Apple Puree as a Natural Fructose Source Provides an Effective Alternative to Artificial Fructose Sources for Fuelling Endurance Cycling Performance in Males
Abstract
1. Introduction
2. Materials and Methods
Experimental Design
3. Results
3.1. Pre-Trial Measures
3.2. Heart Rate, RPE, TS, Blood Glucose/Lactate Responses, and Environmental Conditions
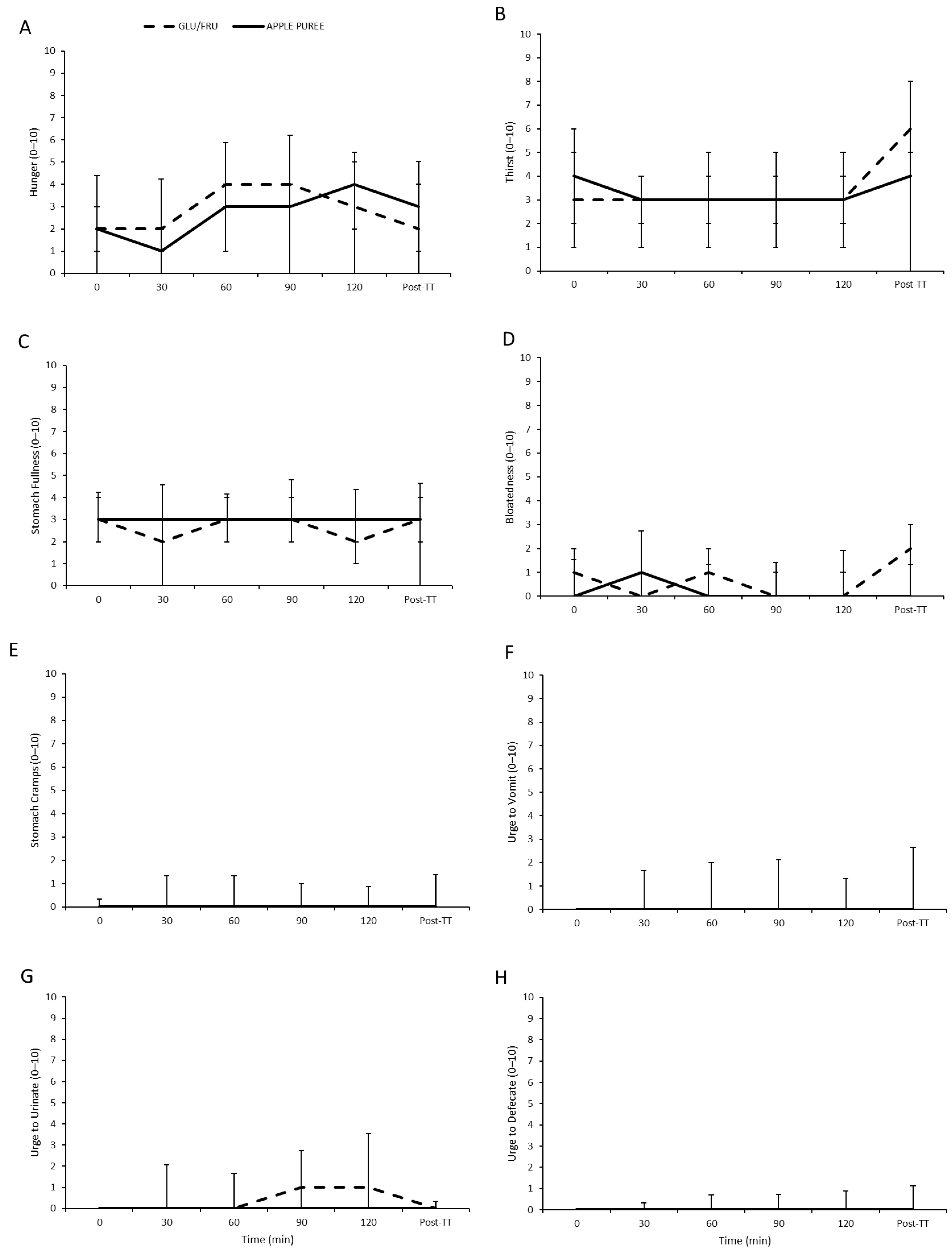
3.3. Gastrointestinal Scale Responses
3.4. Fluid Balance Measures
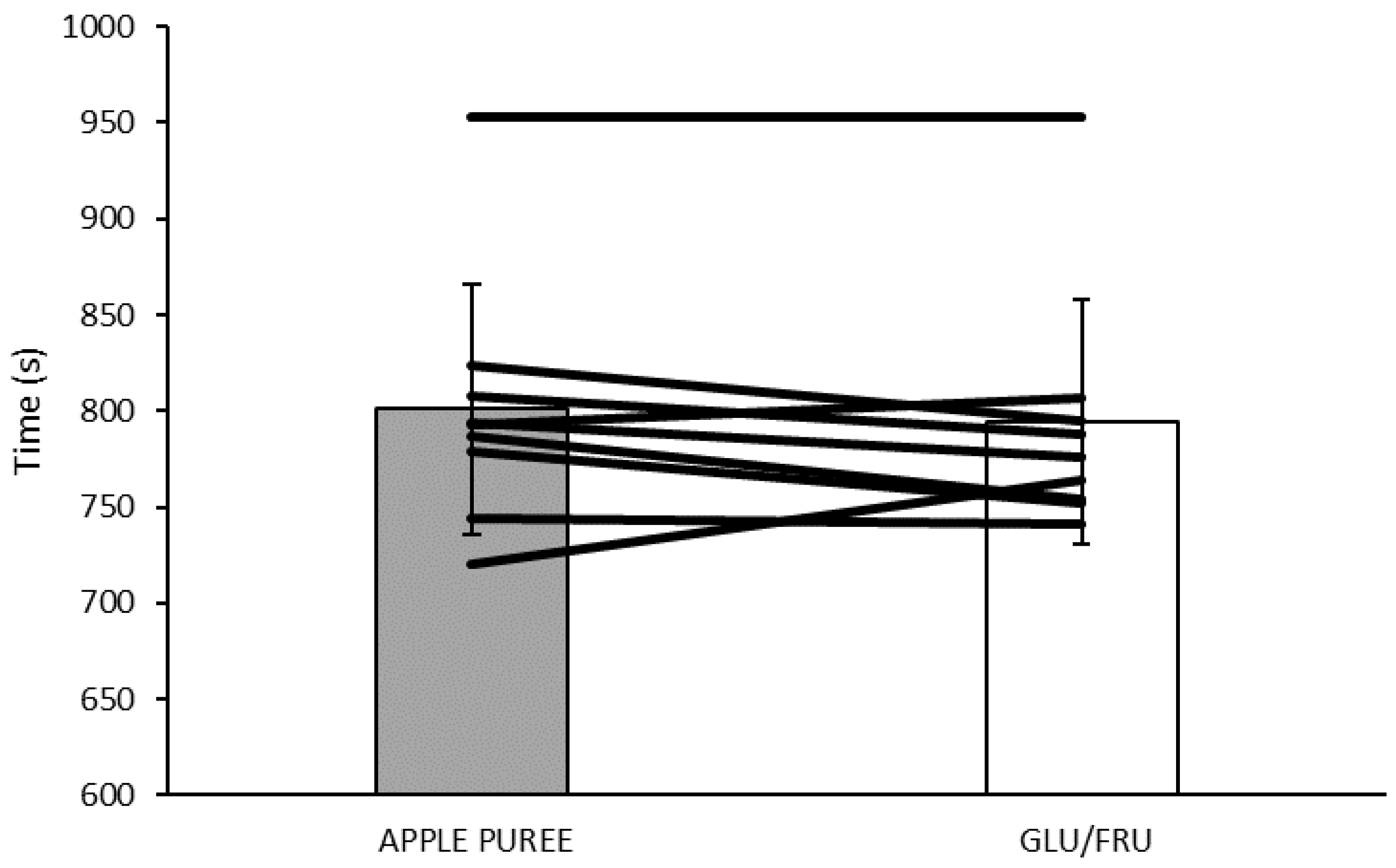
3.5. Time Trial (TT) Performance
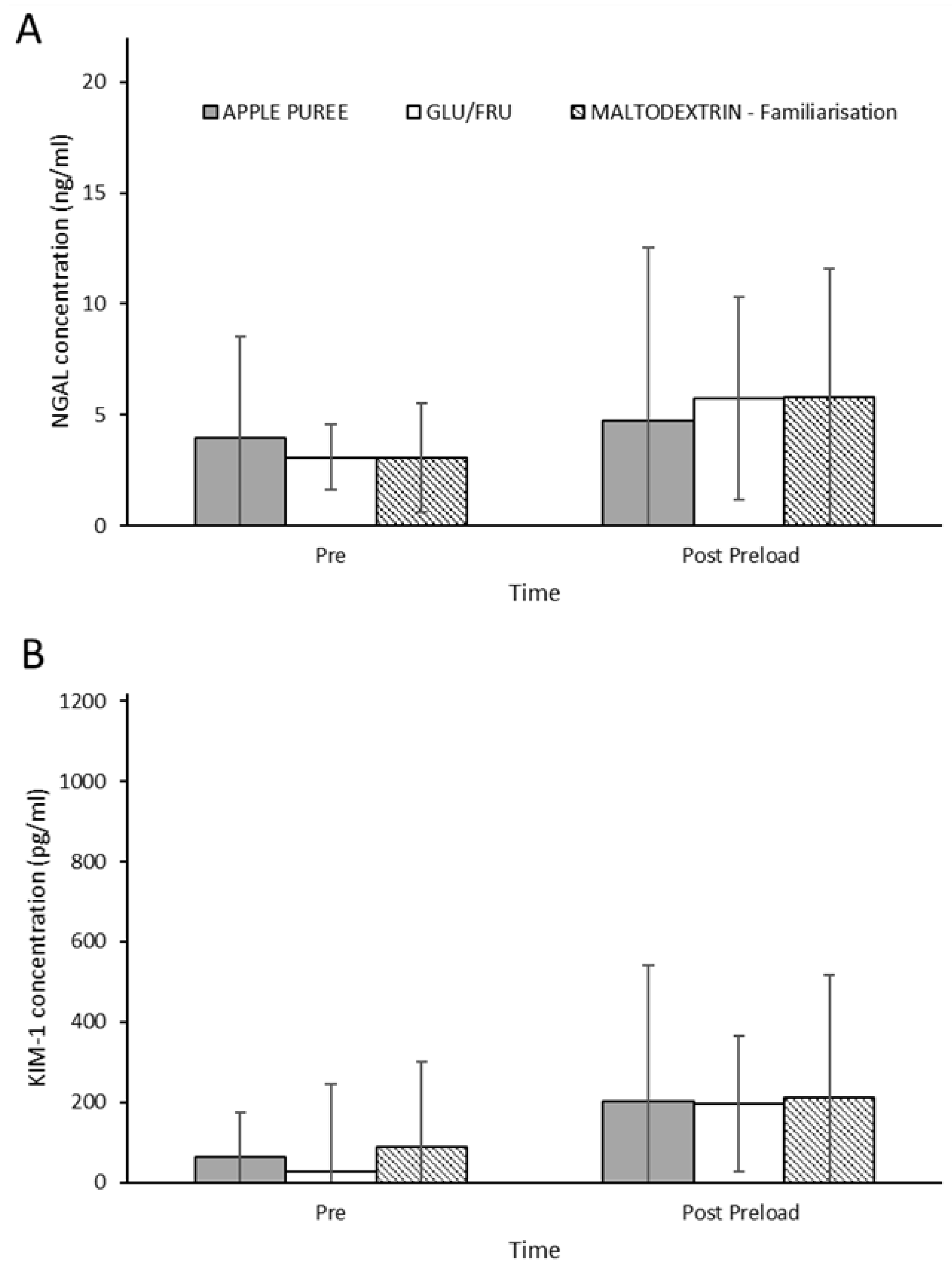
3.6. Renal Injury Biomarkers
3.7. Study Blinding and Trial Order Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stellingwerf, T.; Cox, G.R. Systematic Review: Carbohydrate Supplementation on Exercise Performance or Capacity of Varying Duration. Appl. Physiol. Nutr. Metab. 2014, 39, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Castell, L.M.; Casa, D.J.; Close, G.L.; Costa, R.J.S.; Desbrow, B.; Halson, S.L.; Lis, D.M.; Melin, A.K.; Peeling, P.; et al. International Association of Athletics Federations Consensus Statement 2019: Nutrition for Athletes. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Hawley, J.A.; Wong, S.H.S.; Jeukendrup, A.E. Carbohydrate for Training and Competition. J. Sports Sci. 2011, 29, S17–S27. [Google Scholar] [CrossRef]
- Jeukendrup, A. Nutrition for Endurance Sports: Marathon, Triathlon, and Road Cycling. J. Sports Sci. 2011, 29 (Suppl. 1), S91–S99. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, B.; Stellingwerff, T.; Hodgson, A.B.; Randell, R.; Pöttgen, K.; Res, P.; Jeukendrup, A.E. Nutritional Intake and Gastrointestinal Problems during Competitive Endurance Events. Med. Sci. Sports. Exerc. 2012, 44, 344–351. [Google Scholar] [CrossRef]
- Kelishadi, R.; Mansourian, M.; Heidari-Beni, M. Association of fructose consumption and components of metabolic syndrome in human studies: A systematic review and meta-analysis. Nutrition 2014, 30, 503–510. [Google Scholar] [CrossRef]
- Liu, Q.; Ayoub-Charette, S.; Khan, T.A.; Au-Yeung, F.; Mejia, S.B.; De Souza, R.J.; Wolever, T.M.; Leiter, L.A.; Kendall, C.W.; Sievenpiper, J.L. Important food sources of fructose-containing sugars and incident hypertension: A systematic review and dose-response meta-analysis of prospective cohort studies. J. Am. Heart Assoc. 2019, 8, e010977. [Google Scholar] [CrossRef]
- Chapman, C.L.; Johnson, B.D.; Sackett, J.R.; Parker, M.D.; Schlader, Z.J. Soft drink consumption during and following exercise in the heat elevates biomarkers of acute kidney injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 316, R189–R198. [Google Scholar] [CrossRef]
- Hyson, D.A. A review and critical analysis of the scientific literature related to 100% fruit juice and human health. Adv. Nutr. 2015, 6, 37–51. [Google Scholar] [CrossRef]
- Aprea, E.; Charles, M.; Endrizzi, I.; Corollaro, M.L.; Betta, E.; Biasioli, F.; Gasperi, F. Sweet taste in apple: The role of sorbitol, individual sugars, organic acids and volatile compounds. Sci. Rep. 2017, 7, 44950. [Google Scholar] [CrossRef]
- Fabiani, R.; Minelli, L.; Rosignoli, P. Apple intake and cancer risk: A systematic review and meta-analysis of observational studies. Public Health Nutr. 2016, 19, 2603–2617. [Google Scholar] [CrossRef] [PubMed]
- Hyson, D.A. A comprehensive review of apples and apple components and their relationship to human health. Adv. Nutr. 2011, 2, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Ramírez, B.A.; Catalán, Ú.; Calderón-Pérez, L.; Companys, J.; Pla-Pagà, L.; Ludwig, I.A.; Romero, M.P.; Solà, R. The effects and associations of whole-apple intake on diverse cardiovascular risk factors. A narrative review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3862–3875. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, K.M.; Clifford, T.; Mears, S.A.; James, L.J. A Food First Approach to Carbohydrate Supplementation in Endurance Exercise: A Systematic Review. Int. J. Sport Nutr. Exerc. Metab. 2022, 32, 296–310. [Google Scholar] [CrossRef]
- Guillochon, M.; Rowlands, D.S. Solid, Gel, and Liquid Carbohydrate Format Effects on Gut Comfort and Performance. Int. J. Sport Nutr. Exerc. Metab. 2011, 27, 247–254. [Google Scholar] [CrossRef]
- Ishihara, K.; Taniguchi, H.; Akiyama, N.; Asami, Y. Easy to Swallow Rice Cake as a Carbohydrate Source during Endurance Exercise Suppressed Feelings of Thirst and Hunger without Changing Exercise Performance. J. Nutr. Sci. Vitaminol. 2020, 66, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.W.; Maughan, R.J.; Shirreffs, S.M.; Watson, P. Effects of Milk Ingestion on Prolonged Exercise Capacity in Young, Healthy Men. Nutrition 2008, 24, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Gillitt, N.D.; Henson, D.A.; Sha, W.; Shanely, R.A.; Knab, A.M.; Cialdella-Kam, L.; Jin, F. Bananas as an Energy Source during Exercise: A Metabolomics Approach. PLoS ONE 2012, 7, E37479. [Google Scholar] [CrossRef]
- Nieman, D.C.; Gillitt, N.D.; Sha, W.; Esposito, D.; Ramamoorthy, S. Metabolic Recovery from Heavy Exertion Following Banana Compared to Sugar Beverage or Water Only Ingestion: A Randomized, Crossover Trial. PLoS ONE 2018, 13, E0194843. [Google Scholar] [CrossRef]
- Salvador, A.F.; Mckenna, C.F.; Alamilla, R.A.; Cloud, R.M.T.; Keeble, A.R.; Miltko, A.; Scaroni, S.E.; Beals, J.W.; Ulanov, A.V.; Dilger, R.N.; et al. Potato Ingestion Is as Effective as Carbohydrate Gels to Support Prolonged Cycling Performance. J. Appl. Physiol. 2019, 127, 1651–1659. [Google Scholar] [CrossRef]
- Pugh, J.N.; Kirk, B.; Fearn, R.; Morton, J.P.; Close, G.L. Prevalence, Severity and Potential Nutritional Causes of Gastrointestinal Symptoms during a Marathon in Recreational Runners. Nutrients 2018, 10, 811. [Google Scholar] [CrossRef] [PubMed]
- Drust, B.; Waterhouse, J.; Atkinson, G.; Edwards, B.; Reilly, T. Circadian rhythms in sports performance—An update. Chronobiol. Int. 2005, 22, 21–44. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.; Saris, W.H.; Brouns, F.J.P.H.; Kester, A.D. A new validated endurance performance test. Med. Sci. Sports Exerc. 1996, 28, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Kenefick, R.W.; Cheuvront, S.N.; Palombo, L.J.; Ely, B.R.; Sawka, M.N. Skin temperature modifies the impact of hypohydration on aerobic performance. J. Appl. Physiol. 2010, 109, 79–86. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Vet-Joop, K.; Sturk, A.; Stegen, J.H.J.C.; Senden, J.; Saris, W.H.M.; Wagenmakers, A.J.M. Relationship between gastro-intestinal complaints and endotoxaemia, cytokine release and the acute-phase reaction during and after a long-distance triathlon in highly trained men. Clin. Sci. 2000, 98, 47–55. [Google Scholar] [CrossRef]
- Field, A.P. Discovering Statistics Using IBM SPSS: And Sex and Drugs and Rock’n’Roll; SAGE: London, UK, 2013. [Google Scholar]
- Rietschier, H.L.; Henagan, T.M.; Earnest, C.P.; Baker, B.L.; Cortez, C.C.; Stewart, L.K. Sun-Dried Raisins Are a Cost-Effective Alternative to Sports Jelly Beans in Prolonged Cycling. J. Strength Cond. Res. 2011, 25, 3150. [Google Scholar] [CrossRef]
- Kern, M.; Heslin, C.J.; Rezende, R.S. Metabolic and Performance Effects of Raisins Versus Sports Gel as Pre-Exercise Feedings in Cyclists. J. Strength Cond. Res. 2007, 21, 1204–1207. [Google Scholar]
- Too, B.W.; Cicai, S.; Hockett, K.R.; Applegate, E.; Davis, B.A.; Casazza, G.A. Natural Versus Commercial Carbohydrate Supplementation and Endurance Running Performance. J. Int. Soc. Sports Nutr. 2012, 9, 27. [Google Scholar] [CrossRef]
- Tiller, N.B.; Roberts, J.D.; Beasley, L.; Chapman, S.; Pinto, J.M.; Smith, L.; Wiffin, M.; Russell, M.; Sparks, S.A.; Duckworth, L.; et al. International Society of Sports Nutrition Position Stand: Nutritional Considerations for Single-Stage Ultra-Marathon Training and Racing. J. Int. Soc. Sports Nutr. 2019, 16, 50. [Google Scholar] [CrossRef]
- McIntyre, A.; Vincent, R.M.; Perkins, A.C.; Spiller, R.C. Effect of bran, ispaghula, and inert plastic particles on gastric emptying and small bowel transit in humans: The role of physical factors. Gut 1997, 40, 223–227. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Babio, N.; Balanza, R.; Basulto, J.; Bulló, M.; Salas-Salvadó, J. Dietary fibre: Influence on body weight, glycemic control and plasma cholesterol profile. Nutr. Hosp. 2010, 25, 327–340. [Google Scholar] [PubMed]
- Jeukendrup, A.E. Training the Gut for Athletes. Sports Med. 2017, 47, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Miall, A.; Khoo, A.; Rauch, C.; Snipe, R.M.J.; Camões-Costa, V.L.; Gibson, P.R.; Costa, R.J.S. Two Weeks of Repetitive Gut-Challenge Reduce Exercise-Associated Gastrointestinal Symptoms and Malabsorption. Scand. J. Med. Sci. Sports 2018, 28, 630–640. [Google Scholar] [CrossRef]
- Robinson, T.A.; Hawley, J.A.; Palmer, G.S.; Wilson, G.R.; Gray, D.A.; Noakes, T.D.; Dennis, S.C. Water ingestion does not improve 1-h cycling performance in moderate ambient temperatures. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 71, 153–160. [Google Scholar] [CrossRef]
- Backx, K.; van Someren, K.A.; Palmer, G.S. One hour cycling performance is not affected by ingested fluid volume. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 333–342. [Google Scholar] [CrossRef]
- Funnell, M.P.; Mears, S.A.; Bergin-Taylor, K.; James, L.J. Blinded and Unblinded Hypohydration Similarly Impair Cycling Time Trial Performance in the Heat in Trained Cyclists. J. Appl. Physiol. 2019, 126, 870–879. [Google Scholar] [CrossRef]
- James, L.J.; Funnell, M.P.; James, R.M.; Mears, S.A. Does Hypohydration Really Impair Endurance Performance? Methodological Considerations for Interpreting Hydration Research. Sports Med. 2019, 49, 103–114. [Google Scholar] [CrossRef]
- Eastwood, M.A.; Kay, R.M. An hypothesis for the action of dietary fiber along the gastrointestinal tract. Am. J. Clin. Nutr. 1979, 32, 364–367. [Google Scholar] [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. American college of sports medicine joint position statement. nutrition and athletic performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar]
- Juett, L.A.; James, L.J.; Mears, S.A. Effects of exercise on acute kidney injury biomarkers and the potential influence of fluid intake. Ann. Nutr. Metab. 2020, 76, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Datz, F.L.; Christian, P.E.; Moore, J. Gender-Related Differences in Gastric Emptying. J. Nucl. Med. 1987, 28, 1204–1207. [Google Scholar] [PubMed]
- Ten Haaf, D.S.; van der Worp, M.P.; Groenewoud, H.M.; Leij-Halfwerk, S.; Nijhuis-van der Sanden, M.W.; Verbeek, A.L.; Staal, J.B. Nutritional Indicators for Gastrointestinal Symptoms in Female Runners: The ‘Marikenloop Study’. BMJ Open 2014, 4, e005780. [Google Scholar] [CrossRef] [PubMed]

| 0 | 120 | Post-TT | |
| Urine Specific Gravity | |||
| GLU/FRU | 1.008 ± 0.01 | 1.009 ± 0.00 | 1.010 ± 0.01 |
| APPLE PUREE | 1.010 ± 0.01 | 1.012 ± 0.00 | 1.014 ± 0.01 * |
| Body Mass (kg) | |||
| GLU/FRU | 72.69 ± 6.50 | 72.36 ± 6.60 | 71.71 ± 6.63 |
| APPLE PUREE | 72.58 ± 6.66 | 72.27 ± 6.76 | 71.78 ± 6.73 |
| Total Cumulative Sweat Loss (L) | |||
| GLU/FRU | 1.68 ± 0.35 | 2.13 ± 0.50 | |
| APPLE PUREE | 1.68 ± 0.30 | 2.09 ± 0.38 | |
| Dehydration (%) | |||
| GLU/FRU | −0.47 ± 0.56 | −1.28 ± 0.56 | |
| APPLE PUREE | −0.43 ± 0.35 | −1.12 ± 0.38 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reynolds, K.M.; Juett, L.A.; Cobb, J.; Hulston, C.J.; Mears, S.A.; James, L.J. Apple Puree as a Natural Fructose Source Provides an Effective Alternative to Artificial Fructose Sources for Fuelling Endurance Cycling Performance in Males. Nutraceuticals 2022, 2, 205-217. https://doi.org/10.3390/nutraceuticals2030015
Reynolds KM, Juett LA, Cobb J, Hulston CJ, Mears SA, James LJ. Apple Puree as a Natural Fructose Source Provides an Effective Alternative to Artificial Fructose Sources for Fuelling Endurance Cycling Performance in Males. Nutraceuticals. 2022; 2(3):205-217. https://doi.org/10.3390/nutraceuticals2030015
Chicago/Turabian StyleReynolds, Kirsty M., Loris A. Juett, James Cobb, Carl J. Hulston, Stephen A. Mears, and Lewis J. James. 2022. "Apple Puree as a Natural Fructose Source Provides an Effective Alternative to Artificial Fructose Sources for Fuelling Endurance Cycling Performance in Males" Nutraceuticals 2, no. 3: 205-217. https://doi.org/10.3390/nutraceuticals2030015
APA StyleReynolds, K. M., Juett, L. A., Cobb, J., Hulston, C. J., Mears, S. A., & James, L. J. (2022). Apple Puree as a Natural Fructose Source Provides an Effective Alternative to Artificial Fructose Sources for Fuelling Endurance Cycling Performance in Males. Nutraceuticals, 2(3), 205-217. https://doi.org/10.3390/nutraceuticals2030015






