Abstract
Carbohydrate consumption during exercise enhances endurance performance. A food-focused approach may offer an alternative, ‘healthier’ approach given the potential health concerns associated with artificial fructose sources, but food-based carbohydrate sources may increase gastrointestinal (GI) symptoms. This study compared the cycling performance and GI comfort of two different fructose sources (fruit and artificial) ingested during exercise. Nine trained male cyclists (age 24 ± 7 years; VO2peak 65 ± 6 mL/kg/min) completed a familiarisation and two experimental trials (60 g/h carbohydrate, 120 min at 55% Wmax and ~15 min time trial). In the two experimental trials, carbohydrate was ingested in a 2:1 glucose-to-fructose ratio, with fructose provided as artificial crystalline fructose (GLU/FRU) or natural apple puree (APPLE PUREE) and maltodextrin added to provide sufficient glucose. Time trial (TT) performance was not different between trials (GLU/FRU 792 ± 68 s, APPLE PUREE 800 ± 65 s; p = 0.313). No GI symptoms were significantly different between trials (p ≥ 0.085). Heart rate, blood glucose/lactate concentrations, and RPE were not different between trials, but all, excluding blood glucose concentration, increased from rest to exercise and further increased post-TT. Apple puree as a natural fructose source provides an alternative to artificial fructose sources without influencing cycling performance or GI symptoms.
1. Introduction
Carbohydrate is an important macronutrient for daily living and is the preferred exogenous fuel source during endurance exercise [1], as it has been demonstrated to benefit performance by maintaining blood glucose concentrations and delaying fatigue with exercise for >60 min. Carbohydrate is deemed to be a more efficient fuel source than fat during high-intensity or prolonged endurance exercise due to its ability to provide more adenosine triphosphate (ATP) for a given amount of oxygen [2]. Furthermore, ATP can be produced from carbohydrate in the absence of oxygen using glycolytic pathways [2]. Carbohydrate is stored in the body in the form of muscle and liver glycogen [2]; however, these stores are limited. Therefore, the provision of exogenous carbohydrate before and during exercise helps to preserve glycogen stores and is fundamental for optimal endurance performance. Current guidelines [3,4] suggest that during prolonged endurance exercise of >2 h, the intake of 60–90 g/h from mixed carbohydrate sources (i.e., glucose and fructose) is recommended, which should not cause acute gastrointestinal (GI) upset in the majority of athletes [5].
Whilst the current carbohydrate guidelines provide useful information on carbohydrate dosage and sugar type, they fail to offer suggestions and evidence on the source or form of the carbohydrate. Common sources of fructose used in commercial carbohydrate supplements are high-fructose corn syrup, isolated (crystalline) fructose, and sucrose. However, for some individuals, there may be potential long-term negative implications of high-fructose corn syrup and crystalline fructose ingestion [6,7]. These artificial fructose sources have been associated with increased levels of obesity and metabolic disease [6,7], possibly making them less attractive to some exercisers. Additionally, the consumption of high fructose drinks during and after exercise may exacerbate markers of acute kidney injury (AKI) [8].
However, natural sources of fructose, such as those in fruit, may not have the same potential negative health consequences and thus may provide ‘healthier’ options to the athlete/exerciser [9]. For example, apples naturally contain fructose (~60%) [10], and apple consumption has been related to a reduced risk of cancer [11,12] and cardiovascular disease [13]. The suggested mechanisms link back to the presence of phytochemicals and antioxidants contained within apples reducing oxidative damage. Thus, carbohydrate in the form of apple puree has the potential to provide health benefits, as well as energy intake during endurance exercise. There is a move towards more natural products, and our recent systematic review [14] concluded that whilst performance does not appear to be positively or negatively affected by carbohydrate sources (food vs. supplement), food carbohydrate sources might increase GI symptoms. Eight studies included in the systematic review assessed GI symptoms, and six of those studies reported increased negative symptoms in the food-based trial [15,16,17,18,19,20].
The increased GI symptoms experienced could occur due to the additional plant components and/or the greater volume of food that must be consumed to meet carbohydrate requirements for prolonged exercise. For example, many food sources of carbohydrate, particularly natural foods, such as fruits or vegetables, have a lower carbohydrate density, so a larger volume of food must be consumed to meet recommended intakes during prolonged exercise, e.g., [20]. This is an important consideration given the high prevalence of GI symptoms in endurance athletes when consuming high doses of carbohydrate [5,21]. Indeed, GI symptoms might impair performance or even lead to the failure to complete races [5]. Whole-food sources typically contain additional nutrients to carbohydrate (i.e., dietary fibre, fat, protein, vitamins, minerals, etc.). Whilst many of these nutrients might confer health benefits, some of them may also affect carbohydrate delivery to the working muscles or trigger negative effects on the GI system. However, nutrition in the form of a fruit puree may offer the carbohydrate delivery profile of a gel whilst minimising additional plant components that could upset the GI system, offering a natural and ‘healthier’ fructose source.
Research has tended to focus on the direct comparison between total carbohydrate sources from food or supplements and has not yet focused specifically on the sources of sugars, such as fructose (i.e., natural versus artificial sources), and whether they achieve the same endurance performance benefits without inducing adverse GI effects that may limit sporting outcomes [5]. Investigating natural versus artificial sugar sources is the novel aspect of our study, as it remains unknown whether such carbohydrate sources will have a negative impact on physiological function and performance. Therefore, the aim of this study was to examine endurance performance, GI comfort, and markers of renal injury associated with two different fructose sources (fruit and artificial) ingested during prolonged cycling exercise.
2. Materials and Methods
Participants. The study inclusion criteria included being a cyclist/triathlete (male or female, although no females volunteered), age 18–45 years, having ≥1 year of cycling experience, completing a minimum of 3 h cycling training per week, having previous experience ingesting carbohydrate during exercise. Individuals were not eligible if they had any conditions that could influence carbohydrate metabolism. Participants were healthy and free from injury. Nine trained male cyclists/triathletes completed the study, with an age (mean ± standard deviation) of 24 ± 7 years, a height of 179 ± 9 cm, a mass of 72.7 ± 6.5 kg (Adam CFW-150, Milton Keynes, UK), a VO2peak of 65 ± 6 mL/kg/min, and a Wmax of 373 ± 49 W. Initially, five additional participants (all male) started the study; four completed visit one, and one completed visits one and two. A National Lockdown in response to the COVID-19 pandemic meant that these participants were no longer able to participate in the study and limited some of the intended measures for the study. The study gained institutional ethical approval from the Loughborough University Ethics Approvals (Human Participants) Sub-Committee, and participants gave written informed consent and completed a medical screening questionnaire prior to testing.
Experimental Design
VO2peaktest and familiarisation. During the first visit, the participants completed a peak oxygen uptake (VO2peak) test, and after a short break, they practised the time trial (TT). The VO2peak test was completed on a cycle ergometer (Lode Excalibur Sport, Groningen, The Netherlands) starting at 95 W and increasing by 35 W every 3 min until volitional exhaustion. An expired breath sample was taken in the final 60 s to determine VO2peak. The second visit consisted of a full familiarisation trial, which was identical to the experimental trials (detailed below). Carbohydrate was provided as 60 g/h of maltodextrin (MyProtein, Northwich, UK) only. Bike set-up was determined during the initial visit and then replicated for all trials.
Pre-trial Standardisation. Participants were required to complete an activity and food diary in the 24 h before the familiarisation trial, which was replicated before each subsequent trial. They were asked to abstain from alcohol and strenuous exercise for 24 h prior to each trial. Participants were instructed to consume at least 40 mL/kg body mass from beverages the day before the trials. A standardised breakfast providing 1.5 g carbohydrate/kg body mass and 8 mL water/kg body mass was consumed 2 h prior to arrival at the laboratory (consisting of cereal bars (Nutrigrain, Kellogg’s, Salford, UK), orange juice (Tesco, Welwyn Garden City, UK), and water). Compliance with the pre-trial breakfast was assessed by photographic record, whilst compliance with other pre-trial standardisation procedures was verbally confirmed by participants on arrival. Trials started between 09:00 and 10:00, with each participant starting all trials at the same time to control for circadian rhythm and diurnal variation in performance [22].
Protocol. The study utilised a repeated-measures design. Participants completed the two experimental trials in a randomised order, separated by at least seven days. The exercise protocol consisted of 2 h at ~55% Wmax (preload), followed by a TT designed to last ~15 min. A fan was provided in front of the participants providing a windspeed of ~4.7 m/s. For both trials, carbohydrate was provided at 60 g/h, (15 g at 0 and then every 15 min; ~22% carbohydrate solution) via a 50 mL syringe in a glucose-to-fructose ratio of 2:1, but the sources of fructose differed. No specific information was given to the participants about what the carbohydrate sources were. In the APPLE PUREE trial, the carbohydrate source was apple puree (BIONA ORGANIC; Surrey, UK), which provided natural sources of fructose and glucose (per 100 g of puree: 237 kcal, glucose 3.28 g, fructose 7.08 g, sucrose 2.15 g, dietary fibre 1.3 g, fat 0.1 g, protein 0.2 g). The puree was analysed for full nutritional breakdown by Campden BRI (Campden, UK). Maltodextrin was added to the apple puree (7.3 g maltodextrin and 61.3 g apple puree; total 68.6 g per 15 g carbohydrate serving) to achieve the 2:1 glucose:fructose ratio. In the GLU/FRU trial, the carbohydrate sources and water content were matched to the APPLE PUREE (67.9 g per 15 g carbohydrate serving) and consisted of maltodextrin (MyProtein, Northwich, UK) and crystalline fructose (Bulk Powders, Colchester, UK), providing an artificial source of fructose. Apple flavouring (MyProtein, Northwich, UK) was added to both carbohydrate sources. Tap water was provided ad libitum at room temperature during the preload and before the TT, but no fluid was ingested during the TT.
Each participant completed a workload-directed TT based on delivering 0.75 × Wpeak × 900, meaning it would take ~15 min to complete the target amount of work (kJ) if the participant cycled at ~75% Wpeak. The cycle ergometer was in linear mode, whereby the participants’ preferred cadence would generate ~75% Wpeak [23]. Participants were blinded to all visual feedback during the TT, except target workload and work completed. On completion of each 25% of the target workload, participants were verbally informed using set wording, but there was no further interaction with the investigators, who stood behind the participant to reduce peripheral distractions.
Study Blinding. Participants were informed that the purpose of the study was to investigate the effect of different types of carbohydrate, both providing an optimal dosage, and that they would be debriefed on the specific carbohydrate supplements at the end of the study.
Measures. At rest, every 30 min during the preload, and at the end of the TT, ambient temperature and humidity (Kestrel 4400; Nielsen-Kellerman) were recorded. On arrival, participants provided a total void urine sample, and baseline nude body mass was measured, with additional total void urine samples provided and nude body mass measures made post-preload and post-TT. Participants completed 5 min rest on the cycle ergometer before baseline measures were recorded. Heart Rate (HR) was measured using a chest strap (worn at position T9; Polar M400; Polar Electro Oy, Kempele, Finland) at rest every 30 min during the preload and after each 25% of the TT. Rating of perceived exertion (RPE; 6–20 scale; [24]), thermal sensation (TS; −10 to +10 Scale; [25]), and GI scales (all 11-point Likert scales assessing hunger, thirst, stomach fullness, stomach bloatedness, stomach cramps, urge to vomit, urge to urinate, and urge to defecate [26]) were recorded at rest, every 30 min during the preload, and immediately post-TT. Ad libitum water intake was recorded for each hour of the preload. The time to complete each 25% interval during the TT was recorded. A fingertip capillary blood sample was collected pre-exercise (~320 µL), with additional fingertip capillary blood samples (20 µL) collected every 30 min during the preload and post-TT.
Unfortunately, due to the COVID-19 pandemic, we were unable to collect gas samples to determine VO2 or substrate use during exercise.
Sample Processing and Analysis. For each urine sample, the volume was determined and urine specific gravity was measured using a hand-held analyser (Ceti, Refractometer) before the sample was stored at −80 °C until analysis for biomarkers of kidney damage, KIM-1 (kidney injury molecule-1), and NGAL (neutrophil gelatinase-associated lipocalin), by enzyme-linked immunosorbent assay (ELISA) (Human, NGAL ELISA kit, Bioporto, Hellerup, Denmark; Human KIM-1, ELISA kit ADI-900-226-001, Enzo Life Sciences, Lausen, Switzerland) in the pre- and post-preload urine samples only. Samples from the familiarisation trial (maltodextrin only) were included in this analysis to provide a fructose-free comparison.
From the pre-exercise blood sample, 300 µL was collected in a tube containing a clotting catalyst (Sarstedt 100 microvette, Sarstedt AG & Co., Nümbrecht, Germany). This tube was centrifuged at 2500× g, 4 °C for 20 min before serum was aliquoted and stored at −80 °C until analysis for osmolality by freezing point depression (Gonotec Osmomat 030, Cryoscopic Osmometer; Gonotec). At each blood sampling time point, blood was collected in a 20 µL capillary tube before being mixed with 1 mL of haemolysing solution (EKF Diagnostics, Cardiff, UK) and analysed for lactate and glucose concentrations (Biosen C-Line; EKF Diagnostics, Cardiff, UK).
Statistical Analysis Statistical analyses were completed using Statistical Package for Social Sciences (SPSS) for Windows version 25 SPSS; Chicago, IL, USA). Statistical significance was set at p ≤ 0.05, and results are presented as mean ± SD, except for GI scale responses and renal injury biomarkers, which are presented as medians (IQR). The normality of data was assessed using Shapiro–Wilk tests. Two-way repeated measures ANOVA was used to analyse data containing two factors (trial × time), whilst paired t-tests or Wilcoxon signed rank tests were used, as appropriate, to analyse data containing one factor. To correct for violation of sphericity, the degrees of freedom were corrected using Greenhouse–Geisser (ε > 0.75) or Huynh–Felt (ε < 0.75) [27].
3. Results
3.1. Pre-Trial Measures
There were no differences between trials for pre-trial body mass (Table 1; p = 0.526), urine specific gravity (Table 1; p = 0.195), serum osmolality (GLU/FRU 288 ± 2 mosmol/kg; APPLE PUREE 288 ± 2 mosmol/kg; p = 0.864), heart rate (Figure 1; p = 0.483) or any subjective variable (Figure 2; p ≥ 0.447), indicating that participants started trials in a similar state.

Table 1.
Urine specific gravity; body mass (kg) at rest, after 120 min preload, and post time trial (TT). Sweat loss (L) and dehydration (%) accrued after 120 min preload and post time trial (TT). Data presented as mean ± SD. * denotes a significant difference between carbohydrate supplements (p > 0.05).
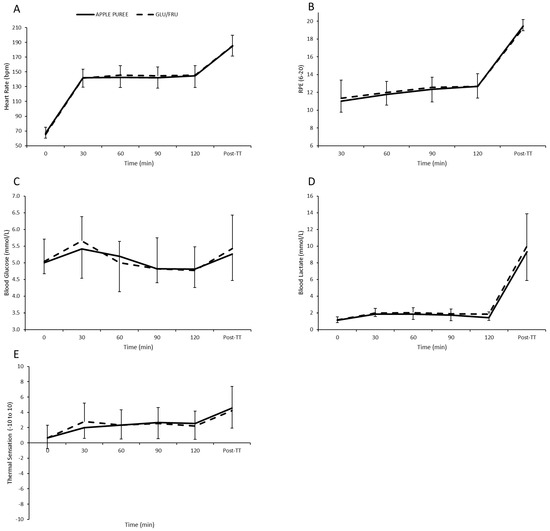
Figure 1.
Heart rate (beat/min), (A); rating of perceived exertion (RPE) (6–20), (B); blood glucose (mmol/L), (C); blood lactate (mmol/L), (D); and thermal sensation (−10–10), (E) over the 2 h preload and post time trial (TT) for both carbohydrate supplements. Data presented as mean ± SD.
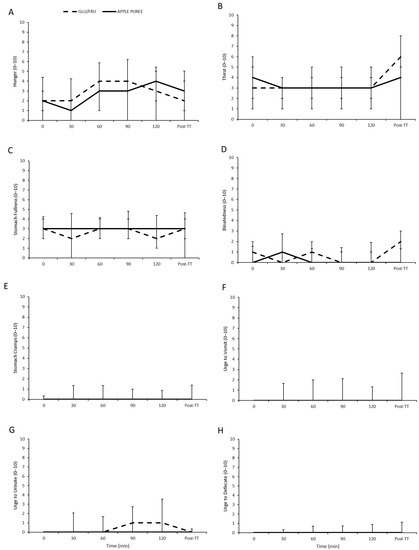
Figure 2.
Gastrointestinal symptoms (hunger, (A); thirst, (B); stomach fullness, (C); bloatedness, (D); stomach cramps, (E); urge to vomit, (F); urge to urinate, (G); and urge to defecate, (H)) over the 2 h preload and post time trial (TT) for both carbohydrate supplements. Data presented as median ± IQR.
3.2. Heart Rate, RPE, TS, Blood Glucose/Lactate Responses, and Environmental Conditions
Laboratory conditions were not different between trials (GLU/FRU 21.8 ± 2.1 °C; APPLE PUREE 21.7 ± 1.7 °C; p = 0.556), relative humidity (GLU/FRU 35.4 ± 11.9%; APPLE PUREE 37.5± 14.4%; p = 0.272), or barometric pressure (GLU/FRU 751 ± 12 mmHg and APPLE PUREE 758 ± 8 mmHg; p = 0.128).
Heart rate (trial effect p = 0.670; interaction effect p = 0.325), blood glucose concentration (trial effect p = 0.837; interaction effect p = 0.622), blood lactate concentration (trial effect p = 0.228; interaction effect p = 0.510), RPE (trial effect p = 0.468; interaction effect p = 0.613), and TS (trial effect p > 0.999; interaction effect p = 0.240) were not different between trials (Figure 1). There were, however, main effects of time for heart rate, blood lactate concentration, RPE, and TS (p ≤ 0.001), with an increase from rest to exercise and a further increase post-TT.
3.3. Gastrointestinal Scale Responses
GI scales for all symptoms were not significantly different between trials (trial effect p ≥ 0.052 interaction effect p ≥ 0.085; Figure 2). On average, no symptoms were classed as severe (≥5 out of 10), although some individual participants did report symptoms ≥ 5, including hunger (GLU/FRU 4 participants; APPLE PUREE 4 participants), thirst (GLU/FRU 6 participants; APPLE PUREE 4 participants), stomach fullness (GLU/FRU 3 participants; APPLE PUREE 4 participants), stomach cramps (GLU/FRU 1 participant; APPLE PUREE 0 participants), bloatedness (GLU/FRU 0 participants; APPLE PUREE 1 participant), urge to vomit (GLU/FRU 1 participant; APPLE PUREE 3 participants), and urge to urinate (GLU/FRU 3 participants; APPLE PUREE 1 participants). Thirst also increased over time (p = 0.007).
3.4. Fluid Balance Measures
There was no difference in ad libitum water intake (GLU/FRU 977 ± 464 g; APPLE PUREE 1018 ± 426 g; p = 0.722; Table 1), total fluid intake (GLU/FRU 1453 ± 464 g; APPLE PUREE 1493 ± 426 mL; p = 0.685), or sweat rate (p = 0.576; Table 1) between trials or in the dehydration accrued in trials (p = 0.555; Table 1). There was a significant trial effect for USG (p = 0.010) but no significant interaction (p = 0.968). Whilst USG was not different between trials upon arrival to the laboratory and after the 2 h preload, after the TT, USG values were slightly but significantly higher in the APPLE PUREE condition (p = 0.010; Table 1).
3.5. Time Trial (TT) Performance
The total time to complete the TT was not significantly different between the two trials (GLU/FRU 792 ± 68 s, APPLE PUREE 800 ± 65 s; p = 0.313, Figure 3). There was no influence on pacing between the trials or across each 25% section (condition × trial interaction p = 0.909).
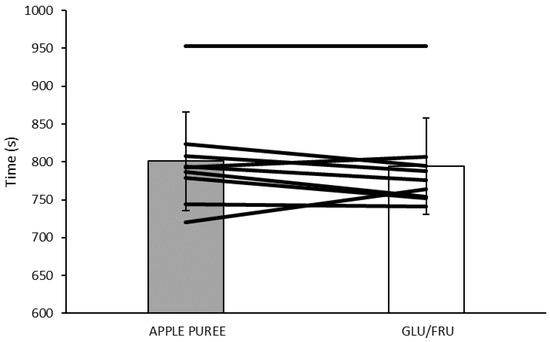
Figure 3.
Time trial performance during GLU/FRU and APPLE PUREE. Bars are means ± SD. Lines are individual participant times.
3.6. Renal Injury Biomarkers
Only seven participants’ data were included for the NGAL and KIM-1 analysis due to storage/analysis issues. Urine samples from familiarisation were also analysed, as they provided a fructose-free control. There were no significant differences for NGAL (trial effect p = 0.538; interaction effect p = 0.974) or KIM1 (trial effect p = 0.591; interaction effect p = 0.440) between trials (Figure 4) and no changes over time (p ≥ 0.069).
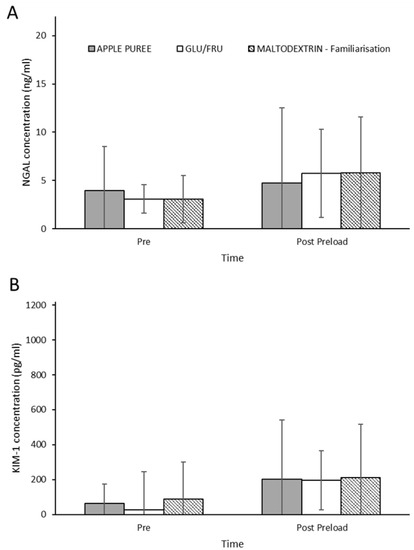
Figure 4.
NGAL (ng/mL; (A)) and KIM-1 (pg/mL; (B)) urinary concentrations at rest and after the 2 h preload for both carbohydrate supplements and maltodextrin (fructose-free control). Data presented as median ± IQR. n = 7.
3.7. Study Blinding and Trial Order Effects
No participant correctly identified both carbohydrate supplements or the aim of the study (i.e., to look at natural/fruit sources of fructose). Generally, participants thought the experiment was comparing the effects of different carbohydrate types on performance (n = 7) or different carbohydrate dosages (n = 1). One participant mentioned that he thought the study aim was something related to fructose but specified this to be in the breakfast content rather than in the carbohydrate provided during exercise. This suggests that the participants were successfully blinded to the study aims. Additionally, there was no trial order effect for any of the measured variables (p ≥ 0.055), including the time to complete the time trial during the first and second visits (Trial 1, 788 ± 67 s, Trial 2, 804 ± 61 s), indicating that there was no learning effect over the trials.
4. Discussion
The main findings of this study were that cycling TT performance and GI comfort were not different between fruit and artificial sources of fructose ingested during prolonged cycling. From a performance perspective, these results align with previous studies that have compared fruit or other whole-food sources with supplemental carbohydrate sources, including bananas [18,19], raisins [28,29,30], and pureed potato [20]. However, in contrast to many previous studies comparing supplemental and whole-food carbohydrate sources [15,16,17,18,19,20], there was no significant increase in GI disturbances with the consumption of apple puree. These results are of important practical relevance to the athlete/exerciser as they suggest that apple puree may be used to provide a more natural source of fructose in sports supplements without negatively impacting exercise performance or GI symptoms. Whole foods are more likely to delay taste and texture fatigue that can be associated with gels and drinks [31], and therefore, alternative carbohydrate sources may play an important role in encouraging athletes to meet fueling guidelines. Research suggests many endurance athletes do not meet the guidelines for carbohydrate intake during exercise, even in competition [5], meaning that widening the scope of appropriate sources through which athletes can obtain carbohydrate might be an important step in facilitating optimal carbohydrate intake during training/competition.
To meet the guidelines for carbohydrate dosage, the volume of whole foods needs to be considered, along with the potential for such volumes to cause GI upset. For example, when comparing potato consumption to a carbohydrate gel [20], 1028 g of potato puree was equitable to consuming 184 g of carbohydrate gel, which increased GI symptoms reported in the potato trial, likely due to the greater volume of food consumed. In the current study, the total amount consumed was similar between supplements (271.6 g/h in GLU/FRU vs. 274.4 g/h in APPLE PUREE), which may account for the lack of a difference in GI symptoms observed between trials, with all symptoms low, on average, in both trials. The current study provided carbohydrate at 60 g/h, whereas for longer durations, some athletes may consume carbohydrate at a higher intake rate (90g/h or more), and at higher dosages, there is the potential that GI symptoms may be increased. GI upset is often associated with increased exercise duration and intensity, and therefore, the finding that the high-intensity TT did not lead to any further exacerbation of GI symptoms in the apple puree trial is important.
The apple puree contained additional nutrients, including small amounts of fibre and protein. Whilst these additional components might be expected to slow gastric emptying [32,33], it seems the very small amounts (3.57 g fibre/h and 0.55 g protein/h) did not slow gastric emptying enough to induce any GI symptoms. This suggests that fruit puree, at least apple puree, might be an attractive option for a natural source of fructose in carbohydrate supplements. Research has suggested that the gut is a trainable organ [34,35], meaning that GI comfort could be improved with experience consuming carbohydrate foods and supplements during exercise with higher-volume feeding strategies. The participants in the current study all had previous experience consuming carbohydrate during exercise, which may have contributed to the low GI symptom scores compared with previous studies [15,16,17,18,19,20] as well as possibly the lack of a difference between trials.
The difference in carbohydrate supplement provided also did not affect other factors that might influence GI symptoms during exercise. High water intake during exercise can induce GI discomfort [36,37], and it is possible that the more viscous nature of the apple puree supplement could have induced increased drinking. However, neither ad libitum water intake throughout the preload nor subjective thirst were different between trials. These observations suggest that participants did not feel the need to drink more with the apple puree, which could have increased stomach volume and, consequently, GI symptoms. Indeed, hydration status was generally well maintained over the trial, with participants losing, on average, <0.5% body mass over the preload and <1.3% over the whole trial and no participant reaching >2% body mass loss during the preload when ad libitum drinking was permitted. Neither sweat rate nor dehydration accrued was different between trials, which was expected since the exercise intensity was the same. Hypohydration can impair endurance exercise performance [38,39], so this study suggests that ad libitum drinking is sufficient to prevent significant hypohydration from accruing during ~2 h of indoor cycling in a temperate environment.
Since it was not possible to measure carbohydrate oxidation, there is no definitive evidence of carbohydrate utilisation, but the performance and blood glucose data indicated no differences in carbohydrate availability or utilisation between trials. Therefore, the inclusion of the additional plant components and nutrients within the apple puree did not seem to hinder the bioavailability of carbohydrate during endurance exercise. However, the inclusion of fibre could explain the small but significant increase in USG in the apple puree trial (although there was no interaction effect). The presence of fibre in the gut has been linked to increased water retention in the lumen [40]. Although this increase was small and within a range considered euhydrated, it may be an important consideration for longer exercise durations or exercise in the heat, conditions in which there is a greater risk of dehydration [41]. Future studies should examine such conditions as well as measure changes in blood/plasma volume to ascertain the effects.
Urinary biomarkers of renal injury, KIM-1 and NGAL, have been shown to increase following various forms of exercise [42]. In the present study, urinary NGAL and KIM-1 concentrations only showed small, non-significant increases from rest to the end of the preload and were not significantly different between trials. It may be that hotter ambient temperatures and/or greater intensity/duration of exercise are required to increase renal injury, particularly with cycling (due to the lack of eccentric muscle contractions and thus muscle damage). Additionally, there is evidence to suggest that the consumption of high-fructose drinks during and after exercise heat stress increases biomarkers of renal injury [8], but that was not apparent in the present study. However, the fructose consumption was significantly greater in the Chapman study [8] (~234 g) compared with that in the present study (40 g). Whilst this may be a concern for individuals exercising for a prolonged duration in hot environments with high fructose intakes, most exercising recommendations suggest that intake rates for athletes have an upper fructose limit of ~30 g/h, which is less likely to produce negative effects.
As with all studies, the current study was not without limitations. The study was open to female participants, but none volunteered, which was very disappointing. Thus, these findings may not be transferable to females since females tend to have slower gastric emptying rates [43] and are more prone to experiencing GI symptoms during prolonged exercise [44].
5. Conclusions
Results from the current study indicate that apple puree as a fructose source may provide a suitable alternative to carbohydrate supplements when ingested during endurance cycling, with no difference in performance outcomes (GLU/FRU 792 ± 68 s, APPLE PUREE 800 ± 65 s; p = 0.313) or GI symptoms. Future research should examine running performance since runners are more prone to experience GI symptoms when exercising, and this could be exacerbated with natural carbohydrate sources. Additionally, further research on females is needed, as this group is poorly represented in studies and may be more prone to increased incidence of GI symptoms.
Author Contributions
The study was designed by K.M.R., C.J.H., S.A.M. and L.J.J.; K.M.R., J.C. and L.J.J. collected the data; K.M.R., L.A.J. and J.C. performed biochemical/data analysis; K.M.R. wrote the manuscript with assistance from L.A.J., J.C., C.J.H., S.A.M. and L.J.J. All authors have read and agreed to the published version of the manuscript.
Funding
K.M.R. is a doctoral researcher funded by Decathlon SA (Principal Investigator L.J.J.) and Loughborough University. Decathlon SA has read the final manuscript but played no role in the data collection, analysis, or writing of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of Loughborough University (protocol code R2020-P008, approved February 2020).
Informed Consent Statement
Informed consent was obtained from all participants involved in the study.
Data Availability Statement
The data for this study is not publicly available.
Conflicts of Interest
L.J.J. is part of the National Institute for Health Research’s Leicester Biomedical Research Centre, which is a partnership between the University Hospitals of Leicester NHS Trust, Loughborough University, and the University of Leicester. This report is independent research by the National Institute for Health Research. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health. L.J.J. has current/previous funding from Entrinsic Beverage Company LLP, Herbalife Europe Ltd., Bridge Farm Nurseries, Decathlon SA, PepsiCo Inc., and Volac International; has performed consultancy for PepsiCo Inc. and Lucozade, Ribena Suntory; and has received conference fees from PepsiCo Inc. and Danone Nutricia. In all cases, monies were paid to L.J.J.’s institution and not directly to L.J.J. SAM has current/previous funding from Entrinsic Beverage Company LLP and Decathlon SA. The authors declare no other conflict of interest.
References
- Stellingwerf, T.; Cox, G.R. Systematic Review: Carbohydrate Supplementation on Exercise Performance or Capacity of Varying Duration. Appl. Physiol. Nutr. Metab. 2014, 39, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Castell, L.M.; Casa, D.J.; Close, G.L.; Costa, R.J.S.; Desbrow, B.; Halson, S.L.; Lis, D.M.; Melin, A.K.; Peeling, P.; et al. International Association of Athletics Federations Consensus Statement 2019: Nutrition for Athletes. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Hawley, J.A.; Wong, S.H.S.; Jeukendrup, A.E. Carbohydrate for Training and Competition. J. Sports Sci. 2011, 29, S17–S27. [Google Scholar] [CrossRef]
- Jeukendrup, A. Nutrition for Endurance Sports: Marathon, Triathlon, and Road Cycling. J. Sports Sci. 2011, 29 (Suppl. 1), S91–S99. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, B.; Stellingwerff, T.; Hodgson, A.B.; Randell, R.; Pöttgen, K.; Res, P.; Jeukendrup, A.E. Nutritional Intake and Gastrointestinal Problems during Competitive Endurance Events. Med. Sci. Sports. Exerc. 2012, 44, 344–351. [Google Scholar] [CrossRef]
- Kelishadi, R.; Mansourian, M.; Heidari-Beni, M. Association of fructose consumption and components of metabolic syndrome in human studies: A systematic review and meta-analysis. Nutrition 2014, 30, 503–510. [Google Scholar] [CrossRef]
- Liu, Q.; Ayoub-Charette, S.; Khan, T.A.; Au-Yeung, F.; Mejia, S.B.; De Souza, R.J.; Wolever, T.M.; Leiter, L.A.; Kendall, C.W.; Sievenpiper, J.L. Important food sources of fructose-containing sugars and incident hypertension: A systematic review and dose-response meta-analysis of prospective cohort studies. J. Am. Heart Assoc. 2019, 8, e010977. [Google Scholar] [CrossRef]
- Chapman, C.L.; Johnson, B.D.; Sackett, J.R.; Parker, M.D.; Schlader, Z.J. Soft drink consumption during and following exercise in the heat elevates biomarkers of acute kidney injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 316, R189–R198. [Google Scholar] [CrossRef]
- Hyson, D.A. A review and critical analysis of the scientific literature related to 100% fruit juice and human health. Adv. Nutr. 2015, 6, 37–51. [Google Scholar] [CrossRef]
- Aprea, E.; Charles, M.; Endrizzi, I.; Corollaro, M.L.; Betta, E.; Biasioli, F.; Gasperi, F. Sweet taste in apple: The role of sorbitol, individual sugars, organic acids and volatile compounds. Sci. Rep. 2017, 7, 44950. [Google Scholar] [CrossRef]
- Fabiani, R.; Minelli, L.; Rosignoli, P. Apple intake and cancer risk: A systematic review and meta-analysis of observational studies. Public Health Nutr. 2016, 19, 2603–2617. [Google Scholar] [CrossRef] [PubMed]
- Hyson, D.A. A comprehensive review of apples and apple components and their relationship to human health. Adv. Nutr. 2011, 2, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Ramírez, B.A.; Catalán, Ú.; Calderón-Pérez, L.; Companys, J.; Pla-Pagà, L.; Ludwig, I.A.; Romero, M.P.; Solà, R. The effects and associations of whole-apple intake on diverse cardiovascular risk factors. A narrative review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3862–3875. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, K.M.; Clifford, T.; Mears, S.A.; James, L.J. A Food First Approach to Carbohydrate Supplementation in Endurance Exercise: A Systematic Review. Int. J. Sport Nutr. Exerc. Metab. 2022, 32, 296–310. [Google Scholar] [CrossRef]
- Guillochon, M.; Rowlands, D.S. Solid, Gel, and Liquid Carbohydrate Format Effects on Gut Comfort and Performance. Int. J. Sport Nutr. Exerc. Metab. 2011, 27, 247–254. [Google Scholar] [CrossRef]
- Ishihara, K.; Taniguchi, H.; Akiyama, N.; Asami, Y. Easy to Swallow Rice Cake as a Carbohydrate Source during Endurance Exercise Suppressed Feelings of Thirst and Hunger without Changing Exercise Performance. J. Nutr. Sci. Vitaminol. 2020, 66, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.W.; Maughan, R.J.; Shirreffs, S.M.; Watson, P. Effects of Milk Ingestion on Prolonged Exercise Capacity in Young, Healthy Men. Nutrition 2008, 24, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Gillitt, N.D.; Henson, D.A.; Sha, W.; Shanely, R.A.; Knab, A.M.; Cialdella-Kam, L.; Jin, F. Bananas as an Energy Source during Exercise: A Metabolomics Approach. PLoS ONE 2012, 7, E37479. [Google Scholar] [CrossRef]
- Nieman, D.C.; Gillitt, N.D.; Sha, W.; Esposito, D.; Ramamoorthy, S. Metabolic Recovery from Heavy Exertion Following Banana Compared to Sugar Beverage or Water Only Ingestion: A Randomized, Crossover Trial. PLoS ONE 2018, 13, E0194843. [Google Scholar] [CrossRef]
- Salvador, A.F.; Mckenna, C.F.; Alamilla, R.A.; Cloud, R.M.T.; Keeble, A.R.; Miltko, A.; Scaroni, S.E.; Beals, J.W.; Ulanov, A.V.; Dilger, R.N.; et al. Potato Ingestion Is as Effective as Carbohydrate Gels to Support Prolonged Cycling Performance. J. Appl. Physiol. 2019, 127, 1651–1659. [Google Scholar] [CrossRef]
- Pugh, J.N.; Kirk, B.; Fearn, R.; Morton, J.P.; Close, G.L. Prevalence, Severity and Potential Nutritional Causes of Gastrointestinal Symptoms during a Marathon in Recreational Runners. Nutrients 2018, 10, 811. [Google Scholar] [CrossRef] [PubMed]
- Drust, B.; Waterhouse, J.; Atkinson, G.; Edwards, B.; Reilly, T. Circadian rhythms in sports performance—An update. Chronobiol. Int. 2005, 22, 21–44. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.; Saris, W.H.; Brouns, F.J.P.H.; Kester, A.D. A new validated endurance performance test. Med. Sci. Sports Exerc. 1996, 28, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Kenefick, R.W.; Cheuvront, S.N.; Palombo, L.J.; Ely, B.R.; Sawka, M.N. Skin temperature modifies the impact of hypohydration on aerobic performance. J. Appl. Physiol. 2010, 109, 79–86. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Vet-Joop, K.; Sturk, A.; Stegen, J.H.J.C.; Senden, J.; Saris, W.H.M.; Wagenmakers, A.J.M. Relationship between gastro-intestinal complaints and endotoxaemia, cytokine release and the acute-phase reaction during and after a long-distance triathlon in highly trained men. Clin. Sci. 2000, 98, 47–55. [Google Scholar] [CrossRef]
- Field, A.P. Discovering Statistics Using IBM SPSS: And Sex and Drugs and Rock’n’Roll; SAGE: London, UK, 2013. [Google Scholar]
- Rietschier, H.L.; Henagan, T.M.; Earnest, C.P.; Baker, B.L.; Cortez, C.C.; Stewart, L.K. Sun-Dried Raisins Are a Cost-Effective Alternative to Sports Jelly Beans in Prolonged Cycling. J. Strength Cond. Res. 2011, 25, 3150. [Google Scholar] [CrossRef]
- Kern, M.; Heslin, C.J.; Rezende, R.S. Metabolic and Performance Effects of Raisins Versus Sports Gel as Pre-Exercise Feedings in Cyclists. J. Strength Cond. Res. 2007, 21, 1204–1207. [Google Scholar]
- Too, B.W.; Cicai, S.; Hockett, K.R.; Applegate, E.; Davis, B.A.; Casazza, G.A. Natural Versus Commercial Carbohydrate Supplementation and Endurance Running Performance. J. Int. Soc. Sports Nutr. 2012, 9, 27. [Google Scholar] [CrossRef]
- Tiller, N.B.; Roberts, J.D.; Beasley, L.; Chapman, S.; Pinto, J.M.; Smith, L.; Wiffin, M.; Russell, M.; Sparks, S.A.; Duckworth, L.; et al. International Society of Sports Nutrition Position Stand: Nutritional Considerations for Single-Stage Ultra-Marathon Training and Racing. J. Int. Soc. Sports Nutr. 2019, 16, 50. [Google Scholar] [CrossRef]
- McIntyre, A.; Vincent, R.M.; Perkins, A.C.; Spiller, R.C. Effect of bran, ispaghula, and inert plastic particles on gastric emptying and small bowel transit in humans: The role of physical factors. Gut 1997, 40, 223–227. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Babio, N.; Balanza, R.; Basulto, J.; Bulló, M.; Salas-Salvadó, J. Dietary fibre: Influence on body weight, glycemic control and plasma cholesterol profile. Nutr. Hosp. 2010, 25, 327–340. [Google Scholar] [PubMed]
- Jeukendrup, A.E. Training the Gut for Athletes. Sports Med. 2017, 47, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Miall, A.; Khoo, A.; Rauch, C.; Snipe, R.M.J.; Camões-Costa, V.L.; Gibson, P.R.; Costa, R.J.S. Two Weeks of Repetitive Gut-Challenge Reduce Exercise-Associated Gastrointestinal Symptoms and Malabsorption. Scand. J. Med. Sci. Sports 2018, 28, 630–640. [Google Scholar] [CrossRef]
- Robinson, T.A.; Hawley, J.A.; Palmer, G.S.; Wilson, G.R.; Gray, D.A.; Noakes, T.D.; Dennis, S.C. Water ingestion does not improve 1-h cycling performance in moderate ambient temperatures. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 71, 153–160. [Google Scholar] [CrossRef]
- Backx, K.; van Someren, K.A.; Palmer, G.S. One hour cycling performance is not affected by ingested fluid volume. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 333–342. [Google Scholar] [CrossRef]
- Funnell, M.P.; Mears, S.A.; Bergin-Taylor, K.; James, L.J. Blinded and Unblinded Hypohydration Similarly Impair Cycling Time Trial Performance in the Heat in Trained Cyclists. J. Appl. Physiol. 2019, 126, 870–879. [Google Scholar] [CrossRef]
- James, L.J.; Funnell, M.P.; James, R.M.; Mears, S.A. Does Hypohydration Really Impair Endurance Performance? Methodological Considerations for Interpreting Hydration Research. Sports Med. 2019, 49, 103–114. [Google Scholar] [CrossRef]
- Eastwood, M.A.; Kay, R.M. An hypothesis for the action of dietary fiber along the gastrointestinal tract. Am. J. Clin. Nutr. 1979, 32, 364–367. [Google Scholar] [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. American college of sports medicine joint position statement. nutrition and athletic performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar]
- Juett, L.A.; James, L.J.; Mears, S.A. Effects of exercise on acute kidney injury biomarkers and the potential influence of fluid intake. Ann. Nutr. Metab. 2020, 76, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Datz, F.L.; Christian, P.E.; Moore, J. Gender-Related Differences in Gastric Emptying. J. Nucl. Med. 1987, 28, 1204–1207. [Google Scholar] [PubMed]
- Ten Haaf, D.S.; van der Worp, M.P.; Groenewoud, H.M.; Leij-Halfwerk, S.; Nijhuis-van der Sanden, M.W.; Verbeek, A.L.; Staal, J.B. Nutritional Indicators for Gastrointestinal Symptoms in Female Runners: The ‘Marikenloop Study’. BMJ Open 2014, 4, e005780. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).