Effects of Zembrin® (Sceletium tortuosum) Supplementation on Mood, Soreness, and Performance Following Unaccustomed Resistance Exercise: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Supplementation
2.4. Muscle Damage Biomarker (Plasma Lactate Dehydrogenase Activity)
2.5. Procedures
2.6. Data Analysis
3. Results
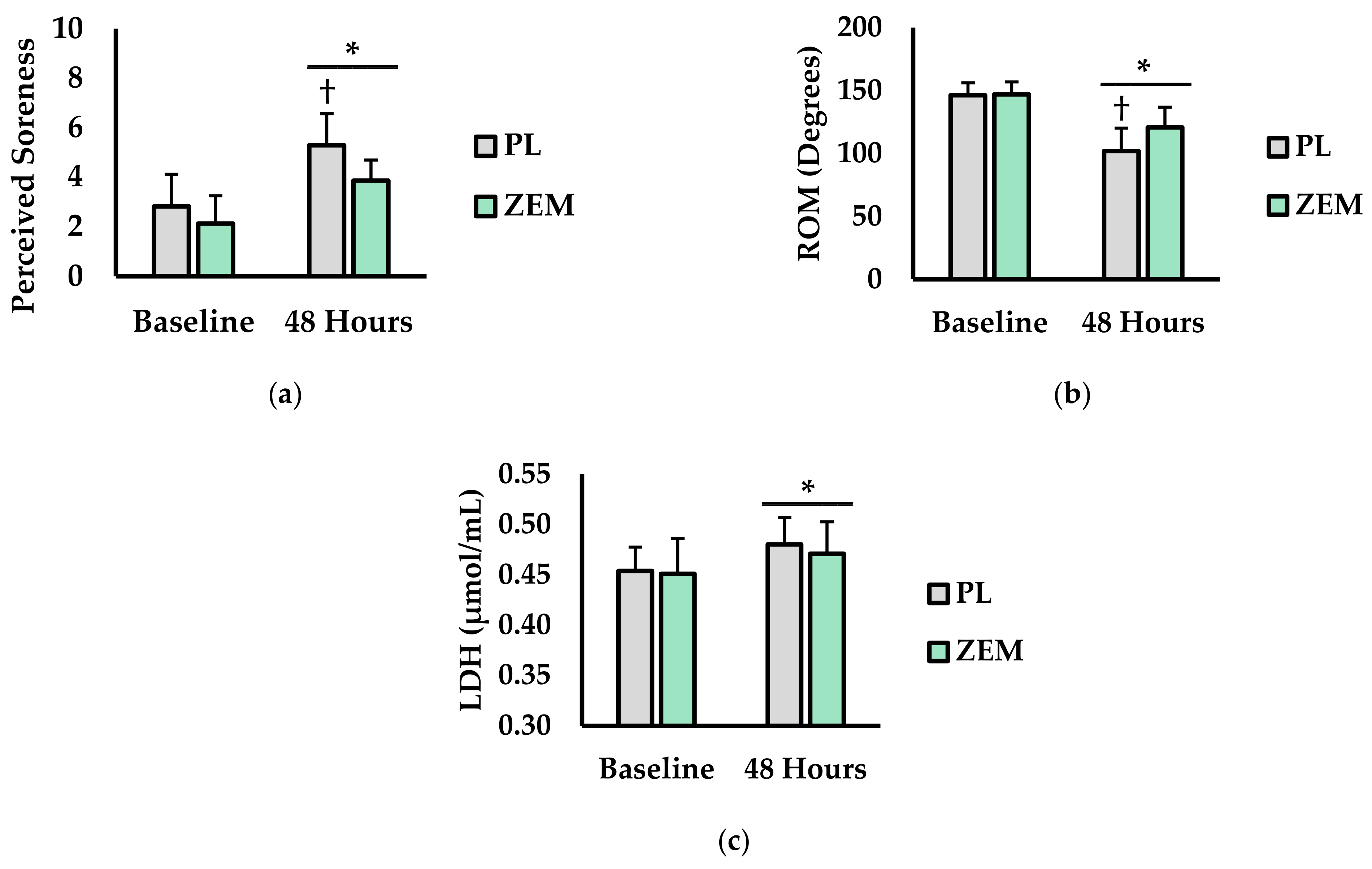
3.1. Perceived Soreness, ROM, and Plasma LDH Levels
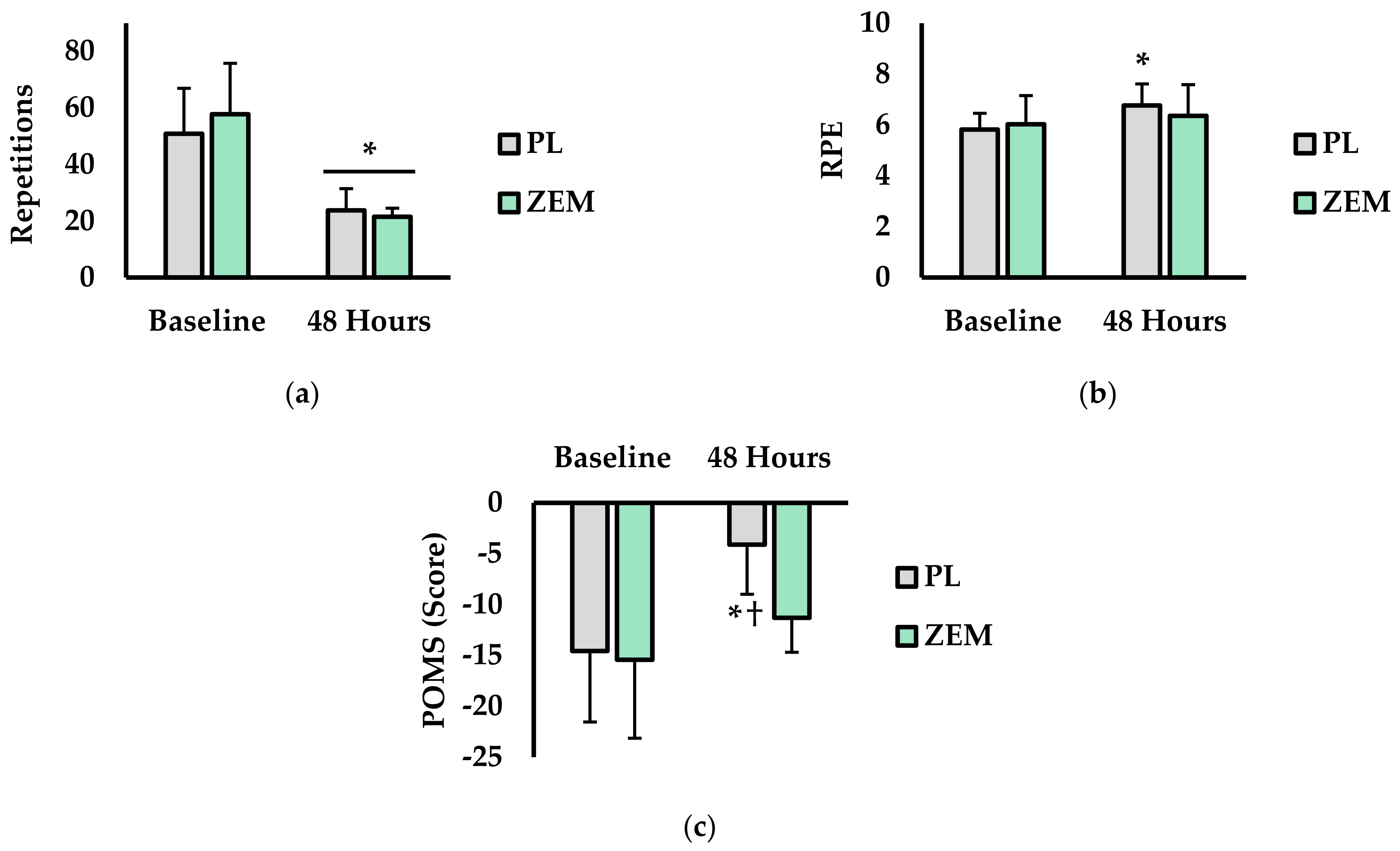
3.2. Total Repetitions, RPE, and Mood
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gericke, O.; Gericke, N.; Stein, D.J. Sceletium Tortuosum; American Psychiatric Association Publishing: Arlington, VA, USA, 2017. [Google Scholar]
- Gericke, N.; Viljoen, A.M. Sceletium—A review update. J. Ethnopharmacol. 2008, 119, 653–663. [Google Scholar] [CrossRef]
- Terburg, D.; Syal, S.; Rosenberger, L.A.; Heany, S.; Phillips, N.; Gericke, N.; Stein, D.J.; van Honk, J. Acute effects of Sceletium tortuosum (Zembrin), a dual 5-HT reuptake and PDE4 inhibitor, in the human amygdala and its connection to the hypothalamus. Neuropsychopharmacology 2013, 38, 2708–2716. [Google Scholar] [CrossRef]
- Reay, J.; Wetherell, M.A.; Morton, E.; Lillis, J.; Badmaev, V. Sceletium tortuosum (Zembrin®) ameliorates experimentally induced anxiety in healthy volunteers. Hum. Psychopharmacol. Clin. Exp. 2020, 35, 1–7. [Google Scholar] [CrossRef]
- Dimpfel, W.; Gericke, N.; Suliman, S.; Dipah, G.N.C. Effect of Zembrin® on Brain Electrical Activity in 60 Older Subjects after 6 Weeks of Daily Intake. A Prospective, Randomized, Double-Blind, Placebo-Controlled, 3-Armed Study in a Parallel Design. World J. Neurosci. 2016, 7, 140–171. [Google Scholar] [CrossRef] [Green Version]
- Hardy, L. Stress, anxiety and performance. J. Sci. Med. Sport 1999, 2, 227–233. [Google Scholar] [CrossRef]
- George, S.Z.; Dover, G.C.; Fillingim, R.B. Fear of pain influences outcomes after exercise-induced delayed onset muscle soreness at the shoulder. Clin. J. Pain 2007, 23, 76–84. [Google Scholar] [CrossRef]
- Harvey, A.L.; Young, L.C.; Viljoen, A.M.; Gericke, N.P. Pharmacological actions of the South African medicinal and functional food plant Sceletium tortuosum and its principal alkaloids. J. Ethnopharmacol. 2011, 137, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Cheng, Y.; Wang, C.; Wu, J.; Zou, Z.; Niu, B.; Yu, H.; Wang, H.; Xu, J. FFPM, a PDE4 inhibitor, reverses learning and memory deficits in APP/PS1 transgenic mice via cAMP/PKA/CREB signaling and anti-inflammatory effects. Neuropharmacology 2017, 116, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Hirose, R.; Manabe, H.; Yanagawa, K.; Ohshima, E.; Ichimura, M. Differential effects of PDE4 inhibitors on cortical neurons and T-lymphocytes. J. Pharmacol. Sci. 2008, 106, 310–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, M.; Richards, E.M.; Niciu, M.J.; Ionescu, D.F.; Zoghbi, S.S.; Hong, J.; Telu, S.; Hines, C.S.; Pike, V.W.; Zarate, C.A. cAMP signaling in brain is decreased in unmedicated depressed patients and increased by treatment with a selective serotonin reuptake inhibitor. Mol. Psychiatry 2017, 22, 754–759. [Google Scholar] [CrossRef] [Green Version]
- Napoletano, M.; Fraire, C.; Santangelo, F.; Moriggi, E. Mesembrine Is an Inhibitor of PDE4 That Follows Structure-Activity Relationship of Rolipram. 2001. Available online: https://ssrn.com/abstract=2969480 (accessed on 16 September 2021).
- Coetzee, D.D.; López, V.; Smith, C. High-mesembrine Sceletium extract (Trimesemine™) is a monoamine releasing agent, rather than only a selective serotonin reuptake inhibitor. J. Ethnopharmacol. 2016, 177, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Pringle, A.; Browning, M.; Cowen, P.; Harmer, C. A cognitive neuropsychological model of antidepressant drug action. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 1586–1592. [Google Scholar] [CrossRef]
- Cashman, J.R.; Voelker, T.; Johnson, R.; Janowsky, A. Stereoselective inhibition of serotonin re-uptake and phosphodiesterase by dual inhibitors as potential agents for depression. Bioorg. Med. Chem. 2009, 17, 337–343. [Google Scholar] [CrossRef]
- Bennett, A.C.; Smith, C. Immunomodulatory effects of Sceletium tortuosum (Trimesemine™) elucidated in vitro: Implications for chronic disease. J. Ethnopharmacol. 2018, 214, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Loria, M.J.; Ali, Z.; Abe, N.; Sufka, K.J.; Khan, I.A. Effects of Sceletium tortuosum in rats. J. Ethnopharmacol. 2014, 155, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.; Gericke, N.; Farina-Woodbury, M.; Badmaev, V.; Raheb, H.; Terpstra, K.; Antongiorgi, J.; Bureau, Y.; Cernovsky, Z.; Hou, J. Proof-of-concept randomized controlled study of cognition effects of the proprietary extract sceletium tortuosum (Zembrin) targeting phosphodiesterase-4 in cognitively healthy subjects: Implications for Alzheimer’s dementia. Evid.-Based Complement. Altern. Med. 2014, 2014, 682014. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Markus, I.; Dubnov-Raz, G.; Gepner, Y. Ergogenic effects of 8 Days of sceletium tortuosum supplementation on mood, visual tracking, and reaction in recreationally trained men and women. J. Strength Cond. Res. 2020, 34, 2476–2481. [Google Scholar] [CrossRef]
- McLemore, B.H.; McLemore, S.G.; Rogers, R.R.; Pederson, J.A.; Williams, T.D.; Marshall, M.R.; Ballmann, C.G. Nocebo Effects on Perceived Muscle Soreness and Exercise Performance Following Unaccustomed Resistance Exercise: A Pilot Study. J. Funct. Morphol. Kinesiol. 2020, 5, 40. [Google Scholar] [CrossRef]
- Smith, L.L. Causes of delayed onset muscle soreness and the impact on athletic performance: A review. J. Strength Cond. Res. 1992, 6, 135–141. [Google Scholar]
- Raglin, J.S. Psychological factors in sport performance. Sports Med. 2001, 31, 875–890. [Google Scholar] [CrossRef]
- Riebe, D.; Ehrman, J.K.; Liguori, G.; Magal, M. ACSM’s Guidelines for Exercise Testing and Prescription; Wolters Kluwer: Riverwood, IL, USA, 2018. [Google Scholar]
- Ballmann, C.G.; Maze, S.B.; Wells, A.C.; Marshall, M.M.; Rogers, R.R. Effects of short-term Rhodiola Rosea (Golden Root Extract) supplementation on anaerobic exercise performance. J. Sports Sci. 2019, 37, 998–1003. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Y.; Liu, G. Protective effect of Barbaloin in a rat model of myocardial ischemia reperfusion injury through the regulation of the CNPY2-PERK pathway. Int. J. Mol. Med. 2019, 43, 2015–2023. [Google Scholar] [CrossRef]
- Ustunova, S.; Takir, S.; Yilmazer, N.; Bulut, H.; Altindirek, D.; Ng, O.H.; Tansel, C.D.; Dogan, B.S.U.; Ozbek, U.; Armutak, E.I. Hydrogen sulphide and nitric oxide cooperate in cardioprotection against ischemia/reperfusion injury in isolated rat heart. In Vivo 2020, 34, 2507–2516. [Google Scholar] [CrossRef] [PubMed]
- Grove, J.R.; Prapavessis, H. Preliminary evidence for the reliability and validity of an abbreviated profile of mood states. Int. J. Sport Psychol. 1992, 4, 45. [Google Scholar]
- Covington, A.C.; Rogers, R.R.; Kopec, T.J.; Ballmann, C.G. The Effect of Walking an Unfamiliar Versus Companion Dog on Mood, Exercise Enjoyment, and Heart Rate: A Pilot Field. Top. Exerc. Sci. Kinesiol. 2021, 2, 3. [Google Scholar]
- Allen, J.D.; Mattacola, C.G.; Perrin, D.H. Effect of microcurrent stimulation on delayed-onset muscle soreness: A double-blind comparison. J. Athl. Train. 1999, 34, 334. [Google Scholar] [PubMed]
- Connolly, D.A.; Sayers, S.P.; McHugh, M.P. Treatment and prevention of delayed onset muscle soreness. J. Strength Cond. Res. 2003, 17, 197–208. [Google Scholar]
- Wei, J.; Carroll, R.J.; Harden, K.K.; Wu, G. Comparisons of treatment means when factors do not interact in two-factorial studies. Amino Acids 2012, 42, 2031–2035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum Associates: Hillsdale, MI, USA, 1988. [Google Scholar]
- Megale, R.Z.; Deveza, L.A.; Blyth, F.M.; Naganathan, V.; Ferreira, P.H.; McLachlan, A.J.; Ferreira, M.L. Efficacy and safety of oral and transdermal opioid analgesics for musculoskeletal pain in older adults: A systematic review of randomized, placebo-controlled trials. J. Pain 2018, 19, 475.e1–475.e24. [Google Scholar] [CrossRef]
- Kindler, S.; Samietz, S.; Houshmand, M.; Grabe, H.J.; Bernhardt, O.; Biffar, R.; Kocher, T.; Meyer, G.; Völzke, H.; Metelmann, H.-R. Depressive and anxiety symptoms as risk factors for temporomandibular joint pain: A prospective cohort study in the general population. J. Pain 2012, 13, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Reif, M.; Field, T.; Krasnegor, J.; Theakston, H. Lower back pain is reduced and range of motion increased after massage therapy. Int. J. Neurosci. 2001, 106, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Kent, J.M.; Coplan, J.D.; Gorman, J.M. Clinical utility of the selective serotonin reuptake inhibitors in the spectrum of anxiety. Biol. Psychiatry 1998, 44, 812–824. [Google Scholar] [CrossRef]
- Izumi, T.; Kitaichi, Y.; An, Y.; Inoue, T.; Yoshioka, M. The Amygdala Is the Target Brain Site of Anxiolytic Effects of SSRIs; Nova Biomedical Books: Hauppauge, NY, USA, 2018. [Google Scholar]
- Peñailillo, L.; Blazevich, A.; Numazawa, H.; Nosaka, K. Rate of force development as a measure of muscle damage. Scand. J. Med. Sci. Sports 2015, 25, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Morgan, W.P.; Costill, D.L.; Flynn, M.G.; Raglin, J.S.; O’Connor, P.J. Mood disturbance following increased training in swimmers. Med. Sci. Sports Exerc. 1988, 44, 125. [Google Scholar] [CrossRef]
- Hieronymus, F.; Lisinski, A.; Nilsson, S.; Eriksson, E. Influence of baseline severity on the effects of SSRIs in depression: An item-based, patient-level post-hoc analysis. Lancet Psychiatry 2019, 6, 745–752. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berry, A.R.; Langley, H.N.; Rogers, R.R.; Benjamin, C.L.; Williams, T.D.; Ballmann, C.G. Effects of Zembrin® (Sceletium tortuosum) Supplementation on Mood, Soreness, and Performance Following Unaccustomed Resistance Exercise: A Pilot Study. Nutraceuticals 2021, 1, 2-11. https://doi.org/10.3390/nutraceuticals1010002
Berry AR, Langley HN, Rogers RR, Benjamin CL, Williams TD, Ballmann CG. Effects of Zembrin® (Sceletium tortuosum) Supplementation on Mood, Soreness, and Performance Following Unaccustomed Resistance Exercise: A Pilot Study. Nutraceuticals. 2021; 1(1):2-11. https://doi.org/10.3390/nutraceuticals1010002
Chicago/Turabian StyleBerry, Angela R., Haley N. Langley, Rebecca R. Rogers, Courteney L. Benjamin, Tyler D. Williams, and Christopher G. Ballmann. 2021. "Effects of Zembrin® (Sceletium tortuosum) Supplementation on Mood, Soreness, and Performance Following Unaccustomed Resistance Exercise: A Pilot Study" Nutraceuticals 1, no. 1: 2-11. https://doi.org/10.3390/nutraceuticals1010002
APA StyleBerry, A. R., Langley, H. N., Rogers, R. R., Benjamin, C. L., Williams, T. D., & Ballmann, C. G. (2021). Effects of Zembrin® (Sceletium tortuosum) Supplementation on Mood, Soreness, and Performance Following Unaccustomed Resistance Exercise: A Pilot Study. Nutraceuticals, 1(1), 2-11. https://doi.org/10.3390/nutraceuticals1010002







