Exploring the Efficacy of Extracts for Cosmetic Creams: In Vivo and In Vitro Assessments
Abstract
:1. Introduction
2. Materials and Methods
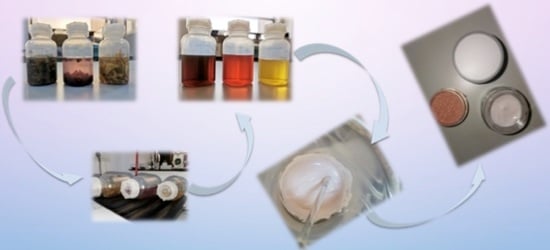
2.1. Biological Material
2.2. Determination of Bioactive Compounds
2.2.1. Determination of Phenol Quantity
2.2.2. Determination of Flavonoid Quantity
2.2.3. HPLC Assay
2.3. Cosmetic Cream Formulation
2.4. Determination of the In Vivo Antioxidant Potential under Blue Light
2.5. Determination of the In Vitro Antioxidant Capacity of the Extracts
2.6. Human Patch Test under Dermatological Control
2.7. Microbial Contamination Testing
2.8. Statistical Analysis
3. Results
3.1. Determination of Major Bioactive Compounds
3.2. Determination of the Antioxidant Effect In Vitro
3.3. Determination of the Antioxidant Effect In Vivo
3.4. Human Patch Test under Dermatological Control
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petran, M.; Dragos, D.; Gilca, M. Historical ethnobotanical review of medicinal plants used to treat children diseases in Romania (1860s–1970s). J. Ethnobiol. Ethnomed. 2020, 16, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.; Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyal, A.; Sharma, A.; Kaur, J.; Kumari, S.; Garg, M.; Sindhu, R.K.; Rahman, M.H.; Akhtar, M.F.; Tagde, P.; Najda, A.; et al. Bioactive-Based Cosmeceuticals: An Update on Emerging Trends. Molecules 2022, 27, 828. [Google Scholar] [CrossRef]
- Sator, P.G. Skin treatments and dermatological procedures to promote youthful skin. Clin. Interv. Aging 2006, 1, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Vulić, J.; Šeregelj, V.; Kalušević, A.; Lević, S.; Nedović, V.; Tumbas Šaponjac, V.; Čanadanović-Brunet, J.; Ćetković, G. Biodisponibilitatea și bioactivitatea fenolicelor încapsulate și a carotenoizilor izolați din deșeurile de ardei roșu. Molecules 2019, 24, 2837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vamanu, E.; Gatea, F.; Sârbu, I. In Vitro Ecological Response of the Human Gut Microbiome to Bioactive Extracts from Edible Wild Mushrooms. Molecules 2018, 23, 2128. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.B.; Hassan, S.; Waheed, M.; Javed, A.; Farooq, M.A.; Tahir, A. Biodisponibilitatea și Calea Metabolică a Compușilor Fenolici, Aspecte Fiziologice ale Plantelor ale Compușilor Fenolici; IntechOpen: London, UK, 2019. [Google Scholar]
- Garbossa, W.A.C.; Berardo, P.M.; Campos, G.M. Euterpe oleracea, Matricaria chamomilla, and Camellia sinensis as promising ingredients for development of skin care formulations. Ind. Crops Prod. 2016, 83, 1–10. [Google Scholar] [CrossRef]
- Kostic, M.; Ivanov, M.; Fernandes, Â.; Calhelha, R.C.; Glamoclija, J.; Barros, L.; Sokovi’c, M.; Ciric, A. A Comparative Study of Lactarius Mushrooms: Chemical Characterization, Antibacterial, Antibiofilm, Antioxidant and Cytotoxic Activity. J. Fungi 2023, 9, 70. [Google Scholar] [CrossRef]
- Choi, E.J.; Park, B.; Lee, J.; Kim, J. Anti-atopic dermatitis properties of Cordyceps militaris on TNFα/IFNγ-stimulated HaCaT cells and experimentally induced atopic dermatitis in mice. Phys. Act. Nutr. 2020, 4, 7–14. [Google Scholar] [CrossRef]
- Vamanu, E.; Gatea, F.; Sârbu, I.; Pelinescu, D. An In Vitro Study of the Influence of Curcuma longa Extracts on the Microbiota Modulation Process, In Patients with Hypertension. Pharmaceutics 2019, 11, 191. [Google Scholar] [CrossRef] [Green Version]
- Uddin, R.; Saha, M.R.; Subhan, N.; Hossain, H.; Jahan, I.A.; Akter, R.; Alam, A. HPLC-Analysis of Polyphenolic Compounds in Gardenia jasminoides and Determination of Antioxidant Activity by Using Free Radical Scavenging Assays. Adv. Pharm. Bul. 2014, 4, 273–281. [Google Scholar] [CrossRef]
- Saidi, A.; Hambaba, L.; Bensaad, M.S.; Melakhessou, M.A.; Bensouici, C.; Ferhat, N.; Kahoul, M.A.; Helal, M.; Sami, R.; Alharthy, S.A.; et al. Phenolic Characterization Using cLC-DAD Analysis and Evaluation of In Vitro and In Vivo Pharmacological Activities of Ruta tuberculata Forssk. Antioxidants 2022, 11, 1351. [Google Scholar] [CrossRef]
- Vamanu, E. Biological activities of the polysaccharides produced in submerged culture of two edible Pleurotus ostreatus mushrooms. J Biomed. Biotechnol. 2012, 2012, 565974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vamanu, E.; Dinu, L.D.; Luntraru, C.M.; Suciu, A. In Vitro Coliform Resistance to Bioactive Compounds in Urinary Infection, Assessed in a Lab Catheterization Model. Appl. Sci. 2021, 11, 4315. [Google Scholar] [CrossRef]
- Smaoui, S.; Hlima, H.B.; Chobba, I.B.; Kadri, A. Development and stability studies of sunscreen cream formulations containing three photo-protective filters. Arab. J. Chem. 2017, 10, S1216–S1222. [Google Scholar] [CrossRef] [Green Version]
- Soto, M.L.; Parada, M.; Falqué, E.; Domínguez, H. Personal-Care Products Formulated with Natural Antioxidant Extracts. Cosmetics 2018, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Butkeviciute, A.; Ramanauskiene, K.; Janulis, V. Formulation of Gels and Emulgels with Malus domestica Borkh: Apple Extracts and Their Biopharmaceutical Evaluation In Vitro. Antioxidants 2022, 11, 373. [Google Scholar] [CrossRef]
- Blue Light Facts: Is Light Blue Bad for Your Eyes? Available online: https://www.allaboutvision.com/cvs/blue-light.htm (accessed on 6 March 2023).
- Zlabiene, U.; Baranauskaite, J.; Kopustinskiene, D.M.; Bernatoniene, J. In Vitro and Clinical Safety Assessment of the Multiple W/O/W Emulsion Based on the Active Ingredients from Rosmarinus officinalis L., Avena sativa L. and Linum usitatissimum L. Pharmaceutics 2021, 13, 732. [Google Scholar] [CrossRef] [PubMed]
- Dabulici, C.M.; Sârbu, I.; Vamanu, E. The Bioactive Potential of Functional Products and Bioavailability of Phenolic Compounds. Foods 2020, 9, 953. [Google Scholar] [CrossRef]
- Bashir, A.; Lambert, P. Microbiological study of used cosmetic products: Highlighting possible impact on consumer health. J Appl. Microbiol. 2020, 128, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Vamanu, E.; Nita, S. Antioxidant capacity and the correlation with major phenolic compounds, anthocyanin, and tocopherol content in various extracts from the wild edible Boletus edulis mushroom. Biomed. Res. Int. 2013, 2013, 313905. [Google Scholar] [CrossRef] [Green Version]
- Vreeburg, R.A.; Airianah, O.B.; Fry, S.C. Fingerprinting of hydroxyl radical-attacked polysaccharides by N-isopropyl-2-aminoacridone labelling. Biochem. J. 2014, 463, 225–237. [Google Scholar] [CrossRef] [Green Version]
- Roncea, F.; Cazacincu, R.; Mireşan, H.; Roșca, R.A. Nutricosmetics. Updates and perspectives. Farmacist 2019, 6, 191. [Google Scholar] [CrossRef]
- Laneri, S.; Di Lorenzo, R.M.; Bernardi, A.; Sacchi, A.; Dini, I. Aloe barbadensis: A Plant of Nutricosmetic Interest. Nat. Prod. Commun. 2020, 15, 7. [Google Scholar] [CrossRef]
- Wu, Y.; Choi, M.-H.; Li, J.; Yang, H.; Shin, H.-J. Mushroom Cosmetics: The Present and Future. Cosmetics 2016, 3, 22. [Google Scholar] [CrossRef]
- Gigliobianco, M.R.; Cortese, M.; Nannini, S.; Di Nicolantonio, L.; Peregrina, D.V.; Lupidi, G.; Vitali, L.A.; Bocchietto, E.; Di Martino, P.; Censi, R. Chemical, Antioxidant, and Antimicrobial Properties of the Peel and Male Flower By-Products of Four Varieties of Punica granatum L. Cultivated in the Marche Region for Their Use in Cosmetic Products. Antioxidants 2022, 11, 768. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- de Andrade Arruda Fernandes, I.; Maciel, G.M.; Ribeiro, V.R.; Rossetto, R.; Pedro, A.C.; Haminiuk, C.W.I. The role of bacterial cellulose loaded with plant phenolics in prevention of UV-induced skin damage. Carbohyd. Pol. Technol. Appl. 2021, 2, 100122. [Google Scholar] [CrossRef]
- Alharbi, K.L.; Raman, J.; Shin, H.-J. Date Fruit and Seed in Nutricosmetics. Cosmetics 2021, 8, 59. [Google Scholar] [CrossRef]
- Ma, X.; Yang, M.; He, Y.; Zhai, C.; Li, C. A review on the production, structure, bioactivities and applications of Tremella polysaccharides. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211000541. [Google Scholar] [CrossRef]
- Kosanic, M.; Petrović, N.; Milosevic-Djordjevic, O.; Grujičić, D.; Tubic, J.; Marković, A.; Stanojkovic, T.P. The health promoting effects of the fruiting bodies extract of the peppery milk cap mushroom Lactarius piperatus (Agaricomycetes) from Serbia. Int. J. Med. Mushrooms 2020, 22, 4. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Jakovljevic, D.; Todorovic, N.; Vunduk, J.; Petrović, P.; Niksic, M.; Vrvic, M.M.; Van Griensven, L. Antioxidants of Edible Mushrooms. Molecules 2015, 20, 19489–19525. [Google Scholar] [CrossRef] [Green Version]
- El-Wahed, A.A.A.; Khalifa, S.A.M.; Elashal, M.H.; Musharraf, S.G.; Saeed, A.; Khatib, A.; Tahir, H.E.; Zou, X.; Naggar, Y.A.; Mehmood, A.; et al. Cosmetic Applications of Bee Venom. Toxins 2021, 13, 810. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papa, C.M.; Suciu, A.; Dopcea, I.; Ene, N.; Singh, S.K.; Vamanu, E. Exploring the Efficacy of Extracts for Cosmetic Creams: In Vivo and In Vitro Assessments. Nutraceuticals 2023, 3, 306-314. https://doi.org/10.3390/nutraceuticals3030024
Papa CM, Suciu A, Dopcea I, Ene N, Singh SK, Vamanu E. Exploring the Efficacy of Extracts for Cosmetic Creams: In Vivo and In Vitro Assessments. Nutraceuticals. 2023; 3(3):306-314. https://doi.org/10.3390/nutraceuticals3030024
Chicago/Turabian StylePapa, Cristina Monica, Alexandru Suciu, Ioan Dopcea, Nicoleta Ene, Sandeep Kumar Singh, and Emanuel Vamanu. 2023. "Exploring the Efficacy of Extracts for Cosmetic Creams: In Vivo and In Vitro Assessments" Nutraceuticals 3, no. 3: 306-314. https://doi.org/10.3390/nutraceuticals3030024
APA StylePapa, C. M., Suciu, A., Dopcea, I., Ene, N., Singh, S. K., & Vamanu, E. (2023). Exploring the Efficacy of Extracts for Cosmetic Creams: In Vivo and In Vitro Assessments. Nutraceuticals, 3(3), 306-314. https://doi.org/10.3390/nutraceuticals3030024