Anti-Inflammatory Effects, Protection of Gut Barrier Integrity and Stimulation of Phagocytosis of Postbiotic Combination ABB C1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Investigational Product
2.2. Anti-Inflammatory Effect of ABB C1® on Intestinal Cells/Epithelial Signalling Assay
2.3. Gut Barrier Integrity Assay
2.4. Stimulation of Phagocytosis of Peripheral Blood Monocytes and Leukocytes and Peritoneal Macrophages in Mice
2.5. Statistical Analysis
3. Results
3.1. Anti-Inflammatory Effect on Intestinal Cells
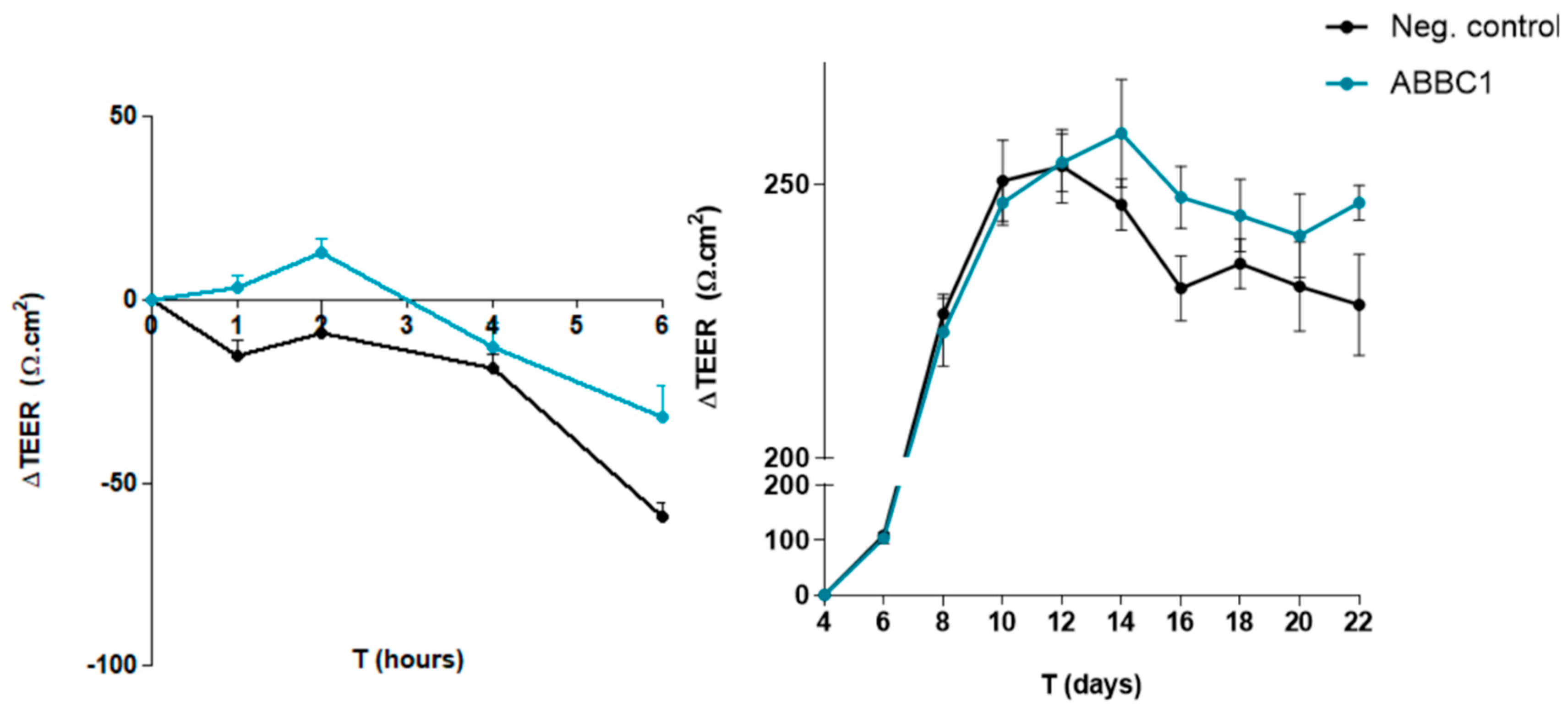
3.2. Gut Barrier Integrity Assay
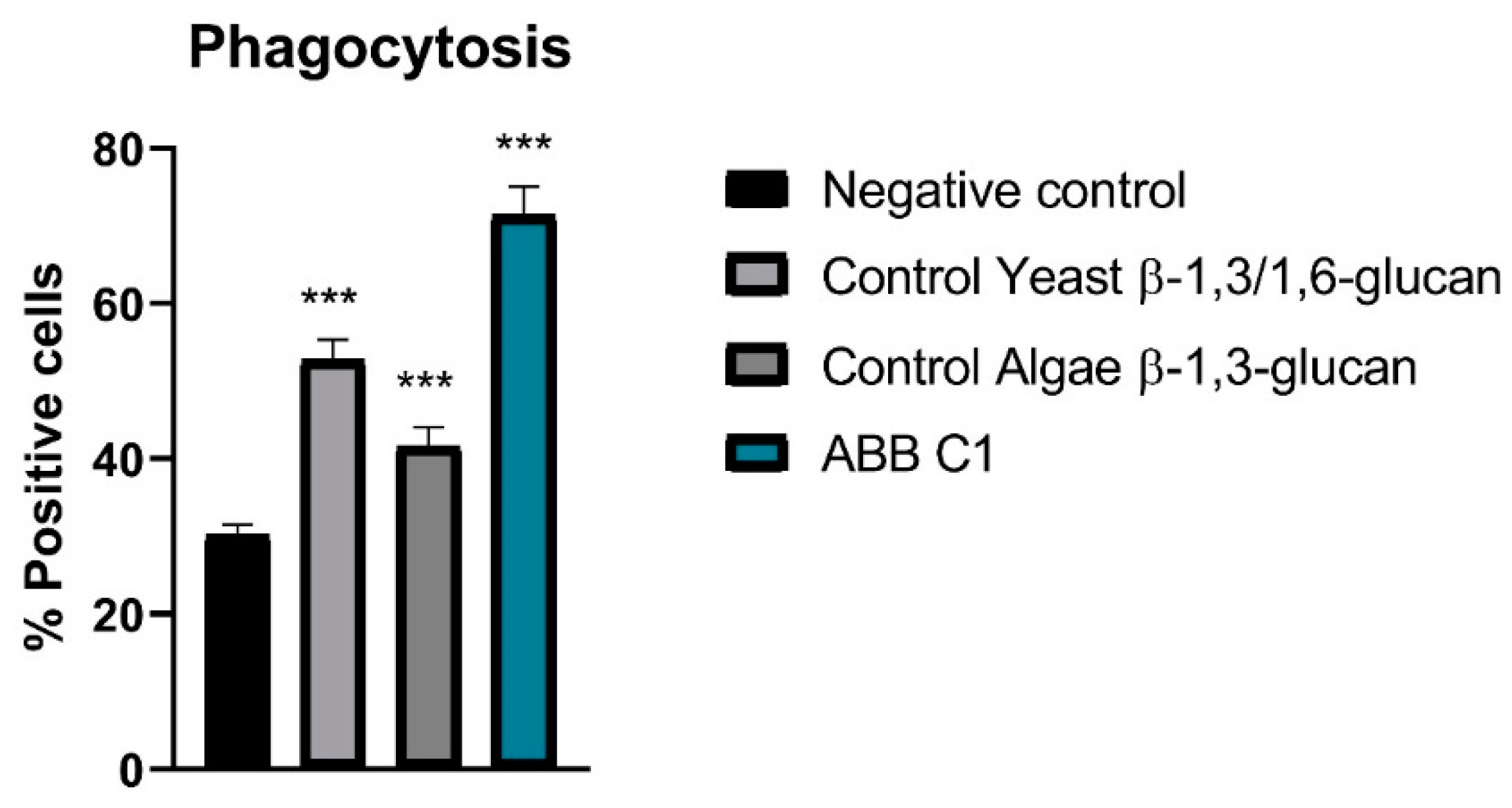
3.3. Stimulation of Phagocytosis of Peripheral Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dinarello, C.A. Proinflammatory cytokines. Chest 2000, 118, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [PubMed]
- Stow, J.L.; Murray, R.Z. Intracellular trafficking and secretion of inflammatory cytokines. Cytokine Growth Factor Rev. 2013, 24, 227–239. [Google Scholar] [CrossRef]
- Geuking, M.B.; Köller, Y.; Rupp, S.; McCoy, K.D. The interplay between the gut microbiota and the immune system. Gut Microbes 2014, 5, 411–418. [Google Scholar] [CrossRef]
- Hosseini, A.; Hashemi, V.; Shomali, N.; Asghari, F.; Gharibi, T.; Akbari, M.; Gholizadeh, S.; Jafari, A. Innate and adaptive immune responses against coronavirus. Biomed. Pharmacother. 2020, 132, 110859. [Google Scholar] [CrossRef]
- Lotfi, R.; Kalmarzi, R.N.; Roghani, S.A. A review on the immune responses against novel emerging coronavirus (SARS-CoV-2). Immunol. Res. 2021, 69, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Channappanavar, R.; Kanneganti, T.D. Coronaviruses: Innate immunity, inflammasome activation, inflammatory cell death, and cytokines. Trends Immunol. 2020, 41, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, F.; Fallahi, P. IP-10 in occupational asthma: Review of the literature and case-control study. Clin. Ter. 2017, 168, e151–e157. [Google Scholar] [CrossRef]
- Di Bari, F. Atopic dermatitis and alpha-chemokines. Clin. Ter. 2015, 166, e182–e187. [Google Scholar] [CrossRef] [PubMed]
- Holla, L.I.; Mrazek, F.; Petrek, M. MCP-1 and CCR2 gene polymorphisms in Czech patients with allergic disorders. Int. J. Immunogenet. 2009, 36, 69–72. [Google Scholar] [CrossRef]
- Mai, W.; Lu, D.; Liu, X.; Chen, L. MCP-1 produced by keratinocytes is associated with leucocyte recruitment during elicitation of nickel-induced occupational allergic contact dermatitis. Toxicol. Ind. Health 2018, 34, 36–43. [Google Scholar] [CrossRef]
- Groschwitz, K.R.; Hogan, S.-P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef]
- Hamulka, J.; Jeruszka-Bielak, M.; Górnicka, M.; Drywień, M.E.; Zielinska-Pukos, M.A. Dietary supplements during COVID-19 outbreak. Results of Google Trends Analysis Supported by PLifeCOVID-19 Online Studies. Nutrients 2020, 13, 54. [Google Scholar] [CrossRef]
- Costagliola, G.; Spada, E.; Comberiati, P.; Peroni, D.G. Could nutritional supplements act as therapeutic adjuvants in COVID-19? Ital. J. Pediatr. 2021, 47, 32. [Google Scholar] [CrossRef]
- Suleiman, W.; Helal, E. Chemical constituents and potential pleiotropic activities of Foeniculum vulgare (Fennel) ethanolic extract; in vitro approach. Egypt. J. Chem. 2022, 65, 617–626. [Google Scholar] [CrossRef]
- El-Naggar, H.A.; Bashar, M.A.E.; Rady, I.; El-Wetidy, M.S.; Suleiman, W.B.; Al-Otibi, F.O.; Al-Rashed, S.A.; Abd El-Maoula, L.M.; Salem, E.-S.S.; Attia, E.M.H.; et al. Two Red Sea Sponge Extracts (Negombata magnifica and Callyspongia siphonella) Induced Anticancer and Antimicrobial Activity. Appl. Sci. 2022, 12, 1400. [Google Scholar] [CrossRef]
- Suleiman, W.B.; Shehata, R.M.; Younis, A.M. In vitro assessment of multipotential therapeutic importance of Hericium erinaceus mushroom extracts using different solvents. Bioresour. Bioprocess. 2022, 9, 99. [Google Scholar] [CrossRef]
- Generoso, S.V.; Viana, M.; Santos, R.; Martins, F.S.; Machado, J.A.; Arantes, R.M.; Nicoli, J.R.; Correia, M.I.; Cardoso, V.N. Saccharomyces cerevisiae strain UFMG 905 protects against bacterial translocation, preserves gut barrier integrity and stimulates the immune system in a murine intestinal obstruction model. Arch. Microbiol. 2010, 192, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Generoso, S.V.; Viana, M.; Santos, R.; Martins, F.S.; Machado, J.A.; Arantes, R.M.; Nicoli, J.R.; Correia, M.I.; Cardoso, V.N. Protection against increased intestinal permeability and bacterial translocation induced by intestinal obstruction in mice treated with viable and heat-killed Saccharomyces boulardii. Eur. J. Nutr. 2011, 50, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.A.M.; Bifano, M.; Roca Goma, E.; Plasencia, C.M.; Torralba, A.O.; Font, M.S.; Millán, P.R. Effect and tolerability of a nutritional supplement based on a synergistic combination of β-glucans and selenium- and zinc-enriched Saccharomyces cerevisiae (ABB C1®) in volunteers receiving the influenza or the COVID-19 vaccine: A randomized, double-blind, placebo-controlled study. Nutrients 2021, 13, 4347. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Kim, H. Mini-Review on the roles of vitamin C, vitamin D, and selenium in the immune system against COVID-19. Molecules 2020, 25, 5346. [Google Scholar] [CrossRef] [PubMed]
- Fakhrolmobasheri, M.; Mazaheri-Tehrani, S.; Kieliszek, M.; Zeinalian, M.; Abbasi, M.; Karimi, F.; Mozafari, A.M. COVID-19 and selenium deficiency: A systematic review. Biol. Trace Elem. Res. 2022, 200, 3945–3956. [Google Scholar] [CrossRef]
- Sales Martinez, S.; Huang, Y.; Acuna, L.; Laverde, E.; Trujillo, D.; Barbieri, M.A.; Tamargo, J.; Campa, A.; Baum, M. Role of Selenium in Viral Infections with a Major Focus on SARS-CoV-2. Int. J. Mol. Sci. 2022, 23, 280. [Google Scholar] [CrossRef]
- Tabatabaeizadeh, S.A. Zinc supplementation and COVID-19 mortality: A meta-analysis. Eur. J. Med. Res. 2022, 27, 70. [Google Scholar] [CrossRef]
- Jawhara, S. How to boost the immune defence prior to respiratory virus infections with the special focus on coronavirus infections. Gut Pathog. 2020, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Geller, A.; Yan, J. Could the induction of trained immunity by β-glucan serve as a defense against COVID-19? Front. Immunol. 2020, 11, 1782. [Google Scholar] [CrossRef]
- Bashir, K.M.I.; Choi, J.S. Clinical and Physiological Perspectives of β-Glucans: The Past, Present, and Future. Int. J. Mol. Sci. 2017, 18, 1906. [Google Scholar] [CrossRef] [PubMed]
- Van De Walle, J.; Hendrickx, A.; Romier, B.; Larondelle, Y.; Schneider, Y.-J. Inflammatory parameters in Caco-2 cells: Effect of stimuli nature, concentration, combination and cell differentiation. Toxicol. Vitr. 2010, 24, 1441–1449. [Google Scholar] [CrossRef]
- Chen, S.; Einspanier, R.; Schoen, J. Transepithelial electrical resistance (TEER): A functional parameter to monitor the quality of oviduct epithelial cells cultured on filter supports. Histochem. Cell Biol. 2015, 144, 509–515. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Liu, C.; Su, L.; Zhang, D.; Fan, J.; Yang, Y.; Xiao, M.; Xie, J.; Xu, Y.; et al. IP-10 and MCP-1 as biomarkers associated with disease severity of COVID-19. Mol. Med. 2020, 26, 97. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chin, C.-h.; Liu, S.-F.; Wu, C.-C.; Tsen, C.-C.; Wang, Y.-H.; Chao, T.-Y.; Lie, C.-H.; Chen, C.-j.; Wang, C.-C.; et al. Prognostic values of serum IP-10 and IL-17 in patients with pulmonary tuberculosis. Dis. Markers 2011, 31, 938794. [Google Scholar] [CrossRef]
- Pons, M.J.; Ymaña, B.; Mayanga-Herrera, A.; Sáenz, Y.; Alvarez-Erviti, L.; Tapia-Rojas, S.; Gamarra, R.; Blanco, A.B.; Moncunill, G.; Ugarte-Gil, M.F. Cytokine profiles associated with worse prognosis in a hospitalized Peruvian COVID-19 cohort. Front. Immunol. 2021, 12, 700921. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef] [PubMed]
- Zanza, C.; Romenskaya, T.; Manetti, A.C.; Franceschi, F.; La Russa, R.; Bertozzi, G.; Maiese, A.; Savioli, G.; Volonnino, G.; Longhitano, Y. Cytokine Storm in COVID-19: Immunopathogenesis and therapy. Medicina 2022, 58, 144. [Google Scholar] [CrossRef] [PubMed]
- Kumboyono, K.; Chomsy, I.N.; Iskandar, A.; Aryati, A.; Parwati, I.; Wihastuti, T.A. The potential predictive role of tumour necrosis factor-α, interleukin-1β, and monocyte chemoattractant protein-1 for COVID-19 patients survival. Infect. Drug Resist. 2022, 15, 821–829. [Google Scholar] [CrossRef]
- Liu, M.; Guo, S.; Hibbert, J.M.; Jain, V.; Singh, N.; Wilson, N.O.; Stiles, J.K. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev. 2011, 22, 121–130. [Google Scholar] [CrossRef]
- Lu, Q.; Zhu, Z.; Tan, C.; Zhou, H.; Hu, Y.; Shen, G.; Zhu, P.; Yang, G.; Xie, X. Changes of serum IL-10, IL-1β, IL-6, MCP-1, TNF-α, IP-10 and IL-4 in COVID-19 patients. Int. J. Clin. Pract. 2021, 75, e14462. [Google Scholar] [CrossRef]
- Prasad, R.; Patton, M.J.; Floyd, J.L.; Vieira, C.P.; Fortmann, S.; DuPont, M.; Harbour, A.; Jeremy, C.S.; Wright, J.; Lamendella, R.; et al. Plasma microbiome in COVID-19 subjects: An indicator of gut barrier defects and dysbiosis. bioRxiv 2021. [Google Scholar] [CrossRef]
- Ferreira, C.; Viana, S.D.; Reis, F. Gut microbiota dysbiosis-immune hyperresponse-inflammation triad in coronavirus disease 2019 (COVID-19): Impact of pharmacological and nutraceutical approaches. Microorganisms 2020, 8, 1514. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef]
- Wesemann, D.R.; Nagler, C.R. The Microbiome, Timing, and Barrier Function in the Context of Allergic Disease. Immunity 2016, 44, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Raa, J. Immune modulation by non-digestible and non-absorbable beta-1,3/1,6-glucan. Microb. Ecl. Health Dis. 2015, 26, 27824. [Google Scholar] [CrossRef] [PubMed]
- Goodridge, H.S.; Wolf, A.J.; Underhill, D.M. Beta-glucan recognition by the innate immune system. Immunol. Rev. 2009, 230, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Zhao, J.; Wang, J.; Song, Q.; Zhao, C. The phagocytic receptors of β-glucan. Int. J. Biol. Macromol. 2022, 205, 430–441. [Google Scholar] [CrossRef] [PubMed]
| Experimental Conditions | Mean ± SD | ANOVA Test p-Value |
|---|---|---|
| IP-10 levels, pg/mL | ||
| ABB C1® | 169.08 ± 4.33 | p< 0.0001 |
| Control | 225.87 ± 15.39 | |
| ABB C1® with TNF-α/IFN-ɣ challenge | 42,369.44 ± 13,446.14 | p= 0.0004 |
| Control with TNF-α/IFN-ɣ challenge | 62,363.65 ± 4611.93 | |
| MCP-1 levels, pg/mL | ||
| ABB C1® | 2.87 (0.63) | p= 0.0104 |
| Control | 4.36 (0.53) | |
| ABB C1® with TNF-α/IFN-ɣ challenge | 19.50 (7.62) | p< 0.0001 |
| Control with TNF-α/IFN-ɣ challenge | 30.62 (2.80) |
| ∆TEER Ω/cm2 | Experimental Condition | ||
|---|---|---|---|
| ABB C1® | Control | t-Test | |
| Mean (SD) | Mean (SD) | p-Value | |
| t = 0 | 0 | 0 | - |
| t = 1 | 3.33 (5.77) | −15.33 (7.51) | 0.030 |
| t = 2 | 12.67 (6.66) | −9.33 (16.01) | 0.126 |
| t = 4 | −13.0 (11.14) | −19.00 (7.21) | 0.484 |
| t = 6 | −32.33 (15.57) | −59.33 (6.81) | 0.078 |
| AUC | |||
| Area of negative peaks | 198.53 (57.38) | 97.62 (53.56) | 0.090 |
| ∆TEER (Ω/cm2) | Experimental Condition | ||
|---|---|---|---|
| ABB C1® | Negative Control | t-Test | |
| Mean (SD) | Mean (SD) | p-Value | |
| t = 4 | 0 | 0 | - |
| t = 6 | 104.33 (17.62) | 109.0 (17.69) | 0.940 |
| t = 8 | 223.0 (10.82) | 226.33 (8.82) | 0.497 |
| t = 10 | 246.67 (7.10) | 250.67 (18.11) | 0.464 |
| t = 12 | 254.0 (9.17) | 253.33 (16.51) | 0.780 |
| t = 14 | 259.33 (17.10) | 246.33 (11.43) | 0.455 |
| t = 16 | 247.67 (9.82) | 231.0 (14.45) | 0.169 |
| t = 18 | 244.33 (11.50) | 235.50 (11.11) | 0.315 |
| t = 20 | 240.67 (13.20) | 231.33 (19.90) | 0.530 |
| t = 22 | 246.67 (5.51) | 228.0 (22.70) | 0.141 |
| AUC | |||
| Area of positive peaks | 1913.00 (62.48) | 1943.67 (48.00) | 0.451 |
| Experimental Condition | Percentage of Positive Cells, Mean ± SD | t-Test p-Value vs. Control |
|---|---|---|
| ABB C1® | 71.37 (3.68) | p < 0.001 |
| Control yeast β-1,3/1,6 glucan | 52.75 (2.61) | p < 0.001 |
| Control algae β-1,3-glucan | 41.63 (2.44) | p < 0.001 |
| Negative control | 30.13 (1.40) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tintoré, M.; Cuñé, J.; Vetvicka, V.; de Lecea, C. Anti-Inflammatory Effects, Protection of Gut Barrier Integrity and Stimulation of Phagocytosis of Postbiotic Combination ABB C1. Nutraceuticals 2023, 3, 109-118. https://doi.org/10.3390/nutraceuticals3010009
Tintoré M, Cuñé J, Vetvicka V, de Lecea C. Anti-Inflammatory Effects, Protection of Gut Barrier Integrity and Stimulation of Phagocytosis of Postbiotic Combination ABB C1. Nutraceuticals. 2023; 3(1):109-118. https://doi.org/10.3390/nutraceuticals3010009
Chicago/Turabian StyleTintoré, Maria, Jordi Cuñé, Vaclav Vetvicka, and Carlos de Lecea. 2023. "Anti-Inflammatory Effects, Protection of Gut Barrier Integrity and Stimulation of Phagocytosis of Postbiotic Combination ABB C1" Nutraceuticals 3, no. 1: 109-118. https://doi.org/10.3390/nutraceuticals3010009
APA StyleTintoré, M., Cuñé, J., Vetvicka, V., & de Lecea, C. (2023). Anti-Inflammatory Effects, Protection of Gut Barrier Integrity and Stimulation of Phagocytosis of Postbiotic Combination ABB C1. Nutraceuticals, 3(1), 109-118. https://doi.org/10.3390/nutraceuticals3010009





