luiken, K. A., Lehman, G. Y., Kaylor, J. C., Simpson, E. G., Westbrooks, S. B., Miller, M. J., Benjamin, C. L., & Williams, T. D. (2024). Yohimbine Ingestion Mitigates Morning-Associated Decrements in High-Intensity Exercise Performance. Nutraceuticals, 4(1), 23-34. https://doi.org/10.3390/nutraceuticals4010002







