Associations Between 10-Year Physical Performance and Activities of Daily Living Trajectories and Physical Behaviors in Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Adult Changes in Thought (ACT) Cohort
2.2. Physical Performance Exposure
2.3. Activities of Daily Living (ADL) Exposure
2.4. Physical Behavior Outcome Variables
2.5. Demographic and Health Characteristics
2.6. Statistical Methods
3. Results
3.1. Physical Performance Trajectories
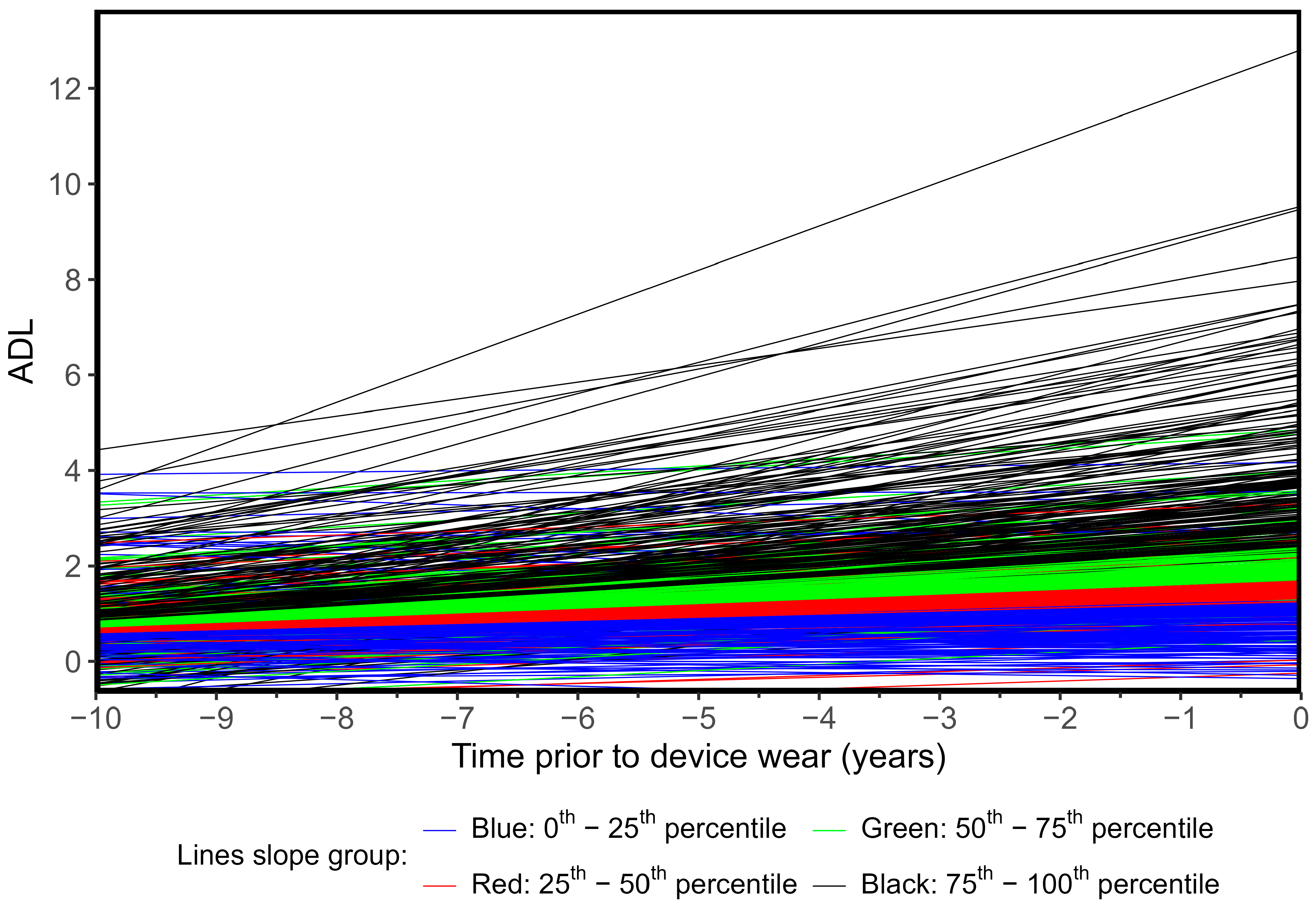
3.2. Activities of Daily Living Trajectories
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, R.Y. A New Strategic Approach to Successful Aging and Healthy Aging. Geriatrics 2018, 3, 86. [Google Scholar] [CrossRef]
- Rosenberger, M.E.; Fulton, J.E.; Buman, M.P.; Troiano, R.P.; Grandner, M.A.; Buchner, D.M.; Haskell, W.L. The 24-Hour Activity Cycle: A New Paradigm for Physical Activity. Med. Sci. Sports Exerc. 2019, 51, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Wingood, M.; Bonnell, L.; LaCroix, A.Z.; Rosenberg, D.; Walker, R.; Bellettiere, J.; Greenwood-Hickman, M.A.; Wing, D.; Gell, N. Community-Dwelling Older Adults and Physical Activity Recommendations: Patterns of Aerobic, Strengthening, and Balance Activities. J. Aging Phys. Act. 2022, 30, 653–665. [Google Scholar] [CrossRef]
- Rosenberg, D.; Walker, R.; Greenwood-Hickman, M.A.; Bellettiere, J.; Xiang, Y.; Richmire, K.; Higgins, M.; Wing, D.; Larson, E.B.; Crane, P.K.; et al. Device-assessed physical activity and sedentary behavior in a community-based cohort of older adults. BMC Public Health 2020, 20, 1256. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Rosenberg, D.E.; Greenwood-Hickman, M.A.; McCurry, S.M.; Proust-Lima, C.; Nelson, J.C.; Crane, P.K.; LaCroix, A.Z.; Larson, E.B.; Shaw, P.A. Analysis of the 24-h activity cycle: An illustration examining the association with cognitive function in the Adult Changes in Thought study. Front. Psychol. 2023, 14, 1083344. [Google Scholar] [CrossRef]
- Greenwood-Hickman, M.A.; Walker, R.L.; Idu, A.E.; Bellettiere, J.; Wing, D.; McCurry, S.M.; Crane, P.K.; Larson, E.B.; Rosenberg, D.E.; LaCroix, A.Z. Current and historic patterns of chronic disease burden are associated with physical activity and sedentary behavior in older adults: An observational study. BMC Public Health 2025, 25, 1032. [Google Scholar] [CrossRef] [PubMed]
- Makino, K.; Lee, S.; Lee, S.; Bae, S.; Jung, S.; Shinkai, Y.; Shimada, H. Daily Physical Activity and Functional Disability Incidence in Community-Dwelling Older Adults with Chronic Pain: A Prospective Cohort Study. Pain. Med. Malden Mass. 2019, 20, 1702–1710. [Google Scholar] [CrossRef]
- He, L.; Rabinowitz, J.A.; An, Y.; Jackson, C.; Hellinger, R.; Wanigatunga, S.; Schrack, J.; Ferrucci, L.; Simonsick, E.M.; Koehler, K.; et al. Age and objectively measured sleep: Investigating associations and interactions by sex and race in middle-aged and older adults. Sleep Adv. J. Sleep Res. Soc. 2024, 5, zpae045. [Google Scholar] [CrossRef]
- Li, J.; Vitiello, M.V.; Gooneratne, N. Sleep in Normal Aging. Sleep. Med. Clin. 2018, 13, 1–11. [Google Scholar] [CrossRef]
- Marzetti, E.; Calvani, R.; Tosato, M.; Troiano, R.P.; Grandner, M.A.; Buchner, D.M.; Haskell, W.L. Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging Clin. Exp. Res. 2017, 29, 35–42. [Google Scholar] [CrossRef]
- Bogen, B.; Aaslund, M.K.; Ranhoff, A.H.; Taraldsen, K.; Moe-Nilssen, R. The Association between Daily Walking Behavior and Self-Reported Physical Function in Community-Dwelling Older Adults. J. Frailty Aging 2017, 6, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Chmelo, E.; Nicklas, B.; Davis, C.; Miller, G.D.; Legault, C.; Messier, S. Physical Activity and Physical Function in Older Adults With Knee Osteoarthritis. J. Phys. Act. Health 2013, 10, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Dondzila, C.J.; Gennuso, K.P.; Swartz, A.M.; Tarima, S.; Lenz, E.K.; Stein, S.S.; Kohl, R.J.; Strath, S.J. Dose-response walking activity and physical function in older adults. J. Aging Phys. Act. 2015, 23, 194–199. [Google Scholar] [CrossRef]
- Copeland, J.L.; Ashe, M.C.; Biddle, S.J.; Brown, W.J.; Buman, M.P.; Chastin, S.; Gardiner, P.A.; Inoue, S.; Jefferis, B.J.; Oka, K.; et al. Sedentary time in older adults: A critical review of measurement, associations with health, and interventions. Br. J. Sports Med. 2017, 51, 1539. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.G.; Fox, K.R.; Stathi, A.; Trayers, T.; Thompson, J.L.; Cooper, A.R. Objectively measured sedentary time and its association with physical function in older adults. J. Aging Phys. Act. 2014, 22, 474–481. [Google Scholar] [CrossRef]
- Reuter, C.; Bellettiere, J.; Liles, S.; Di, C.; Sears, D.D.; LaMonte, M.J.; Stefanick, M.L.; LaCroix, A.Z.; Natarajan, L. Diurnal patterns of sedentary behavior and changes in physical function over time among older women: A prospective cohort study. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 88. [Google Scholar] [CrossRef]
- Schrack, J.A.; Kuo, P.L.; Wanigatunga, A.A.; Di, J.; Simonsick, E.M.; Spira, A.P.; Ferrucci, L.; Zipunnikov, V. Active-to-Sedentary Behavior Transitions, Fatigability, and Physical Functioning in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 560–567. [Google Scholar] [CrossRef]
- Walker, R.L.; Greenwood-Hickman, M.A.; Bellettiere, J.; LaCroix, A.Z.; Wing, D.; Higgins, M.; Richmire, K.; Larson, E.B.; Crane, P.K.; Rosenberg, D.E. Associations between physical function and device-based measures of physical activity and sedentary behavior patterns in older adults: Moving beyond moderate-to-vigorous intensity physical activity. BMC Geriatr. 2021, 21, 216. [Google Scholar] [CrossRef]
- Arias-Fernández, L.; Smith-Plaza, A.M.; Barrera-Castillo, M.; Prado-Suárez, J.; Lopez-Garcia, E.; Rodríguez-Artalejo, F.; Lana, A. Sleep patterns and physical function in older adults attending primary health care. Fam. Pract. 2021, 38, 146–152. [Google Scholar] [CrossRef]
- Lorenz, R.A.; Budhathoki, C.B.; Kalra, G.K.; Richards, K.C. The relationship between sleep and physical function in community-dwelling adults: A pilot study. Fam. Community Health 2014, 37, 298–306. [Google Scholar] [CrossRef]
- Thorpe, R.J.; Gamaldo, A.A.; Salas, R.E.; Gamaldo, C.E.; Whitfield, K.E. Relationship between Physical Function and Sleep Quality in African Americans. J. Clin. Sleep. Med. JCSM Off. Publ. Am. Acad. Sleep. Med. 2016, 12, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Campanini, M.Z.; Mesas, A.E.; Carnicero-Carreño, J.A.; Rodríguez-Artalejo, F.; Lopez-Garcia, E. Duration and Quality of Sleep and Risk of Physical Function Impairment and Disability in Older Adults: Results from the ENRICA and ELSA Cohorts. Aging Dis. 2019, 10, 557–569. [Google Scholar] [CrossRef]
- Baniak, L.M.; Yang, K.; Choi, J.; Chasens, E.R. Long Sleep Duration Is Associated with Increased Frailty Risk In Older Community-Dwelling Adults. J. Aging Health 2020, 32, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Nakakubo, S.; Makizako, H.; Doi, T.; Tsutsumimoto, K.; Hotta, R.; Lee, S.; Bae, S.; Makino, K.; Suzuki, T.; Shimada, H. Long and Short Sleep Duration and Physical Frailty in Community-Dwelling Older Adults. J. Nutr. Health Aging 2018, 22, 1066–1071. [Google Scholar] [CrossRef]
- Chien, M.Y.; Chen, H.C. Poor Sleep Quality is Independently Associated with Physical Disability in Older Adults. J. Clin. Sleep. Med. JCSM Off. Publ. Am. Acad. Sleep. Med. 2015, 11, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Vega Rde la Miró, J.; Esteve, R.; Ramírez-Maestre, C.; López-Martínez, A.E.; Jensen, M.P. Sleep disturbance in individuals with physical disabilities and chronic pain: The role of physical, emotional and cognitive factors. Disabil. Health J. 2019, 12, 588–593. [Google Scholar] [CrossRef]
- Wang, L.; van Belle, G.; Kukull, W.B.; Larson, E.B. Predictors of functional change: A longitudinal study of nondemented people aged 65 and older. J. Am. Geriatr. Soc. 2002, 50, 1525–1534. [Google Scholar] [CrossRef]
- Wang, L.; Larson, E.B.; Bowen, J.D.; van Belle, G. Performance-based physical function and future dementia in older people. Arch. Intern. Med. 2006, 166, 1115–1120. [Google Scholar] [CrossRef]
- McCurry, S.M.; Gibbons, L.E.; Bond, G.E.; Teri, L.; Kukull, W.A.; Higdon, R.; Bowen, J.D.; McCormick, W.C.; Larson, E.B.; Rice, M.M.; et al. Older adults and functional decline: A cross-cultural comparison. Int. Psychogeriatr. 2002, 14, 161–179. [Google Scholar] [CrossRef]
- Branch, L.G.; Katz, S.; Kniepmann, K.; Papsidero, J.A. A prospective study of functional status among community elders. Am. J. Public Health 1984, 74, 266–268. [Google Scholar] [CrossRef]
- Katz, S.; Akpom, C.A. A measure of primary sociobiological functions. Int. J. Health Serv. Plan. Adm. Eval. 1976, 6, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Nagi, S.Z. An epidemiology of disability among adults in the United States. Milbank Mem. Fund. Q. Health Soc. 1976, 54, 439–467. [Google Scholar] [CrossRef]
- Rosow, I.; Breslau, N. A Guttman health scale for the aged. J. Gerontol. 1966, 21, 556–559. [Google Scholar] [CrossRef]
- Evenson, K.R.; Wen, F.; Herring, A.H.; Di, C.; LaMonte, M.J.; Tinker, L.F.; Lee, I.-M.; Rillamas-Sun, E.; LaCroix, A.Z.; Buchner, D.M. Calibrating physical activity intensity for hip-worn accelerometry in women age 60 to 91 years: The Women’s Health Initiative OPACH Calibration Study. Prev. Med. Rep. 2015, 2, 750–756. [Google Scholar] [CrossRef]
- Buysse, D.J.; Yu, L.; Moul, D.E.; Germain, A.; Stover, A.; Dodds, N.E.; Johnston, K.L.; Shablesky-Cade, M.A.; Pilkonis, P.A. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep 2010, 33, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Buysse, D.J.; Germain, A.; Moul, D.E.; Stover, A.; Dodds, N.E.; Johnston, K.L.; Pilkonis, P.A. Development of short forms from the PROMISTM sleep disturbance and Sleep-Related Impairment item banks. Behav. Sleep. Med. 2011, 10, 6–24. [Google Scholar] [CrossRef]
- Lewinsohn, P.M.; Seeley, J.R.; Roberts, R.E.; Allen, N.B. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol. Aging 1997, 12, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Moser, N.; Sahiti, F.; Gelbrich, G.; Cejka, V.; Kerwagen, F.; Albert, J.; Frantz, S.; Heuschmann, P.U.; Störk, S.; Morbach, C. Association between self-reported and objectively assessed physical functioning in the general population. Sci. Rep. 2024, 14, 16236. [Google Scholar] [CrossRef]
- Wollesen, B.; Schott, N.; Klotzbier, T.; Bischoff, L.L.; Cordes, T.; Rudisch, J.; Otto, A.-K.; Zwingmann, K.; Hildebrand, C.; Joellenbeck, T.; et al. Cognitive, physical and emotional determinants of activities of daily living in nursing home residents—A cross-sectional study within the PROCARE-project. Eur. Rev. Aging Phys. Act. 2023, 20, 17. [Google Scholar] [CrossRef]
- Paluch, A.E.; Bajpai, S.; Bassett, D.R.; Carnethon, M.R.; Ekelund, U.; Evenson, K.R.; Galuska, D.A.; Jefferis, B.J.; Kraus, W.E.; Lee, I.-M.; et al. Daily steps and all-cause mortality: A meta-analysis of 15 international cohorts. Lancet Public Health 2022, 7, e219–e228. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, J.; Ferrari, G.; Rey-Lopez, J.P.; Rezende, L.F.M. Association of Physical Activity Intensity with Mortality: A National Cohort Study of 403 681 US Adults. JAMA Intern. Med. 2021, 181, 203–211. [Google Scholar] [CrossRef]
- Xu, C.; Jia, J.; Zhao, B.; Yuan, M.; Luo, N.; Zhang, F.; Wang, H. Objectively measured daily steps and health outcomes: An umbrella review of the systematic review and meta-analysis of observational studies. BMJ Open 2024, 14, e088524. [Google Scholar] [CrossRef] [PubMed]
- Young, D.R.; Haskell, W.L. Accumulation of Moderate-to-Vigorous Physical Activity and All-Cause Mortality. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2018, 7, e008929. [Google Scholar] [CrossRef]
- Vaughan, L.; Leng, X.; La Monte, M.J.; Tindle, H.A.; Cochrane, B.B.; Shumaker, S.A. Functional Independence in Late-Life: Maintaining Physical Functioning in Older Adulthood Predicts Daily Life Function after Age 80. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71 (Suppl. S1), S79–S86. [Google Scholar] [CrossRef] [PubMed]
- Maintaining Mobility and Preventing Disability Are Key to Living Independently as We Age. National Institute on Aging. 30 November 2020. Available online: https://www.nia.nih.gov/news/maintaining-mobility-and-preventing-disability-are-key-living-independently-we-age (accessed on 4 April 2025).
- Celis-Morales, C.A.; Perez-Bravo, F.; Ibañez, L.; Salas, C.; Bailey, M.E.S.; Gill, J.M.R. Objective vs. self-reported physical activity and sedentary time: Effects of measurement method on relationships with risk biomarkers. PLoS ONE 2012, 7, e36345. [Google Scholar] [CrossRef]
- Nelson, M.C.; Taylor, K.; Vella, C.A. Comparison of Self-Reported and Objectively Measured Sedentary Behavior and Physical Activity in Undergraduate Students. Meas. Phys. Educ. Exerc. Sci. 2019, 23, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Prince, S.A.; Cardilli, L.; Reed, J.L.; Saunders, T.J.; Kite, C.; Douillette, K.; Fournier, K.; Buckley, J.P. A comparison of self-reported and device measured sedentary behaviour in adults: A systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 31. [Google Scholar] [CrossRef]
- Boe, L.A.; Mossavar-Rahmani, Y.; Sotres-Alvarez, D.; Daviglus, M.L.; Durazo-Arvizu, R.A.; Thyagarajan, B.; Kaplan, R.C.; Shaw, P.A. Nutritional Blood Concentration Biomarkers in the Hispanic Community Health Study/Study of Latinos: Measurement Characteristics and Power. Am. J. Epidemiol. 2023, 192, 1288–1303. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]

| Activity Monitor ACT Cohort | sPPF: 0–8 | sPPF: 9+ | |
|---|---|---|---|
| N = 905 a | N = 334 a | N = 571 a | |
| N (%) Mean (SD) Median [Q1, Q3] | N (%) Mean (SD) Median [Q1, Q3] | N (%) Mean (SD) Median [Q1, Q3] | |
| Age (years) category, n (%) | |||
| 65–74 | 378 (41.8%) | 91 (27.2%) | 287 (50.3%) |
| 74–84 | 381 (42.1%) | 151 (45.2%) | 230 (40.3%) |
| 85+ | 146 (16.1%) | 92 (27.5%) | 54 (9.5%) |
| Age (years), mean (SD) | 77.6 (6.9) | 80.3 (7.4) | 76.0 (6.1) |
| Gender, n (%) | |||
| Female | 502 (55.5%) | 200 (59.9%) | 302 (52.9%) |
| Male | 403 (44.5%) | 134 (40.1%) | 269 (47.1%) |
| Race, n (%) | |||
| Asian | 28 (3.1%) | 13 (3.9%) | 15 (2.6%) |
| Black | 13 (1.4%) | 8 (2.4%) | 5 (0.9%) |
| White | 820 (90.6%) | 291 (87.1%) | 529 (92.6%) |
| Other or mixed b | 43 (4.8%) | 22 (6.6%) | 22 (3.9%) |
| Latino/Hispanic ethnicity, n (%) | 12 (1.3%) | 4 (1.2%) | 8 (1.4%) |
| Currently work for pay, n (%) | 168 (18.6%) | 46 (13.8%) | 122 (21.4%) |
| Education level 16+ years, n (%) | 677 (74.8%) | 224 (67.1%) | 453 (79.3%) |
| Live alone, n (%) | 308 (34.0%) | 131 (39.2%) | 177 (31.0%) |
| Self-rated health, n (%) | |||
| Excellent | 178 (19.7%) | 37 (11.1%) | 141 (24.7%) |
| Very good | 393 (43.4%) | 127 (38.0%) | 266 (46.6%) |
| Good | 271 (29.9%) | 128 (38.3%) | 143 (25.0%) |
| Fair/poor | 63 (7.0%) | 42 (12.6%) | 21 (3.7%) |
| Depressive symptoms CES-D Score ≥ 10, n (%) b | 77 (8.5%) | 37 (11.1%) | 40 (7.0%) |
| Depressive symptoms (CES-D) b | 2 [1, 5] | 3 [1, 6] | 2 [0, 5] |
| Charlson Comorbidity Index | 0 [0, 2] | 1 [0, 2] | 0 [0, 1] |
| Activity of Daily Living (ADL) score | 1 [0, 2] | 2 [1, 4] | 0 [0, 1] |
| Short Performance-Based Physical Function (sPPF) score | 9 [7, 11] | 7 [5, 8] | 10 [9, 11] |
| CASI score, mean (SD) | 0.6 (1.0) | 0.3 (1.0) | 0.7 (0.9) |
| BMI, Mean (SD) | 26.9 (4.8) | 27.5 (5.5) | 26.6 (4.3) |
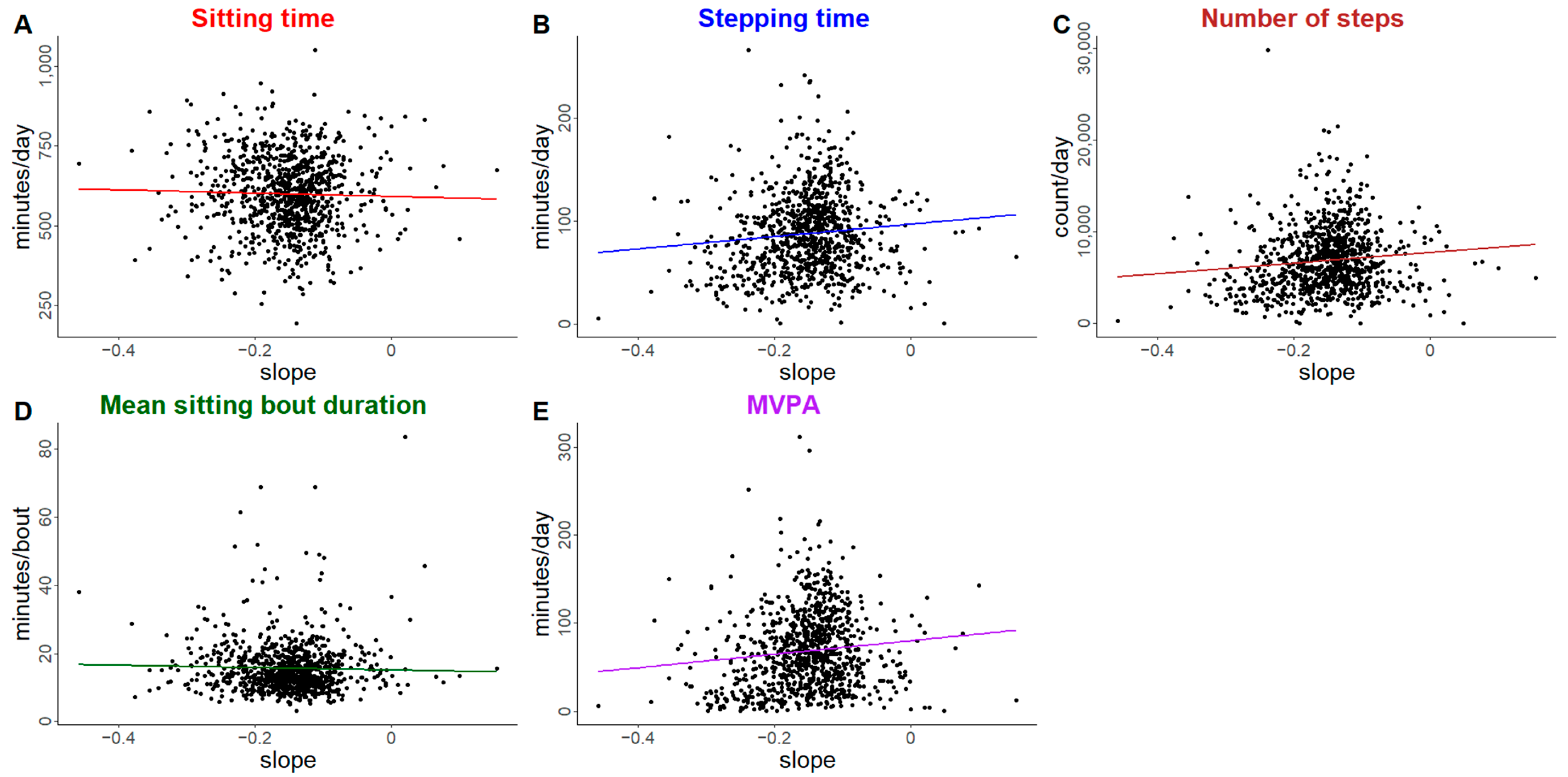
| Outcome | Individual Trajectory Features | Est b | 95% CI c |
|---|---|---|---|
| ActivPAL | |||
| Sitting time (min/day) † | Intercept (baseline) | 6.6 | (1.2, 11.7) |
| Slope | 6.6 | (−32.1, 52.0) | |
| Standing time (min/day) † | Intercept (baseline) | −2.5 | (−7.0, 2.0) |
| Slope | 5.3 | (−29.1, 38.7) | |
| Stepping time (min/day) † | Intercept (baseline) | −4.1 | (−5.7, −2.6) |
| Slope | −11.8 | (−29.7, 0.1) | |
| Number of steps (count/day) † | Intercept (baseline) | −359 | (−502, −226) |
| Slope | −1180 | (−2853, −185) | |
| Mean sitting bout duration (min/day) † | Intercept (baseline) | 0.4 | (0.05, 0.8) |
| Slope | 0.2 | (−3.4, 3.3) | |
| ActiGraph | |||
| Light activity (min/day) ‡ | Intercept (baseline) | −1.6 | (−4.9, 1.6) |
| Slope | −2.1 | (−26.3, 21.9) | |
| Moderate-to-vigorous physical activity (min/day) ‡ | Intercept (baseline) | −4.4 | (−6.1, −3.0) |
| Slope | −15.7 | (−35.6, −2.3) | |
| Self-Reported Sleep | |||
| Time-in-bed (min/day) | Intercept (baseline) | 1.1 | (−2.1, 4.3) |
| Slope | 6.0 | (−15.0, 30.3) | |
| PROMIS Sleep Disturbance Score d | Intercept (baseline) | 0.2 | (−0.2, 0.5) |
| Slope | 1.6 | (−0.6, 4.4) | |
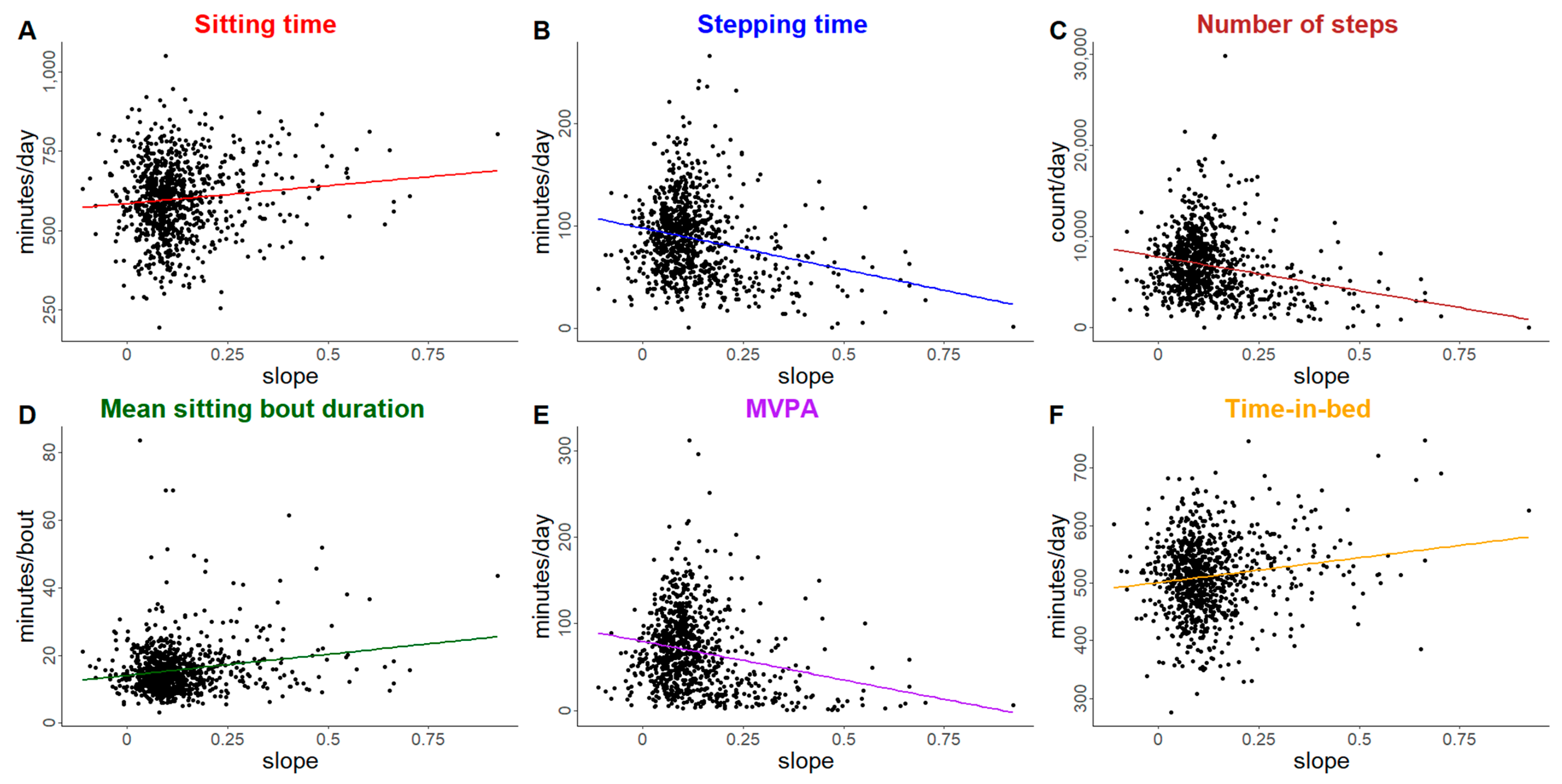
| Outcome | Individual Trajectory Features | Est b | 95% CI c |
|---|---|---|---|
| ActivPAL Outcomes | |||
| Sitting time (min/day) † | Intercept (baseline) | 8.8 | (2.9, 13.9) |
| Slope | 35.0 | (4.3, 65.0) | |
| Standing time (min/day) † | Intercept (baseline) | −5.1 | (−9.2, 0.01) |
| Slope | −20.6 | (−43.1, 4.4) | |
| Stepping time (min/day) † | Intercept (baseline) | −3.7 | (−5.2, −2.2) |
| Slope | −14.4 | (−24.2, −5.6) | |
| Number of steps (count/day) † | Intercept (baseline) | −348 | (−478, −225) |
| Slope | −1372 | (−2223, −638) | |
| Mean sitting bout duration (min/day) † | Intercept (baseline) | 0.8 | (0.3, 1.3) |
| Slope | 3.5 | (0.8, 6.2) | |
| ActiGraph | |||
| Light activity (min/day) ‡ | Intercept (baseline) | −0.9 | (−4.1, 2.7) |
| Slope | −8.0 | (−23.9, 9.8) | |
| Moderate-to-vigorous physical activity (min/day) ‡ | Intercept (baseline) | −3.3 | (−5.0, −1.7) |
| Slope | −13.0 | (−22.6, −5.0) | |
| Self-Reported Sleep | |||
| Time-in-bed (min/day) | Intercept (baseline) | 4.5 | (1.0, 8.2) |
| Slope | 25.5 | (6.5, 43.5) | |
| PROMIS Sleep Disturbance Score d | Intercept (baseline) | 0.2 | (−0.1, 0.6) |
| Slope | 1.0 | (−0.9, 3.1) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greenwood-Hickman, M.A.; Zhu, W.; Idu, A.; Harrington, L.B.; McCurry, S.M.; LaCroix, A.Z.; Shaw, P.A.; Rosenberg, D.E. Associations Between 10-Year Physical Performance and Activities of Daily Living Trajectories and Physical Behaviors in Older Adults. Int. J. Environ. Res. Public Health 2025, 22, 704. https://doi.org/10.3390/ijerph22050704
Greenwood-Hickman MA, Zhu W, Idu A, Harrington LB, McCurry SM, LaCroix AZ, Shaw PA, Rosenberg DE. Associations Between 10-Year Physical Performance and Activities of Daily Living Trajectories and Physical Behaviors in Older Adults. International Journal of Environmental Research and Public Health. 2025; 22(5):704. https://doi.org/10.3390/ijerph22050704
Chicago/Turabian StyleGreenwood-Hickman, Mikael Anne, Weiwei Zhu, Abisola Idu, Laura B. Harrington, Susan M. McCurry, Andrea Z. LaCroix, Pamela A. Shaw, and Dori E. Rosenberg. 2025. "Associations Between 10-Year Physical Performance and Activities of Daily Living Trajectories and Physical Behaviors in Older Adults" International Journal of Environmental Research and Public Health 22, no. 5: 704. https://doi.org/10.3390/ijerph22050704
APA StyleGreenwood-Hickman, M. A., Zhu, W., Idu, A., Harrington, L. B., McCurry, S. M., LaCroix, A. Z., Shaw, P. A., & Rosenberg, D. E. (2025). Associations Between 10-Year Physical Performance and Activities of Daily Living Trajectories and Physical Behaviors in Older Adults. International Journal of Environmental Research and Public Health, 22(5), 704. https://doi.org/10.3390/ijerph22050704







