Friends or Foes? Cytotoxicity, HPTLC and NMR Analyses of Some Important Naturally Occurring Hydroxyanthraquinones
Abstract
:1. Introduction
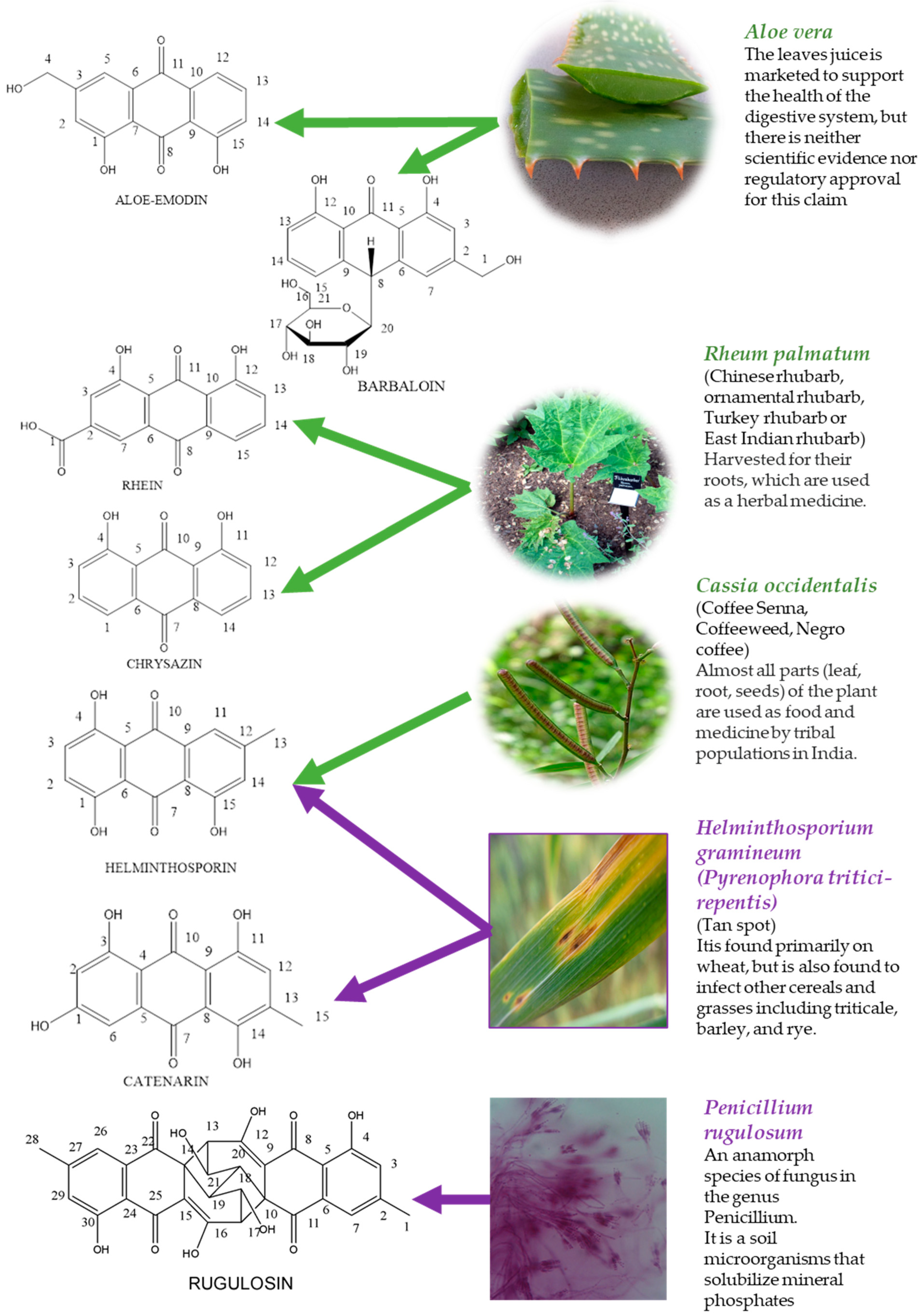
1.1. Aloe-Emodin
1.2. Barbaloin
1.3. Catenarin
1.4. Chrysazin
1.5. Helminthosporin
1.6. Rhein
1.7. Rugulosin
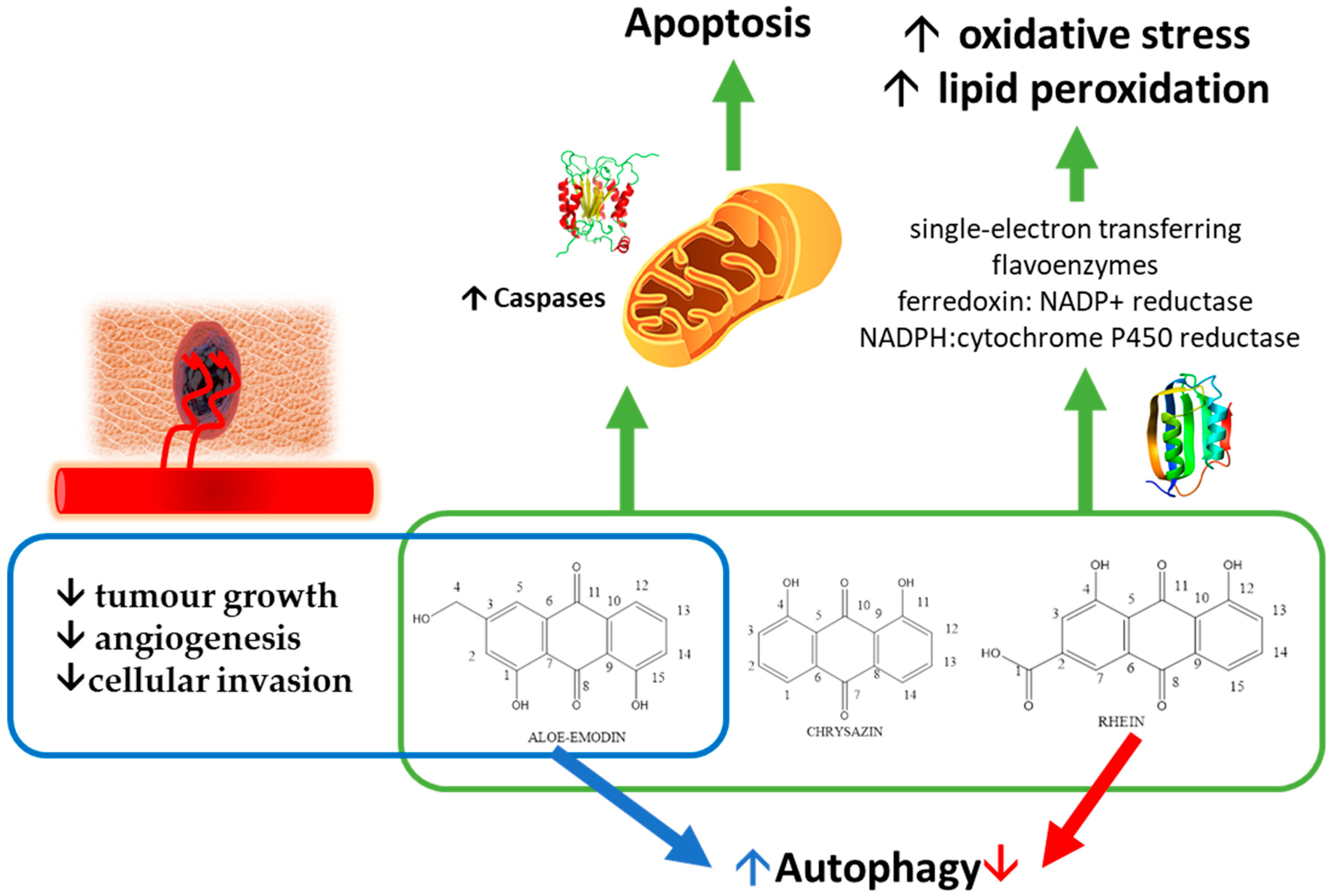
1.8. Overall Mechanisms of Action
1.9. Aims
2. Material and Methods
2.1. Compounds and Reagents
2.2. Thin Layer Chromatography
2.3. High-Performance Thin-Layer Chromatography
2.4. Nuclear Magnetic Resonance
2.5. Anti-Proliferative Activity
3. Results
3.1. Development of High-Performance Thin-Layer Chromatography Systems for the Determination of the Purity of Hydroxyantharquimones
3.2. NMR Studies
3.2.1. NMR Spectroscopic Characteristics of Aged Aloe-Emodin
3.2.2. NMR Spectroscopic Characteristics of Aged Barbaloin
3.2.3. NMR Spectroscopic Characteristics of Aged Catenarin
3.2.4. NMR Spectroscopic Characteristics of Aged Chrysazin
3.2.5. NMR Spectroscopic Characteristics of Aged Helminthosporin
3.2.6. NMR Study of Aged Rhein
3.2.7. NMR Spectroscopic Characteristics of Aged Rugulosin
3.3. Anti-Proliferative Activity
4. Discussion
4.1. Purity and Identity of the Compounds as a Proxy to Determine Their Long Term Stability
4.2. General Toxicity of Selected Anthraquinones, Their Activity on Caco-2 Cells and Cytotoxicity in Other Cancer Cells as a Proxy to Determine Their Nutraceutical Interest
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Betts, T.J.; Fairbairn, J.W.; Mital, V.K. Vegetable purgatives containing anthracene derivatives: Part viii.—the paper chromatography of certain anthraquinones and their glycosides. J. Pharm. Pharmacol. 1976, 10, 436–441. [Google Scholar] [CrossRef]
- Akilandeswari, P.; Pradeep, B.V. Exploration of industrially important pigments from soil fungi. Appl. Microbiol. Biotechnol. 2016, 100, 1631–1643. [Google Scholar] [CrossRef]
- Kuzuya, M.; Noguchi, A.; Kawai, K.; Mori, H. Quantum chemical study for genotoxic and antitumor activities of hydroxyanthraquinones. Regul. Toxicol. Pharm. 1991, 13, 185–194. [Google Scholar] [CrossRef]
- Tian, W.; Wang, C.; Li, D.; Hou, H. Novel anthraquinone compounds as anticancer agents and their potential mechanism. Future Med. Chem. 2020, 12, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, C.; Capasso, R. Constipation and Botanical Medicines: An Overview. Phytother. Res. 2015, 29, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Su, M.; Liang, S.; Sun, H. Investigation of six bioactive anthraquinones in slimming tea by accelerated solvent extraction and high performance capillary electrophoresis with diode-array detection. Food Chem. 2016, 199, 7. [Google Scholar] [CrossRef] [PubMed]
- Cosmetic Ingredient Review Expert Panel. Final report on the safety assessment of AloeAndongensis Extract, Aloe Andongensis Leaf Juice, Aloe Arborescens Leaf Extract, Aloe Arborescens Leaf Juice, Aloe Arborescens Leaf Protoplasts, Aloe Barbadensis Flower Extract, Aloe Barbadensis Leaf, Aloe Barbadensis Leaf Extract, Aloe Barbadensis Leaf Juice, Aloe Barbadensis Leaf Polysaccharides, Aloe Barbadensis Leaf Water, Aloe Ferox Leaf Extract, Aloe Ferox Leaf Juice, and Aloe Ferox Leaf Juice Extract. Int. J. Toxicol. 2007, 26, 1–50. [Google Scholar]
- Caro, Y.; Anamale, L.; Fouillaud, M.; Laurent, P.; Petit, T.; Dufosse, L. Natural hydroxyanthraquinoid pigments as potent food grade colorants: An overview. Nat. Prod. Bioprospect. 2012, 2, 174–193. [Google Scholar] [CrossRef]
- Rossi, M.; Mirbagheri, S.; Keshavarzian, A.; Bishehsari, F. Nutraceuticals in colorectal cancer: A mechanistic approach. Eur. J. Pharm. 2018, 833, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, S.N.; Tollefsbol, T.O. The Role of Nutraceuticals in Chemoprevention and Chemotherapy and Their Clinical Outcomes. J. Oncol. 2012, 2012, 192464. [Google Scholar] [CrossRef] [Green Version]
- Velíšek, J.; Cejpek, K. Pigments of higher fungi-a review. Czech J. Food Sci. 2011, 29, 87–102. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.P. A review of the genetic effects of naturally occurring flavonoids, anthraquinones and related compounds. Mutat. Res./Rev. Genet. Toxicol. 1980, 75, 243–277. [Google Scholar] [CrossRef]
- Yusuf, S.; Agunu, A.; Diana, M. The effect of Aloe vera A. Berger (Liliaceae) on gastric acid secretion and acute gastric mucosal injury in rats. J. Ethnopharmacol. 2004, 93, 33–37. [Google Scholar] [CrossRef]
- Dewick, P.M.; MyiLibrary. Medicinal Natural Products a Biosynthetic Approach, 3rd ed.; Wiley & Sons, Ltd: Chichester, West Sussex, UK, 2009; pp. 103–110. [Google Scholar]
- Shukla, V.; Asthana, S.; Gupta, P.; Dwivedi, P.D.; Tripathi, A.; Das, M. Chapter One—Toxicity of Naturally Occurring Anthraquinones. In Advances in Molecular Toxicology; Fishbein, J.C., Heilman, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 11, pp. 1–50. [Google Scholar]
- Duval, J.; Pecher, V.; Poujol, M.; Lesellier, E. Research advances for the extraction, analysis and uses of anthraquinones: A review. Ind. Crop. Prod. 2016, 94, 812–833. [Google Scholar] [CrossRef]
- Dufossé, L. Anthraquinones, the Dr Jekyll and Mr Hyde of the food pigment family. Food Res. Int. 2014, 65, 132–136. [Google Scholar] [CrossRef]
- Jiang, W.; Tian, X.; Wang, Y.; Sun, Z.; Dong, P.; Wang, C.; Huo, X.; Zhang, B.; Huang, S.; Deng, S.; et al. The natural anthraquinones from Rheum palmatum induced the metabolic disorder of melatonin by inhibiting human CYP and SULT enzymes. Toxicol. Lett. 2016, 262, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Consulting, A.R.A. Anthraquinone Market Worth US$2.2 Billion By 2025: Acumen Research and Consulting. Available online: https://www.globenewswire.com/news-release/2019/01/11/1690490/0/en/Anthraquinone-Market-Worth-US-2-2-Billion-By-2025-Acumen-Research-and-Consulting.html (accessed on 10 May 2021).
- Ghosh, A.; Jose, D.A.; Kaushik, R. Anthraquinones as versatile colorimetric reagent for anions. Sens. Actuators B Chem. 2016, 229, 545–560. [Google Scholar] [CrossRef]
- Taher, A.T.; Hegazy, G.H. Synthesis of novel bis-anthraquinone derivatives and their biological evaluation as antitumor agents. Arch. Pharmacal Res. 2013, 36, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Winter, R.; Cornell, K.A.; Johnson, L.L.; Isabelle, L.M.; Hinrichs, D.J.; Riscoe, M.K.J.B.; Letters, M.C. Hydroxy-anthraquinones as antimalarial agents. Bioorgan. Med. Chem. Lett. 1995, 5, 1927–1932. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Andrés-Lacueva, C. Polyphenols and Health: Current State and Progress. J. Agric. Food Chem. 2012, 60, 8773–8775. [Google Scholar] [CrossRef]
- Tikhomirov, A.S.; Shtil, A.A.; Shchekotikhin, A.E. Advances in the Discovery of Anthraquinone-Based Anticancer Agents. Recent Pat. Anti-Cancer Drug Discov. 2018, 13, 159–183. [Google Scholar] [CrossRef]
- Wang, D.; Wang, X.-H.; Yu, X.; Cao, F.; Cai, X.; Chen, P.; Li, M.; Feng, Y.; Li, H.; Wang, X. Pharmacokinetics of Anthraquinones from Medicinal Plants. Front. Pharmacol. 2021, 12, 638993. [Google Scholar]
- Westendorf, J.; Marquardt, H.; Poginsky, B.; Dominiak, M.; Schmidt, J.; Marquardt, H. Genotoxicity of naturally occurring hydroxyanthraquinones. Mutat. Res./Genet. Toxicol. 1990, 240, 12. [Google Scholar] [CrossRef]
- Wölfle, D.; Schmutte, C.; Westendorf, J.; Marquardt, H. Hydroxyanthraquinones as Tumor Promoters: Enhancement of Malignant Transformation of C3H Mouse Fibroblasts and Growth Stimulation of Primary Rat Hepatocytes. Cancer Res. 1990, 50, 6540–6544. [Google Scholar] [PubMed]
- Lown, J.W. Anthracycline and anthraquinone anticancer agents: Current status and recent developments. Pharmacol. Ther. 1993, 60, 185–214. [Google Scholar] [CrossRef] [PubMed]
- Siddamurthi, S.; Gutti, G.; Jana, S.; Kumar, A.; Singh, S.K. Anthraquinone: A promising scaffold for the discovery and development of therapeutic agents in cancer therapy. Future Med. Chem. 2020, 12, 1037–1069. [Google Scholar] [PubMed]
- Matsuda, H.; Shimoda, H.; Morikawa, T.; Yoshikawa, M.J.B. Phytoestrogens from the roots of Polygonum cuspidatum (Polygonaceae): Structure-requirement of hydroxyanthraquinones for estrogenic activity. Bioorgan. Med. Chem. Lett. 2001, 11, 1839–1842. [Google Scholar] [CrossRef]
- Acevedo-Duncan, M.; Russell, C.; Patel, S.; Patel, R. Aloe-emodin modulates PKC isozymes, inhibits proliferation, and induces apoptosis in U-373MG glioma cells. Int. Immunopharmacol. 2004, 4, 1775–1784. [Google Scholar] [CrossRef]
- Kuo, P.L.; Lin, T.C.; Lin, C.C. The antiproliferative activity of aloe-emodin is through p53-dependent and p21-dependent apoptotic pathway in human hepatoma cell lines. Life Sci. 2002, 71, 1879–1892. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Chiang, S.Y.; Lin, J.G.; Ma, Y.S.; Liao, C.L.; Weng, S.W.; Lai, T.Y.; Chung, J.G. Emodin, aloe-emodin and rhein inhibit migration and invasion in human tongue cancer SCC-4 cells through the inhibition of gene expression of matrix metalloproteinase-9. Int. J. Oncol. 2010, 36, 1113–1120. [Google Scholar] [PubMed]
- Dorsey, J.F.; Kao, G.D. Aloe(-emodin) for cancer? More than just a comforting salve. Cancer Biol. 2007, 6, 89–90. [Google Scholar] [CrossRef] [Green Version]
- Lin, K.-Y.; Uen, Y.-H. Aloe-emodin, an anthraquinone, in vitro inhibits proliferation and induces apoptosis in human colon carcinoma cells. Oncol. Lett. 2010, 1, 541–547. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.-Y.; Zhang, J.-H.; Gao, J.-H.; Li, Y.-S. Aloe-emodin (AE) nanoparticles suppresses proliferation and induces apoptosis in human lung squamous carcinoma via ROS generation in vitro and in vivo. Biochem. Biophys. Res. Commun. 2017, 490, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-Z.; Hsu, S.-L.; Liu, M.-C.; Wu, C.-H. Effects and mechanisms of aloe-emodin on cell death in human lung squamous cell carcinoma. Eur. J. Pharmacol. 2001, 431, 287–295. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Fukai, T.; Sakagami, H.; Kuroda, J.; Miyaoka, R.; Tamura, M.; Yoshida, N.; Nomura, T. Cytotoxic and DNA damage-inducing activities of low molecular weight phenols from rhubarb. Anticancer Res. 2001, 21, 2847–2853. [Google Scholar] [PubMed]
- Chen, Y.Y.; Chiang, S.Y.; Lin, J.G.; Yang, J.S.; Ma, Y.S.; Liao, C.L.; Lai, T.Y.; Tang, N.Y.; Chung, J.G. Emodin, aloe-emodin and rhein induced DNA damage and inhibited DNA repair gene expression in SCC-4 human tongue cancer cells. Anticancer Res. 2010, 30, 945–951. [Google Scholar] [PubMed]
- Chiu, T.H.; Lai, W.W.; Hsia, T.C.; Yang, J.S.; Lai, T.Y.; Wu, P.P.; Ma, C.Y.; Yeh, C.C.; Ho, C.C.; Lu, H.F.; et al. Aloe-emodin induces cell death through S-phase arrest and caspase-dependent pathways in human tongue squamous cancer SCC-4 cells. Anticancer Res. 2009, 29, 4503–4511. [Google Scholar]
- Tabolacci, C.; Cordella, M.; Turcano, L.; Rossi, S.; Lentini, A.; Mariotti, S.; Nisini, R.; Sette, G.; Eramo, A.; Piredda, L.; et al. Aloe-emodin exerts a potent anticancer and immunomodulatory activity on BRAF-mutated human melanoma cells. Eur. J. Pharmacol. 2015, 762, 283–292. [Google Scholar] [CrossRef]
- Tabolacci, C.; Lentini, A.; Mattioli, P.; Provenzano, B.; Oliverio, S.; Carlomosti, F.; Beninati, S. Antitumor properties of aloe-emodin and induction of transglutaminase 2 activity in B16–F10 melanoma cells. Life Sci. 2010, 87, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Tabolacci, C.; Oliverio, S.; Lentini, A.; Rossi, S.; Galbiati, A.; Montesano, C.; Mattioli, P.; Provenzano, B.; Facchiano, F.; Beninati, S. Aloe-emodin as antiproliferative and differentiating agent on human U937 monoblastic leukemia cells. Life Sci. 2011, 89, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Pecere, T.; Gazzola, M.V.; Mucignat, C.; Parolin, C.; Vecchia, F.D.; Cavaggioni, A.; Basso, G.; Diaspro, A.; Salvato, B.; Carli, M.; et al. Aloe-emodin is a new type of anticancer agent with selective activity against neuroectodermal tumors. Cancer Res. 2000, 60, 2800–2804. [Google Scholar] [PubMed]
- Mijatovic, S.; Maksimovic-Ivanic, D.; Radovic, J.; Popadic, D.; Momcilovic, M.; Harhaji, L.; Miljkovic, D.; Trajkovic, V. Aloe-emodin prevents cytokine-induced tumor cell death: The inhibition of auto-toxic nitric oxide release as a potential mechanism. Cell. Mol. Life Sci. 2004, 61, 1805–1815. [Google Scholar] [CrossRef] [PubMed]
- El-Shemy, H.A.; Aboul-Soud, M.A.; Nassr-Allah, A.A.; Aboul-Enein, K.M.; Kabash, A.; Yagi, A. Antitumor properties and modulation of antioxidant enzymes’ activity by Aloe vera leaf active principles isolated via supercritical carbon dioxide extraction. Curr. Med. Chem. 2010, 17, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Groom, Q.J.; Reynolds, T. Barbaloin in Aloe Species. Planta. Med. 1987, 53, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Patel, K.; Tahilyani, V. Barbaloin: A concise report of its pharmacological and analytical aspects. Asian Pac. J. Trop. Biomed. 2012, 2, 835–838. [Google Scholar] [CrossRef] [Green Version]
- Chandrasenan, K.; Neelakantan, S.; Seshadri, T.R. A new synthesis of catenarin and erythroglaucin. Proc. Indian Acad. Sci.-Sect. A 1960, 51, 296–300. [Google Scholar] [CrossRef]
- Wakuliński, W.; Kachlicki, P.; Sobiczewski, P.; Schollenberger, M.; Zamorski, C.; Łotocka, B.; Sarova, J. Catenarin Production by Isolates of Pyrenophora tritici-repentis (Died.) Drechsler and its Antimicrobial Activity. J. Phytopathol. 2003, 151, 74–79. [Google Scholar] [CrossRef]
- Du, L.; Zhu, T.; Liu, H.; Fang, Y.; Zhu, W.; Gu, Q. Cytotoxic polyketides from a marine-derived fungus Aspergillus glaucus. J. Nat. Prod. 2008, 71, 1837–1842. [Google Scholar] [CrossRef]
- Drugbank. Dantron. Available online: https://go.drugbank.com/drugs/DB04816 (accessed on 10 May 2021).
- Nemeikaite-Ceniene, A.; Sergediene, E.; Nivinskas, H.; Cenas, N. Cytotoxicity of natural hydroxyanthraquinones: Role of oxidative stress. Z Nat. C J. Biosci. 2002, 57, 822–827. [Google Scholar]
- National Toxicology Program. Danthron. In The Report on Carcinogens; USA Department of Health and Human Services: Morrisville, NC, USA, 2016. [Google Scholar]
- Sugie, S.; Mori, H.; Niwa, K.; Takahashi, M.; Kawai, K. Induction of intestinal tumours in rats by chrysazin. Br. J. Cancer 1985, 52, 781–783. [Google Scholar]
- van Gorkom, B.A.P.; Timmer-Bosscha, H.; de Jong, S.; van der Kolk, D.M.; Kleibeuker, J.H.; de Vries, E.G.E. Cytotoxicity of rhein, the active metabolite of sennoside laxatives, is reduced by multidrug resistance-associated protein 1. Br. J. Cancer 2002, 86, 1494–1500. [Google Scholar] [CrossRef] [Green Version]
- Wijeratne, E.M.K.; Turbyville, T.J.; Fritz, A.; Whitesell, L.; Gunatilaka, A.A.L. A new dihydroxanthenone from a plant-associated strain of the fungus Chaetomium globosum demonstrates anticancer activity. Bioorg. Med. Chem. 2006, 14, 7917–7923. [Google Scholar] [CrossRef] [PubMed]
- Engström, K.; Brishammar, S.; Svensson, C.; Bengtsson, M.; Andersson, R. Anthraquinones from some Drechslera species and Bipolaris sorokiniana. Mycol. Res. 1993, 97, 381–384. [Google Scholar] [CrossRef]
- Fouillaud, M.; Venkatachalam, M.; Girard-Valenciennes, E.; Caro, Y.; Dufossé, L. Anthraquinones and Derivatives from Marine-Derived Fungi: Structural Diversity and Selected Biological Activities. Mar. Drugs 2016, 14, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dave, H.; Ledwani, L. A review on anthraquinones isolated from Cassia species and their applications. Res. J. 2012, 3, 291–319. [Google Scholar]
- Fozia, A.A. Phytochemical Investigation of Aloe Turkanensis for Anticancer Activity. Master’ Thesis, University of Nairobi, Nairobi, Kenya, 2014. [Google Scholar]
- Tang, N.; Chang, J.; Lu, H.-C.; Zhuang, Z.; Cheng, H.-L.; Shi, J.-X.; Rao, J. Rhein induces apoptosis and autophagy in human and rat glioma cells and mediates cell differentiation by ERK inhibition. Microb. Pathog. 2017, 113, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lv, P.; Liao, R.; Zhao, Y.; Yang, B. Synthesis, characterization and biological activity of Rhein-cyclodextrin conjugate. J. Mol. Struct. 2017, 1128, 239–244. [Google Scholar] [CrossRef]
- He, Z.H.; Zhou, R.; He, M.F.; Lau, C.B.; Yue, G.G.; Ge, W.; But, P.P. Anti-angiogenic effect and mechanism of rhein from Rhizoma Rhei. Phytomedicine 2011, 18, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.-y.; Ye, M.-y.; Huang, R.-z.; Li, Y.-j.; Pan, Y.-m.; Xu, Q.; Liao, Z.-X.; Wang, H.-S. Synthesis and antitumor activities of novel rhein α-aminophosphonates conjugates. Bioorg. Med. Chem. Lett. 2014, 24, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-L.; Chung, J.-G.; Lu, Y.-C.; Yang, C.-Y.; Chen, S.-S. Rhein inhibits invasion and migration of human nasopharyngeal carcinoma cells in vitro by down-regulation of matrix metalloproteinases-9 and vascular endothelial growth factor. Oral Oncol. 2009, 45, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Bounda, G.-A.; Zhou, W.; Wang, D.-D.; Yu, F. Rhein Elicits In Vitro Cytotoxicity in Primary Human Liver HL-7702 Cells by Inducing Apoptosis through Mitochondria-Mediated Pathway. Evid.-Based Complement. Altern. Med. 2015, 2015, 329831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, X.; Luo, X.; Chen, Y.; Li, M.; Jiang, S.; Wang, X. Rhein induces apoptosis of HCT-116 human colon cancer cells via activation of the intrinsic apoptotic pathway. Afr. J. Biotechnol. 2011, 10, 13244–13251. [Google Scholar]
- Deitersen, J.; El-Kashef, D.H.; Proksch, P.; Stork, B. Anthraquinones and autophagy—Three rings to rule them all? Bioorg. Med. Chem. 2019, 27, 115042. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.B.; Bai, W.; Ding, C.X.; Liang, J.; Wu, S.-H.; Tan, R.X. Intertwined Biosynthesis of Skyrin and Rugulosin A Underlies the Formation of Cage-Structured Bisanthraquinones. J. Am. Chem. Soc. 2021, 143, 14218–14226. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.-X.; Mo, S.-Y.; He, H.-X.; Shi, J.-G.; Ye, W.-C.; Liu, Z.; Jiang, R.-W. Molecular structure and tautomerization of the 1:1 complex of luteoskyrin and rugulosin. J. Mol. Struct. 2010, 979, 86–91. [Google Scholar] [CrossRef]
- Watts, P.; Kittakoop, P.; Veeranondha, S.; Wanasith, S.; Thongwichian, R.; Saisaha, P.; Intamas, S.; Hywel-Jones, N.L. Cytotoxicity against insect cells of entomopathogenic fungi of the genera Hypocrella (anamorph Aschersonia): Possible agents for biological control. Mycol. Res. 2003, 107, 581–586. [Google Scholar] [CrossRef]
- Ahmad, A.; Li, Y.; Bao, B.; Kong, D.; Sarkar, F.H. Epigenetic regulation of miRNA-cancer stem cells nexus by nutraceuticals. Mol. Nutr. Food Res. 2014, 58, 79–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Divella, R.; Daniele, A.; Savino, E.; Paradiso, A. Anticancer Effects of Nutraceuticals in the Mediterranean Diet: An Epigenetic Diet Model. Cancer Genom. Proteom. 2020, 17, 335–350. [Google Scholar] [CrossRef]
- Switzer, R.L.; Medrano, J.; Reedel, D.A.; Weiss, J. Substituted anthraquinones represent a potential scaffold for DNA methyltransferase 1-specific inhibitors. PLoS ONE 2019, 14, e0219830. [Google Scholar] [CrossRef] [Green Version]
- Fairbairn, J.W. Chemical structure, mode of action and therapeutical activity of anthraquinone glycosides. Pharm. Weekbl. 1965, 100, 1493–1499. [Google Scholar]
- Fairbairn, J.W. The anthraquinone laxatives. Biological assay and its relation to chemical structure. Pharmacology 1976, 14, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Fairbairn, J.W.; El-Muhtadi, F.J. Chemotaxonomy of anthraquinones in Rumex. Phytochemistry 1972, 11, 263–268. [Google Scholar] [CrossRef]
- Fairbairn, J.W.; Shrestha, A.B. The distribution of anthraquinone glycosides in Cassia senna L. Phytochemistry 1967, 6, 1203–1207. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. JNCI J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- AAT Bioquest, Inc. Quest Graph.™ IC50 Calculator. Available online: https://www.aatbio.com/tools/ic50-calculator (accessed on 12 October 2021).
- Arup, U.; Ekman, S.; Lindblom, L.; Mattsson, J.-E. High performance thin layer chromatography (HPTLC), an improved technique for screening lichen substances. Lichenologist 1993, 25, 61–71. [Google Scholar] [CrossRef]
- Gomathi, D.; Ravikumar, G.; Kalaiselvi, M.; Vidya, B.; Uma, C. HPTLC fingerprinting analysis of Evolvulus alsinoides (L.) L. J. Acute Med. 2012, 2, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Marston, A. Role of advances in chromatographic techniques in phytochemistry. Phytochemistry 2007, 68, 2786–2798. [Google Scholar] [CrossRef] [PubMed]
- Megeressa, M.; Bisrat, D.; Mazumder, A.; Asres, K. Structural elucidation of some antimicrobial constituents from the leaf latex of Aloe trigonantha L.C. Leach. BMC Complement. Altern Med. 2015, 15, 270. [Google Scholar] [CrossRef] [Green Version]
- Nile, S.H.; Park, S.W. HPTLC Analysis, Antioxidant and Antigout Activity of Indian Plants. Iran. J. Pharm. Res. IJPR 2014, 13, 531–539. [Google Scholar]
- Singhvi, G.; Shukla, V.K.; Ukawala, R.; Gampa, G.; Saha, R.N. Development of a new, rapid and sensitive HPTLC method for estimation of Milnacipran in bulk, formulation and compatibility study. Arab. J. Chem. 2017, 10, S2417–S2423. [Google Scholar] [CrossRef] [Green Version]
- Wagner, H.; Bladt, S. Plant Drug Analysis: A Thin Layer Chromatography Atlas; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Sanchez, J.F.; Entwistle, R.; Hung, J.-H.; Yaegashi, J.; Jain, S.; Chiang, Y.-M.; Wang, C.C.C.; Oakley, B.R. Genome-Based Deletion Analysis Reveals the Prenyl Xanthone Biosynthesis Pathway in Aspergillus nidulans. J. Am. Chem. Soc. 2011, 133, 4010–4017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalidhar, S.B. Structural elucidation in anthraquinones using 1H NMR glycosylation and alkylation shifts. Phytochemistry 1989, 28, 3459–3463. [Google Scholar] [CrossRef]
- Pullella, G.A.; Wild, D.A.; Nealon, G.L.; Elyashberg, M.; Piggott, M.J. What Is the Structure of the Antitubercular Natural Product Eucapsitrione? J. Org. Chem. 2017, 82, 7287–7299. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, K.; Aksnes, D.W.; Francis, G.W. NMR study of some anthraquinones from rhubarb. Magn. Reson. Chem. 1992, 30, 359–360. [Google Scholar] [CrossRef]
- Velarde, G.; Ait-Aissa, S.; Gillet, C.; Rogerieux, F.; LaMbre, C.; Vindimian, E.; Porcher, J.M. Use of the CaCo-2 Model in the Screening of Polluting Substance Toxicity. Toxicol. Vitr. 1999, 13, 719–722. [Google Scholar] [CrossRef]
- IARC. Dantron (chrysazin; 1,8-dihydroxyanthraquinone). Pharmaceutical Drugs. In IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans; International Agency for Research on Cancer: Lyon, France, 1990; Volume 50, pp. 265–275. [Google Scholar]
- Sugie, S.; Mori, Y.; Okumura, A.; Yoshimi, N.; Okamoto, K.; Sato, S.; Tanaka, T.; Mori, H. Promoting and synergistic effects of chrysazin on 1,2-dimethylhydrazine-induced carcinogenesis in male ICR/CD-1 mice. Carcinogenesis 1994, 15, 1175–1179. [Google Scholar] [CrossRef]
- Lin, L.-C.; Chou, C.-J.; Kuo, Y.-C. Cytotoxic Principles from Ventilago leiocarpa. J. Nat. Prod. 2001, 64, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.M.; Selby, P.J.; Deacon, J.; Chilvers, C.; McElwain, T.J. Anthraquinone laxatives and human cancer: An association in one case. Postgrad. Med. J. 1989, 65, 216–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanz, P.; Reig, R. Clinical and pathological findings in fatal plant oxalosis. A review. Am. J. Forensic Med. Pathol. 1992, 13, 342–345. [Google Scholar] [CrossRef]
- Qu, K.; Shen, N.-Y.; Xu, X.-S.; Su, H.-B.; Wei, J.-C.; Tai, M.-H.; Meng, F.-D.; Zhou, L.; Zhang, Y.-L.; Liu, C. Emodin induces human T cell apoptosis in vitro by ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction. Acta Pharmacol. Sin. 2013, 34, 1217–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brkanac, S.R.; Gerić, M.; Gajski, G.; Vujčić, V.; Garaj-Vrhovac, V.; Kremer, D.; Domijan, A.M. Toxicity and antioxidant capacity of Frangula alnus Mill. bark and its active component emodin. Regul. Toxicol. Pharmacol. RTP 2015, 73, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Ren, X.; Mason, A.S.; Liu, H.; Xiao, M.; Li, J.; Fu, D. Horizontal gene transfer in plants. Funct. Integr. Genom. 2014, 14, 23–29. [Google Scholar] [CrossRef] [PubMed]
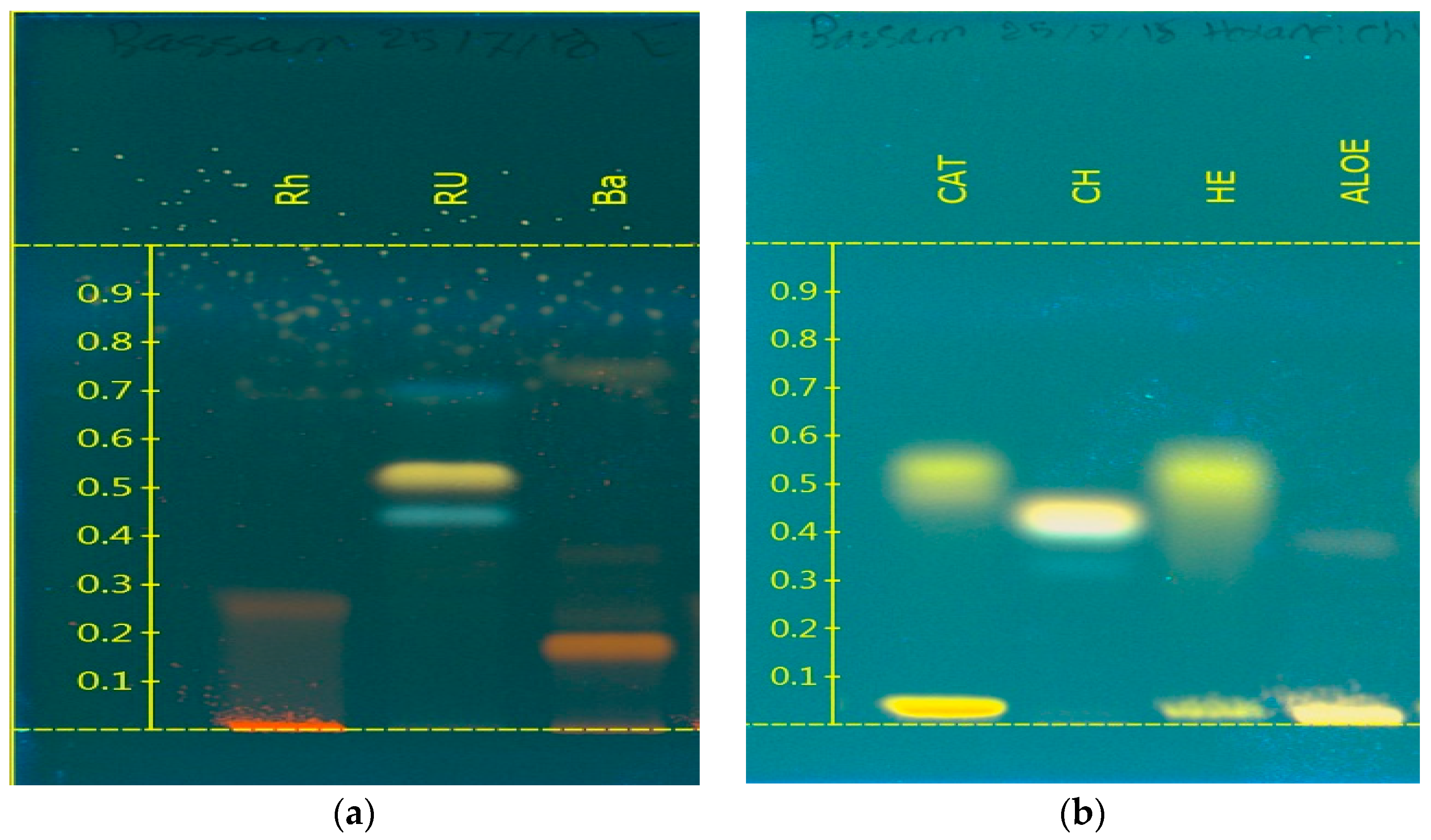
| Compounds | System 1 | System 2 |
|---|---|---|
| Rugulosin | 0.68, 0.74 and 0.79 | - |
| Rhein | 0.38 | - |
| Barbaloin | 0.28 and 0.79 | - |
| Aloe-emodin | - | 0.10 |
| Catenarin | - | 0.35 and 0.44 |
| Chrysazin | - | 0.14 |
| Helminthosporin | - | 0.22 and 0.30 |
| Position | Experimental (500 MHz, DMSO-d6) | Prediction (ChemDraw) (300 MHz, DMSO-d6) | Literature Data [89] (500 MHz, DMSO-d6) | ||||
|---|---|---|---|---|---|---|---|
| 1H | Peak Shape | 13C | 1H | 13C | 1H | 13C | |
| 1 | -- | -- | 161.62 | -- | 162.1 | -- | 162.3 |
| 2 | 7.29 | S | 120.64 | 7.11 | 120.6 | 7.29 | 121.4 |
| 3 | -- | -- | 153.9 | -- | 153.2 | -- | 154.4 |
| 4 | 4.62 | S | 62.12 | 4.61 | 65.0 | 4.62 | 62.7 |
| 5 | 7.69 | S | 117.01 | 7.34 | 118.3 | 7.69 | 117.8 |
| 6 | -- | -- | 133.09 | -- | 133.6 | -- | 133.9 |
| 7 | -- | -- | 114.48 | -- | 115.2 | -- | 115.2 |
| 8 | -- | -- | 191.62 | -- | 188.0 | -- | 192.4 |
| 9 | -- | -- | 115.91 | -- | 116.3 | -- | 116.7 |
| 10 | -- | -- | 133.31 | -- | 133.1 | -- | 134.1 |
| 11 | -- | -- | 181.46 | -- | 182.1 | -- | 182.2 |
| 12 | 7.71 | D | 119.34 | 7.74 | 119.4 | 7.72 | 120.0 |
| 13 | 7.80 | T | 137.74 | 7.65 | 136.2 | 7.80 | 138.0 |
| 14 | 7.38 | D | 124.38 | 7.06 | 124.1 | 7.38 | 125.1 |
| 15 | -- | -- | 161.33 | -- | 161.9 | -- | 162.0 |
| Position | Experimental (500 MHz, DMSO-d6) | Prediction (ChemDraw) (300 MHz, DMSO-d6) | Literature Data [90] (400 MHz, DMSO-d6) | ||||
|---|---|---|---|---|---|---|---|
| 1H | Peak Shape | 13C | 1H | 13C | 1H | 13C | |
| 1 | -- | -- | 164.43 | -- | 163.3 | NO Data | |
| 2 | 6.58 | ds | 108.19 | 6.48 | 107.0 | 6.66 | |
| 3 | -- | -- | 165.51 | -- | 164.5 | ||
| 4 | -- | -- | 108.97 | -- | 108.9 | ||
| 5 | -- | -- | 134.70 | -- | 136.4 | ||
| 6 | 7.13 | ds | 108.30 | 6.75 | 108.3 | 7.13 | |
| 7 | -- | -- | 186.0 | -- | 186.4 | ||
| 8 | -- | -- | 110.07 | -- | 111.7 | ||
| 9 | -- | -- | 111.43 | -- | 111.7 | ||
| 10 | -- | -- | 187.71 | -- | 188.0 | ||
| 11 | -- | -- | 156.04 | -- | 157.4 | ||
| 12 | 7.24 | s | 129.09 | 6.82 | 129.4 | 7.32 | |
| 13 | -- | -- | 139.75 | -- | 140.9 | ||
| 14 | -- | -- | 156.74 | -- | 158.0 | ||
| 15 | 2.25 | S | 15.87 | 2.15 | 15.4 | 2.35 | |
| Position | Experimental (500 MHz, DMSO-d6) | Prediction (ChemDraw) (300 MHz, DMSO-d6) | Literature Data [91] (500 MHz, DMSO-d6) | ||||
|---|---|---|---|---|---|---|---|
| 1H | Peak Shape | 13C | 1H | 13C | 1H | 13C | |
| 1 | 7.71 | DD | 119.29 | 7.74 | 119.4 | 7.70 | 120.2 |
| 2 | 7.81 | DD | 137.44 | 7.65 | 136.2 | 7.80 | 138.0 |
| 3 | 7.39 | DD | 124.39 | 7.06 | 124.1 | 7.37 | 125.1 |
| 4 | -- | -- | 161.30 | -- | 161.9 | -- | 163.0 |
| 5 | -- | -- | 115.94 | -- | 116.3 | -- | 116.7 |
| 6 | -- | -- | 133.28 | -- | 133.1 | -- | 134.4 |
| 7 | -- | -- | 181.38 | -- | 182.1 | -- | 182.0 |
| 8 | -- | -- | 133.28 | -- | 133.1 | -- | 134.4 |
| 9 | -- | -- | 115.94 | -- | 116.3 | -- | 116.7 |
| 10 | -- | -- | 192.01 | -- | 188.0 | -- | 193.4 |
| 11 | -- | -- | 161.30 | -- | 161.9 | -- | 163.0 |
| 12 | 7.39 | DD | 124.39 | 7.06 | 124.1 | 7.37 | 125.1 |
| 13 | 7.81 | DD | 137.44 | 7.65 | 136.2 | 7.80 | 138.0 |
| 14 | 7.71 | DD | 119.29 | 7.74 | 119.4 | 7.70 | 120.2 |
| Position | Experimental (500 MHz, DMSO-d6) | Prediction (ChemDraw) (300 MHz, DMSO-d6) | Literature Data [58] (500 MHz, DMSO-d6) | ||||
|---|---|---|---|---|---|---|---|
| 1H | Peak Shape | 13C | 1H | 13C | 1H | 13C | |
| 1 | -- | -- | 157.06 | -- | 157.3 | -- | 158.2 |
| 2 | 7.44 | S | 129.43 | 7.37 | 129.5 | 7.44 | 129.5 |
| 3 | 7.44 | S | 129.69 | 7.37 | 129.5 | 7.44 | 129.6 |
| 4 | -- | -- | 156.39 | -- | 157.3 | -- | 157.6 |
| 5 | -- | -- | 112.71 | -- | 114.7 | -- | 112.8 |
| 6 | -- | -- | 112.55 | -- | 114.7 | -- | 112.5 |
| 7 | -- | -- | 189.94 | -- | 188.0 | -- | 190.6 |
| 8 | -- | -- | 113.84 | -- | 113.3 | -- | 114.0 |
| 9 | -- | -- | 132.88 | - | 133.3 | -- | 133.2 |
| 10 | -- | -- | 186.36 | -- | 185.5 | -- | 186.6 |
| 11 | 7.64 | S | 120.29 | 7.22 | 120.2 | 7.65 | 120.8 |
| 12 | -- | -- | 149.14 | -- | 147.6 | -- | 149.1 |
| 13 | 2.46 | S | 21.63 | 2.36 | 21.6 | 2.45 | 22.3 |
| 14 | 7.26 | S | 124.3 | 6.66 | 122.7 | 7.26 | 124.6 |
| 15 | -- | -- | 161.68 | -- | 161.8 | -- | 162.8 |
| Position | Experimental (500 MHz, DMSO-d6) | Prediction (ChemDraw) (300 MHz, DMSO-d6) | Literature Data [92] (500 MHz, DMSO-d6) | ||||
|---|---|---|---|---|---|---|---|
| 1H | Peak Shape | 13C | 1H | 13C | 1H | 13C | |
| 1 | -- | -- | 165.55 | -- | 169.3 | -- | 165.52 |
| 2 | -- | -- | 128.01 | -- | 135.0 | -- | 138.20 |
| 3 | 7.76 | DS | 123.93 | 7.81 | 124.5 | 7.77 | 124.21 |
| 4 | -- | -- | 161.10 | -- | 160.1 | -- | 161.51 |
| 5 | -- | -- | 119.6 | -- | 121.5 | -- | 118.48 |
| 6 | -- | -- | 132.95 | -- | 131.5 | -- | 133.61 |
| 7 | 8.13 | DS | 118.03 | 7.56 | 120.6 | 8.14 | 119.05 |
| 8 | -- | -- | 181.7 | -- | 182.1 | -- | 181.25 |
| 9 | -- | -- | 134.8 | -- | 133.1 | -- | 133.41 |
| 10 | -- | -- | 120.63 | -- | 116.3 | -- | 116.33 |
| 11 | -- | -- | 187.38 | -- | 188.0 | -- | 191.49 |
| 12 | -- | -- | 158.31 | -- | 161.9 | -- | 161.27 |
| 13 | 7.74 | DD | 122.42 | 7.06 | 124.1 | 7.41 | 124.64 |
| 14 | 7.91 | -- | 136.26 | 7.65 | 136.2 | 7.84 | 137.63 |
| 15 | 7.90 | -- | 120.57 | 7.74 | 119.4 | 7.75 | 119.48 |
| Compound | GI50 (µg/mL) | GI50 (µM) | (CI 95%) |
|---|---|---|---|
| Aloe-emodin | 55.34 | 204.8 | (174.1–237.6) |
| Catenarin | 27.29 | 95.3 | (81.0–110.5) |
| Chrysazin | 15.26 | 63.5 | (54.0–73.7) |
| Helminthosporin | 52.91 | 195.8 | (166.4–227.1) |
| Rhein | 49.55 | 174.3 | (148.2–202.2) |
| Paclitaxel | 1.78 | 2.1 | (1.8–2.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Kazman, B.S.M.; Prieto, J.M. Friends or Foes? Cytotoxicity, HPTLC and NMR Analyses of Some Important Naturally Occurring Hydroxyanthraquinones. Nutraceuticals 2021, 1, 13-30. https://doi.org/10.3390/nutraceuticals1010004
Al Kazman BSM, Prieto JM. Friends or Foes? Cytotoxicity, HPTLC and NMR Analyses of Some Important Naturally Occurring Hydroxyanthraquinones. Nutraceuticals. 2021; 1(1):13-30. https://doi.org/10.3390/nutraceuticals1010004
Chicago/Turabian StyleAl Kazman, Bassam S. M., and Jose M. Prieto. 2021. "Friends or Foes? Cytotoxicity, HPTLC and NMR Analyses of Some Important Naturally Occurring Hydroxyanthraquinones" Nutraceuticals 1, no. 1: 13-30. https://doi.org/10.3390/nutraceuticals1010004
APA StyleAl Kazman, B. S. M., & Prieto, J. M. (2021). Friends or Foes? Cytotoxicity, HPTLC and NMR Analyses of Some Important Naturally Occurring Hydroxyanthraquinones. Nutraceuticals, 1(1), 13-30. https://doi.org/10.3390/nutraceuticals1010004







