Abstract
The rapid aging of the Western countries’ populations makes increasingly necessary the promotion of healthy lifestyles in order to prevent/delay the onset of age-related diseases. The use of functional foods can significantly help to achieve this aim, thanks to the contribution of biologically active compounds suitable to protect cellular and metabolic homeostasis from damage caused by stress factors. Indeed, the excessive production of reactive oxygen species (ROS), favored by incorrect eating and behavioral habits, are considered causal elements of oxidative stress, which in turn favors tissue and organism aging. Microalgae represent a convenient and suitable functional food because of their extraordinary ability to concentrate various active compounds, comprising omega-3 polyunsaturated fatty acids, sterols, phenolic compounds, carotenoids and others. Within cells, mitochondria are the cellular organelles most affected by the accumulation of molecular damage produced by oxidative stress. Since, in addition to producing the chemical energy for cellular metabolism, mitochondria control numerous cell cycle regulation processes, including intrinsic apoptosis, responses to inflammatory signals and other biochemical pathways, their dysfunction is considered decisive for many pathologies. Among these, some degenerative diseases of the nervous system, cardiovascular system, kidney function and even cancer are found. From this viewpoint, bioactive compounds of microalgae, in addition to possessing high antioxidant properties, can enhance mitochondrial functionality by modulating the expression of numerous protective factors and enzymes, which in turn regulate some essential biochemical pathways for the preservation of the functional integrity of the cell. Here, we summarize the current knowledge on the role played by microalgal compounds in the regulation of the mitochondrial life cycle, expression of protective and reparative enzymes, regulation of intrinsic apoptosis and modulation of some key biochemical pathways. Special attention was paid to the composition of some cultivable microalgae strains selected for their high content of active compounds suitable to protect and improve mitochondrial functions.
Keywords:
microalgae; mitochondria; oxidative stress; antioxidants; PUFA; carotenoids; sterols; polyphenols; chronic diseases 1. Introduction
In the last 70 years, the world population has grown from 2.5 billion to over 7 billion, but a general aging process has been occurring since 1980, albeit geographically uneven, which will lead to the number of people aged between 15 and 59 in developed countries halving by 2100 [1]. Among the consequent impacts, healthcare costs represent a problem deserving special attention, both for the present situation and, in perspective, for the future evolution of the world population [2,3,4,5]. In particular, long-term chronic-degenerative diseases increase with the fraction of the elderly population [2], and the adoption of policies aimed at delaying the onset and severity of these diseases represents the best strategy for managing their social and economic impact [3,4,5].
There is a general scientific consensus considering the diet as one of the main factors promoting and maintaining human health status, especially aiming at the prevention of diseases due to aging [6,7]. Unfortunately, the Western diet constitutes a primary risk factor, together with insufficient physical activity, being characterized by an excessive intake of calories, ultra-processed foods, saturated fats, carbohydrates with a high glycemic index and salt [8]. At the same time, it is often deficient in healthy compounds which are abundant in plants, i.e., antioxidants, minerals, vitamins and fibers [9], and which can significantly contribute to the reduction of certain diseases and extend lifespan [7].
As part of a synergistic effort of scientific research and sustainable economic development aimed at promoting healthy aging, the identification and characterization of functional foods assume great relevance. These, even taken in modest amounts, can effectively contribute to favor the improvement of the diet of populations with a high aging rate while, at the same time, supporting economic policies oriented towards a sustainable development model [10,11,12]. In this context, many microalgae are now considered innovative functional foods which could play an important role in delaying aging and also in reducing the impact of many age-related diseases [13,14,15,16]. The literature reports on a huge variety of studies highlighting the value of microalgae as a source of healthful substances, especially antioxidant compounds [14,16,17,18,19,20,21]. Indeed, oxidative stress represents one of the main mechanisms of alteration of cellular and tissue homeostasis at the basis of aging and many important diseases, including various types of cancer and neurological diseases [22,23].
Mitochondria represent the cellular components in which the highest number of redox reactions take place and are, therefore, the organelles most exposed to oxidative damage. The significant endowment of enzymes aimed at preventing oxidative damage and the related functional implications confers a primary role to the mitochondrion in the protection of the cell from oxidative stress and related pathologies [24,25,26]. Notwithstanding, the importance of proper nutrition for preserving the mitochondrion’s functional integrity is still an insufficiently understood topic, despite being increasingly at the center of clinical research. In this short review, the potential benefits of microalgal compounds in the prevention of functional mitochondrial disorders are specifically discussed. In particular, we tried to summarize some mechanisms of action of the main active compounds present in microalgae, highlighting how their action is not only due to mere chemical protection from oxidant compounds but also to their ability to modulate the life cycle of mitochondria and their main biochemical pathways.
This contribution is dedicated to the memory of Prof. Mario Roberto Tredici (University of Florence) in recognition of his irreplaceable contribution to the development of microalgae biotechnology and of his passionate dedication, which he passed to those who had the fortune to collaborate with him.
2. Functions of Mitochondria
Mitochondria are involved in many other essential metabolic activities, including the control of cellular apoptosis, in addition to being responsible for the processes of cellular respiration and ATP synthesis.
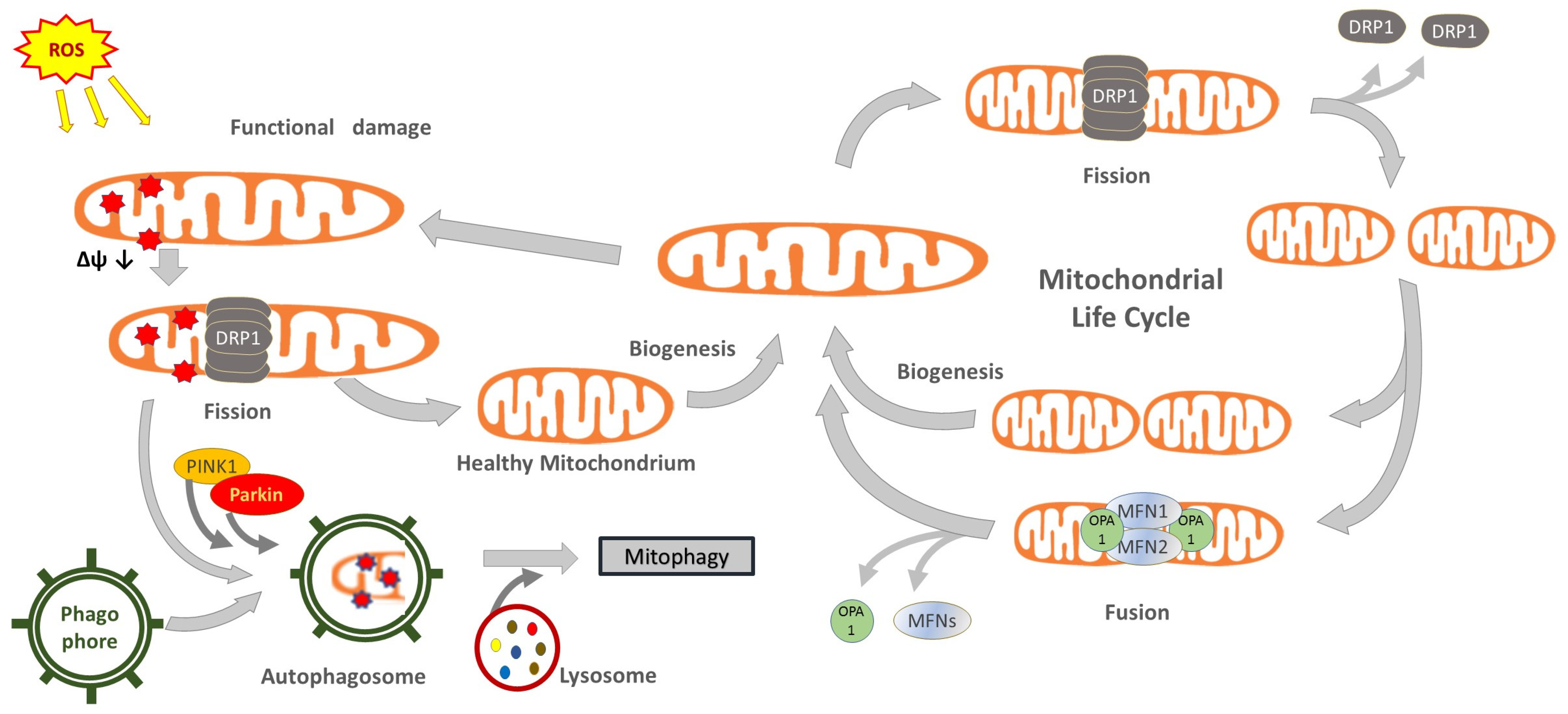
Their outer membrane delimits a space further compartmentalized by the inner membrane, very developed and folded into ridges, where numerous enzymes are integrated, such as those constituting the respiratory chain, also known as the electron transport chain. The inner membrane delimits an internal matrix in which a specific DNA strand and ribosomes can be found, allowing the synthesis of part of mitochondrial structural and enzymatic proteins. However, most of them must be synthesized in the cytoplasm and then imported by specific transporters. Mitochondria follow a complex life cycle within the cell, the main phases of which are defined as biogenesis, fusion, fission and mitophagy (Figure 1). These phases, also called mitochondrial dynamics, are characterized by interactive processes among the organelles, which behave as an integrated mitochondrial reticulum [27]. During the biogenesis phase, mitochondria modulate their mass, function, size and morphology through processes controlled by a network of transcription factors and coregulators (cf. Table 1) which, in addition to the fewer but essential genes of the mtDNA, also require the coordinated transcription of a large number of genes in the nucleus [28,29,30]. Furthermore, mitochondria can mix their content and generate extended organelle networks through a process named fusion [27]. This phase implies the fusion of the outer membrane controlled by mitofusins, i.e., large Mfn1 and Mfn2 GTPases, whereas OPA1 Mitochondrial Dynamin Like GTPase (OPA1) intermembrane protein is required for inner membrane fusion [31,32].
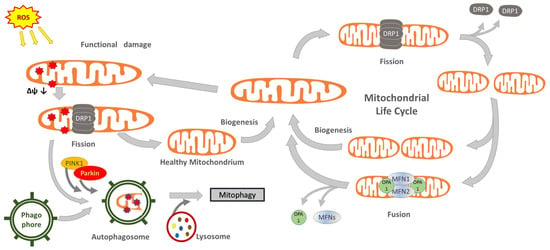
Figure 1.
Conceptual illustration of the mitochondrial life cycle and the contribution of mitochondrial dynamics and mitophagy to maintain organelle shape, size and functionality. Abbreviations: Drp1, dynamin-related-protein 1; Mfn, mitofusin; OPA1, mitochondrial dynamin like GTPase; parkin, E3 ubiquitin-protein ligase parkin; Pink1, PTEN-induced putative kinase protein 1.
Following the replication of their single chromosome, mitochondria can proceed to the division phase, called fission [33]. This allows for the replacement of dysfunctional mitochondria or, in the case of cell replication, provides an adequate number of organelles to be segregated between daughter cells. Fission requires the intervention of dynamin-related protein 1 (Drp1), a cytosolic GTPase that translocates to the outer mitochondrial membrane for assembling multimeric rings, which induce mitochondrial division [27,34,35,36]. Importantly, fission also allows the separation of dysfunctional parts of the mitochondrion [37]. The degradation of dysfunctional parts, or entire damaged mitochondria, occurs through a special autophagy clearance phase known as mitophagy. This phase is controlled by the PTEN-induced putative kinase protein 1 (PINK1), a protein kinase activated by the depolarization of the organelle membrane and which recruits the E3 ubiquitin-protein ligase parkin (parkin) to the outer mitochondrial membrane, which in turn promotes mitophagy by binding ubiquitin moieties as degradation signal (ubiquitination) [38,39,40]. An alternative pathway is regulated by the mitophagic receptors BCL-2 interacting protein 3 Like (Bnip3L alias Nix) and BCL-2 interacting protein 3 (Bnip3) [40]. Mitophagy, however, is a complex phase involving selective autophagic events, in which mitochondrial and other cytoplasmic materials are sequestered in double-membrane-delimited vesicles, called autophagosomes, then fused to lysosomes to form autolysosomes where materials are degraded. Several enzymes, membrane transporters and receptors are involved, among which a pivotal function is attributed to processing regulators, such as autophagy related 5 (Atg5) and beclin-1 (BECN1) [40].

Table 1.
Compounds known for their modulatory activities on the main mitochondrial cycle phases and on their main regulatory enzymes/pathways (the compounds found in microalgae are underlined). Abbreviations: Atg5, autophagy related 5; BCN1, beclin-1; Bnip3, BCL-2 interacting protein 3; DHA, docosahexaenoic acid; Drp1, dynamin-related-protein 1; EPA, eicosapentaenoic acid; Fis1, mitochondrial fission 1 protein; Mfns, mitofusins; MT, mitochondrion; NRF1, nuclear respiratory factor-1; parkin, E3 ubiquitin-protein ligase parkin; Nrf2, nuclear factor erythroid 2-related factor 2; OPA1, mitochondrial dynamin like GTPase; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator-1α; Pink1, PTEN-induced kinase 1; Prk2, protein kinase C-related kinase 2; SIRT1, silent information regulator-1; TFAM, transcription factor A mitochondrial; TFBM, transcription factor B mitochondrial.
Table 1.
Compounds known for their modulatory activities on the main mitochondrial cycle phases and on their main regulatory enzymes/pathways (the compounds found in microalgae are underlined). Abbreviations: Atg5, autophagy related 5; BCN1, beclin-1; Bnip3, BCL-2 interacting protein 3; DHA, docosahexaenoic acid; Drp1, dynamin-related-protein 1; EPA, eicosapentaenoic acid; Fis1, mitochondrial fission 1 protein; Mfns, mitofusins; MT, mitochondrion; NRF1, nuclear respiratory factor-1; parkin, E3 ubiquitin-protein ligase parkin; Nrf2, nuclear factor erythroid 2-related factor 2; OPA1, mitochondrial dynamin like GTPase; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator-1α; Pink1, PTEN-induced kinase 1; Prk2, protein kinase C-related kinase 2; SIRT1, silent information regulator-1; TFAM, transcription factor A mitochondrial; TFBM, transcription factor B mitochondrial.
| Modulatory Active Compound | Ref. | Key Regulatory Enzymes or Pathways | Cycle Phase | Description |
|---|---|---|---|---|
| Astaxanthin | [41,42] | PGC-1α, Tfam | Biogenesis | MTs increase by organelle division. The organelles undergo mtDNA replication and subsequent division. |
| EPA | [43] | NRF-1, TFAM, COXIV, SIRT1, PGC-1α | ||
| EPA/DHA, curcumin | [44,45,46] | PGC-1α, NRF1 | ||
| Fucoxanthin | [47] | NRF1, NRF2 | ||
| Quercetin, resveratrol | [45] | Nrf2 | ||
| Salidroside (Rhodiola) | [48] | SIRT1 | ||
| Fucoxanthin, curcumin | [45,47,49] | PGC1α, Tfam | ||
| Fucoxanthin | [47] | Mfns; Opa1 | Fusion | Coordinated fusion of the inner and outer membranes between two organelles aimed to merge intact and slightly dysfunctional MTs. It is particularly useful in case of damaged mtDNA. |
| Omega-3 fatty acids | [46] | Mfns; Opa1 | ||
| Resveratrol | [32] | Mfn2 | ||
| Omega-3 fatty acids | [46] | Drp1, Fis1 | Fission | Separation of the MT into two smaller units. Fission allows the isolation of damaged MT parts for elimination, but it becomes massive in the case of apoptosis. |
| 1H-pyrrole-2-carboxamide compounds (synthetic) | [34] | Drp1 | ||
| Astaxanthin | [42,50] | Drp1 | ||
| Curcumin, astaxanthin, resveratrol, hydroxytyrosol, oleuropein, spermidine | [51] | Modulation of several mitophagy mediators | Mitophagy | Autophagic degradation of irreversibly damaged MTs or part of them. |
| Astaxanthin | [42] | PINK, parkin | ||
| Fucoxanthin | [49] | Pink1, Prk2, Bnip3, BECN1, Atg5 |
The regulation of mitochondrial dynamics can directly affect the mass and functionality of the mitochondrial reticulum. Many compounds, some reported in Table 1, modulate mitochondrial dynamics by acting on key enzymes or regulating the expression of relevant cytosolic and mitochondrial factors. Among these, central functions are performed by peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), nuclear respiratory factor-1 (NRF1), nuclear factor erythroid 2-related factor 2 (Nrf2), transcription factor A mitochondrial (TFAM) and transcription factor B mitochondrial (TFBM), which are all positive regulators of the mitochondrial biogenesis.
PGC-1α interacts with many cell functions and is regulated at both transcription and post-translation levels, e.g., activated by deacetylation by silent information regulator-1 (SIRT1). Among other effects, it modulates mitochondrial biogenesis by interacting with both NRF1 and Nrf2 nuclear respiratory factors [29]. In turn, NRF1 and Nrf2 regulate the expression of subunits of the electron transfer chain and promote mtDNA transcription [29]. NRF1 is also a promoter factor of the expression of TFAM and of other promoters required for the basal transcription of mitochondrial DNA, in particular of TFB1M and TFB2M (transcription factor B1 and B2, mitochondrial, respectively) [29,52]. In the activated form, Nrf2 translocates to the nucleus, where it regulates the expression of several genes by binding to the antioxidant response elements (AREs) included in their promoter regions, four of which are also in the NRF1 promoter [29].
As the powerhouse of the cell, the mitochondrial enzymatic machinery carries out the redox processes by which the chemical energy of organic compounds is transferred to ATP molecules (glycolysis, Krebs cycle and beta-oxidation of fatty acids), then directly used as an energy source for the cell. The transfer of electrons through the respiratory chain implies the production of a high amount of reactive oxygen species (ROS), i.e., the main actors involved in the oxidative stress, which in turn is believed to be the main cause of the molecular damage at the base of cellular aging processes [24,53,54,55]. Oxidative damage produced by ROS is kept under control by several enzymes (Table 2), which perform two essential functions: the prevention of the excessive concentration or persistence of ROS and the repair of molecular damage produced by these reactive compounds.
The biochemical network of protection mechanisms against oxidative damage and related signaling pathways is indicated by the term “mitohormesis” [30]. Despite defense mechanisms, the progressive accumulation of oxidative damage leads to dysfunctionalities of the mitochondrial reticulum, which is considered to be among the main causes of aging [56,57] and of numerous chronic degenerative noncommunicable diseases (NCDs), such as nonalcoholic steatohepatitis (NASH) [58,59,60,61], muscle insulin resistance related to obesity [62,63], kidney pathologies [64], cardiovascular pathologies [25,65,66], Parkinson’s disease [67], Huntington’s disease [68], and Alzheimer’s disease [54,69]. The study of the correlation among the morpho-functional alteration of mitochondria, the aging processes and the onset of numerous chronic and degenerative diseases made it possible to highlight the importance of diet in the preservation of mitohormesis. In particular, the intake of foods containing compounds suitable to enhance the protective mechanisms of mitochondrial functionality is now considered a primary factor for delaying aging and for preventing related diseases [51]. Table 1 and Table 2 list some compounds which have been shown to modulate protective enzymes or specific processes of the mitochondrial life cycle. Notably, most of these molecules present antioxidant properties in vitro, therefore, they are able to inactivate ROS by direct chemical interaction, and this has often led to the belief that their primary biological activity is due to this mechanism. However, even if it is evident that antioxidant activity is, to varying degrees, involved in the effects produced by antioxidants, it was demonstrated that these compounds often trigger much more complex interactions and show biological properties different from those just attributable to antioxidant activity. As an illustrative example, the highly studied case of resveratrol can be considered referring to the so-called “French paradox” [70]. This consists in the low incidence of coronary heart disease observed in French people, while having a diet relatively rich in saturated fats. According to authors’ hypothesis, this was attributed to the protective effect of resveratrol, taken through a moderate consumption of red wine. Resveratrol is a polyphenol with antioxidant properties modulating various cellular and even mitochondrial processes (cf. Table 1 and Table 2), producing a protective action against cardiovascular pathologies. However, this protection occurs even assuming very small amounts of this compound, which would not justify a significant effect in terms of chemical antioxidant action.

Table 2.
Factors/proteins/compounds known as regulators of activity or gene expression of mitochondrial enzymes involved in the prevention and repair of damage due to ROS. Abbreviations: AP-1, activator protein 1; AP-2, adaptor protein complex 2; ARE, antioxidant response element; C/EPB, CCAAT/enhancer-binding protein; CREB, cAMP response element-binding protein; DHA, docosahexaenoic acid; Egr1, Early growth response protein 1; FOXO3a, forkhead box O3a; HIF-1, hypoxia-inducible factor 1; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NRF-1, nuclear respiratory factor-1; Nrf2, nuclear factor erythroid 2-related factor 2; Oct-1, POU domain, class 2, transcription factor 1; p53, cellular tumor antigen p53; PIG3, p53 inducible gene 3; PPARγ, peroxisome proliferator activated receptor γ; SIRT, sirtuin; Sp1, specificity protein 1.
Table 2.
Factors/proteins/compounds known as regulators of activity or gene expression of mitochondrial enzymes involved in the prevention and repair of damage due to ROS. Abbreviations: AP-1, activator protein 1; AP-2, adaptor protein complex 2; ARE, antioxidant response element; C/EPB, CCAAT/enhancer-binding protein; CREB, cAMP response element-binding protein; DHA, docosahexaenoic acid; Egr1, Early growth response protein 1; FOXO3a, forkhead box O3a; HIF-1, hypoxia-inducible factor 1; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NRF-1, nuclear respiratory factor-1; Nrf2, nuclear factor erythroid 2-related factor 2; Oct-1, POU domain, class 2, transcription factor 1; p53, cellular tumor antigen p53; PIG3, p53 inducible gene 3; PPARγ, peroxisome proliferator activated receptor γ; SIRT, sirtuin; Sp1, specificity protein 1.
| Factor/Protein/Compound Able to Regulate Target Enzyme or Its Gene Expression | Effect | Ref. | Target Enzyme | Target Enzyme Function |
|---|---|---|---|---|
| PPARγ | Up | [71] | Catalase | Hydrogen peroxide (H2O2) decomposition to oxygen (O2) and water (H2O). |
| Oct-1 | Up | [71] | ||
| Astaxanthin | Up | [72] | ||
| DHA (via Nrf2) | Up | [73] | ||
| p53, PIG3 | Down | [74] | ||
| MicroRNA-30b | Up | [75] | ||
| NF-κB, (Sp1), C/EBP, SIRT, FOXO3a, CREB | Up | [73,76,77,78] | Mn-Superoxide dismutase (SOD2) | Manganese enzyme expressed in the inner matrix catalyzes the dismutation of the superoxide radical (O2•−) into ordinary molecular oxygen (O2) and hydrogen peroxide (H2O2). |
| AP-1 | Up | [79] | ||
| AP-2 | Down | [76] | ||
| p53, p50 | Down | [78,80] | ||
| miR-146a | Down | [81] | ||
| Quercetin | Down | [82] | ||
| Curcumin | Up | [83] | ||
| Astaxanthin | Up | [72] | ||
| Sp1, C/EBP, Egr1, Nrf2, NF-κB, ELAV-like proteins, resveratrol | Up | [84,85,86,87] | Cu,Zn-Superoxyde dismutase (SOD1) | Copper-zinc enzyme with the same function as SOD-2 but expressed in the inter-membrane space [77,88]. |
| AP-1 | Down | [78,89] | ||
| Quercetin | Down | [82] | ||
| ARE/EpRE | Up | [90] | Peroxiredoxins (Prx3) | Enzymes are able to catalyze the oxidation of the redox-active cysteine (i.e., peroxidatic cysteine) to a sulfenic acid by the peroxide substrate. |
| Angiotensin II | Down | [91] | ||
| SOD2 | Up | [92] | Thioredoxin (TNX2) | Enzymes are expressed by a nuclear gene and imported into the mitochondrion, which carries out ROS scavenging activity with the concomitant anti-apoptotic effect [93]. |
| Curcumin | Up | [83] | ||
| Resveratrol | Up | [85] | Glutathione peroxidase-1(GPx-1) | A selenocysteine-containing enzyme involved in the reductive detoxification of peroxides. Its expression seems stimulated by the epidermal growth factor (EGF). |
| Genistein | Up | [94] | ||
| Quercetin | Down | [82] | ||
| Resveratrol | Up | [85] | Glutathione (GSH) | It protects the cell from respiration-induced reactive oxygen species and detoxifies lipid hydroperoxides and electrophiles. |
| Quercetin | Down | [45] | ||
| Nrf2/Nrf1 via ARE, AP-1 and NF-kB | Up | [95] | ||
| Procyanidin B2 (upregulation of P1 isoform via nuclear translocation of Nrf2) | Up | [96] | Glutathione-S- transferases (GSTs) | Mitochondrial GSTs display both GSH transferase and peroxidase activities for the detoxification of harmful byproducts [97]. |
| Obtusilactone A (OA) and (−)-sesamin | Down | [98] | Lon proteases | They decompose damaged and misfolded proteins tagged for degradation at their –COOH or –NH3 terminus [57]. Mitochondrial biogenesis in mammalian cells is partly regulated by the matrix Lon protease [99]. |
| Acute stressors, such as heat shock, serum starvation, and oxidative stress (Nrf-2, HIF-1) | Up | [100] | ||
| Only synthetic molecules are known: β-lactones (A2-32-01); Phenyl esters (AV167, TG42, TG53); α-aminoboronic acid | Down | [101] | Clp proteases | Variants of chaperon ATPase subunits (ClpA, ClpC, ClpE etc.) combined with a proteolytic subunit (ClpP) [57]. |
| Only synthetic molecules are known: Acyldepsipeptide analogs (ADEP-41); imipridones (ONC201, ONC212, TR57) | Up | [101] |
Mitochondrial Apoptosis and the Role of BCL-2 Family Proteins
Many compounds found in microalgae show to affect the regulation of apoptosis, a cell death process in which mitochondria are key players. Apoptosis is a complex and still not fully understood process, the discussion of which is beyond the scope of the present review. However, some essential mechanisms of action are summarized here since they will be frequently mentioned in the following discussion of the bioactivities of microalgal compounds.
In healthy subjects, apoptosis is a physiological mechanism by which the transformation of cells into cancerous cells is prevented; therefore, its regulation assumes relevant therapeutic applications. Mitochondrial activation of apoptosis is controlled by proteins of the BCL-2 family, i.e., proteins sharing the BCL-2 homology (BH) domains, numbered from 1 to 4, and playing both pro- and anti-apoptotic roles. The antiapoptotic proteins which contain four BH domains (BH1-BH4) include B-cell lymphoma 2 (BCL-2), B-cell lymphoma-extra-large (BCL-xL), BCL-2-like protein 2 (BCL2L2 alias BCL-w), BCL-2 family protein myeloid cell leukemia-1 (Mcl-1), and BCL-2-related protein A1 (A1) [102]. Proapoptotic BH1-BH4 effectors, which directly promote the mitochondrial outer membrane permeabilization, consist of BCL-2-associated X protein (Bax) and BCL-2 homologous antagonist killer (Bak) [102]. The BCL-2 family also includes pro-apoptotic BH-3 only proteins: BH3 interacting-domain death agonist (Bid), BCL-2-like protein 11 (Bim), BCL-2-interacting killer (Bik), BCL-2-associated death promoter (Bad), BCL-2-modifying factor (Bmf), harakiri BCL-2 interacting protein (Hrk), phorbol-12-myristate-13-acetate-induced protein 1 (PMAIP1, generally mentioned with the alias Noxa), and p53-upregulated modulator of apoptosis (Puma) [103,104].
The interaction among these apoptosis actors and regulators is complicated and not completely elucidated, but some recent reviews with complementary insights were published by Peña-Blanco and Garcıa-Sáez [102], Kale et al. [105], and Roufayel et al. [103], from which some of the following concepts were here synthesized.
As a very schematic model, Bak and Bax represent the apoptotic effectors, which translocate from cytosol to the mitochondrial outer membrane following their activation, where they can accumulate. There, they induce the formation of pores though which activators of the caspase cascade, such as SMAC/DIABLO protein and cytochrome c (Cyt-c), are released to the cytosol, triggering cell death in a short time. Concerning the apoptotic event, Bak/Bax can be activated by the interaction with BH3-only proteins or by dephosphorylation as an effect of specific direct activators. On the contrary, their inhibition occurs by dimerization binding with antiapoptotic BCL-2 proteins. On the other side, BH3-only proteins can antagonize Bak/Bax to bind antiapoptotic BCL-2 proteins and form heterodimers, thus releasing the apoptotic proteins, which can accumulate on the mitochondrial membrane.
Various models describe how BCL-2 family proteins competitively interact with each other to control apoptosis [103]. It is interesting to note that activation/inhibition of different BCL-2 proteins is triggered by some biochemical pathways, with which compounds found in microalgae can interact and by oxidative stress itself. For instance, the regulation of the activity of BCL-2 proteins, which can occur at the gene expression level and/or by phosphorylation/de-phosphorylation, is strongly affected by the ERK1/2 MAP kinase signaling (ERK, extracellular signal-regulated kinase; MAP, mitogen-activated protein) through several and alternative biochemical routes [106].
3. Conceptualization of the Functionality of Foods and Implications of Their Active Compounds
Many compounds with an activity on the protection of mitochondria, and other cellular components, are normally present in the human diet, but only in very small amounts. The recent awareness of their relevance has progressively pushed the research to look for functional foods, i.e., foods particularly rich in suitable molecules for significant diet supplementation. There is no internationally agreed definition of functional food, but the regulations issued by several countries share the assumption that it “has the ability to promote well-being and health beyond basic nutritional properties” [107]. The definition introduced by the European Food Safety Authority (EFSA) specifies that the effects of functional foods must be: “… relevant to either an improved state of health and well-being and/or reduction of risk of disease. […] and it must demonstrate their effects in amounts that can normally be expected to be consumed in the diet”, while experts of the Functional Food Center (USA) specified that active food compounds must “… provide a clinically proven and documented health benefit utilizing specific biomarkers for the prevention, management, or treatment of chronic disease or its symptoms” [10]. Beyond the above definitions, a shared concept is that a functional food must contain compounds considered not essential for human nutrition and yet able to positively modulate metabolic processes and cellular functions as well as to protect from detrimental alterations of the well-being state. The latter can be intended as consistent with the functional decay due to aging or even related to actual pathological conditions. Furthermore, some compounds found in functional foods could be included in a further functional category of relatively recent and fairly uncertain definition, namely nutraceutical compounds. This term implies an intermediate biological activity between that of a nutrient and that of a drug, but the proposed definitions are, once again, grounded on the effectiveness of preventing or treating pathologies or metabolic disorders [108,109]. The significant overlap with the definition of functional food is evident, even if nutraceuticals do not necessarily have to be foods or part of them. Generally, nutraceuticals are provided as one or more natural nutrients in powder or tablet form, therefore similar to typical drug preparations, without however falling into this category [110]. It is worth noting here that the concepts underlying these definitions, often influenced by commercial needs, are not really new, having already been implicitly introduced by various forms of traditional or alternative medicine, such as traditional Chinese medicine, Indian Ayurvedic medicine or, for some aspects, homeopathic medicine [111,112].
The most interesting point of this heritage of concepts, beyond the implicit interpretations of many definitions, relies on a novel approach for the interaction among diet, metabolism and health state. The ever deeper understanding of the mechanisms of action of biologically active substances, such as polyphenols, carotenoids, omega-3 polyunsaturated fatty acids (PUFAs), polysaccharides, alkaloids, etc., has encouraged the research for novel sources of compounds suitable to support a thriving industry focused on the development of food supplements.
In the last two decades, microalgae have been particularly studied for their ability to concentrate in a unique cell, several actives suitable to promote well-being and health, which are often rare in foods from terrestrial ecosystems [13,14,16,113,114,115,116]. Even if some microalgal species can be considered for their relevant contribution to macronutrients, in general, the nutritional interest of these microorganisms is closely related to their supply of active compounds. For instance, the food use of Haematococcus derivatives provides a high amount of astaxanthin (a very active carotenoid) which cannot be compared with any traditional food [19]. Furthermore, an ethical issue concerning the preservation of natural resources should be considered. In a previous review, we showed that a daily intake of 6 g dry weight (DW) of Nannochloropsis can guarantee the intake of 240 mg/day of eicosapentaenoic acid (EPA), as recommended by EFSA, comparable to a weekly consumption of about 236 g fresh weight of seabream or seabass [117]. Fish oil required to produce these prized fishes can be obtained from 800–1200 g of wild forage fish, which appears ecologically unsustainable.
Generally, microalgae are cultivated for the content of their most represented active compound, justifying the claim of their nutritional “functionality”. However, if also all minor active compounds comprised in the same biomass are considered, microalgae should be regarded as “multifunctional” foods, as will become evident in the following part of the present review. Indeed, it is opportune here to mention that microalgae and cyanobacteria are rich in several compounds, not treated in the following discussion, but very useful for human health and even for therapeutic applications. For instance, the cyanobacteria Arthrospira can contain up to 60% DW of proteins rich in essential amino acids [118]. Microalgal polysaccharides of the cell wall can contribute approximately 10% of the DW [119] and comprise different heteropolysaccharides and monosaccharides, with potential and still unexplored bioactivities, whereas some microalgae are known sources of extracellular sulfated polysaccharides with relevant effects on the human metabolism [120]. Moreover, microalgae are a valuable source of vitamins such as tocopherols and important vitamins of the B group (e.g., B6, B9, B12), also comprising some of them poorly found in terrestrial plants, such as vitamins D and K [15]. The high content of minerals is consistent with the composition of ash, which can vary between 13–18% in marine microalgae and 4.5–6.7% in some freshwater species, such as Chlorella and Arthrospira [119]. Some minerals of high interest for nutritional scopes can be abundant: for instance, Phaeodactylum tricornutum contains about 5% DW of Ca and 0.24% DW of Fe [119].
Importantly, fish farmers exploited for decades the exceptional nutritional properties of some microalgae for weaning larvae of fish and shrimps [117]. This provided strong evidence of their potential and safety as a source of bioactive compounds for human nutritional applications, also because microalgae do not share common pathogens with humans. Interestingly, their rigid cell wall can be exploited as a natural encapsulation to protect new-generation therapeutics from the aggressive environment of the stomach, as recently proposed for an edible preparation of S-glycoprotein and soluble angiotensin-converting enzyme 2 (ACE2) for the treatment/inhibition of SARS-CoV-2 [121]. Despite this, still few microalgal strains are Generally Recognized As Safe (GRAS) in the USA or are authorized for human food use in other relevant markets. In this regard, the situation is quite confusing since some microalgae were already approved as raw biomass ingredients, but in other cases, only as a source of edible extracts (generally oils). However, there are inconsistencies among the consulted references, whereas databases of national authorities are not easily searchable. Consequently, some inaccuracies or deficiencies could also be reported in the present work. To the best of our knowledge, only the following species were authorized for human consumption [117,122,123]: Arthrospira platensis in the USA, Canada, the EU, India, Japan and China; Haematococcus pluvialis, and Chlamydomonas reinhardtii in the USA and China; Auxenochlorella pyrenoidosa in the EU and China; A. protothecoides in the USA, the EU and Japan; Chlorella vulgaris in the EU and Japan; C. sorokiniana in Canada and the EU; C. regularis in Canada; Dunaliella bardawil in the USA; Dunaliella salina in Canada and China; Euglena gracilis in Canada, the EU, China and Japan; Schizochytrium sp. as a fermented ingredient in the USA; Aphanizomenon flos-aquae, Parachlorella kessleri, Jaagichlorella luteoviridis, Tetraselmis chui and Odontella aurita in the EU; Nostoc sphaeroides and Microchloropsis gaditana in China. Furthermore, the following algal derivatives (not the algal biomass) were authorized as food ingredients:
- In the EU [117]: β-carotene and a mixture of carotenoids from Dunaliella salina; oil rich in PUFAs from Ulkenia sp.; DHA and EPA ethyl esters oil from Schizochytrium sp.; astaxanthin-rich oleoresin from Haematococcus pluvialis; oil rich in EPA from Phaeodactylum tricornutum;
- In the USA (data by Food and Drug Administration or cited reference): DHA Algal Oil from Schizochytrium sp.; EPA oil from Microchloropsis gaditana [124]; Astaxanthin extracted from Haematococcus pluvialis; algal fat from Prototheca zopfii (syn. moriformis) [125]; oil from Ulkenia sp.;
- National Health Commission (People’s Republic of China): DHA oil from Schizochytrium sp., Ulkenia amoeboida, Crypthecodinium cohnii.
Surprisingly, moreover, the analysis of these microorganisms as potential sources of compounds useful for the maintenance of mitohormesis was still insufficiently investigated.
3.1. Protective Features of Crude Extracts of Microalgae
Studies focused on the use of microalgae as food for their protective action on mitochondria are still very scarce, and most of the information, therefore, comes from the study of individual compounds present in their composition. In this section, the few works to our knowledge were considered, including experimental results obtained on cultivated cells.
An ethanol/water extract obtained from Tetraselmis suecica (phylum: Chlorophyta, class: Chlorodendrophyceae [126]) was tested on a human lung cancer cell line challenged with H2O2 and showed enhanced oxidative stress resilience and cell survival by the upregulation of several enzymes, including mitochondrial SOD2 and GPx1 [127]. Interestingly, the extract treatment also upregulated the expression of SIRT2, a cytosolic deacetylase, which promotes mitochondrial biogenesis, whereas it inhibited Drp1 and the related fission activity [128]. A 10% dietary supplementation with Nannochloropsis gaditana (phylum: Ochrophyta, class: Eustigmatophyceae, currently accepted name: Microchloropsis gaditana [126]) was able to reduce the peroxidation on some mitochondrial components of the liver of diabetic rats, using malondialdehyde and carbonyl proteins as damage markers [129]. These effects mitigated the decreased activities of catalase (CAT) and superoxide dismutase (SOD), as well as the concentration of reduced glutathione (GSH) observed in untreated diabetic rats in comparison to healthy controls. A 70% methanolic extract of Euglena tuba (phylum: Euglenozoa, class: Euglenophyceae [126]) showed antitumoral activity against Dalton’s lymphoma cells in inbred populations of a BALB/c (H2d) mice strain [130]. This result was consistent with the detected alteration of the mitochondrial membrane potential, upregulation of proapoptotic proteins Bax and cellular tumor antigen p53 (p53), while the anti-apoptotic protein regulator BCL-2 was downregulated. Mitochondria-mediated apoptosis in non-small cell lung cancer lines was also demonstrated for hot water extracts of Chlorella sorokiniana (phylum: Chlorophyta, class: Trebouxiophyceae [126]). This conclusion was supported by the downregulation of the anti-apoptotic BCL-2, E3 ubiquitin-protein ligase XIAP (XIAP) and survivin, whereas the proapoptotic caspase-3, caspase-9, and poly [ADP-ribose] polymerase (PARP) were activated by peptide bond cleavage [131]. An anticancer effect due to mitochondrial apoptosis was also proposed for methanol and ethyl acetate extracts of Picochlorum sp. RCC486 [132] (phylum: Chlorophyta, class: Trebouxiophyceae [126]), ethanol extracts of an unclassified Antarctic freshwater of the Botryidiopsidaceae family [133] (phylum: Ochrophyta, class: Xanthophyceae [126]), and ethanol extracts of Chaetoceros calcitrans [134] (phylum: Bacillariophyta, class: Mediophyceae [126]). Most of these anticarcinogenic activities can be explained by the high content of polyphenols and carotenoids in microalgae, suitable to modulate mitochondrial metabolism, reported below. However, it should be kept in mind that part of these results was obtained only on tumor-derived cell lines, and their verification in vivo is recommended for robust confirmation.
Other activities of interest mainly concerned disorders related to aging and chronic diseases. The attenuation of hepatic steatosis was shown in a murine model following the treatment with ethanol extracts of Nitzschia laevis (phylum: Bacillariophyta, class: Bacillariophyceae, accepted name: Nitzschia amabilis [126]) prepared using a Super High Pressure-Low Temperature Flowing Cell Cracker. The disease improvement was due to the enhancement of hepatic mitochondrial function and the repression of fatty acid synthesis by the phosphorylation of acetyl-CoA carboxylase [135]. In another study carried out in vitro, both on primary skin cells and on ex vivo full-thickness skin, an aqueous extract of Scenedesmus rubescens (phylum: Chlorophyta, class: Chlorophyceae, accepted name: Halochlorella rubescens [126]) showed protective effects against UV radiation damage by an increase of both mitochondrial efficiency and of cell proliferation [136].
An interesting finding was obtained using Spirulina platensis (phylum: Cyanobacteria, class: Cyanophyceae, currently accepted name: Arthrospira platensis [126]) treating a horse’s endocrine disorder named Equine Metabolic Syndrome, a severe pathology linked to insulin resistance, oxidative stress, and systemic inflammation [137]. In vivo trials carried out with pelleted Spirulina, as well as in vitro tests on cell line models with a water extract of Spirulina, have shown that this cyanobacterium improved the mitochondrial functionality, downregulated the proapoptotic proteins p21, p53 and Bax, as well as the pro-mitophagic proteins PINK1 and parkin.
3.2. Activity of Long-Chain Polyunsaturated Fatty Acids (PUFAs)
Microalgae are known for their ability to synthesize and concentrate long-chain PUFAs, and in particular EPA and docosahexaenoic acid (DHA), especially if culture conditions aimed at maximizing the accumulation of these compounds are adopted. For instance, DHA can reach up to 40% of the total fatty acids in some Dinophytes, 30% of the total fatty acids in some Haptophytes and even up to 60% of total fatty acids in the form of triacylglycerols in Thraustochytrids belonging to the genus Aurantiochytrium and Schizochytrium [115]. The content of EPA, on the other hand, can reach up to 20% of the fatty acids in Diatoms, e.g., Phaeodactylum tricornutum, while among the Eustigmatophytes, EPA can range between 15 and 30% of the lipo-acidic profile of Nannochloropsis oculata [115].
In addition to presenting an antioxidant effect, PUFAs provide benefits to human health through various mechanisms of action and metabolic pathways. Clinical trials involving PUFAs are generally based on the intake of fish oil, whose EPA and DHA content, in any case, derives from microalgae through the fish food chain. When focusing on the biology of mitochondria, it has been shown that omega-3-rich diets improved mitochondrial dynamics and morphology, increasing the expression of mitofusins (Mfns) and OPA1, while a downregulation of Drp1 and mitochondrial fission 1 protein (Fis1) was observed [46]. The overall effect was to promote mitochondrial fusion and inhibit fission processes, thus increasing the mitochondrial reticulum and enhancing its functionality.
It is worth mentioning that a diet rich in EPA and DHA modifies the composition of the mitochondrial membrane phospholipids by partially replacing arachidonic acid [138], so improving stress tolerance and reducing the incidence of heart attack [65,139]. Among the lipids almost exclusively synthesized in mitochondria, cardiolipins constitute approximately 15–20% of the total mitochondrial phospholipids. Among many functions, they regulate mitochondrial dynamics stabilizing the interaction of key enzymes with the organelle membrane, especially Drp1 and OPA1 [65]. Cardiolipins anchor Cyt-c to the internal mitochondrial membrane, and their integrity seems to be fundamental for the prevention of the “mitochondrial permeability transition pore” opening, which triggers the release of Cyt-c into the cytosol and cell apoptosis [65]. However, cardiolipins’ integrity is threatened by oxidative reactions, to which they are particularly exposed due to their position with respect to oxidative phosphorylation enzymes. Several studies have shown that PUFAs play an important role in the functionality and protection of cardiolipins (see the review by Paradies et al. [65]). Dietary supplementation with DHA and EPA can increase the total cardiolipin content in cardiac mitochondria [139]. In hearts subjected to prolonged ischemic conditions, DHA has been shown to counteract the decline of tetralinoleyl cardiolipin, i.e., the primary moiety of cardiolipin, preventing cardiomyocyte death by the formation of the mitochondrial permeability transition pore [65,139].
Studies focused on overweight subjects show that EPA-treated adipocytes improved their mitochondrial reticulum and its functionality through the upregulation of mRNA expression of NRF-1, MTFA and Cyt-c oxidase [43]. These effects were accompanied by the upregulation of genes involved in mitochondrial biogenesis, such as sirtuin 1, PGC1-α and 5′-AMP-activated protein kinase (AMPK). Moreover, mitochondrial biogenesis was stimulated via upregulation of PGC1-α and NRF-1 also by treating C57BL/6J mice with a high-fat diet comprising an EPA and DHA concentrate (6% EPA, 51% DHA) [44]. At the same time, a tissue-specific modulation of lipid metabolism was observed. Indeed, the expression of carnitine palmitoyltransferase 1A and fatty acid catabolism were increased in epididymal, but not subcutaneous, fat cells.
Interestingly, it has long been known that EPA can be found in low amounts in the fatty liver of diabetic patients [140]. A study carried out on myotubes prepared using cells obtained from obese type 2 diabetic patients have shown an impaired mitochondrial capacity for fatty acid and glucose oxidation. The treatment with EPA (100 μmol/L) enhanced glucose oxidation, whereas positive effects on lipid metabolism were also observed, but with less clear results [141]. Furthermore, in rats, EPA significantly increased the expression of thermogenic genes, such as uncoupling protein 1-3 (UCP1-3), cell death-inducing DFFA-like effector A (CIDEA) and vascular endothelial growth factor A (VEGFα) [142], consistent with the promotion of the beige-like adipocytes differentiation observed in humans [43].
3.3. Sterols
These compounds are synthesized in microalgae mainly as components of the cell membrane, in quantities that can vary in response to environmental conditions, such as salinity, temperature and light intensity [143]. Several microalgal sterol compounds have been described, including campesterol, stigmasterol, beta-sitosterol, monomethylsterol, brassicasterol, ergosterol and cholesterol [143,144]. Some molecules of this group are quite uncommon if compared to those occurring among higher plants, such as poriferasterol and clionasterol, identified in Chlorella pringsheimii (phylum: Chlorophyta, class: Trebouxiophyceae, currently accepted name: Pseudochlorella pringsheimii [126]), or ergosterol and chondrillasterol in Chlorella fusca (phylum: Chlorophyta, class: Chlorophyceae, currently accepted name: Desmodesmus abundans [126]) [18]. Interestingly, appreciable contents of β-sitosterol and stigmasterol were detected in Nannochloropsis sp. [145].
The interactions of sterols with the biology of mitochondria have been studied mainly as modulators of the apoptotic process and, in general, for the related effects on cancer prevention. Stigmasterol, a sterol extracted from Navicula incerta (phylum: Bacillariophyta, class: Bacillariophyceae [126]), promoted the apoptosis of hepatocarcinoma cells (HepG2) by upregulating the expression of BAX and P53, both powerful pro-apoptotic genes, and by downregulating the anti-apoptotic BCL2 gene [146]. The activation of cellular apoptosis via the mitochondrial route suggests possible applications of microalgal sterols for the treatment of cancer, but also potential uses aimed at promoting the clearance of dysfunctional cells, which could be candidates to transform into neoplastic cells, thus favoring the renewal of tissue composition.
In a cellular model of colon cancer, the effect of different phytosterols, pure substances or mixtures, was also studied in combination with a carotenoid, i.e., β-cryptoxanthin, by treatments at concentrations comparable to values present in human serum after the intake of functional drinks. Various effects were detected at the mitochondrial level with consequent apoptosis activation and inhibition of cell proliferation, according to the following efficacy scale: phytosterols-mix > stigmasterol > β-cryptoxanthin + phytosterols-mix > campesterol > β-cryptoxanthin [147].
3.4. Phenolic Compounds
Phenolic compounds are secondary metabolites with antioxidant properties and several biological activities. They are abundant in plants and comprise phenolic acids and polyphenols (i.e., flavonoids and tannins), stilbenes, lignans and lignins [148]. Considering the data reported in the literature for this group of compounds, it should be kept in mind that the quantification of phenolic compounds is a challenging topic since they generally show limited solubility in water, and the extraction efficiency varies significantly with the method and solvent used. The commonest methodologies are based on the extraction using hydrophilic solvents, or their mixtures, such as ethanol, methanol, or water (in the latter case, preferably with a microwave-assisted process), but various other techniques are currently also available [149,150]. The composition of phenolic compounds in different microalgal species, however, should be critically considered for representing their actual total amount. In general, results obtained by a specific extraction process, even if deemed sufficiently efficient, still represent an underestimate of the actual content of phenolic compounds, as will be evident in some examples below. Importantly, the quantification is generally expressed in terms of standard antioxidant capacity, conventionally established in μmol or, more frequently, in mg of gallic acid equivalents (mg GAE) per gram of sample. However, the latter conventional concentration unit can be applied just for the antioxidant capacity, and it is not suitable for representing the impact of these substances on biological functions, such as the regulation of enzymes and nuclear factors.
Goiris et al. [151] studied the fractional extraction of phenolic compounds from microalgae, obtaining the highest quantities with an ethanol–water mixture, followed by hot water, and only modest amounts with hexane or ethyl acetate. This is consistent with the abundance of polar hydroxyl groups in the molecules of phenolic compounds, which therefore confer a prevailingly hydrophilic behavior, as also demonstrated by studies on the partitioning between aqueous and olive oil phases of phenolic compounds of olive fruits [152]. In work carried out on biomasses of Nannochlorospis sp. and of Spirulina sp., Scaglioni et al. [153] quantified the insoluble part of phenolic compounds, believed to be bound to cell walls after extraction of the soluble fraction using methanol and/or ethanol. Extractions of Spirulina using the two alcohols produced quantitatively similar extracts, while the ethanolic fraction obtained from Nannochloropsis was higher than the methanolic fraction by about 40%. Finally, the insoluble polyphenol fraction was quantitatively lower than 4% and 8% of the phenols extracted by ethanol (i.e., the most effective solvent) in Spirulina and Nannochloropsis, respectively.
The few data presented here point out that phenolic composition varies significantly with the adopted extraction technique and the species-specific composition of the microalgal cell wall. This should suggest a reflection on the criticalities inherent in the available literature, but also on the bioavailability of phenolic compounds of microalgae for nutritional purposes. Their effectiveness, in fact, does not only depend on their content and composition in the considered microalga, but also on their relative bioavailability and their uncertain stability throughout the digestive processes of the human intestine. From a biochemical point of view, there are well-founded reasons to consider that the in vitro antioxidant activity of microalgal polyphenols is less relevant than other, more specific, effects of microalgal compounds.
Goh et al. [154] quantified the phenolic compounds obtained by different solvents from Chaetoceros and Nannochloropsis, two algal genera widely used in aquaculture, and then compared their antioxidant activity by means of widely used assays. Surprisingly, this study showed that the extracts with the highest antioxidant activity were not those richest in phenolic compounds. Therefore, antioxidant capacity must have been mainly attributable to different antioxidant compounds, of which many microalgae are rich. It is noteworthy that the antioxidant activity of polyphenols is strongly dependent on their chemical structure, beyond their abundance, and influenced by the arrangements of hydroxyl groups and double bounds in their molecule [155]. Considering the abovementioned results, literature data on microalgae indicate highly variable total contents of phenolic compounds among genera (Table 3). Andriopoulos et al. [156] revised the total phenolic content (TPC) in 35 genera of microalgae and cyanobacteria, showing that these compounds range on average between 0.9 and 38.5 mg GAE/g DW, with few exceptions below the minimum value and most of the data ranging between 2 and 7 mg GAE/g DW. The same authors cultivated and analyzed five species of microalgae (Chlorella minutissima (phylum: Chlorophyta, class: Chlorophyceae, currently accepted name: Mychonastes homosphaera [126]), Dunaliella salina, Nannochloropsis oculata, Tisochrysis lutea and Isochrysis galbana) detecting values of TPC (calculated as the sum of aqueous and methanolic extracts) between 6 and 12 mg GAE/g DW. An example of TPC occurring in some microalgae is shown in Table 3.

Table 3.
Total phenolic content in some microalgae biomasses (mgGAE/g DW).
Table 3.
Total phenolic content in some microalgae biomasses (mgGAE/g DW).
| Species | Total Phenolic Content mg GAE/g Biomass DW | Reference |
|---|---|---|
| Euglena cantabrica | 0.6–12.6 | [157] |
| Demodesmus sp. | 7.7 | [158] |
| Tetraselmis suecica | 4.3 | [151] |
| Dunaliella salina | 4.5 | [158] |
| Haematococcus pluvialis | 1.9 | [151] |
| Nannochloropsis limnetica | 5.8 | [158] |
| Nannochloropsis salina | 6.5 | [158] |
| Galdieria sulphuraria | 1.6–5.3 | [159] |
| Nannochloropsis sp. | 2.2 | [151] |
| Isochrysis sp. | 7.8 | [151] |
| Chaetoceros calcitrans | 2.3 | [151] |
| Porphyridium cruentum | 1 | [151] |
| Phaeodactylum tricornutum | 3.2–6.1 | [151,158] |
| Chlorella sorokiniana | 5.8–5.9 | [158] |
| Auxenochlorella pyrenoidosa | 13.2–25.8 | [160] |
| Arthrospira platensis | 17–43.2 | [160] |
| Arthrospira fusiformis | 47.3–88.5 | [161] |
| Nostoc commune | 0.9 | [162] |
Many phenolic acids and polyphenols can have strong positive effects on human metabolism, and their importance as micronutrients for preserving health is now well documented [163]. The specific activities of these substances on the regulation of mitochondrial functions have been investigated: Chodari et al. [164] reviewed the activities of polyphenols on biogenesis, Sandoval-Acuna et al. [165] their activities not explained by antioxidant effects, and Naoi et al. [166] their modulatory effects on the mitochondrial apoptosis machinery. However, studies relating polyphenols obtained from microalgal extraction on mitochondria are lacking. Note that the mechanisms of action of this group of compounds are far from being well understood and can vary in relation to cell type and metabolic conditions.
Some polyphenols have been shown to modulate the biochemical pathways involved in mitochondrial biogenesis. The disclosed activity of resveratrol as an upregulator of SIRT1, which in turn inactivates the proapoptotic p53 by deacetylation, shined a light on the importance of polyphenols for cell cycle regulation [167]. It was shown that this activity was not explained by the mere antioxidant property and was also observed with quercetin and with other polyphenols, which are not known in microalgae (fisetin, piceatannol, butein, and soliquiritigenin) [167]. As already said, SIRT1 is an activator of PGC-1α, which in turn is a strong enhancer of mitochondrial biogenesis. According to Chodari et al. [164], the activation of AMPK by resveratrol represents a collateral effect of biogenesis activation via PGC-1α. A significant increase of SIRT1, PGC-1α, mtDNA, Cyt-c, and mitochondrial biogenesis was detected in the muscle and brain of mice following dietary administration of quercetin [168]. It should be noted, however, that the treated groups received 12.5 mg/kg or 25 mg/kg, which are considered quite high dosages. Moreover, polyphenols have been shown to increase mitochondrial biogenesis and activation of AREs by upregulating Nrf2, possibly by displacing this factor from the inhibition binding to Kelch ECH associating protein 1 (Keap1) [165]. Apocynin, epicatechin, cyanidin, cyanidin-3-glucoside and curcumin (not all identified in microalgae) have been shown to inhibit or repress some superoxide anion generators, among which are xanthine oxidase and monoamine oxidase (MAO) [165]. In this regard, the inhibition of MAO-B can have relevant neuroprotective effects [166]. Apigenin, biapigenin, curcumin, oroxylin A, ferulic acid, quercetin, resveratrol, and hesperidin were reported to interact with the mitochondrial membrane in complex ways, promoting or inhibiting the opening of the mitochondrial permeability transition pore [166], above discussed considering the role of cardiolipins. Even if it is not possible to explain in detail here the structure and regulation of the mitochondrial permeability transition pore, the overall results of these effects lead to a neuroprotective activity against important neurodegenerative pathologies [166].
As examples of biological activities of polyphenols, hydroxytyrosol, an abundant polyphenol in olives that is not yet identified in microalgae, was reported to stimulate the mitochondrial functionality and biogenesis via PCG-1α and to increase the activity and protein expression of respiratory complexes I, II, III and V in 3T3-L1 adipocytes [169]. The same compound produced a similar upregulation of the respiratory chain in human fibroblast cultures, explained by the activation of protein kinase A (PKA), cAMP response element-binding protein (CREB) and PGC-1α [170]. Moreover, a trial conducted on high-fat, high-sucrose (HFHS) diet-fed rats showed that a polyphenol extract from red wine could significantly enhance the mitochondrial respiratory chain and NADPH oxidase system, reducing oxidative stress in liver and heart tissues, with a strong preventive effect on steatosis development in the liver [171].
Considering some polyphenols found in microalgae (Table 4), ellagic acid, a compound found at a concentration of 860 μg/g DW in Galdieria sulphuraria cultivated under specific conditions [172], regulated ROS production in hepatocytes under oxidative stress, preventing cell damage and consequent apoptosis [173]. Moreover, this substance showed protective activities toward inflammations induced by arsenic on nerve cells via mitochondrial regulation [174] and against BCL-2 protein Bnip3-mediated oxidative stress in cardiomyocytes [175]. In apparent contrast with these cytoprotective activities, the same compound has shown proapoptotic mitochondrial effects in different types of cancer cells, such as human colon adenocarcinoma Caco-2 cells [176], in cancerous B-lymphocytes [177], in bladder cancer cells [178], in pancreatic cancer cells [179] and in lung cancer [180]. A very scholarly review discussing the anticancer mechanisms of action of selected polyphenols was published by Gorlach et al. [181]. Furthermore, apigenin, luteolin, kaempferol, and quercetin inhibited the growth of HepG2 cells and induced morphological changes associated with apoptosis in a dosage- and time-dependent manner by upregulating the expression of the proapoptotic p53-inducible gene 3 (PIG3) [182]. Therefore, even if polyphenols were reported to improve several mitochondrial functions, especially the electron transport chain activity, by modulating the redox state and inhibiting the apoptotic process, they can also promote this last phenomenon depending on their concentration and cell environmental conditions [166,183]. Indeed, the fine-tuning of biochemical mechanisms need to be elucidated in detail, in consideration that many of the reported results, although not all, have been obtained using cell models. In this last case, even if the demonstrated interactions are surely well founded, there are no certainties about the reproducibility in vivo.

Table 4.
Phenolic compounds detected in microalgae species. For each strain, any compound reported in at least one of the cited references was included. Synonyms: Porphyridium purpureum = Porphyridium cruentum; Microchloropsis salina = Nannochloropsis salina; Auxenochlorella pyrenoidosa = Chlorella pyrenoidosa; Arthrospira platensis = Spirulina platensis [126].
Table 4.
Phenolic compounds detected in microalgae species. For each strain, any compound reported in at least one of the cited references was included. Synonyms: Porphyridium purpureum = Porphyridium cruentum; Microchloropsis salina = Nannochloropsis salina; Auxenochlorella pyrenoidosa = Chlorella pyrenoidosa; Arthrospira platensis = Spirulina platensis [126].
| Phenolic Compound | Euglena cantabrica | Desmodesmus sp. | Tetraselmis suecica | Dunaliella salina | Haematoc. pluvialis | Nannochl. limnetica | Microchl. salina | Galdieria sulphuraria | Nannochloropsis sp. | Diacronema lutheri | Porph. purpureum | Phaeod. tricornutum | Chlorella sorokiniana | Auxenoc. pyrenoidosa | Arthrospira platensis | Arthrospira sp. | Nostoc commune |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phloroglucinol | + | + | + | + | + | + | + | + | |||||||||
| Pyrocatechol | + | ||||||||||||||||
| Pyrogallol | + | ||||||||||||||||
| Gallic ac. | + | + | + | + | + | + | + | + | + | + | |||||||
| 4-Hydroxy benzoic ac. | + | + | + | + | |||||||||||||
| 3,4-Dihydroxy benzoic ac. | + | + | + | ||||||||||||||
| Protocatechuic ac. | + | + | + | ||||||||||||||
| Quinic ac. | + | ||||||||||||||||
| Salicylic ac. | + | + | + | + | + | ||||||||||||
| Syringic ac. | + | + | + | + | |||||||||||||
| Vanillic ac. | + | + | |||||||||||||||
| Vanillin | + | + | + | ||||||||||||||
| 4-Aminobenzoic ac. | + | ||||||||||||||||
| Caffeic ac. | + | + | + | + | + | + | + | + | |||||||||
| Cinnamic ac. | + | + | + | ||||||||||||||
| Ferulic ac. | + | + | + | + | + | + | + | + | + | + | + | + | |||||
| 2/3/4-Hydroxy-cinnamic ac. | + | ||||||||||||||||
| P-Coumaric ac. | + | + | + | + | + | + | + | + | + | + | + | ||||||
| Chlorogenic ac. | + | + | + | + | + | ||||||||||||
| Phloretin | + | ||||||||||||||||
| Rosmarinic ac. | + | ||||||||||||||||
| Apigenin | + | + | + | + | + | + | |||||||||||
| Catechin hydrate | + | + | + | ||||||||||||||
| Daidzein | + | + | + | ||||||||||||||
| Dihydrokaempferol | + | ||||||||||||||||
| Dihydroquercetin | + | ||||||||||||||||
| Epicatechin | + | + | |||||||||||||||
| Epigallocatechin | + | ||||||||||||||||
| Genistein | + | + | + | ||||||||||||||
| Kaempferol | + | ||||||||||||||||
| Luteolin | + | + | |||||||||||||||
| Naringenin | + | + | |||||||||||||||
| Quercetin | + | + | + | + | + | ||||||||||||
| Ellagic ac. | + | + | |||||||||||||||
| Rutin | + | ||||||||||||||||
| Resveratrol | + | ||||||||||||||||
| References | [150] | [158] | [150,184] | [158] | [184] | [158] | [158] | [172] | [153] | [184] | [184] | [142,150,176] | [158] | [160] | [160,184,185,186] | [153] | [150] |
3.5. Carotenoids
Carotenoids are excellent antioxidants, which can contribute to preventing dysfunctions due to oxidative stress and appear to be particularly active on membranes and the enzymatic machinery of the mitochondria, nuclei and microsomes [120]. It is widely accepted that their intake is able to modulate the metabolism of cancer cells and significantly reduce the incidence of various types of cancer [187]. However, the activity of carotenoids depends on their bioavailability, which appears to be variable; e.g., fucoxanthin was described to be better absorbed than lutein or astaxanthin in mice [120].
Over 1100 naturally occurring carotenoids were estimated [125], among which more than 750 have been identified, whereas only 40 are commonly consumed, mainly represented by β-carotene, lycopene, lutein, β-cryptoxanthin, α-carotene and zeaxanthin [188].
Each microalgal species is generally characterized by a few prevailing carotenoids. For instance, β-carotene is the main metabolite in algae of the genus Dunaliella, astaxanthin in Haematococcus pluvialis (phylum: Chlorophyta; class: Chlorophyceae [126]), violaxanthin and vaucheriaxanthin in Nannochloropsis, accompanied by other compounds of the violaxanthin cycle, i.e., antheraxanthin and zeaxanthin [189] (Table 5).
However, the synthetic pathways of carotenoids lead to the production of several intermediate metabolites. Thus, many of them are detected in the microalgal composition in addition to the main metabolite, albeit at lower concentrations.
The beneficial activity of carotenoids on human health has been studied for only a few of them, and often with insufficient detail. Among these, the most studied are probably: β-carotene, astaxanthin and fucoxanthin. Specifically, β-carotene belongs to the carotene family, i.e., carotenoids which do not contain oxygen, and, possibly, it represents the molecule studied for the longest time as a supplement in the human diet, always being considered an excellent provitamin A.
Some clinical studies associated the intake of β-carotene with several beneficial outcomes, such as a reduced risk of cardiovascular disease, the prevention of several age-related cancers and the improvement of endogenous cellular antioxidants via modulation of Nrf2/ARE pathway in response to oxidative stress conditions [190]. Moreover, β-carotene has been shown to protect mitochondrial membranes from the impairment of mitochondrial import receptor subunit TOM20 homolog (Tom20) due to oxidative stress [190]. Notably, Tom20 is an import receptor of the outer-membrane translocator TOM40 complex and plays an essential role in the import of mitochondrial proteins [191]. Low-doses of β-carotene were shown to modulate autophagy and to downregulate the NF-κB inflammatory factor, whereas mitochondrial biogenesis was stimulated via Nrf2 and apoptotic signals of caspase 3 and 9 were downregulated [190]. Trials carried out on Drosophila melanogaster with 9-cis-β-carotene obtained from Dunaliella salina showed that this carotenoid improved the mitochondrial function in terms of organelle mobility and ATP production, leading to the extension of the mean lifespan [192]. On the other side, adverse effects concerning cardiovascular disease were observed in smoker subjects treated with β-carotene [193]. Furthermore, according to the meta-analysis proposed by Pecollo et al. [194], an increased risk of lung and gastric cancer was observed in smokers and asbestos workers treated with β-carotene at a dosage equal to or above 20 mg/day, whereas no protective effect on other kinds of cancer was observed at lower dosages. However, this topic remains the object of discussion, and some evidence of protective effects at physiological dosages was proposed for breast cancer [195,196], colon cancer [197], esophageal cancer [198] and others.
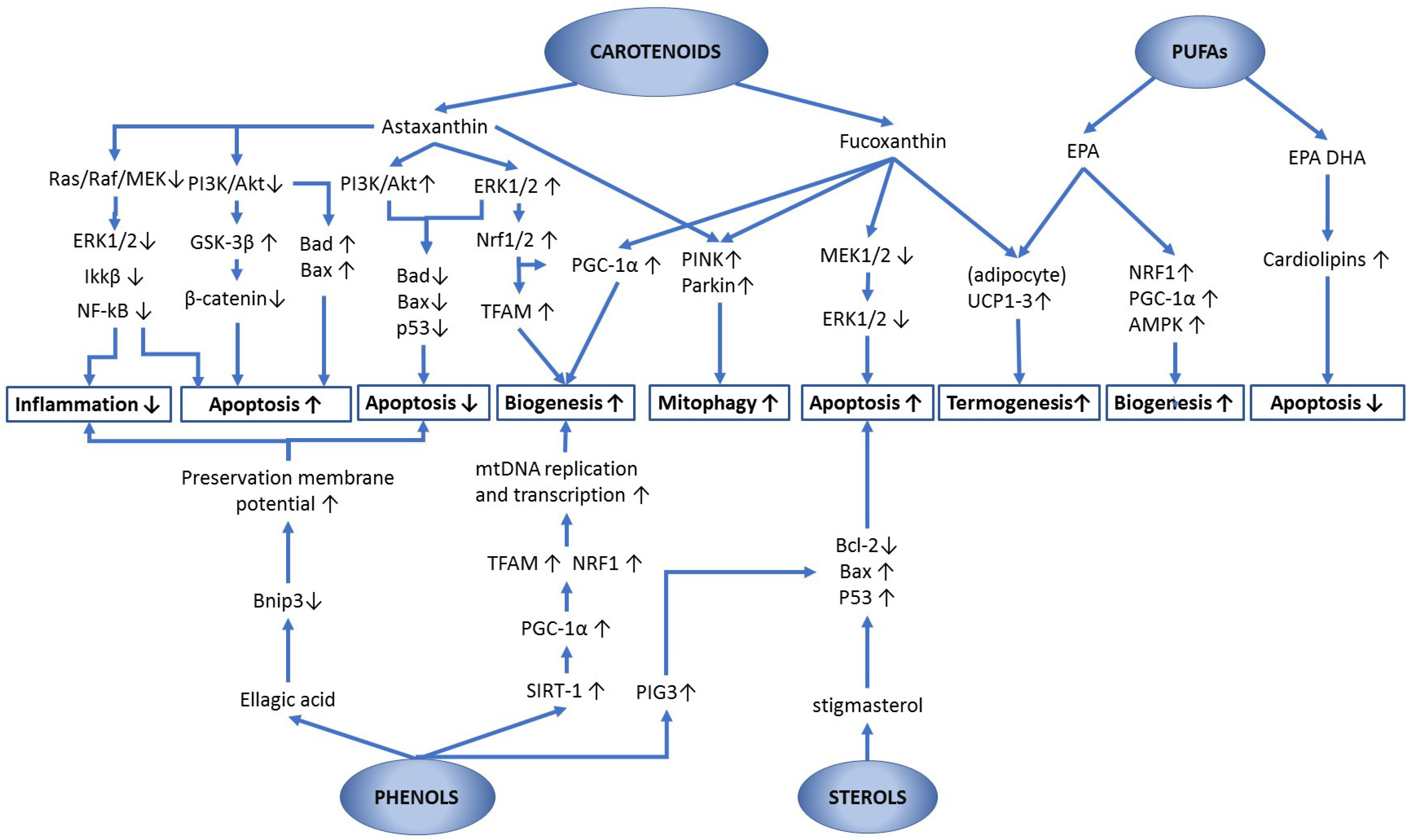
Astaxanthin and fucoxanthin are xanthophylls, i.e., oxygen-containing carotenoids, which derive from carotenes through the carotenoid synthesis pathway [199]. They act by upregulating peroxisome proliferator-activated receptors (PPARs) [188]. Importantly, PPARɣ increases the PGC1α, NRF1/2, and TFAM transcription factors, with consequent positive effects on mitochondrial biogenesis, oxygen consumption, mitochondrial membrane potential, antioxidant defenses, etc. [200].

Table 5.
Carotenoids detected in microalgae species. Some reported data are significantly higher than values expected from standard cultures because of referred biomasses cultivated under stressing conditions or adopting modified methods (including heterotrophic cultures), in order to maximize the synthesis and accumulation of the desired metabolite. Some species names used in the cited references have been updated according to the current taxonomic status reported in AlgaeBase [126].
Table 5.
Carotenoids detected in microalgae species. Some reported data are significantly higher than values expected from standard cultures because of referred biomasses cultivated under stressing conditions or adopting modified methods (including heterotrophic cultures), in order to maximize the synthesis and accumulation of the desired metabolite. Some species names used in the cited references have been updated according to the current taxonomic status reported in AlgaeBase [126].
| Major Component | Species | Compound Concentration | Ref. | Total Carotenoid Concentration | Ref. |
|---|---|---|---|---|---|
| β-carotene | Dunaliella salina | Up to 10–13% DW | [201] | up to 29% | [188] |
| Tetraselmis suecica | 0.1% DW | 0.35–1.1% DW | [202] | ||
| Vischeria stellata | 5.9% DW | [203] | 7.7% DW | [203] | |
| Chromochloris zofingiensis | 0.9% DW | [201] | 0.7–0.88% DW | [204,205] | |
| Astaxanthin | Haematococcus pluvialis | 2.3–7.7% DW | [206] | astaxanthin accounts for 85–90% of total carotenoids [207] | |
| Up to 5% DW | [188] | ||||
| Chromochloris zofingiensis | 0.3–0.6% DW | [208] | 0.7% DW | [204] | |
| 0.53–0.6% DW | [188] | 0.7% DW | [204] | ||
| Canthaxanthin | Coelastrella striolata var. multistriata | 4.75% DW | [205] | 5.6% DW | [205] |
| Fucoxanthin | Isochrysis aff. galbana | 1.7–2.1% DW | [209] | 2% DW | [209] |
| Mallomonas sp. SBV13 | 2.6% | [210] | |||
| Isochrysis galbana | 0.22–1.35% DW | [130,211] | 1.76% DW | [211] | |
| Odontella aurita | up to 2.2% DW | [201] | ~1.5% DW | [212] | |
| Phaeodactylum tricornutum | 0.78–1.65% DW | [201,211] | 0.61–1% DW | [151,211] | |
| Lutein | Auxenochlorella protothecoides | 0.54 DW | [201] | 0.8% DW | [213] |
| Chlorella sorokiniana | 0.21–0.32% DW | [158] | 0.4% DW | [214] | |
| Coelastrella sp. | 0.69% DW | [201] | |||
| Desmodesmus sp. | 0.51% DW | [158] | 0.67% DW | [158] | |
| Chromochloris zofingiensis (mutant strain) | 1.38% DW | [215] | 2.74% DW | [215] | |
| Scenedesmus almeriensis | 0.54% DW | [201] | |||
| Violaxanthin | Nannochloropsis sp. | 0.12–0.58% DW | [117] | 0.30–0.86% DW | [117] |
| Zeaxanthin | Chloroidium saccharophila | 1.1% DW | [216] | 1.6% DW | [217] |
| Chloroidium ellipsoideum | 0.42% DW | [216] | |||
| Chromochloris zofingiensis (mutant strain) | 0.7% DW | [215] | 2.74% DW | [215] | |
| Dunaliella salina (mutant strain) | 0.42–0.59% DW | [218] | 1.1–1.28% DW | [218] | |
Astaxanthin is one of the most studied carotenoids for its high antioxidant power, effective anti-inflammatory activity, and many other effects due to the modulation of several enzymes and genetic factors. Moreover, it was reported to inhibit the generation of mitochondrial-derived ROS by protecting its membranes from lipid peroxidation [219]. The cystic form of Haematococcus pluvialis is the richest source in nature of this compound with about 3% DW [19], which can reach 5–7% DW under special culture conditions (cf. Table 5). Astaxanthin has been shown to significantly extend the lifespan by affecting the biogenesis of the mitochondrial respiratory complex III (CIII), in plants, Caenorhabditis elegans (a nematode often used as a model organism), rodents and humans [220]. Mutant-lines of the animal model Drosophila melanogaster with reduced levels of SOD1, SOD2 and catalase were fed with experimental diets comprising Haematococcus pluvialis extracts, which, at specific concentrations (1 mg/mL), have shown a significant lifespan extension and amelioration of age-related decline of motility [221].
Astaxanthin appears to act on various biochemical pathways, and, in many cases, its action is not attributable to the mere antioxidant activity, of which the review by Sztretye et al. [222] proposed a perusal analysis. Under some experimental conditions, astaxanthin has been shown to protect mitochondrial functionality, possibly by protecting membranes from oxidative damage rather than by preventing excessive ROS production [223]. At the same time, it increased the expression of the PINK-parkin pathway, thus promoting the mitophagic degradation of damaged mitochondria or their dysfunctional parts [42]. In addition to the scavenging of free radicals and membrane integrity preservation, an important activity of astaxanthin as a neuroprotector was observed. This was explained as due to the modulation of Nrf2, FOXO3 (a transcriptional activator which has been shown to promote mtDNA transcription under reduction of nutrient intake [224]) and SIRT1, i.e., key regulators which control the metabolic mechanisms of longevity, directly or indirectly involving mitochondria [225]. It was also reported that, under hypoxic stress, i.e., conditions of special interest for cardiovascular diseases, astaxanthin promoted the phosphorylation of ERK1/2 in response to the concomitant administration of acetyl-L-carnitine and was able to stimulate mitochondrial biogenesis by upregulating PGC-1α, or NRF-1 via upregulation of Nrf2 [156]. Furthermore, several studies show that this carotenoid was able to effectively protect mitochondria from the damage resulting from oxidative stress in experimental models of pathologies, such as myocardial ischemia, homocysteine-induced cardiotoxicity, pulmonary fibrosis, hyperglycemia, hepatic inflammation and fibrosis, and nonalcoholic steatosis [226]. In another study on cells subjected to stress, astaxanthin was shown to stimulate both the mitophagy of dysfunctional mitochondria via the PINK-Parkin pathway and organelle biogenesis for maintaining the overall mitochondrial efficiency via upregulation of TFAM and PGC-1α [42]. Moreover, astaxanthin increased the oxidative metabolism of lipids by promoting the activation of carnitine palmitoyl-transferase I (CPT I) in the muscle cells of endurance athletes, protecting this enzyme from alterations induced by hexanoyl-lysine (an oxidative stress marker) produced during prolonged physical exercise [227]. This mitochondrial enzyme catalyzes the transfer of the acyl group of a long-chain fatty acyl-CoA from coenzyme A to L-carnitine, producing acyl carnitine that is suitable to be moved from the cytosol into the mitochondria and to undergo the oxidative degradation. For this reason, astaxanthin is adopted by athletes to improve their performances, even if the effectiveness can depend on the muscle effort required. In addition to this, several genes suitable for protecting mitochondrial functionality from oxidative stress are upregulated by lycopene and astaxanthin, thanks to the activation of Nrf2 via different pathways, only partially elucidated and possibly including the dissociation of Nrf2 from the Keap1 repressor [219]. KEAP1 forms part of an E3 ubiquitin ligase, which binds NRF2 by targeting it for ubiquitination and proteasome-dependent degradation. The action of lycopene and astaxanthin, if confirmed, would be interesting since it would support the assumption that, under oxidative stress conditions, their reaction with ROS attributes an electrophilic property to these carotenoids. These reaction products could be sufficiently electrophilic to react with cysteine thiol groups of Keap1, which in turn will act as an oxidative stress sensor, weakening its binding to Nrf2. Upon dissociation from Keap1, Nrf2 evades proteasome degradation, accumulates in the cytosol, and finally translocates to the nucleus, where it exerts its regulatory activities.
Among the most important activities of astaxanthin and fucoxanthin, the regulation of apoptosis should be mentioned, with relevant implications for the prevention or reduction of tumors. Although it is generally accepted that carotenoids help cancer prevention, their interaction with several biochemical pathways producing both proapoptotic and antiapoptotic effects, depending on the cell environment, was reported. Sathasivam and Ki [201] reviewed the bioactivities of carotenoids and, within a complex network of reactions, pointed out three key metabolic pathways involving MAP kinases and mitochondrion as organelle effectors:
- Activation of the PI3K/Akt (phosphatidylinositol 3-kinase; AKT-serine/threonine kinase also known as PKB, protein kinase B) survival pathway, which inactivates Bax by phosphorylation and reduces the release of Cyt-c (antiapoptotic effect). Indeed, according to Kale et al. [105], the phosphorylation at residue S184 by Akt inhibits Bax, thus preventing its translocation into the mitochondrion.
- Activation of the p38 MAPK signaling pathway, which promotes the release of Cyt-c and activates the apoptosome (apoptotic effect). Specifically, p38 MAPK can act on the mitochondrial permeability by activating Bim by phosphorylation, which in turn activates Bax or, alternatively, it can phosphorylate p53, which induces the expression of death receptors and can activate members of the BCL-2 family to promote apoptosis [228]. Other proapoptotic mechanisms of action have been hypothesized for p38MAPK, beyond the scope of the present review.
- Stimulation of MEK1/2–ERK1/2 signaling pathway, which in turn activates the pro-survival BCL-2 proteins (antiapoptotic effect), as shortly explained in the first part of this contribution.
Interestingly, the activation of ERK1/2 can also stimulate DRP1 expression and, thus, promote mitochondrial fission [106], which is functional to the segregation of mitochondria between the two daughter cells during mitosis, but also to the separation and subsequent mitophagy of dysfunctional parts of the mitochondrial reticulum. On the other side, a massive activation of mitochondrial fission is consistent with a proapoptotic effect described for ERK1/2 by activation of the pro-death BCL-2 protein, NOXA, which can occur as an alternative route to the classic pro-survival Ras/Raf/MEK/ERK signaling [106]. Therefore, as already remarked concerning the activities of phenolic compounds, it should be noted that carotenoids can produce a complex and variable range of effects, depending on both the considered molecule and the metabolic situation of the cell. For example, it has been shown that fucoxanthin and siphonaxanthin were able to inhibit the activation of ERK1/2 by phosphorylation mediated by FGF-2, in vascular endothelial cells [229]. However, astaxanthin stimulated the activation of ERK1/2 in HCT-116 colon cancer cells as part of an overall pro-apoptotic effect [230]. Again, astaxanthin prevented apoptosis induced by cytotoxic treatments aimed at increasing ROS in the substantia nigra neurons in a mouse model of Parkinson’s disease and in human neuroblastoma SH-SY5Y cells [72]. This effect was due to the increased expression of BCL-2 protein, the downregulation of α-synuclein and Bax, and the inhibition of the cleavage of caspase-3. However, the administration of a diet supplemented with astaxanthin showed pro-apoptotic activity in Syrian hamsters treated with carcinogenic stimuli, producing protective effects against the onset of squamous cell carcinomas [231]. The study showed an antiproliferative effect of astaxanthin by inhibiting the MEK/ERK and PI3K/Akt pathways and the related downstream IKKβ/NF-κB and GSK-3β/Wnt-catenin signaling, promoting intrinsic apoptosis as a final outcome. Notably, in this study, astaxanthin showed opposite effects with respect to the above-reported stimulations of MEK/ERK and PI3/Akt, suggesting that the same receptors can produce alternative modulations on the underling pathways depending on the cellular environmental context. According to Kavitha et al. [231], astaxanthin prevented the inactivation of GSK-3β by phosphorylation, which, therefore, can constitute, with other proteins, the “destruction complex” responsible for the phosphorylation and subsequent degradation of β-catenin. This last protein, therefore, interrupts its translocation into the nucleus and its activation of genes involved in cell proliferation and apoptosis evasion. Besides, in the same context, astaxanthin also inhibited the Akt kinase, responsible, among other activities, for the inactivating phosphorylation of Bad (in addition to the inhibiting phosphorylation of Bax, above mentioned). Bad, in the dephosphorylated form, can translocate to the mitochondrial membrane, selectively displacing Bax from the inhibiting binding to antiapoptotic BCL-2 proteins. In this free form, Bax is then able to initiate the mitochondrial membrane permeability process by inducing the release of Cyt-c and Smac/DIABLO proteins, which in turn trigger the caspase cascade and, finally, apoptosis. Similarly, astaxanthin has shown pro-apototic activities by inhibiting NF-κB and Wnt/β-catenin in human hepatoma cell lines [232]. These cases are of special interest in order to point out the complexity of the response characterizing the intra-cell secondary messages and the related biochemical pathways.
Considering the above-reported studies, it should be noted that NF-kB, a typical pro-inflammatory factor, can act as a pro- or anti-apoptotic intermediate depending on the upstream stimulus [233], with relevant implications on the efficacy of some anticancer chemotherapies [234]. More generally, the pleiotropic action of astaxanthin and its ability to act in different cellular contexts as a promoter of biogenesis and mitochondrial functionality or as a pro-apoptotic and anticarcinogenic agent, depends on its ability to interact with a high number of enzymes involved in cell cycle regulation. The different outcomes depend on the cell metabolic condition and on the concomitant presence of other stimuli. Recent studies suggested that astaxanthin can interact with a number of proteins involved in cell growth pathways, including EGFR (epidermal growth factor receptor), IGF1R (insulin-like growth factor 1 receptor), AKT1, AKT2, ERK1, and ERK2 [235].
Fucoxanthin, another carotenoid synthesized by several microalgae, has been shown to protect mitochondrial function in a cell model treated with pro-inflammatory stimuli, specifically by preserving the membrane potential and by modulating mitochondrial dynamics, i.e., by promoting the mitophagy of dysfunctional mitochondria and, at the same time, stimulating their renewal via biogenesis [49]. This carotenoid is known for its peculiar ability to modulate the metabolism of adipocytes and to reduce the accumulation of fat by promoting the mitochondrial expression of UCP1 and the uptake of glucose in the muscles via increased expression of GLUT4 glucose transporter [236,237]. In addition to this, the improved metabolism of fat also appeared due to the upregulation of PGC-1α and improved mitochondrial dynamics [47]. Importantly, the combination of the antioxidant activity with the regulation of glucose uptake makes fucoxanthin an ideal candidate for the treatment of metabolic syndrome, since there is strong evidence of the important role played by ROS in various types of insulin resistances [238]. Moreover, fucoxanthin has shown neuroprotective activity, in vivo and in vitro, upregulating the expression of the DJ-1 stress-sensing protein in rats and protecting mitochondria from oxidative stress following the treatment with hydrogen peroxide [239].
3.6. Other Bioactive Compounds
Some microalgal compounds have shown important bioactivities, possibly influencing the biology of the mitochondria, although their mechanisms of action are still not sufficiently known. Many of these have been characterized as anticarcinogens [17,240,241,242], even if often by tests conducted only on cell lines, as already pointed out in many above-reported examples. In this regard, it should be kept in mind that if the microalgal preparation has to be taken orally, as happens with functional foods, the data obtained by treating cell cultures should be confirmed in vivo since active molecules may not be absorbed or can be modified during digestion or after absorption. These compounds include, by way of example, polysaccharides (often sulfated), glycosides, terpenoids and galactolipids.
Among polysaccharides, β-glucans or chrysolaminarins are produced by several microalgae and especially by Bacillariophyta [189]. In particular, 1,3-1,6 β-glucans have been shown to improve mitochondrial respiration and functionality in a model of Duchenne muscular dystrophy [243]. A fatty alcohol ester isolated from Phaeodactylum tricornutum, nonyl 8-acetoxy-6-methyloctanoate, was described to promote intrinsic apoptosis in promyeloblast HL-60 cells by activating the pro-apoptotic Bax protein and by suppressing the anti-apoptotic BCL-2-like protein 1 (alias BCL-xL) [244].
Cyclic peptides synthesized by some marine cyanobacteria, such as cyclic depsipeptidesaurilide B and C produced by Lyngbya majusculus, or the cyclic depsipeptide Coibamade A produced by Leptolyngbya sp., showed cytotoxicity in several human tumor cell lines. A perusal revision was proposed by Mondal et al. [240]. Some of these compounds, e.g., aurilides isolated from the cyanobacterium Moorena bouillonii, have been shown to act on mitochondria by binding prohibitin 1 (PHB1) and by stimulating OPA1 synthesis, thus promoting mitochondrial fission and apoptosis [240]. PHB1 regulates the organization and maintenance of the mitochondrial genome by the accurate organization of mitochondrial nucleoids and controls the copy number of mtDNA by stabilizing TFAM [245].
4. Discussion and Conclusions
The interest in the use of microalgae as a source of active compounds is grounded on their ability to produce and concentrate bioactive substances suitable to protect the cell from harmful processes and to preserve some needful functionalities, many of which are related to the functional integrity of mitochondria. As shown in many cases discussed above, the preservation of mitochondrial functionality is essential to prevent damages due to oxidative stress, the main cause of aging. Oxidative stress also promotes many chronic and degenerative diseases, such as cardiovascular diseases, diseases of the nervous system, diabetes, and many types of cancer. In addition to this, these single-cell organisms assume high economic interest because they can be industrially cultivated in purity by means of bioreactors or in semi-purity in suitable open-pond plants [246]. Their culture does not require the use of pesticides or drugs and can also occur in infertile soils, ensuring the logistical and insolation conditions to successfully manage bioreactor plants. Furthermore, microalgae can be produced in wastewater treatment plants, even with some applicative limitations on the use of the biomasses obtained [247]. From a technical point of view, therefore, microalgae are perfect candidates to play a relevant role in the development of an innovative, sustainable and green economy. Moreover, microalgae can be processed in order to obtain dehydrated flours, which can be commercialized as raw materials and easily mixed to obtain a balanced combination of desirable actives. In this sense, they represent an ideal source of ingredients for functional foods and nutritional supplements. In 2020, the global microalgae market was estimated at USD 3.4 billion, and the annual growth rate is estimated at 4.3%, so in 2027, this value is expected to reach USD 4.6 billion [248]. The attention paid to the antioxidant properties of many microalgal active compounds, such as carotenoids, PUFAs, polyphenols, etc., is consistent with the fact that aging is largely derived from molecular damage due to oxidative reactions triggered by free radicals. Importantly, even if many of these active compounds are industrially synthesized at lower costs, there is evidence that the biological activity of synthetic analogs is different, and in some cases considerably lower, than that of natural compounds [249,250,251].
On the other hand, mitochondria, due to their function as cell “powerhouses” and sites of high-intensity redox reactions, represent a fundamental target to protect the functional integrity of the cell. However, from the above-reported data, it is evident that the protective activity exerted by the mentioned bio-actives is only partly related to their antioxidant properties. Indeed, it seems that the relevance of their biological action is broader and correlated with their ability to interact with some biochemical pathways involving inflammation, gene expression of antioxidant enzymes, intrinsic apoptosis and mitochondrial dynamics. Simplified synthesis of some key pathways involving mitochondria as final organelle executors and affected by microalgal compounds are shown in Figure 2. Interestingly, the regulatory action exerted by microalgal bioactive compounds on cell signaling explains how they can be effective, even if taken in limited quantities, compatible with dietary intake. Works currently available in the literature are often focused on the beneficial properties of individual compounds present in microalgae, but studies aimed at verifying the benefits deriving from the natural combination of different active compounds are very scarce.
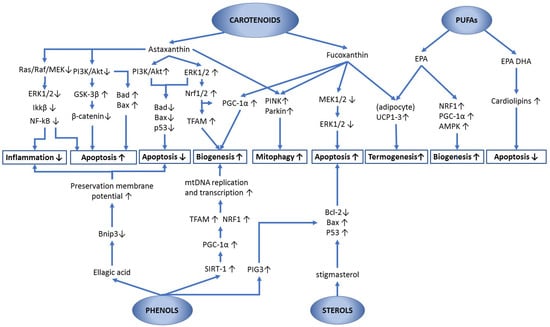
Figure 2.
Simplified scheme of relevant interactions of microalgal bioactive compounds acting on mitochondrial functionality and apoptosis. The direction of the arrows indicates the modulation of enzyme/compound activity, not of gene expression. Abbreviations: AMPK, 5′-AMP-activated protein kinase; Bad, Bcl-2-associated death promoter; Bax, BCL-2-associated X protein; BCL-2, B-cell lymphoma 2; Bnip3, BCL2 interacting protein 3; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; ERKs, extracellular signal-regulated kinases; MEK, MAPK/ERK kinase; NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cells; Nrf1, nuclear respiratory factor-1; Nrf2, nuclear factor erythroid 2-related factor 2; p53, cellular tumor antigen p53; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator-1α; PINK, PTEN-induced kinase; Raf, RAF proto-oncogene serine/threonine-protein kinase (alias rapidly accelerated fibrosarcoma kinase); Ras, KRAS Proto-Oncogene, GTPase; SIRT1, silent information regulator-1; TFAM, transcription factor A mitochondrial; UCPs, uncoupling proteins.
Nevertheless, multifunctionality is one of the most interesting characteristics of microalgae and suggests that they can represent excellent functional foods, potentially suitable for simultaneously providing protection against different types of metabolic disorders and for delaying cellular aging through complementary and synergistic mechanisms of action. However, multifunctional actions can hardly be evaluated using in vitro experimental models, while they would require the execution of long-term clinical trials. Another aspect in which the study of microalgae is still unsatisfactory concerns the chemical characterization of their composition, containing different compounds potentially active in human metabolism. For example, the quantitative comparison between the identified phenolic compounds and the total phenolic content observed in many case studies shows that most of the compounds have not been characterized yet. This is probably due to the fact that analyses were carried out looking for phenolic compounds already known in terrestrial plants, whereas it is known that these microorganisms produce molecules (e.g., carotenoids and long-chain fatty acids) that are rare or even absent in the terrestrial biomes.
Importantly, the use of microalgae for foods would require further development of the downstream processing of cell-mass and products in order to improve the bioavailability of active compounds of interest and, in addition, to ensure preservation until consumption [252]. Indeed, the cell wall of some strains can represent an important impairment to intestinal absorption of part of the actives [119,253,254,255], which, due to their reducing properties, are also susceptible to being modified during their processing or storage. In any case, the potential of microalgae as sources of active compounds deserves intense research efforts.
Even if the chemical characterization of algal biomass is still insufficient, the most important deficiency relies on the current lack of an overall evaluation of the nutraceutical functions attributable to the different microalgal species already authorized for food use and the relative recommended rations. To date, trials on humans are available for a few microalgae and are often based on dietary treatments for a few weeks. In this regard, some relevant findings are available for Phaeodactylum tricornutum (Bacillariophyta) [256], species of the genus Chlorella (Trebouxiophyceae) [257,258,259,260,261,262], Haematococcus pluvialis (Chlorophyceae) [263,264,265,266], species of the genus Tetraselmis (Chlorodendrophyceae) [267], Euglena gracilis (Euglenophyceae) [268], species of the genus Nannochloropsis (Eustigmatophyceae) [269] and cyanobacteria of the genus Arthrospira (Cyanophyceae) [270,271].
In conclusion, authors considered of particular interest the promotion of further trials involving significant numbers of subjects and carried out over a long period of time. Indeed, the effects of functional foods based on the response of biochemical markers can be very useful, but unlike the behavior of drugs for the treatment of specific pathologies, the assessment of a preventive approach requires long-term studies and data based on large sample sizes in order to support robust statistical analyses.
Author Contributions
All authors contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This work did not receive external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bloom, D.E.; Luca, D.L. Chapter 1—The Global Demography of Aging: Facts, Explanations, Future. In Handbook of the Economics of Population Aging; Piggott, J., Woodland, A., Eds.; North-Holland: Amsterdam, The Netherlands, 2016; Volume 1, pp. 3–56. [Google Scholar]
- de Meijer, C.; Wouterse, B.; Polder, J.; Koopmanschap, M. The Effect of Population Aging on Health Expenditure Growth: A Critical Review. Eur. J. Ageing 2013, 10, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; He, Y.; Lyu, J.; Yu, C.; Bian, M.; Lee, L. Aging in China: Perspectives on Public Health. Glob. Health J. 2020, 4, 11–17. [Google Scholar] [CrossRef]
- Kaplan, M.; Inguanzo, M. The Social, Economic, and Public Health Consequences of Global Population Aging: Implications for Social Work Practice and Public Policy. J. Soc. Work Glob. Community 2017, 2, 1–12. [Google Scholar] [CrossRef]
- Vancea, M.; Solé-Casals, J. Population Aging in the European Information Societies: Towards a Comprehensive Research Agenda in EHealth Innovations for Elderly. Aging Dis. 2015, 7, 526–539. [Google Scholar] [CrossRef]
- Meydani, M. Nutrition Interventions in Aging and Age-Associated Disease. Ann. N. Y. Acad. Sci. 2001, 928, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Abe, C.; Burdeos, G.C.; Matsumoto, A.; Toda, M. Food Antioxidants and Aging: Theory, Current Evidence and Perspectives. Nutraceuticals 2022, 2, 181–204. [Google Scholar] [CrossRef]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota–Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and Evolution of the Western Diet: Health Implications for the 21st Century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- Castillo, M.; Iriondo-DeHond, A.; Martirosyan, D. Are Functional Foods Essential for Sustainable Health? Ann. Nutr. Food Sci. 2018, 2, 1015. [Google Scholar]
- Milner, J.A. Functional Foods and Health Promotion. J. Nutr. 1999, 129, 1395S–1397S. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Böhm, V.; Borge, G.I.A.; Cano, M.P.; Fikselová, M.; Gruskiene, R.; Lavelli, V.; Loizzo, M.R.; Mandić, A.I.; Brahm, P.M.; et al. Carotenoids: Considerations for Their Use in Functional Foods, Nutraceuticals, Nutricosmetics, Supplements, Botanicals, and Novel Foods in the Context of Sustainability, Circular Economy, and Climate Change. Annu. Rev. Food Sci. Technol. 2021, 12, 433–460. [Google Scholar] [CrossRef]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for High-Value Products Towards Human Health and Nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef]
- Basheer, S.; Huo, S.; Zhu, F.; Qian, J.; Xu, L.; Cui, F.; Zou, B. Microalgae in Human Health and Medicine. In Microalgae Biotechnology for Food, Health and High Value Products; Alam, M.A., Xu, J.-L., Wang, Z., Eds.; Springer: Singapore, 2020; pp. 149–174. ISBN 9789811501692. [Google Scholar]
- Del Mondo, A.; Smerilli, A.; Sané, E.; Sansone, C.; Brunet, C. Challenging Microalgal Vitamins for Human Health. Microb. Cell Factories 2020, 19, 201. [Google Scholar] [CrossRef]
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as Healthy Ingredients for Functional Food: A Review. Food Funct. 2017, 8, 2672–2685. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Abdelnour, S.; Alagawany, M.; Abdo, M.; Sakr, M.A.; Khafaga, A.F.; Mahgoub, S.A.; Elnesr, S.S.; Gebriel, M.G. Microalgae in Modern Cancer Therapy: Current Knowledge. Biomed. Pharmacother. 2019, 111, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.M.; Andrade, C.J.; Dias, M.; Nascimento, C.A.O.; Mendes, M.A. Chlorella and Spirulina Microalgae as Sources of Functional Foods, Nutraceuticals, and Food Supplements; an Overview. MOJ Food Process. Technol. 2018, 6, 45–58. [Google Scholar] [CrossRef]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus Astaxanthin: Applications for Human Health and Nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Napolitano, G.; Fasciolo, G.; Salbitani, G.; Venditti, P. Chlorella sorokiniana Dietary Supplementation Increases Antioxidant Capacities and Reduces ROS Release in Mitochondria of Hyperthyroid Rat Liver. Antioxidants 2020, 9, 883. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as Nutritional and Functional Food Sources: Revisiting Our Understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S. Chapter Two—Oxidative Stress, Inflammation, and Disease. In Oxidative Stress and Biomaterials; Dziubla, T., Butterfield, D.A., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 35–58. ISBN 978-0-12-803269-5. [Google Scholar]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative Stress and Metabolic Disorders: Pathogenesis and Therapeutic Strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Ardehali, H.; Balaban, R.S.; DiLisa, F.; Dorn, G.W.; Kitsis, R.N.; Otsu, K.; Ping, P.; Rizzuto, R.; Sack, M.N.; et al. Mitochondrial Function, Biology, and Role in Disease. Circ. Res. 2016, 118, 1960–1991. [Google Scholar] [CrossRef]
- Murphy, M.P.; Hartley, R.C. Mitochondria as a Therapeutic Target for Common Pathologies. Nat. Rev. Drug Discov. 2018, 17, 865–886. [Google Scholar] [CrossRef]
- Sukhorukov, V.M.; Dikov, D.; Reichert, A.S.; Meyer-Hermann, M. Emergence of the Mitochondrial Reticulum from Fission and Fusion Dynamics. PLoS Comput. Biol. 2012, 8, e1002745. [Google Scholar] [CrossRef]
- Hock, M.B.; Kralli, A. Transcriptional Control of Mitochondrial Biogenesis and Function. Annu. Rev. Physiol. 2009, 71, 177–203. [Google Scholar] [CrossRef]
- Gureev, A.P.; Shaforostova, E.A.; Popov, V.N. Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction Between the Nrf2 and PGC-1α Signaling Pathways. Front. Genet. 2019, 10, 435. [Google Scholar] [CrossRef]
- Yun, J.; Finkel, T. Mitohormesis. Cell Metab. 2014, 19, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Westermann, B. Bioenergetic Role of Mitochondrial Fusion and Fission. Biochim. Biophys. Acta BBA Bioenerg. 2012, 1817, 1833–1838. [Google Scholar] [CrossRef]
- Robb, E.L.; Moradi, F.; Maddalena, L.A.; Valente, A.J.F.; Fonseca, J.; Stuart, J.A. Resveratrol Stimulates Mitochondrial Fusion by a Mechanism Requiring Mitofusin-2. Biochem. Biophys. Res. Commun. 2017, 485, 249–254. [Google Scholar] [CrossRef]
- Nicholls, T.J.; Gustafsson, C.M. Separating and Segregating the Human Mitochondrial Genome. Trends Biochem. Sci. 2018, 43, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Mallat, A.; Uchiyama, L.F.; Lewis, S.C.; Fredenburg, R.A.; Terada, Y.; Ji, N.; Nunnari, J.; Tseng, C.C. Discovery and Characterization of Selective Small Molecule Inhibitors of the Mammalian Mitochondrial Division Dynamin, DRP1. Biochem. Biophys. Res. Commun. 2018, 499, 556–562. [Google Scholar] [CrossRef]
- Otera, H.; Ishihara, N.; Mihara, K. New Insights into the Function and Regulation of Mitochondrial Fission. Biochim. Biophys. Acta BBA Mol. Cell Res. 2013, 1833, 1256–1268. [Google Scholar] [CrossRef]
- Kageyama, Y.; Zhang, Z.; Sesaki, H. Mitochondrial Division: Molecular Machinery and Physiological Functions. Curr. Opin. Cell Biol. 2011, 23, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.; Duvezin-Caubet, S.; Koob, S.; Occhipinti, A.; Jagasia, R.; Petcherski, A.; Ruonala, M.O.; Priault, M.; Salin, B.; Reichert, A.S. Mitophagy Is Triggered by Mild Oxidative Stress in a Mitochondrial Fission Dependent Manner. Biochim. Biophys. Acta BBA Mol. Cell Res. 2012, 1823, 2297–2310. [Google Scholar] [CrossRef]
- Youle, R.J.; Narendra, D.P. Mechanisms of Mitophagy. Nat. Rev. Mol. Cell Biol. 2011, 12, 9–14. [Google Scholar] [CrossRef]
- Tanaka, K. The PINK1–Parkin Axis: An Overview. Neurosci. Res. 2020, 159, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.-X.; Yin, X.-M. Mitophagy: Mechanisms, Pathophysiological Roles, and Analysis. Biol. Chem. 2012, 393, 547–564. [Google Scholar] [CrossRef]
- Liu, P.H.; Aoi, W.; Takami, M.; Terajima, H.; Tanimura, Y.; Naito, Y.; Itoh, Y.; Yoshikawa, T. The Astaxanthin-Induced Improvement in Lipid Metabolism during Exercise Is Mediated by a PGC-1α Increase in Skeletal Muscle. J. Clin. Biochem. Nutr. 2014, 54, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, S.; Guo, Y.; Yu, H.; Bao, Y.; Xin, X.; Yang, H.; Ni, X.; Wu, N.; Jia, D. Astaxanthin Attenuates Hypertensive Vascular Remodeling by Protecting Vascular Smooth Muscle Cells from Oxidative Stress-Induced Mitochondrial Dysfunction. Oxidative Med. Cell. Longev. 2020, 2020, 4629189. [Google Scholar] [CrossRef]
- Laiglesia, L.M.; Lorente-Cebrián, S.; Prieto-Hontoria, P.L.; Fernández-Galilea, M.; Ribeiro, S.M.R.; Sáinz, N.; Martínez, J.A.; Moreno-Aliaga, M.J. Eicosapentaenoic Acid Promotes Mitochondrial Biogenesis and Beige-like Features in Subcutaneous Adipocytes from Overweight Subjects. J. Nutr. Biochem. 2016, 37, 76–82. [Google Scholar] [CrossRef]
- Flachs, P.; Horakova, O.; Brauner, P.; Rossmeisl, M.; Pecina, P.; Franssen-van Hal, N.; Ruzickova, J.; Sponarova, J.; Drahota, Z.; Vlcek, C.; et al. Polyunsaturated Fatty Acids of Marine Origin Upregulate Mitochondrial Biogenesis and Induce β-Oxidation in White Fat. Diabetologia 2005, 48, 2365–2375. [Google Scholar] [CrossRef]
- Gibellini, L.; Bianchini, E.; De Biasi, S.; Nasi, M.; Cossarizza, A.; Pinti, M. Natural Compounds Modulating Mitochondrial Functions. Evid.-Based Complement. Altern. Med. ECAM 2015, 2015, 527209. [Google Scholar] [CrossRef]
- Lepretti, M.; Martucciello, S.; Burgos Aceves, M.A.; Putti, R.; Lionetti, L. Omega-3 Fatty Acids and Insulin Resistance: Focus on the Regulation of Mitochondria and Endoplasmic Reticulum Stress. Nutrients 2018, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-T.; Chou, H.-N.; Huang, C. Dietary Fucoxanthin Increases Metabolic Rate and Upregulated MRNA Expressions of the PGC-1alpha Network, Mitochondrial Biogenesis and Fusion Genes in White Adipose Tissues of Mice. Mar. Drugs 2014, 12, 964–982. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.-X.; Xu, X.-G.; Wang, S.-Y.; Li, H.-F.; Zhang, J.; Zhang, Z.-S.; Su, H.-L.; Chen, S.-S.; Xing, W.-M.; Wang, Y.-Z.; et al. Salidroside Delays Cellular Senescence by Stimulating Mitochondrial Biogenesis Partly through a MiR-22/SIRT-1 Pathway. Oxid. Med. Cell. Longev. 2019, 2019, e5276096. [Google Scholar] [CrossRef]
- Li, S.; Ren, X.; Wang, Y.; Hu, J.; Wu, H.; Song, S.; Yan, C. Fucoxanthin Alleviates Palmitate-Induced Inflammation in RAW 264.7 Cells through Improving Lipid Metabolism and Attenuating Mitochondrial Dysfunction. Food Funct. 2020, 11, 3361–3370. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, P.; Wang, Y.; Wang, M.; Li, H.; Lin, S.; Mao, C.; Wang, B.; Song, X.; Lv, C. Astaxanthin Prevents Pulmonary Fibrosis by Promoting Myofibroblast Apoptosis Dependent on Drp1-Mediated Mitochondrial Fission. J. Cell. Mol. Med. 2015, 19, 2215–2231. [Google Scholar] [CrossRef]
- Varghese, N.; Werner, S.; Grimm, A.; Eckert, A. Dietary Mitophagy Enhancer: A Strategy for Healthy Brain Aging? Antioxidants 2020, 9, 932. [Google Scholar] [CrossRef] [PubMed]
- Gleyzer, N.; Vercauteren, K.; Scarpulla, R.C. Control of Mitochondrial Transcription Specificity Factors (TFB1M and TFB2M) by Nuclear Respiratory Factors (NRF-1 and NRF-2) and PGC-1 Family Coactivators. Mol. Cell. Biol. 2005, 25, 1354–1366. [Google Scholar] [CrossRef]
- Nicholls, D.G. Mitochondrial Function and Dysfunction in the Cell: Its Relevance to Aging and Aging-Related Disease. Int. J. Biochem. Cell Biol. 2002, 34, 1372–1381. [Google Scholar] [CrossRef]
- Ortiz, G.G.; Mireles-Ramírez, M.A.; González-Usigli, H.; Macías-Islas, M.A.; Bitzer-Quintero, O.K.; DheniTorres-Sánchez, E.; Sánchez-López, A.L.; Ramírez-Jirano, J.; Ríos-Silva, M.; Torres-Mendoza, B. Mitochondrial Aging and Metabolism: The Importance of a Good Relationship in the Central Nervous System; IntechOpen: London, UK, 2018; ISBN 978-1-78984-266-1. [Google Scholar]
- Srivastava, S. The Mitochondrial Basis of Aging and Age-Related Disorders. Genes 2017, 8, 398. [Google Scholar] [CrossRef]
- Brand, M.D.; Orr, A.L.; Perevoshchikova, I.V.; Quinlan, C.L. The Role of Mitochondrial Function and Cellular Bioenergetics in Ageing and Disease. Br. J. Dermatol. 2013, 169, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hamon, M.-P.; Bulteau, A.-L.; Friguet, B. Mitochondrial Proteases and Protein Quality Control in Ageing and Longevity. Ageing Res. Rev. 2015, 23, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Sastre, J.; Serviddio, G.; Pereda, J.; Minana, J.B.; Arduini, A.; Vendemiale, G.; Poli, G.; Pallardo, F.V.; Vina, J. Mitochondrial Function in Liver Disease. Front. Biosci.-Landmark 2007, 12, 1200–1209. [Google Scholar] [CrossRef]
- Serviddio, G.; Bellanti, F.; Vendemiale, G.; Altomare, E. Mitochondrial Dysfunction in Nonalcoholic Steatohepatitis. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Song, B.-J.; Moon, K.-H.; Olsson, N.U.; Salem, N. Prevention of Alcoholic Fatty Liver and Mitochondrial Dysfunction in the Rat by Long-Chain Polyunsaturated Fatty Acids. J. Hepatol. 2008, 49, 262–273. [Google Scholar] [CrossRef]
- Videla, L.A.; Rodrigo, R.; Araya, J.; Poniachik, J. Oxidative Stress and Depletion of Hepatic Long-Chain Polyunsaturated Fatty Acids May Contribute to Nonalcoholic Fatty Liver Disease. Free Radic. Biol. Med. 2004, 37, 1499–1507. [Google Scholar] [CrossRef]
- Hirabara, S.M.; Curi, R.; Maechler, P. Saturated Fatty Acid-Induced Insulin Resistance Is Associated with Mitochondrial Dysfunction in Skeletal Muscle Cells. J. Cell. Physiol. 2010, 222, 187–194. [Google Scholar] [CrossRef]
- Jheng, H.-F.; Tsai, P.-J.; Guo, S.-M.; Kuo, L.-H.; Chang, C.-S.; Su, I.-J.; Chang, C.-R.; Tsai, Y.-S. Mitochondrial Fission Contributes to Mitochondrial Dysfunction and Insulin Resistance in Skeletal Muscle. Mol. Cell. Biol. 2012, 32, 309–319. [Google Scholar] [CrossRef]
- Che, R.; Yuan, Y.; Huang, S.; Zhang, A. Mitochondrial Dysfunction in the Pathophysiology of Renal Diseases. Am. J. Physiol.-Ren. Physiol. 2014, 306, F367–F378. [Google Scholar] [CrossRef]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Role of Cardiolipin in Mitochondrial Function and Dynamics in Health and Disease: Molecular and Pharmacological Aspects. Cells 2019, 8, 728. [Google Scholar] [CrossRef] [PubMed]
- Zharikov, S.; Shiva, S. Platelet Mitochondrial Function: From Regulation of Thrombosis to Biomarker of Disease. Biochem. Soc. Trans. 2013, 41, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P. Altered Mitochondrial Function, Iron Metabolism and Glutathione Levels in Parkinson’s Disease. Acta Neurol. Scand. 1993, 87, 6–13. [Google Scholar] [CrossRef]
- Turner, C.; Cooper, J.M.; Schapira, A.H.V. Clinical Correlates of Mitochondrial Function in Huntington’s Disease Muscle. Mov. Disord. 2007, 22, 1715–1721. [Google Scholar] [CrossRef]
- Eckert, G.P.; Renner, K.; Eckert, S.H.; Eckmann, J.; Hagl, S.; Abdel-Kader, R.M.; Kurz, C.; Leuner, K.; Muller, W.E. Mitochondrial Dysfunction—A Pharmacological Target in Alzheimer’s Disease. Mol. Neurobiol. 2012, 46, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Catalgol, B.; Batirel, S.; Taga, Y.; Ozer, N. Resveratrol: French Paradox Revisited. Front. Pharmacol. 2012, 3, 141. [Google Scholar] [CrossRef]
- Kodydková, J.; Vávrová, L.; Kocík, M.; Žák, A. Human Catalase, Its Polymorphisms, Regulation and Changes of Its Activity in Different Diseases. Folia Biol. 2014, 60, 153–167. [Google Scholar]
- Lee, D.-H.; Kim, C.-S.; Lee, Y.J. Astaxanthin Protects against MPTP/MPP+-Induced Mitochondrial Dysfunction and ROS Production in Vivo and in Vitro. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2011, 49, 271–280. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Sun, D.; He, Y.; Jiang, Y.; Cheng, K.-W.; Chen, F. DHA Protects against Monosodium Urate-Induced Inflammation through Modulation of Oxidative Stress. Food Funct. 2019, 10, 4010–4021. [Google Scholar] [CrossRef]
- Kang, M.Y.; Kim, H.-B.; Piao, C.; Lee, K.H.; Hyun, J.W.; Chang, I.-Y.; You, H.J. The Critical Role of Catalase in Prooxidant and Antioxidant Function of P53. Cell Death Differ. 2013, 20, 117–129. [Google Scholar] [CrossRef]
- Haque, R.; Chun, E.; Howell, J.C.; Sengupta, T.; Chen, D.; Kim, H. MicroRNA-30b-Mediated Regulation of Catalase Expression in Human ARPE-19 Cells. PLoS ONE 2012, 7, e42542. [Google Scholar] [CrossRef]
- Xu, Y.; Porntadavity, S.; St Clair, D.K. Transcriptional Regulation of the Human Manganese Superoxide Dismutase Gene: The Role of Specificity Protein 1 (Sp1) and Activating Protein-2 (AP-2). Biochem. J. 2002, 362, 401–412. [Google Scholar] [CrossRef]
- Kim, Y.S.; Gupta Vallur, P.; Phaëton, R.; Mythreye, K.; Hempel, N. Insights into the Dichotomous Regulation of SOD2 in Cancer. Antioxidants 2017, 6, 86. [Google Scholar] [CrossRef]
- Miao, L.; St. Clair, D.K. Regulation of Superoxide Dismutase Genes: Implications in Diseases. Free Radic. Biol. Med. 2009, 47, 344–356. [Google Scholar] [CrossRef]
- Rui, T.; Kvietys, P.R. NFkappaB and AP-1 Differentially Contribute to the Induction of Mn-SOD and ENOS during the Development of Oxidant Tolerance. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2005, 19, 1908–1910. [Google Scholar] [CrossRef]
- Drane, P.; Bravard, A.; Bouvard, V.; May, E. Reciprocal Down-Regulation of P53 and SOD2 Gene Expression–Implication in P53 Mediated Apoptosis. Oncogene 2001, 20, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Lv, K.; Chen, H.; Wang, T.; Wang, Y.; Zhao, D.; Qu, L.; Li, Y. MiR-146a Regulates SOD2 Expression in H2O2 Stimulated PC12 Cells. PLoS ONE 2013, 8, e69351. [Google Scholar] [CrossRef] [PubMed]
- Röhrdanz, E.; Bittner, A.; Tran-Thi, Q.-H.; Kahl, R. The Effect of Quercetin on the MRNA Expression of Different Antioxidant Enzymes in Hepatoma Cells. Arch. Toxicol. 2003, 77, 506–510. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, K.W.; He, J.; Niu, Y.; Lu, Y.; Zhang, L.; Wang, T. Curcumin Attenuates Hepatic Mitochondrial Dysfunction through the Maintenance of Thiol Pool, Inhibition of MtDNA Damage, and Stimulation of the Mitochondrial Thioredoxin System in Heat-Stressed Broilers. J. Anim. Sci. 2018, 96, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Milani, P.; Amadio, M.; Laforenza, U.; Dell’Orco, M.; Diamanti, L.; Sardone, V.; Gagliardi, S.; Govoni, S.; Ceroni, M.; Pascale, A.; et al. Posttranscriptional Regulation of SOD1 Gene Expression under Oxidative Stress: Potential Role of ELAV Proteins in Sporadic ALS. Neurobiol. Dis. 2013, 60, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Spanier, G.; Xu, H.; Xia, N.; Tobias, S.; Deng, S.; Wojnowski, L.; Forstermann, U.; Li, H. Resveratrol Reduces Endothelial Oxidative Stress by Modulating the Gene Expression of Superoxide Dismutase 1 (SOD1), Glutathione Peroxidase 1 (GPx1) and NADPH Oxidase Subunit (Nox4). J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2009, 60 (Suppl. 4), 111–116. [Google Scholar]
- Seo, S.J.; Kim, H.T.; Cho, G.; Rho, H.M.; Jung, G. Spl and C/EBP-Related Factor Regulate the Transcription of Human Cu/Zn SOD Gene. Gene 1996, 178, 177–185. [Google Scholar] [CrossRef]
- Minc, E.; de Coppet, P.; Masson, P.; Thiery, L.; Dutertre, S.; Amor-Guéret, M.; Jaulin, C. The Human Copper-Zinc Superoxide Dismutase Gene (SOD1) Proximal Promoter Is Regulated by Sp1, Egr-1, and WT1 via Non-Canonical Binding Sites*. J. Biol. Chem. 1999, 274, 503–509. [Google Scholar] [CrossRef]
- Okado-Matsumoto, A.; Fridovich, I. Subcellular Distribution of Superoxide Dismutases (SOD) in Rat Liver: Cu,Zn-SOD in Mitochondria. J. Biol. Chem. 2001, 276, 38388–38393. [Google Scholar] [CrossRef]
- Riera, H.; Afonso, V.; Collin, P.; Lomri, A. A Central Role for JNK/AP-1 Pathway in the Pro-Oxidant Effect of Pyrrolidine Dithiocarbamate through Superoxide Dismutase 1 Gene Repression and Reactive Oxygen Species Generation in Hematopoietic Human Cancer Cell Line U937. PLoS ONE 2015, 10, e0127571. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Yanagawa, T. Stress-Induced Peroxiredoxins. In Peroxiredoxin Systems: Structures and Functions; Flohé, L., Harris, J.R., Eds.; Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2007; pp. 375–384. ISBN 978-1-4020-6051-9. [Google Scholar]
- Lijnen, P.J.; Piccart, Y.; Coenen, T.; Prihadi, J.S. Angiotensin II-Induced Mitochondrial Reactive Oxygen Species and Peroxiredoxin-3 Expression in Cardiac Fibroblasts. J. Hypertens. 2012, 30, 1986–1991. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Joseph, S.; Khan, A.; Epstein, C.J.; Sobel, R.; Huang, T.-T. Enhanced Expression of Mitochondrial Superoxide Dismutase Leads to Prolonged in Vivo Cell Cycle Progression and Up-Regulation of Mitochondrial Thioredoxin. Free Radic. Biol. Med. 2010, 48, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Li, K.-Y.; Xiang, X.-J.; Song, L.; Chen, J.; Luo, B.; Wen, Q.-X.; Zhong, B.-R.; Zhou, G.-F.; Deng, X.-J.; Ma, Y.-L.; et al. Mitochondrial TXN2 Attenuates Amyloidogenesis via Selective Inhibition of BACE1 Expression. J. Neurochem. 2021, 157, 1351–1365. [Google Scholar] [CrossRef]
- Suzuki, K.; Koike, H.; Matsui, H.; Ono, Y.; Hasumi, M.; Nakazato, H.; Okugi, H.; Sekine, Y.; Oki, K.; Ito, K.; et al. Genistein, a Soy Isoflavone, Induces Glutathione Peroxidase in the Human Prostate Cancer Cell Lines LNCaP and PC-3. Int. J. Cancer 2002, 99, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Regulation of Glutathione Synthesis. Mol. Aspects Med. 2009, 30, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ramiro, I.; Ramos, S.; Bravo, L.; Goya, L.; Martín, M.Á. Procyanidin B2 Induces Nrf2 Translocation and Glutathione S-Transferase P1 Expression via ERKs and P38-MAPK Pathways and Protect Human Colonic Cells against Oxidative Stress. Eur. J. Nutr. 2012, 51, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Ribas, V.; García-Ruiz, C.; Fernández-Checa, J.C. Glutathione and Mitochondria. Front. Pharmacol. 2014, 5, 151. [Google Scholar] [CrossRef]
- Wang, H.-M.; Cheng, K.-C.; Lin, C.-J.; Hsu, S.-W.; Fang, W.-C.; Hsu, T.-F.; Chiu, C.-C.; Chang, H.-W.; Hsu, C.-H.; Lee, A.Y.-L. Obtusilactone A and (−)-Sesamin Induce Apoptosis in Human Lung Cancer Cells by Inhibiting Mitochondrial Lon Protease and Activating DNA Damage Checkpoints. Cancer Sci. 2010, 101, 2612–2620. [Google Scholar] [CrossRef]
- Luciakova, K.; Sokolikova, B.; Chloupkova, M.; Nelson, B.D. Enhanced Mitochondrial Biogenesis Is Associated with Increased Expression of the Mitochondrial ATP-Dependent Lon Protease. FEBS Lett. 1999, 444, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Bota, D.A.; Davies, K.J.A. Mitochondrial Lon Protease in Human Disease and Aging: Including an Etiologic Classification of Lon-Related Diseases and Disorders. Free Radic. Biol. Med. 2016, 100, 188–198. [Google Scholar] [CrossRef]
- Nouri, K.; Feng, Y.; Schimmer, A.D. Mitochondrial ClpP Serine Protease-Biological Function and Emerging Target for Cancer Therapy. Cell Death Dis. 2020, 11, 841. [Google Scholar] [CrossRef]
- Peña-Blanco, A.; García-Sáez, A.J. Bax, Bak and beyond—Mitochondrial Performance in Apoptosis. FEBS J. 2018, 285, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Roufayel, R.; Younes, K.; Al-Sabi, A.; Murshid, N. BH3-Only Proteins Noxa and Puma Are Key Regulators of Induced Apoptosis. Life 2022, 12, 256. [Google Scholar] [CrossRef]
- Happo, L.; Strasser, A.; Cory, S. BH3-Only Proteins in Apoptosis at a Glance. J. Cell Sci. 2012, 125, 1081–1087. [Google Scholar] [CrossRef]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 Family Proteins: Changing Partners in the Dance towards Death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef]
- Cook, S.J.; Stuart, K.; Gilley, R.; Sale, M.J. Control of Cell Death and Mitochondrial Fission by ERK1/2 MAP Kinase Signalling. FEBS J. 2017, 284, 4177–4195. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.J. Functional Foods. Eur. J. Clin. Nutr. 2010, 64, 657–659. [Google Scholar] [CrossRef]
- Domínguez Díaz, L.; Fernández-Ruiz, V.; Cámara, M. The Frontier between Nutrition and Pharma: The International Regulatory Framework of Functional Foods, Food Supplements and Nutraceuticals. Crit. Rev. Food Sci. Nutr. 2020, 60, 1738–1746. [Google Scholar] [CrossRef]
- Kalra, E.K. Nutraceutical-Definition and Introduction. AAPS PharmSci 2003, 5, 27–28. [Google Scholar] [CrossRef] [PubMed]
- Hardy, G. Nutraceuticals and Functional Foods: Introduction and Meaning. Nutrition 2000, 16, 688–689. [Google Scholar] [CrossRef]
- Basu, S.; Thomas, J.; Acharya, S. Prospects for Growth in Global Nutraceutical and Functional Food Markets: A Canadian Perspective. Aust. J. Basic Appl. Sci. 2007, 1, 637–649. [Google Scholar]
- Kwak, N.-S.; Jukes, D.J. Functional Foods. Part 1: The Development of a Regulatory Concept. Food Control 2001, 12, 99–107. [Google Scholar] [CrossRef]
- Guleri, S.; Tiwari, A. Algae and Ageing. In Microalgae Biotechnology for Food, Health and High Value Products; Alam, M.A., Xu, J.-L., Wang, Z., Eds.; Springer: Singapore, 2020; pp. 267–293. ISBN 9789811501692. [Google Scholar]
- Hachicha, R.; Elleuch, F.; Ben Hlima, H.; Dubessay, P.; de Baynast, H.; Delattre, C.; Pierre, G.; Hachicha, R.; Abdelkafi, S.; Michaud, P.; et al. Biomolecules from Microalgae and Cyanobacteria: Applications and Market Survey. Appl. Sci. 2022, 12, 1924. [Google Scholar] [CrossRef]
- Remize, M.; Brunel, Y.; Silva, J.L.; Berthon, J.-Y.; Filaire, E. Microalgae N-3 PUFAs Production and Use in Food and Feed Industries. Mar. Drugs 2021, 19, 113. [Google Scholar] [CrossRef]
- Ampofo, J.; Abbey, L. Microalgae: Bioactive Composition, Health Benefits, Safety and Prospects as Potential High-Value Ingredients for the Functional Food Industry. Foods 2022, 11, 1744. [Google Scholar] [CrossRef] [PubMed]
- Zanella, L.; Vianello, F. Microalgae of the Genus Nannochloropsis: Chemical Composition and Functional Implications for Human Nutrition. J. Funct. Foods 2020, 68, 103919. [Google Scholar] [CrossRef]
- Chen, C.; Tang, T.; Shi, Q.; Zhou, Z.; Fan, J. The Potential and Challenge of Microalgae as Promising Future Food Sources. Trends Food Sci. Technol. 2022, 126, 99–112. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Gheysen, L.; Kyomugasho, C.; Jamsazzadeh Kermani, Z.; Vandionant, S.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. Comparison of Microalgal Biomasses as Functional Food Ingredients: Focus on the Composition of Cell Wall Related Polysaccharides. Algal Res. 2018, 32, 150–161. [Google Scholar] [CrossRef]
- Raposo, M.F.D.J.; De Morais, A.M.M.B.; De Morais, R.M.S.C. Carotenoids from Marine Microalgae: A Valuable Natural Source for the Prevention of Chronic Diseases. Mar. Drugs 2015, 13, 5128–5155. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, J.; Movafeghi, A.; Mathieu-Rivet, E.; Mati-Baouche, N.; Calbo, S.; Lerouge, P.; Bardor, M. Microalgae as an Efficient Vehicle for the Production and Targeted Delivery of Therapeutic Glycoproteins against SARS-CoV-2 Variants. Mar. Drugs 2022, 20, 657. [Google Scholar] [CrossRef] [PubMed]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a Future Food Source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef]
- Prüser, T.F.; Braun, P.G.; Wiacek, C. Microalgae as a novel food. Potential and legal framework. Ernahrungs Umsch. 2021, 68, 78–85. [Google Scholar]
- Cecchin, M.; Cazzaniga, S.; Martini, F.; Paltrinieri, S.; Bossi, S.; Maffei, M.E.; Ballottari, M. Astaxanthin and Eicosapentaenoic Acid Production by S4, a New Mutant Strain of Nannochloropsis gaditana. Microb. Cell Factories 2022, 21, 117. [Google Scholar] [CrossRef]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.-F. Microalgal Carotenoids: A Review of Production, Current Markets, Regulations, and Future Direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase World-Wide Electronic Publication, National University of Ireland, Galway. Available online: https://www.algaebase.org (accessed on 23 December 2022).
- Sansone, C.; Galasso, C.; Orefice, I.; Nuzzo, G.; Luongo, E.; Cutignano, A.; Romano, G.; Brunet, C.; Fontana, A.; Esposito, F.; et al. The Green Microalga Tetraselmis suecica Reduces Oxidative Stress and Induces Repairing Mechanisms in Human Cells. Sci. Rep. 2017, 7, 41215. [Google Scholar] [CrossRef]
- Cha, Y.; Kim, T.; Jeon, J.; Jang, Y.; Kim, P.B.; Lopes, C.; Leblanc, P.; Cohen, B.M.; Kim, K.-S. SIRT2 Regulates Mitochondrial Dynamics and Reprogramming via MEK1-ERK-DRP1 and AKT1-DRP1 Axes. Cell Rep. 2021, 37, 110155. [Google Scholar] [CrossRef]
- Nacer, W.; Baba Ahmed, F.Z.; Merzouk, H.; Benyagoub, O.; Bouanane, S. Evaluation of the Anti-Inflammatory and Antioxidant Effects of the Microalgae Nannochloropsis gaditana in Streptozotocin-Induced Diabetic Rats. J. Diabetes Metab. Disord. 2020, 19, 1483–1490. [Google Scholar] [CrossRef]
- Gupta, S.P.; Siddiqi, N.J.; Khan, H.A.; Alrokayan, S.H.; Alhomida, A.S.; Singh, R.K.; Verma, P.K.; Kumar, S.; Acharya, A.; Sharma, B. Phytochemical Profiling of Microalgae Euglena tuba and Its Anticancer Activity in Dalton’s Lymphoma Cells. Front. Biosci.-Landmark 2022, 27, 120. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-Y.; Tsai, C.-T.; Chuang, W.-L.; Chao, Y.-H.; Pan, I.-H.; Chen, Y.-K.; Lin, C.-C.; Wang, B.-Y. Chlorella sorokiniana Induces Mitochondrial-Mediated Apoptosis in Human Non-Small Cell Lung Cancer Cells and Inhibits Xenograft Tumor Growth In Vivo. BMC Complement. Altern. Med. 2017, 17, 88. [Google Scholar] [CrossRef] [PubMed]
- Abolhasani, M.H.; Safavi, M.; Goodarzi, M.T.; Kassaee, S.M.; Azin, M. Identification and Anti-Cancer Activity in 2D and 3D Cell Culture Evaluation of an Iranian Isolated Marine Microalgae Picochlorum sp. RCC486. DARU J. Pharm. Sci. 2018, 26, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.-S.; Kim, S.-M.; Kim, J.E.; Hong, J.-M.; Lee, S.G.; Youn, U.J.; Han, S.J.; Kim, I.-C.; Kim, S. Anticancer Activities of Ethanol Extract from the Antarctic Freshwater Microalga, Botryidiopsidaceae sp. BMC Complement. Altern. Med. 2017, 17, 509. [Google Scholar] [CrossRef]
- Ebrahimi Nigjeh, S.; Yusoff, F.M.; Mohamed Alitheen, N.B.; Rasoli, M.; Keong, Y.S.; bin Omar, A.R. Cytotoxic Effect of Ethanol Extract of Microalga, Chaetoceros calcitrans, and Its Mechanisms in Inducing Apoptosis in Human Breast Cancer Cell Line. BioMed Res. Int. 2012, 2013, e783690. [Google Scholar] [CrossRef]
- Guo, B.; Zhou, Y.; Liu, B.; He, Y.; Chen, F.; Cheng, K.-W. Lipid-Lowering Bioactivity of Microalga Nitzschia laevis Extract Containing Fucoxanthin in Murine Model and Carcinomic Hepatocytes. Pharmaceuticals 2021, 14, 1004. [Google Scholar] [CrossRef]
- Campiche, R.; Sandau, P.; Kurth, E.; Massironi, M.; Imfeld, D.; Schuetz, R. Protective Effects of an Extract of the Freshwater Microalga Scenedesmus rubescens on UV-Irradiated Skin Cells. Int. J. Cosmet. Sci. 2018, 40, 187–192. [Google Scholar] [CrossRef]
- Nawrocka, D.; Kornicka, K.; Śmieszek, A.; Marycz, K. Spirulina platensis Improves Mitochondrial Function Impaired by Elevated Oxidative Stress in Adipose-Derived Mesenchymal Stromal Cells (ASCs) and Intestinal Epithelial Cells (IECs), and Enhances Insulin Sensitivity in Equine Metabolic Syndrome (EMS) Horses. Mar. Drugs 2017, 15, 237. [Google Scholar] [CrossRef] [PubMed]
- Stanley, W.C.; Khairallah, R.J.; Dabkowski, E.R. Update on Lipids and Mitochondrial Function: Impact of Dietary n-3 Polyunsaturated Fatty Acids. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 122–126. [Google Scholar] [CrossRef]
- Khairallah, R.J.; Sparagna, G.C.; Khanna, N.; O’Shea, K.M.; Hecker, P.A.; Kristian, T.; Fiskum, G.; Des Rosiers, C.; Polster, B.M.; Stanley, W.C. Dietary Supplementation with Docosahexaenoic Acid, but Not Eicosapentaenoic Acid, Dramatically Alters Cardiac Mitochondrial Phospholipid Fatty Acid Composition and Prevents Permeability Transition. Biochim. Biophys. Acta BBA Bioenerg. 2010, 1797, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Honigmann, G.; Schliack, V. Decrease of Eicosapentaenoic Acid in Fatty Liver of Diabetic Subjects. Prostaglandins Med. 1980, 5, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Wensaas, A.J.; Rustan, A.C.; Just, M.; Berge, R.K.; Drevon, C.A.; Gaster, M. Fatty Acid Incubation of Myotubes From Humans With Type 2 Diabetes Leads to Enhanced Release of β-Oxidation Products Because of Impaired Fatty Acid Oxidation: Effects of Tetradecylthioacetic Acid and Eicosapentaenoic Acid. Diabetes 2009, 58, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chen, X. Eicosapentaenoic Acid Promotes Thermogenic and Fatty Acid Storage Capacity in Mouse Subcutaneous Adipocytes. Biochem. Biophys. Res. Commun. 2014, 450, 1446–1451. [Google Scholar] [CrossRef]
- Zhou, L.; Li, K.; Duan, X.; Hill, D.; Barrow, C.; Dunshea, F.; Martin, G.; Suleria, H. Bioactive Compounds in Microalgae and Their Potential Health Benefits. Food Biosci. 2022, 49, 101932. [Google Scholar] [CrossRef]
- Randhir, A.; Laird, D.W.; Maker, G.; Trengove, R.; Moheimani, N.R. Microalgae: A Potential Sustainable Commercial Source of Sterols. Algal Res. 2020, 46, 101772. [Google Scholar] [CrossRef]
- Fithriani, D.; Ambarwaty, D.; Nurhayati, N. Identification of Bioactive Compounds from Nannochloropsis sp. IOP Conf. Series: Earth Environ. Sci. 2020, 404, 012064. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Li, X.-F.; Kang, K.-H.; Ryu, B.; Kim, S.K. Stigmasterol Isolated from Marine Microalgae Navicula incerta Induces Apoptosis in Human Hepatoma HepG2 Cells. BMB Rep. 2014, 47, 433–438. [Google Scholar] [CrossRef]
- Cilla, A.; Attanzio, A.; Barberá, R.; Tesoriere, L.; Livrea, M.A. Anti-Proliferative Effect of Main Dietary Phytosterols and β-Cryptoxanthin Alone or Combined in Human Colon Cancer Caco-2 Cells through Cytosolic Ca+2–and Oxidative Stress-Induced Apoptosis. J. Funct. Foods 2015, 12, 282–293. [Google Scholar] [CrossRef]
- Mamari, H.H.A. Phenolic Compounds: Classification, Chemistry, and Updated Techniques of Analysis and Synthesis; IntechOpen: London, UK, 2021; ISBN 978-1-83969-347-2. [Google Scholar]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of Phenolic Compounds: A Review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, S.; Singh, M.; Srivastava, A.; Chavali, M.; Chandrasekhar, K.; Verma, P. Extraction and Characterization of Microalgae-Derived Phenolics for Pharmaceutical Applications: A Systematic Review. J. Basic Microbiol. 2021, 62, 1044–1063. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant Potential of Microalgae in Relation to Their Phenolic and Carotenoid Content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Rodis, P.S.; Karathanos, V.T.; Mantzavinou, A. Partitioning of Olive Oil Antioxidants between Oil and Water Phases. J. Agric. Food Chem. 2002, 50, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Scaglioni, P.T.; Quadros, L.; de Paula, M.; Furlong, V.B.; Abreu, P.C.; Badiale-Furlong, E. Inhibition of Enzymatic and Oxidative Processes by Phenolic Extracts from Spirulina sp. and Nannochloropsis sp. Food Technol. Biotechnol. 2018, 56, 344–353. [Google Scholar] [CrossRef]
- Goh, S.-H.; Yusoff, F.M.; Loh, S.P. A Comparison of the Antioxidant Properties and Total Phenolic Content in a Diatom, Chaetoceros sp. and a Green Microalga, Nannochloropsis sp. J. Agric. Sci. 2010, 2, p123. [Google Scholar] [CrossRef]
- Parcheta, M.; Świsłocka, R.; Orzechowska, S.; Akimowicz, M.; Choińska, R.; Lewandowski, W. Recent Developments in Effective Antioxidants: The Structure and Antioxidant Properties. Materials 2021, 14, 1984. [Google Scholar] [CrossRef] [PubMed]
- Andriopoulos, V.; Gkioni, M.D.; Koutra, E.; Mastropetros, S.G.; Lamari, F.N.; Hatziantoniou, S.; Kornaros, M. Total Phenolic Content, Biomass Composition, and Antioxidant Activity of Selected Marine Microalgal Species with Potential as Aquaculture Feed. Antioxidants 2022, 11, 1320. [Google Scholar] [CrossRef] [PubMed]
- Muñóz-Almagro, N.; Gilbert-López, B.M.; Carmen, P.-R.; García-Fernandez, Y.; Almeida, C.; Villamiel, M.; Mendiola, J.A.; Ibáñez, E. Exploring the Microalga Euglena cantabrica by Pressurized Liquid Extraction to Obtain Bioactive Compounds. Mar. Drugs 2020, 18, 308. [Google Scholar] [CrossRef]
- Safafar, H.; Van Wagenen, J.; Møller, P.; Jacobsen, C. Carotenoids, Phenolic Compounds and Tocopherols Contribute to the Antioxidative Properties of Some Microalgae Species Grown on Industrial Wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef] [PubMed]
- Massa, M.; Buono, S.; Langellotti, A.L.; Martello, A.; Russo, G.L.; Troise, D.A.; Sacchi, R.; Vitaglione, P.; Fogliano, V. Biochemical Composition and in Vitro Digestibility of Galdieria sulphuraria Grown on Spent Cherry-Brine Liquid. New Biotechnol. 2019, 53, 9–15. [Google Scholar] [CrossRef]
- Machu, L.; Misurcova, L.; Vavra Ambrozova, J.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic Content and Antioxidant Capacity in Algal Food Products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef]
- Michael, A.; Kyewalyanga, M.S.; Mtolera, M.S.; Lugomela, C.V. Antioxidants Activity of the Cyanobacterium, Arthrospira (Spirulina) fusiformis Cultivated in a Low-Cost Medium. Afr. J. Food Sci. 2018, 12, 188–195. [Google Scholar] [CrossRef]
- Martinez-Goss, M.R.; Arguelles, E.D.L.R.; Sapin, A.B.; Almeda, R.A. Chemical Composition and In Vitro Antioxidant and Antibacterial Properties of the Edible Cyanobacterium, Nostoc commune Vaucher. Philipp. Sci. Lett. 2021, 14, 25–35. [Google Scholar]
- Lima, G.P.P.; Vianello, F.; Corrêa, C.R.; Campos, R.A.D.S.; Borguini, M.G. Polyphenols in Fruits and Vegetables and Its Effect on Human Health. Food Nutr. Sci. 2014, 5, 1065–1082. [Google Scholar] [CrossRef]
- Chodari, L.; Dilsiz Aytemir, M.; Vahedi, P.; Alipour, M.; Vahed, S.Z.; Khatibi, S.M.H.; Ahmadian, E.; Ardalan, M.; Eftekhari, A. Targeting Mitochondrial Biogenesis with Polyphenol Compounds. Oxid. Med. Cell. Longev. 2021, 2021, e4946711. [Google Scholar] [CrossRef]
- Sandoval-Acuña, C.; Ferreira, J.; Speisky, H. Polyphenols and Mitochondria: An Update on Their Increasingly Emerging ROS-Scavenging Independent Actions. Arch. Biochem. Biophys. 2014, 559, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Naoi, M.; Wu, Y.; Shamoto-Nagai, M.; Maruyama, W. Mitochondria in Neuroprotection by Phytochemicals: Bioactive Polyphenols Modulate Mitochondrial Apoptosis System, Function and Structure. Int. J. Mol. Sci. 2019, 20, 2451. [Google Scholar] [CrossRef]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.-L.; et al. Small Molecule Activators of Sirtuins Extend Saccharomyces cerevisiae Lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D.; Davis, B. Quercetin Increases Brain and Muscle Mitochondrial Biogenesis and Exercise Tolerance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1071–R1077. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Shen, W.; Yu, G.; Jia, H.; Li, X.; Feng, Z.; Wang, Y.; Weber, P.; Wertz, K.; Sharman, E.; et al. Hydroxytyrosol Promotes Mitochondrial Biogenesis and Mitochondrial Function in 3T3-L1 Adipocytes. J. Nutr. Biochem. 2010, 21, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Signorile, A.; Micelli, L.; De Rasmo, D.; Santeramo, A.; Papa, F.; Ficarella, R.; Gattoni, G.; Scacco, S.; Papa, S. Regulation of the Biogenesis of OXPHOS Complexes in Cell Transition from Replicating to Quiescent State: Involvement of PKA and Effect of Hydroxytyrosol. Biochim. Biophys. Acta 2014, 1843, 675–684. [Google Scholar] [CrossRef]
- Feillet-Coudray, C.; Sutra, T.; Fouret, G.; Ramos, J.; Wrutniak-Cabello, C.; Cabello, G.; Cristol, J.P.; Coudray, C. Oxidative Stress in Rats Fed a High-Fat High-Sucrose Diet and Preventive Effect of Polyphenols: Involvement of Mitochondrial and NAD(P)H Oxidase Systems. Free Radic. Biol. Med. 2009, 46, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Zimermann, J.D.F.; Sydney, E.B.; Cerri, M.L.; de Carvalho, I.K.; Schafranski, K.; Sydney, A.C.N.; Vitali, L.; Gonçalves, S.; Micke, G.A.; Soccol, C.R.; et al. Growth Kinetics, Phenolic Compounds Profile and Pigments Analysis of Galdieria sulphuraria Cultivated in Whey Permeate in Shake-Flasks and Stirred-Tank Bioreactor. J. Water Process Eng. 2020, 38, 101598. [Google Scholar] [CrossRef]
- Hwang, J.M.; Cho, J.S.; Kim, T.H.; Lee, Y.I. Ellagic Acid Protects Hepatocytes from Damage by Inhibiting Mitochondrial Production of Reactive Oxygen Species. Biomed. Pharmacother. 2010, 64, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Firdaus, F.; Zafeer, M.F.; Waseem, M.; Anis, E.; Hossain, M.M.; Afzal, M. Ellagic Acid Mitigates Arsenic-Trioxide-Induced Mitochondrial Dysfunction and Cytotoxicity in SH-SY5Y Cells. J. Biochem. Mol. Toxicol. 2018, 32, e22024. [Google Scholar] [CrossRef]
- Dhingra, A.; Jayas, R.; Afshar, P.; Guberman, M.; Maddaford, G.; Gerstein, J.; Lieberman, B.; Nepon, H.; Margulets, V.; Dhingra, R.; et al. Ellagic Acid Antagonizes Bnip3-Mediated Mitochondrial Injury and Necrotic Cell Death of Cardiac Myocytes. Free Radic. Biol. Med. 2017, 112, 411–422. [Google Scholar] [CrossRef]
- Larrosa, M.; Tomás-Barberán, F.A.; Espín, J.C. The Dietary Hydrolysable Tannin Punicalagin Releases Ellagic Acid That Induces Apoptosis in Human Colon Adenocarcinoma Caco-2 Cells by Using the Mitochondrial Pathway. J. Nutr. Biochem. 2006, 17, 611–625. [Google Scholar] [CrossRef]
- Salimi, A.; Roudkenar, M.H.; Sadeghi, L.; Mohseni, A.; Seydi, E.; Pirahmadi, N.; Pourahmad, J. Ellagic Acid, a Polyphenolic Compound, Selectively Induces ROS-Mediated Apoptosis in Cancerous B-Lymphocytes of CLL Patients by Directly Targeting Mitochondria. Redox Biol. 2015, 6, 461–471. [Google Scholar] [CrossRef]
- Ho, C.-C.; Huang, A.-C.; Yu, C.-S.; Lien, J.-C.; Wu, S.-H.; Huang, Y.-P.; Huang, H.-Y.; Kuo, J.-H.; Liao, W.-Y.; Yang, J.-S.; et al. Ellagic Acid Induces Apoptosis in TSGH8301 Human Bladder Cancer Cells through the Endoplasmic Reticulum Stress- and Mitochondria-Dependent Signaling Pathways. Environ. Toxicol. 2014, 29, 1262–1274. [Google Scholar] [CrossRef]
- Edderkaoui, M.; Odinokova, I.; Ohno, I.; Gukovsky, I.; Go, V.L.W.; Pandol, S.J.; Gukovskaya, A.S. Ellagic Acid Induces Apoptosis through Inhibition of Nuclear Factor Kappa B in Pancreatic Cancer Cells. World J. Gastroenterol. 2008, 14, 3672–3680. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Li, Y.; Gao, H.; Yang, D.; He, X.; Fang, Y.; Zhou, G. Phenolic Compound Ellagic Acid Inhibits Mitochondrial Respiration and Tumor Growth in Lung Cancer. Food Funct. 2020, 11, 6332–6339. [Google Scholar] [CrossRef] [PubMed]
- Gorlach, S.; Fichna, J.; Lewandowska, U. Polyphenols as Mitochondria-Targeted Anticancer Drugs. Cancer Lett. 2015, 366, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cheng, G.; Qiu, H.; Zhu, L.; Ren, Z.; Zhao, W.; Zhang, T.; Liu, L. The P53-Inducible Gene 3 Involved in Flavonoid-Induced Cytotoxicity through the Reactive Oxygen Species-Mediated Mitochondrial Apoptotic Pathway in Human Hepatoma Cells. Food Funct. 2015, 6, 1518–1525. [Google Scholar] [CrossRef]
- de Oliveira, M.R.; Nabavi, S.F.; Manayi, A.; Daglia, M.; Hajheydari, Z.; Nabavi, S.M. Resveratrol and the Mitochondria: From Triggering the Intrinsic Apoptotic Pathway to Inducing Mitochondrial Biogenesis, a Mechanistic View. Biochim. Biophys. Acta BBA Gen. Subj. 2016, 1860, 727–745. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Voorspoels, S.; Noten, B.; De Paepe, D.; E Baart, G.J.; De Cooman, L. Detection of Flavonoids in Microalgae from Different Evolutionary Lineages. J. Phycol. 2014, 50, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Mapoung, S.; Arjsri, P.; Thippraphan, P.; Semmarath, W.; Yodkeeree, S.; Chiewchanvit, S.; Piyamongkol, W.; Limtrakul, P. Photochemoprotective Effects of Spirulina platensis Extract against UVB Irradiated Human Skin Fibroblasts. S. Afr. J. Bot. 2020, 130, 198–207. [Google Scholar] [CrossRef]
- Seghiri, R.; Kharbach, M.; Essamri, A. Functional Composition, Nutritional Properties, and Biological Activities of Moroccan Spirulina Microalga. J. Food Qual. 2019, 2019, e3707219. [Google Scholar] [CrossRef]
- Niranjana, R.; Gayathri, R.; Nimish Mol, S.; Sugawara, T.; Hirata, T.; Miyashita, K.; Ganesan, P. Carotenoids Modulate the Hallmarks of Cancer Cells. J. Funct. Foods 2015, 18, 968–985. [Google Scholar] [CrossRef]
- Le Goff, M.; Le Ferrec, E.; Mayer, C.; Mimouni, V.; Lagadic-Gossmann, D.; Schoefs, B.; Ulmann, L. Microalgal Carotenoids and Phytosterols Regulate Biochemical Mechanisms Involved in Human Health and Disease Prevention. Biochimie 2019, 167, 106–118. [Google Scholar] [CrossRef]
- Zanella, L.; Alam, M.A. Extracts and Bioactives from Microalgae (Sensu Stricto): Opportunities and Challenges for a New Generation of Cosmetics. In Microalgae Biotechnology for Food, Health and High Value Products; Alam, M.A., Xu, J.-L., Wang, Z., Eds.; Springer: Singapore, 2020; pp. 295–349. ISBN 9789811501692. [Google Scholar]
- Lesmana, R.; Yusuf, I.F.; Goenawan, H.; Achadiyani, A.; Khairani, A.F.; Fatimah, S.N.; Supratman, U. Low Dose of β-Carotene Regulates Inflammation, Reduces Caspase Signaling, and Correlates with Autophagy Activation in Cardiomyoblast Cell Lines. Med. Sci. Monit. Basic Res. 2020, 26, e928648-1. [Google Scholar] [CrossRef]
- Yamamoto, H.; Itoh, N.; Kawano, S.; Yatsukawa, Y.; Momose, T.; Makio, T.; Matsunaga, M.; Yokota, M.; Esaki, M.; Shodai, T.; et al. Dual Role of the Receptor Tom20 in Specificity and Efficiency of Protein Import into Mitochondria. Proc. Natl. Acad. Sci. USA 2011, 108, 91–96. [Google Scholar] [CrossRef]
- Weinrich, T.; Xu, Y.; Wosu, C.; Harvey, P.J.; Jeffery, G. Mitochondrial Function, Mobility and Lifespan Are Improved in Drosophila melanogaster by Extracts of 9-Cis-β-Carotene from Dunaliella salina. Mar. Drugs 2019, 17, 279. [Google Scholar] [CrossRef] [PubMed]
- Voutilainen, S.; Nurmi, T.; Mursu, J.; Rissanen, T.H. Carotenoids and Cardiovascular Health. Am. J. Clin. Nutr. 2006, 83, 1265–1271. [Google Scholar] [CrossRef]
- Druesne-Pecollo, N.; Latino-Martel, P.; Norat, T.; Barrandon, E.; Bertrais, S.; Galan, P.; Hercberg, S. Beta-Carotene Supplementation and Cancer Risk: A Systematic Review and Metaanalysis of Randomized Controlled Trials. Int. J. Cancer 2010, 127, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Sowmya Shree, G.; Yogendra Prasad, K.; Arpitha, H.S.; Deepika, U.R.; Nawneet Kumar, K.; Mondal, P.; Ganesan, P. β-Carotene at Physiologically Attainable Concentration Induces Apoptosis and down-Regulates Cell Survival and Antioxidant Markers in Human Breast Cancer (MCF-7) Cells. Mol. Cell. Biochem. 2017, 436, 1–12. [Google Scholar] [CrossRef]
- He, J.; Gu, Y.; Zhang, S. Vitamin A and Breast Cancer Survival: A Systematic Review and Meta-Analysis. Clin. Breast Cancer 2018, 18, e1389–e1400. [Google Scholar] [CrossRef]
- Lee, K.E.; Kwon, M.; Kim, Y.S.; Kim, Y.; Chung, M.G.; Heo, S.C.; Kim, Y. KoreaMed Synapse. Nutr. Res. Pract. 2021, 16, 161–172. [Google Scholar] [CrossRef]
- Li, K.; Zhang, B. The Association of Dietary β-Carotene and Vitamin A Intake on the Risk of Esophageal Cancer: A Meta-Analysis. Rev. Espanola Enfermedades Dig. Organo Of. Soc. Espanola Patol. Dig. 2020, 112, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, S.; Mochida, K.; Suzuki, K. Diverse Biosynthetic Pathways and Protective Functions against Environmental Stress of Antioxidants in Microalgae. Plants 2021, 10, 1250. [Google Scholar] [CrossRef]
- Corona, J.C.; Duchen, M.R. PPARγ as a Therapeutic Target to Rescue Mitochondrial Function in Neurological Disease. Free Radic. Biol. Med. 2016, 100, 153–163. [Google Scholar] [CrossRef]
- Sathasivam, R.; Ki, J.-S. A Review of the Biological Activities of Microalgal Carotenoids and Their Potential Use in Healthcare and Cosmetic Industries. Mar. Drugs 2018, 16, 26. [Google Scholar] [CrossRef]
- Patel, A.K.; Albarico, F.P.J.B.; Perumal, P.K.; Vadrale, A.P.; Nian, C.T.; Chau, H.T.B.; Anwar, C.; Wani, H.M.U.D.; Pal, A.; Saini, R.; et al. Algae as an Emerging Source of Bioactive Pigments. Bioresour. Technol. 2022, 351, 126910. [Google Scholar] [CrossRef]
- Li, Z.; Sun, M.; Li, Q.; Li, A.; Zhang, C. Profiling of Carotenoids in Six Microalgae (Eustigmatophyceae) and Assessment of Their β-Carotene Productions in Bubble Column Photobioreactor. Biotechnol. Lett. 2012, 34, 2049–2053. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.A.; Rodríguez, H.; Moreno, J.; Vargas, M.Á.; Rivas, J.; Guerrero, M.G. Accumulation of Astaxanthin and Lutein in Chlorella zofingiensis (Chlorophyta). Appl. Microbiol. Biotechnol. 2004, 64, 848–854. [Google Scholar] [CrossRef]
- Abe, K.; Hattori, H.; Hirano, M. Accumulation and Antioxidant Activity of Secondary Carotenoids in the Aerial Microalga Coelastrella striolata var. multistriata. Food Chem. 2007, 100, 656–661. [Google Scholar] [CrossRef]
- Kang, C.D.; Lee, J.S.; Park, T.H.; Sim, S.J. Comparison of Heterotrophic and Photoautotrophic Induction on Astaxanthin Production by Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 2005, 68, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Butler, T.; Golan, Y. Astaxanthin Production from Microalgae. In Microalgae Biotechnology for Food, Health and High Value Products; Springer: Singapore, 2020; pp. 175–242. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Z.; Gerken, H.; Liu, Z.; Jiang, Y.; Chen, F. Chlorella zofingiensis as an Alternative Microalgal Producer of Astaxanthin: Biology and Industrial Potential. Mar. Drugs 2014, 12, 3487–3515. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Kang, S.-W.; Kwon, O.-N.; Chung, D.; Pan, C.-H. Fucoxanthin as a Major Carotenoid in Isochrysis aff. galbana: Characterization of Extraction for Commercial Application. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 477–483. [Google Scholar] [CrossRef]
- Petrushkina, M.; Gusev, E.; Sorokin, B.; Zotko, N.; Mamaeva, A.; Filimonova, A.; Kulikovskiy, M.; Maltsev, Y.; Yampolsky, I.; Guglya, E.; et al. Fucoxanthin Production by Heterokont Microalgae. Algal Res. 2017, 24, 387–393. [Google Scholar] [CrossRef]
- Di Lena, G.; Casini, I.; Lucarini, M.; Lombardi-Boccia, G. Carotenoid Profiling of Five Microalgae Species from Large-Scale Production. Food Res. Int. 2019, 120, 810–818. [Google Scholar] [CrossRef]
- Li, Z.; Li, A.-F.; Zhang, C.-W. High Performance Liquid Chromatography Analysis and Supercritical Carbon Dioxide Extraction of Pigments from the Diatom Odontella aurita. Nat. Prod. Res. Dev. 2012, 24, 814–818. [Google Scholar]
- Campenni’, L.; Nobre, B.P.; Santos, C.A.; Oliveira, A.C.; Aires-Barros, M.R.; Palavra, A.M.F.; Gouveia, L. Carotenoid and Lipid Production by the Autotrophic Microalga Chlorella protothecoides under Nutritional, Salinity, and Luminosity Stress Conditions. Appl. Microbiol. Biotechnol. 2013, 97, 1383–1393. [Google Scholar] [CrossRef] [PubMed]
- Diprat, A.B.; Silveira Thys, R.C.; Rodrigues, E.; Rech, R. Chlorella sorokiniana: A New Alternative Source of Carotenoids and Proteins for Gluten-Free Bread. LWT 2020, 134, 109974. [Google Scholar] [CrossRef]
- Huang, W.; Lin, Y.; He, M.; Gong, Y.; Huang, J. Induced High-Yield Production of Zeaxanthin, Lutein, and β-Carotene by a Mutant of Chlorella zofingiensis. J. Agric. Food Chem. 2018, 66, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, L.; Jensen, A.A.; Kavanagh, J.M.; McClure, D.D. Microalgal Production of Zeaxanthin. Algal Res. 2021, 55, 102266. [Google Scholar] [CrossRef]
- Singh, D.; Puri, M.; Wilkens, S.; Mathur, A.S.; Tuli, D.K.; Barrow, C.J. Characterization of a New Zeaxanthin Producing Strain of Chlorella saccharophila Isolated from New Zealand Marine Waters. Bioresour. Technol. 2013, 143, 308–314. [Google Scholar] [CrossRef]
- Jin, E.; Feth, B.; Melis, A. A Mutant of the Green Alga Dunaliella salina Constitutively Accumulates Zeaxanthin under All Growth Conditions. Biotechnol. Bioeng. 2003, 81, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Nawaz, A.; Hecht, K.; Tobe, K. Astaxanthin as a Novel Mitochondrial Regulator: A New Aspect of Carotenoids, beyond Antioxidants. Nutrients 2022, 14, 107. [Google Scholar] [CrossRef]
- Hoffman, R.; Sultan, L.D.; Saada, A.; Hirschberg, J.; Osterzetser-Biran, O.; Gruenbaum, Y. Astaxanthin Extends Lifespan via Altered Biogenesis of the Mitochondrial Respiratory Chain Complex III. bioRxiv 2019. [CrossRef]
- Huangfu, J.; Liu, J.; Sun, Z.; Wang, M.; Jiang, Y.; Chen, Z.-Y.; Chen, F. Antiaging Effects of Astaxanthin-Rich Alga Haematococcus pluvialis on Fruit Flies under Oxidative Stress. J. Agric. Food Chem. 2013, 61, 7800–7804. [Google Scholar] [CrossRef]
- Sztretye, M.; Dienes, B.; Gönczi, M.; Czirják, T.; Csernoch, L.; Dux, L.; Szentesi, P.; Keller-Pintér, A. Astaxanthin: A Potential Mitochondrial-Targeted Antioxidant Treatment in Diseases and with Aging. Oxid. Med. Cell. Longev. 2019, 2019, e3849692. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.M.; Asoh, S.; Hiranuma, H.; Ohsawa, I.; Iio, K.; Satou, A.; Ishikura, M.; Ohta, S. Astaxanthin Protects Mitochondrial Redox State and Functional Integrity against Oxidative Stress. J. Nutr. Biochem. 2010, 21, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Peserico, A.; Chiacchiera, F.; Grossi, V.; Matrone, A.; Latorre, D.; Simonatto, M.; Fusella, A.; Ryall, J.G.; Finley, L.W.S.; Haigis, M.C.; et al. A Novel AMPK-Dependent FoxO3A-SIRT3 Intramitochondrial Complex Sensing Glucose Levels. Cell. Mol. Life Sci. CMLS 2013, 70, 2015–2029. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Davinelli, S.; Scapagnini, G.; Willcox, B.J.; Allsopp, R.C.; Willcox, D.C. Astaxanthin as a Putative Geroprotector: Molecular Basis and Focus on Brain Aging. Mar. Drugs 2020, 18, 351. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H. Inhibitory Effect of Astaxanthin on Oxidative Stress-Induced Mitochondrial Dysfunction-A Mini-Review. Nutrients 2018, 10, 1137. [Google Scholar] [CrossRef]
- Aoi, W.; Naito, Y.; Takanami, Y.; Ishii, T.; Kawai, Y.; Akagiri, S.; Kato, Y.; Osawa, T.; Yoshikawa, T. Astaxanthin Improves Muscle Lipid Metabolism in Exercise via Inhibitory Effect of Oxidative CPT I Modification. Biochem. Biophys. Res. Commun. 2008, 366, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Gräb, J.; Rybniker, J. The Expanding Role of P38 Mitogen-Activated Protein Kinase in Programmed Host Cell Death. Microbiol. Insights 2019, 12, 1178636119864594. [Google Scholar] [CrossRef]
- Ganesan, P.; Matsubara, K.; Sugawara, T.; Hirata, T. Marine Algal Carotenoids Inhibit Angiogenesis by Down-Regulating FGF-2-Mediated Intracellular Signals in Vascular Endothelial Cells. Mol. Cell. Biochem. 2013, 380, 1–9. [Google Scholar] [CrossRef]
- Palozza, P.; Torelli, C.; Boninsegna, A.; Simone, R.; Catalano, A.; Mele, M.C.; Picci, N. Growth-Inhibitory Effects of the Astaxanthin-Rich Alga Haematococcus pluvialis in Human Colon Cancer Cells. Cancer Lett. 2009, 283, 108–117. [Google Scholar] [CrossRef]
- Kavitha, K.; Kowshik, J.; Kishore, T.K.K.; Baba, A.B.; Nagini, S. Astaxanthin Inhibits NF-ΚB and Wnt/β-Catenin Signaling Pathways via Inactivation of Erk/MAPK and PI3K/Akt to Induce Intrinsic Apoptosis in a Hamster Model of Oral Cancer. Biochim. Biophys. Acta 2013, 1830, 4433–4444. [Google Scholar] [CrossRef]
- Li, J.; Dai, W.; Xia, Y.; Chen, K.; Li, S.; Liu, T.; Zhang, R.; Wang, J.; Lu, W.; Zhou, Y.; et al. Astaxanthin Inhibits Proliferation and Induces Apoptosis of Human Hepatocellular Carcinoma Cells via Inhibition of Nf-Κb P65 and Wnt/Β-Catenin in Vitro. Mar. Drugs 2015, 13, 6064–6081. [Google Scholar] [CrossRef] [PubMed]
- Kaltschmidt, B.; Kaltschmidt, C.; Hofmann, T.G.; Hehner, S.P.; Dröge, W.; Schmitz, M.L. The Pro- or Anti-Apoptotic Function of NF-ΚB Is Determined by the Nature of the Apoptotic Stimulus. Eur. J. Biochem. 2000, 267, 3828–3835. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.-L.; Kamata, H.; Karin, M. IKK/NF-ΚB Signaling: Balancing Life and Death—A New Approach to Cancer Therapy. J. Clin. Investig. 2005, 115, 2625–2632. [Google Scholar] [CrossRef]
- Gardaneh, M.; Nayeri, Z.; Akbari, P.; Gardaneh, M.; Tahermansouri, H. Molecular Simulations Identify Target Receptor Kinases Bound by Astaxanthin to Induce Breast Cancer Cell Apoptosis. Arch. Breast Cancer 2020, 7, 72–82. [Google Scholar] [CrossRef]
- Gammone, M.A.; D’Orazio, N. Anti-Obesity Activity of the Marine Carotenoid Fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Nutraceutical Effects of Fucoxanthin for Obesity and Diabetes Therapy: A Review. J. Oleo Sci. 2015, 64, 125–132. [Google Scholar] [CrossRef]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive Oxygen Species Have a Causal Role in Multiple Forms of Insulin Resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, K.A.; Burnett, G.; Scott, M.; Amjad, E.; Bannerman, S.; Park, H.-A. Neuroprotective Function of Fucoxanthin in Oxidative Stress-Mediated Mitochondrial Dysfunction. Curr. Dev. Nutr. 2022, 6, 787. [Google Scholar] [CrossRef]
- Mondal, A.; Bose, S.; Banerjee, S.; Patra, J.K.; Malik, J.; Mandal, S.K.; Kilpatrick, K.L.; Das, G.; Kerry, R.G.; Fimognari, C.; et al. Marine Cyanobacteria and Microalgae Metabolites—A Rich Source of Potential Anticancer Drugs. Mar. Drugs 2020, 18, 476. [Google Scholar] [CrossRef]
- Gugulothu, P.; Bajhaiya, A.K. Bioactive Compound from Micro Algae and Their Anti-Cancer Properties. Biomed. J. Sci. Tech. Res. 2022, 42, 33928–33931. [Google Scholar] [CrossRef]
- Saxena, A.; Raj, A.; Tiwari, A.; Saxena, A.; Raj, A.; Tiwari, A. Exploring the Anti-Cancer Potential of Microalgae; IntechOpen: London, UK, 2022; ISBN 978-1-80356-024-3. [Google Scholar]
- Brogi, L.; Marchese, M.; Cellerino, A.; Licitra, R.; Naef, V.; Mero, S.; Bibbiani, C.; Fronte, B. β-Glucans as Dietary Supplement to Improve Locomotion and Mitochondrial Respiration in a Model of Duchenne Muscular Dystrophy. Nutrients 2021, 13, 1619. [Google Scholar] [CrossRef]
- Samarakoon, K.W.; Ko, J.-Y.; Lee, J.-H.; Kwon, O.-N.; Kim, S.-W.; Jeon, Y.-J. Apoptotic Anticancer Activity of a Novel Fatty Alcohol Ester Isolated from Cultured Marine Diatom, Phaeodactylum tricornutum. J. Funct. Foods 2014, 6, 231–240. [Google Scholar] [CrossRef]
- Kasashima, K.; Sumitani, M.; Satoh, M.; Endo, H. Human Prohibitin 1 Maintains the Organization and Stability of the Mitochondrial Nucleoids. Exp. Cell Res. 2008, 314, 988–996. [Google Scholar] [CrossRef]
- Tredici, M.R. Mass Production of Microalgae: Photobioreactors. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Blackwell Publishing Ltd.: Oxford, UK, 2004; pp. 178–214. [Google Scholar]
- Vishwakarma, R.; Dhaka, V.; Ariyadasa, T.U.; Malik, A. Exploring Algal Technologies for a Circular Bio-Based Economy in Rural Sector. J. Clean. Prod. 2022, 354, 131653. [Google Scholar] [CrossRef]
- Loke Show, P. Global Market and Economic Analysis of Microalgae Technology: Status and Perspectives. Bioresour. Technol. 2022, 357, 127329. [Google Scholar] [CrossRef]
- Brendler, T.; Williamson, E.M. Astaxanthin: How Much Is Too Much? A Safety Review. Phytother. Res. 2019, 33, 3090–3111. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Xing, L.; Lin, H.; Leng, K.; Zhai, Y.; Liu, X. Assessment and Comparison of in Vitro Immunoregulatory Activity of Three Astaxanthin Stereoisomers. J. Ocean Univ. China 2016, 15, 283–287. [Google Scholar] [CrossRef]
- Becker, W. Microalgae in Human and Animal Nutrition. In Handbook of Microalgal Culture; Blackwell: Oxford, UK, 2004; pp. 312–351. [Google Scholar]
- Muhammad, G.; Alam, M.A.; Xiong, W.; Lv, Y.; Xu, J.-L. Microalgae Biomass Production: An Overview of Dynamic Operational Methods. In Microalgae Biotechnology for Food, Health and High Value Products; Alam, M.A., Xu, J.-L., Wang, Z., Eds.; Springer: Singapore, 2020; pp. 415–432. ISBN 9789811501692. [Google Scholar]
- Teuling, E.; Wierenga, P.A.; Agboola, J.O.; Gruppen, H.; Schrama, J.W. Cell Wall Disruption Increases Bioavailability of Nannochloropsis gaditana Nutrients for Juvenile Nile Tilapia (Oreochromis niloticus). Aquaculture 2019, 499, 269–282. [Google Scholar] [CrossRef]
- Machado, L.; Carvalho, G.; Pereira, R.N. Effects of Innovative Processing Methods on Microalgae Cell Wall: Prospects towards Digestibility of Protein-Rich Biomass. Biomass 2022, 2, 80–102. [Google Scholar] [CrossRef]
- Mendes-Pinto, M.M.; Raposo, M.F.J.; Bowen, J.; Young, A.J.; Morais, R. Evaluation of Different Cell Disruption Processes on Encysted Cells of Haematococcus pluvialis: Effects on Astaxanthin Recovery and Implications for Bio-Availability. J. Appl. Phycol. 2001, 13, 19–24. [Google Scholar] [CrossRef]
- Stiefvatter, L.; Lehnert, K.; Frick, K.; Montoya-Arroyo, A.; Frank, J.; Vetter, W.; Schmid-Staiger, U.; Bischoff, S.C. Oral Bioavailability of Omega-3 Fatty Acids and Carotenoids from the Microalgae Phaeodactylum tricornutum in Healthy Young Adults. Mar. Drugs 2021, 19, 700. [Google Scholar] [CrossRef]
- Merchant, R.E.; Andre, C.A. A Review of Recent Clinical Trials of the Nutritional Supplement Chlorella pyrenoidosa in the Treatment of Fibromyalgia, Hypertension, and Ulcerative Colitis. Altern. Ther. Health Med. 2001, 7, 79–91. [Google Scholar]
- Panahi, Y.; Darvishi, B.; Jowzi, N.; Beiraghdar, F.; Sahebkar, A. Chlorella vulgaris: A Multifunctional Dietary Supplement with Diverse Medicinal Properties. Curr. Pharm. Des. 2016, 22, 164–173. [Google Scholar]
- Ebrahimi-Mameghani, M.; Sadeghi, Z.; Abbasalizad Farhangi, M.; Vaghef-Mehrabany, E.; Aliashrafi, S. Glucose Homeostasis, Insulin Resistance and Inflammatory Biomarkers in Patients with Non-Alcoholic Fatty Liver Disease: Beneficial Effects of Supplementation with Microalgae Chlorella vulgaris: A Double-Blind Placebo-Controlled Randomized Clinical Trial. Clin. Nutr. 2017, 36, 1001–1006. [Google Scholar] [CrossRef]
- Panahi, Y.; Ghamarchehreh, M.E.; Beiraghdar, F.; Zare, R.; Jalalian, H.R.; Sahebkar, A. Investigation of the Effects of Chlorella vulgaris Supplementation in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized Clinical Trial. Hepatogastroenterology 2012, 59, 2099–2103. [Google Scholar] [CrossRef] [PubMed]
- Talebi Pour, B.; Jameshorani, M.; Salmani, R.; Chiti, H. The Effect of Chlorella vulgaris vs. Artichoke on Patients with Non-Alcoholic Fatty Liver Disease (NAFLD): A Randomized Clinical Trial. J. Adv. Med. Biomed. Res. 2015, 23, 36–44. [Google Scholar]
- Chiu, H.-F.; Lee, H.-J.; Han, Y.-C.; Venkatakrishnan, K.; Golovinskaia, O.; Wang, C.-K. Beneficial Effect of Chlorella pyrenoidosa Drink on Healthy Subjects: A Randomized, Placebo-Controlled, Double-Blind, Cross-over Clinical Trial. J. Food Biochem. 2021, 45, e13665. [Google Scholar] [CrossRef] [PubMed]
- Spiller, G.A.; Dewell, A. Safety of an Astaxanthin-Rich Haematococcus pluvialis Algal Extract: A Randomized Clinical Trial. J. Med. Food 2003, 6, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, M.; Satoh, A.; Tsuji, S.; Shirasawa, T. Effects of Astaxanthin-Rich Haematococcus pluvialis Extract on Cognitive Function: A Randomised, Double-Blind, Placebo-Controlled Study. J. Clin. Biochem. Nutr. 2012, 51, 102–107. [Google Scholar] [CrossRef]
- Kim, J.H.; Chang, M.J.; Choi, H.D.; Youn, Y.-K.; Kim, J.T.; Oh, J.M.; Shin, W.G. Protective Effects of Haematococcus Astaxanthin on Oxidative Stress in Healthy Smokers. J. Med. Food 2011, 14, 1469–1475. [Google Scholar] [CrossRef]
- Kidd, P. Astaxanthin, Cell Membrane Nutrient with Diverse Clinical Benefits and Anti-Aging Potential. Altern. Med. Rev. J. Clin. Ther. 2011, 16, 355–364. [Google Scholar]
- García, Á.; Toro-Román, V.; Siquier-Coll, J.; Bartolomé, I.; Muñoz, D.; Maynar-Mariño, M. Effects of Tetraselmis chuii Microalgae Supplementation on Anthropometric, Hormonal and Hematological Parameters in Healthy Young Men: A Double-Blind Study. Int. J. Environ. Res. Public. Health 2022, 19, 6060. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Yasuda, K.; Murata, A.; Suzuki, K.; Miura, N. Effects of Euglena gracilis Intake on Mood and Autonomic Activity under Mental Workload, and Subjective Sleep Quality: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2020, 12, 3243. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Briskey, D.; Nalley, J.O.; Ganuza, E. Omega-3 Eicosapentaenoic Acid (EPA) Rich Extract from the Microalga Nannochloropsis Decreases Cholesterol in Healthy Individuals: A Double-Blind, Randomized, Placebo-Controlled, Three-Month Supplementation Study. Nutrients 2020, 12, 1869. [Google Scholar] [CrossRef]
- Karkos, P.D.; Leong, S.C.; Karkos, C.D.; Sivaji, N.; Assimakopoulos, D.A. Spirulina in Clinical Practice: Evidence-Based Human Applications. Evid. Based Complement. Alternat. Med. 2010, 2011, 531053. [Google Scholar] [CrossRef] [PubMed]
- de la Jara, A.; Ruano-Rodriguez, C.; Polifrone, M.; Assunçao, P.; Brito-Casillas, Y.; Wägner, A.M.; Serra-Majem, L. Impact of Dietary Arthrospira (Spirulina) Biomass Consumption on Human Health: Main Health Targets and Systematic Review. J. Appl. Phycol. 2018, 30, 2403–2423. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).