Metabolic Mechanism of Bacillus sp. LM24 under Abamectin Stress
Abstract
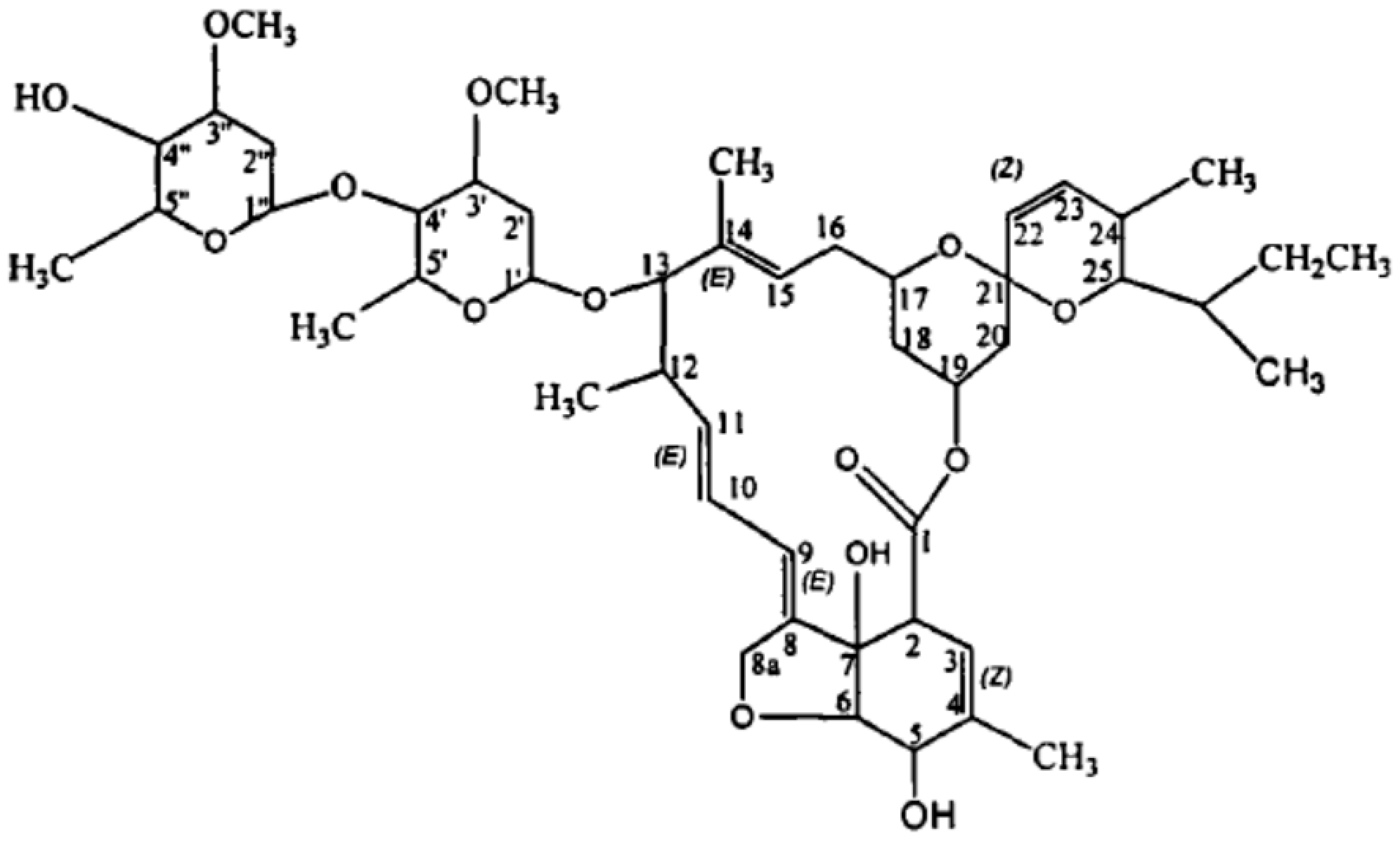
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Microbial Culture
2.3. Effect of Degradation Conditions on Experiments
2.3.1. Effect of Time on ABM Degradation
2.3.2. Effect of Substrate Concentration on ABM Degradation
2.3.3. Effect of Glucose Concentration on ABM Degradation
2.3.4. Effect of Bacteria Concentration on ABM Degradation
2.4. Degradation Experiment Extraction and Liquid Phase Analysis
2.5. Detection of Extracellular Degradation Products
2.6. Metabolic Experiments
2.7. Intracellular Metabolite Analysis
2.8. Analysis Conditions for Metabolite LC-MS
2.8.1. Chromatographic Conditions
2.8.2. Mass Spectrometry Conditions
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effect of Degradation Conditions on Experiments
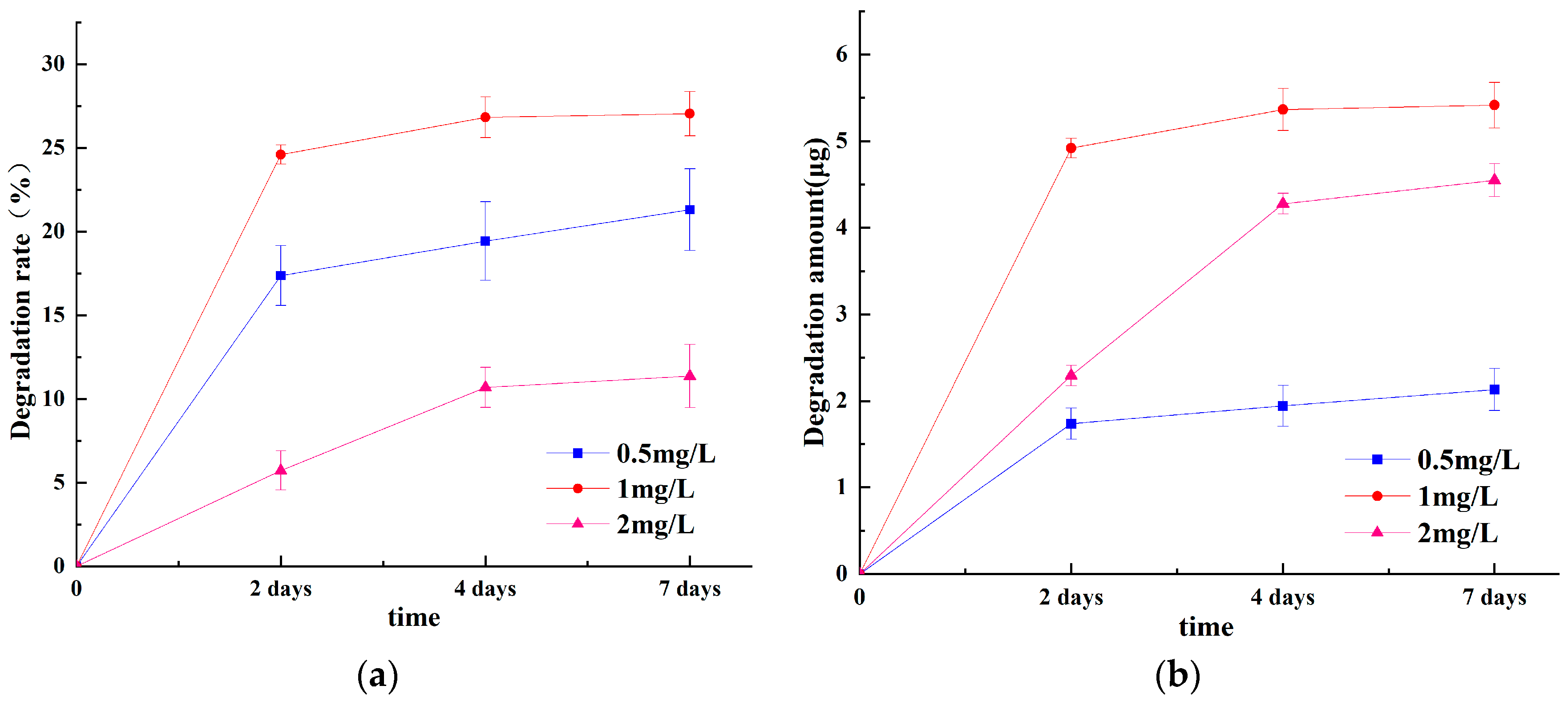
3.1.1. Effect of Time and Substrate Concentration on ABM Degradation
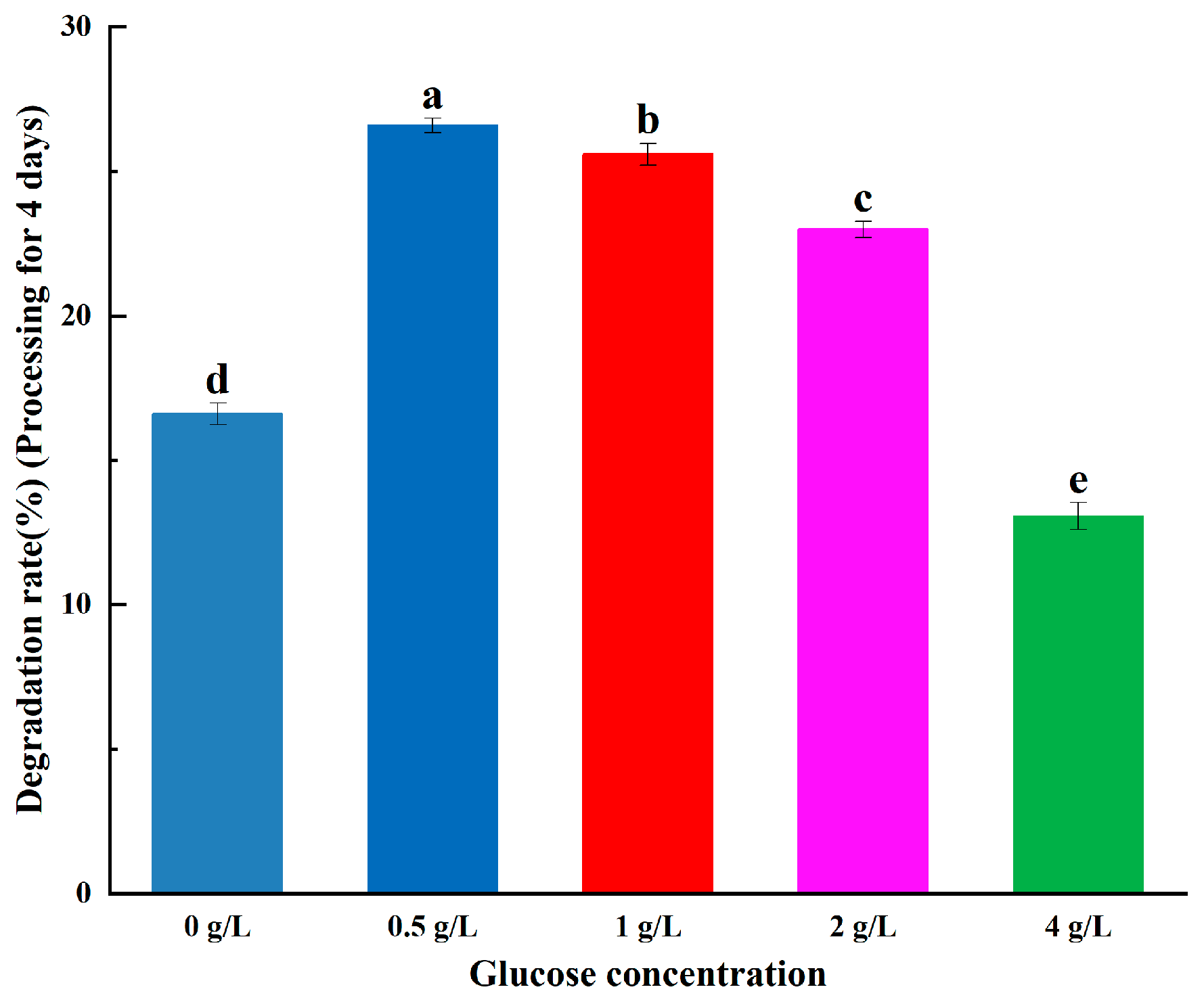
3.1.2. Effect of Glucose Concentration on ABM Degradation
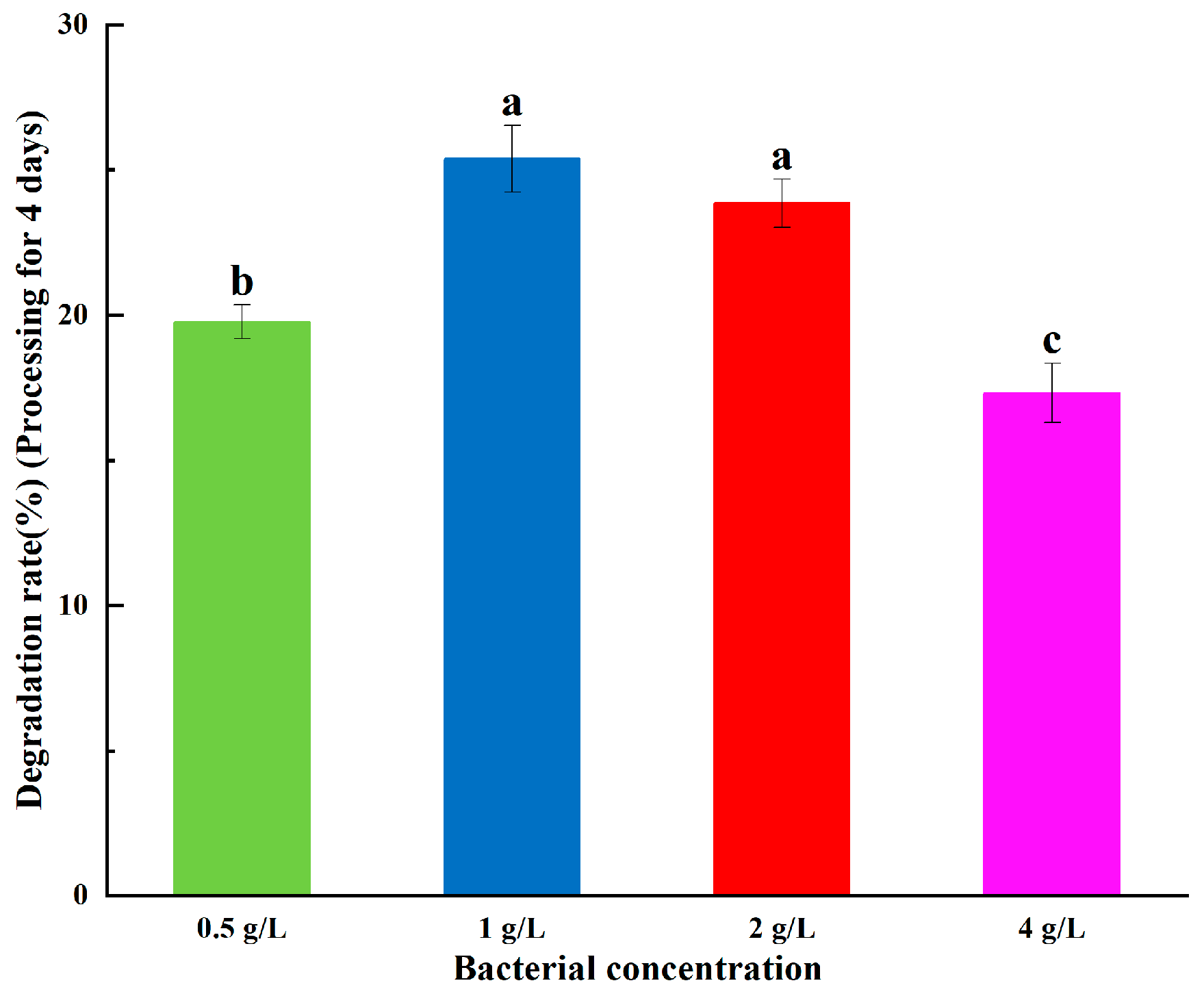
3.1.3. Effect of Bacteria Concentration on ABM Degradation
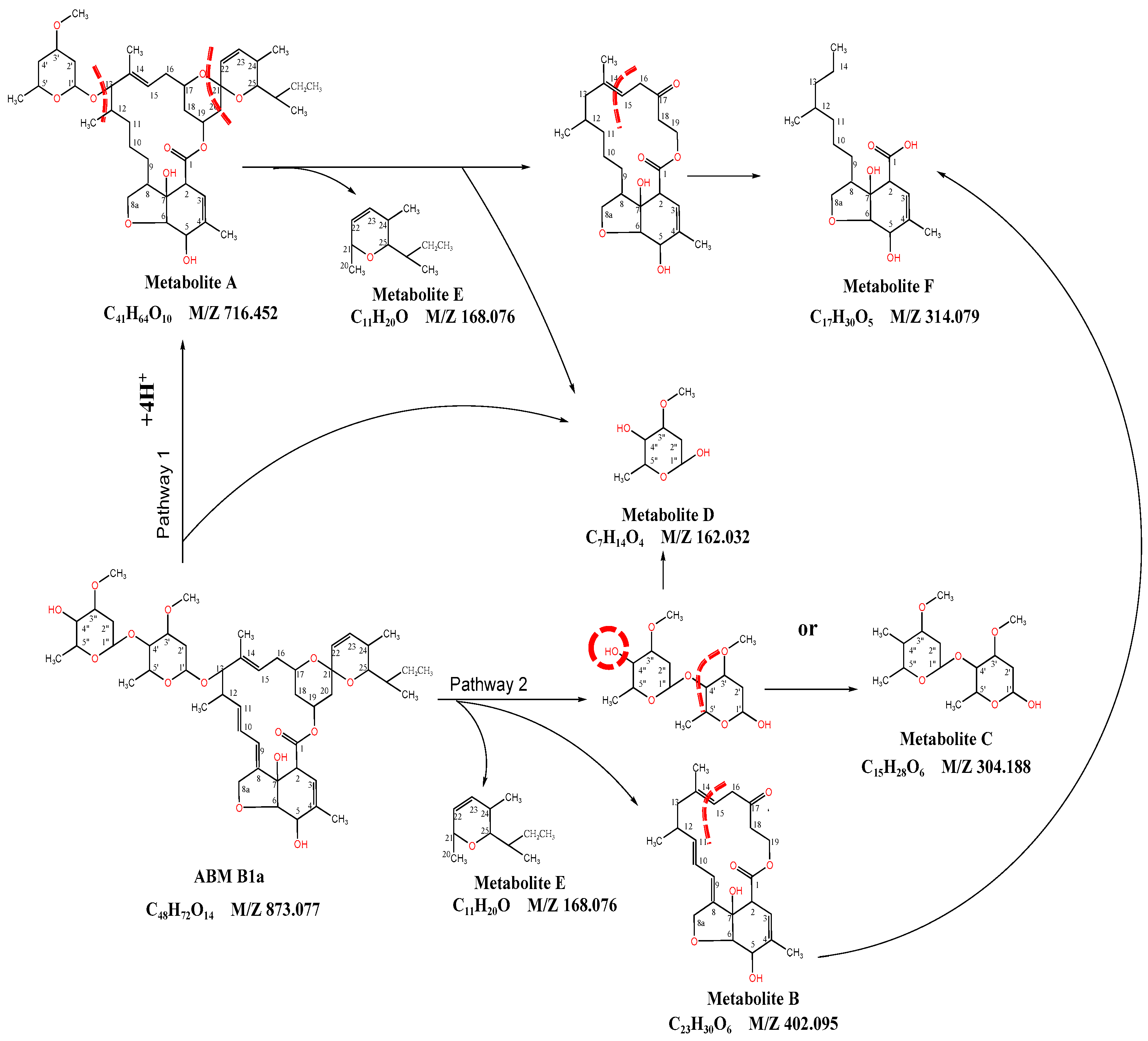
3.2. Extracellular ABM Degradation Products
3.3. Metabolic Effects of 0.5 mg/L (0.5A) ABM on B. sp LM24
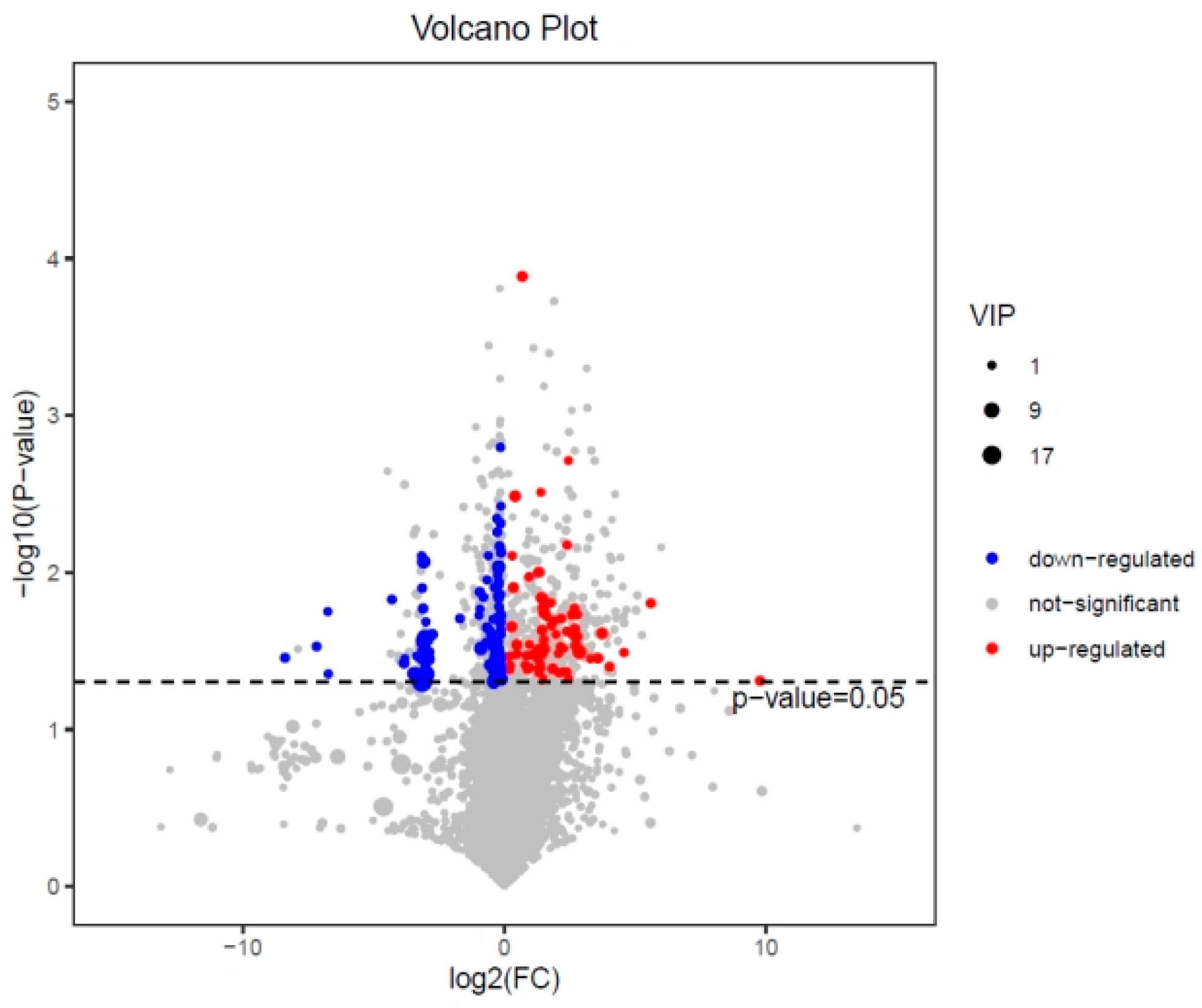
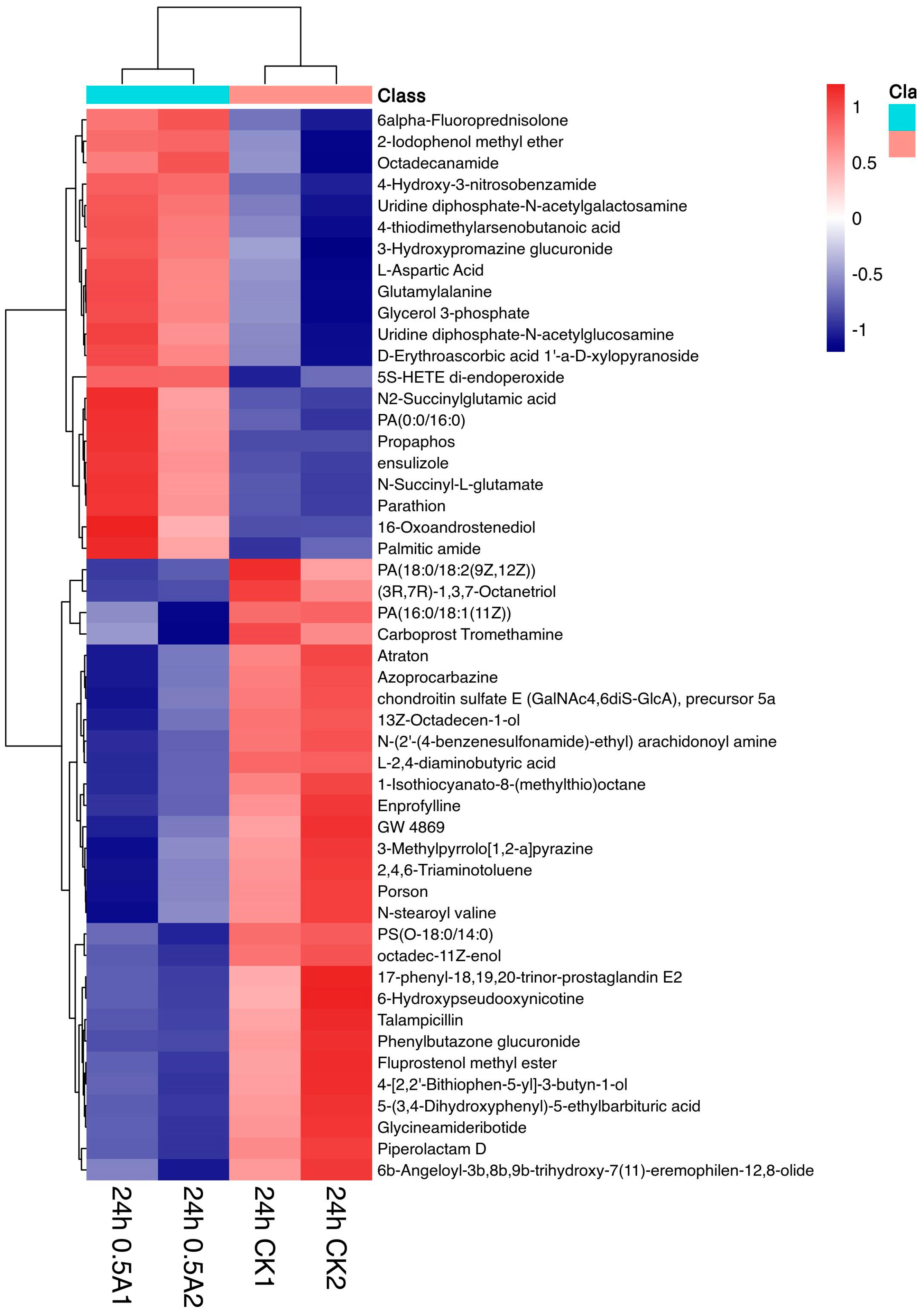
3.3.1. Expression Analysis of Differential Metabolites in the 24 h 0.5 A~24 h CK Group
3.3.2. Expression Analysis of Differential Metabolites in the 48 h 0.5 A-48 h CK Group
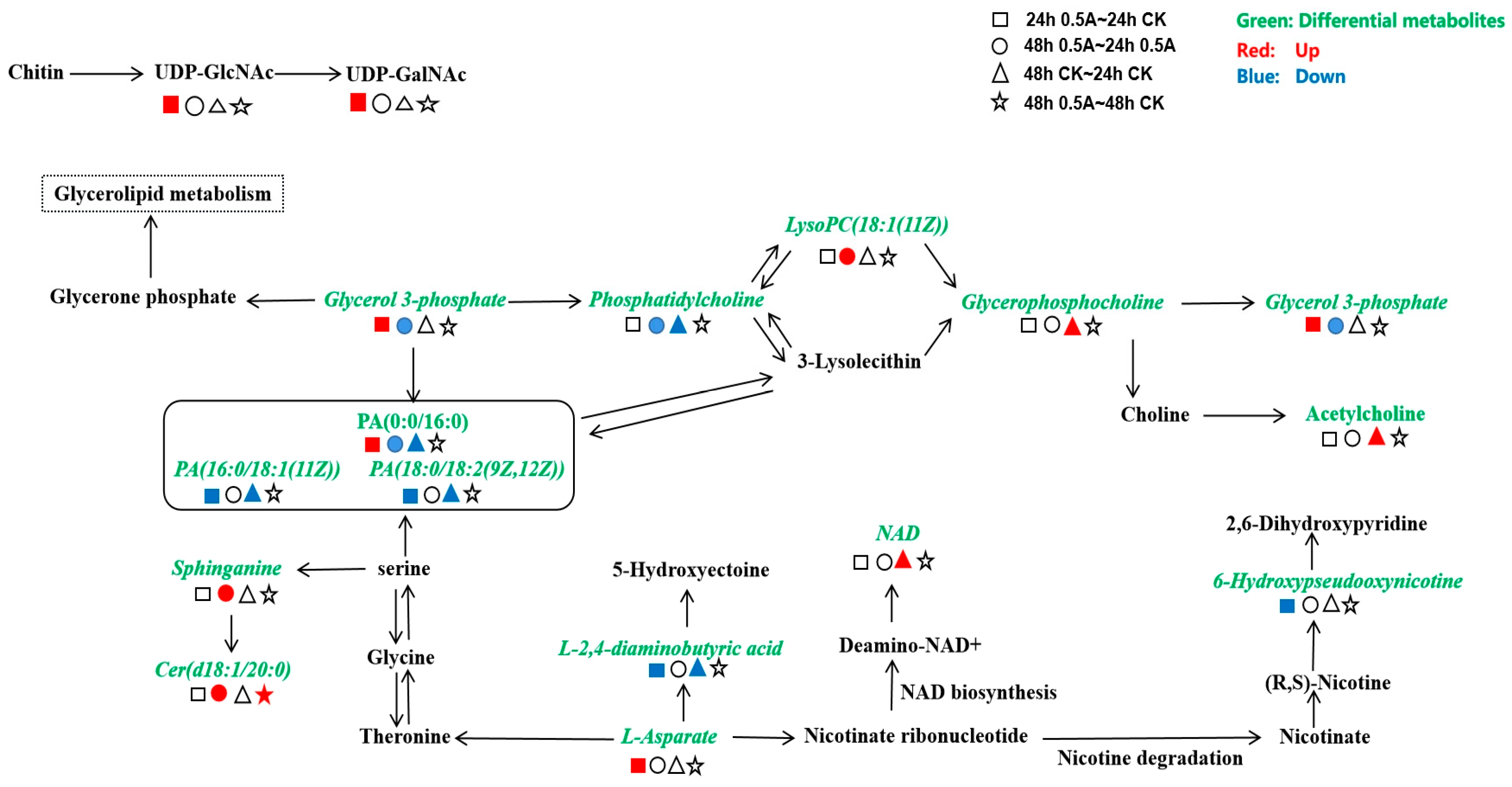
3.3.3. Analysis of Metabolic Pathways in the KEGG (Kyoto Encyclopaedia of Genes and Genomes) Database
3.4. Metabolic Analysis of B. sp LM24 by Degrading 1 mg/L (1A) ABM
3.4.1. Differential Expression Analysis of Metabolites in the 24 h 1 A-24 h CK Group
3.4.2. Expression Analysis of Differential Metabolites in the 48 h 1 A-48 h CK Group
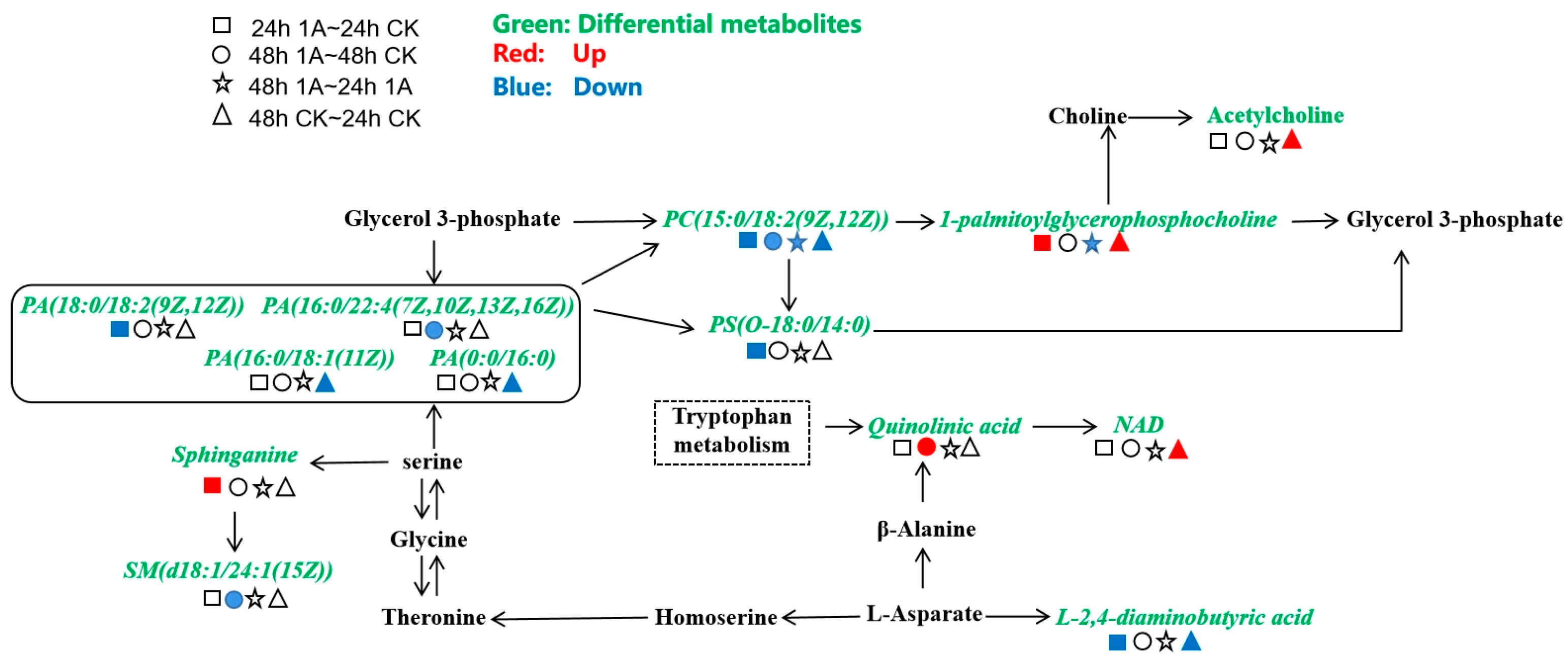
3.4.3. KEGG Database Metabolic Pathway Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, D.M.; Liu, X.H.; Wang, L.; Gong, W.W.; Liu, G.N.; Fu, W.J.; Cheng, M. Seasonal variation and sedimentwater exchange of antibiotics in a shallower large lake in North China. Sci. Total Environ. 2014, 476, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Zheng, X.C.; Hu, Q.W.; Zheng, Y.G. Degradation of abamectin by newly isolated Stenotrophomonas maltophilia ZJB-14120 and characterization of its abamectin-tolerance mechanism. Res. Microbiol. 2015, 166, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Gupta, S.C.; Chander, Y.; Ashok, K.S. Antibiotic use in agriculture and its impact on the terrestrial environment. Adv. Agron. 2005, 87, 1–54. [Google Scholar] [CrossRef]
- Huang, F.Y.; An, Z.Y.; Moran, M.J.; Liu, F. Recognition of typical antibiotic residues in environmental media related to groundwater in China (2009-2019). J. Hazard. Mater. 2020, 399, 12213. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Rajapaksha, A.U.; Lim, J.E.; Vu, N.T.; Kim, S.; Kang, H.M.; Lee, S.S.; Ok, Y.S. Distribution and accumulative pattern of tetracyclines and sulfonamides in edible vegetables of cucumber, tomato, and lettuce. J. Agric. Food Chem. 2015, 63, 398–405. [Google Scholar] [CrossRef]
- Boy-Roura, M.; Mas-Pla, J.; Petrovic, M.; Gros, M.; Soler, D.; Brusi, D.; Menció, A. Towards the understanding of antibiotic occurrence and transport in groundwater: Findings from the Baix Fluvià alluvial aquifer (NE Catalonia, Spain). Sci. Total Environ. 2018, 612, 1387–1406. [Google Scholar] [CrossRef]
- Feng, L.; Cheng, Y.R.; Zhang, Y.Y.; Li, Z.W.; Yu, Y.C.; Feng, L.; Zhang, S.; Xu, L.J. Distribution and human health risk assessment of antibiotic residues in large-scale drinking water sources in Chongqing area of the Yangtze River. Environ Res. 2020, 185, 109386. [Google Scholar] [CrossRef]
- Bai, S.H.; Ogbourne, S. Eco-toxicological effects of the avermectin family with a focus on abamectin and ivermectin. Chemosphere 2016, 154, 204–214. [Google Scholar] [CrossRef]
- Zhang, T.M.; Dong, Z.H.; Liu, F.X.; Pan, E.Z.; He, N.N.; Ma, F.F.; Wang, G.L.; Wang, Y.; Dong, J.Q. Avermectin induces carp neurotoxicity by mediating blood-brain barrier dysfunction, oxidative stress, inflammation, and apoptosis through PI3K/Akt and NF-κB pathways. Ecotoxicol. Environ. Saf. 2022, 243, 113961. [Google Scholar] [CrossRef]
- Liu, L.X.; Wang, H.Y.; Xie, P. Gut microbiota: A new insight into neuropsychiatric disorders (in Chinese). Sci Sin Vitae. 2022, 52, 1097. [Google Scholar] [CrossRef]
- Zou, Y.Q.; Xue, W.B.; Luo, G.W.; Deng, Z.Q.; Qin, P.P.; Guo, R.J.; Sun, H.P.; Xia, Y.; Liang, S.S.; Dai, Y.; et al. 1,520 reference genomes from cultivated human gut bacteria enable functional microbiome analyses. Nat. Biotechnol. 2019, 37, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Escalada, J.P.; Gianotti, J.; Pajares, A.; Massad, W.A.; Amat-Guerri, F.; Garcia, N.A. Photodegradation of the acaricide abamectin: A kinetic study. J. Agr. Food Chem. 2008, 56, 7355–7359. [Google Scholar] [CrossRef] [PubMed]
- Windels, E.M.; Michiels, J.E.; Den Bergh, B.V.; Fauvart, M.; Michiels, J. Antibiotics: Combatting Tolerance To Stop Resistance. Mbio 2019, 10, e02095-19. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Plesiat, P.; Nikaido, H. The Challenge of Efflux-Mediated Antibiotic Resistance in Gram-Negative Bacteria. Clin. Microbiol. Rev. 2015, 28, 337–418. [Google Scholar] [CrossRef]
- Kester, J.C.; Fortune, S.M. Persisters and beyond: Mechanisms of phenotypic drug resistance and drug tolerance in bacteria. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 91–101. [Google Scholar] [CrossRef]
- Wilmes, P.; Heintzbuschart, A.; Bond, P.L. A decade of metaproteomics: Where we stand andwhat the future holds. Proteomics 2015, 15, 3409–3417. [Google Scholar] [CrossRef]
- Ali, S.W.; Li, R.; Zhou, W.Y.; Sun, J.Q.; Guo, P.; Ma, J.P.; Li, S.P. Isolation and characterization of an abamectin-degrading Burkholderia cepacia-like GB-01 strain. Biodegradation 2010, 21, 441–452. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, K.F.; Cai, L.K.; Ma, W.W.; Fang, S.F. Study on microbial degradationof Abamectin and mechanism. Ecol. Environ. Sci. 2007, 16, 421–424. [Google Scholar] [CrossRef]
- Ke, Y.; Gonthier, R.; Labrie, F. The conversion of 16β hydroxyldehydroepiandrosterone in human serum. Steroids 2016, 109, 50–55. [Google Scholar] [CrossRef]
- Velikanova, L.I.; Shafigullina, Z.R.; Vorokhobina, N.V.; Malevanaya, E.V. Gas Chromatography-Mass Spectrometry Analysis of Urinary Steroid Metabolomics for Detection of Early Signs of Adrenal Neoplasm Malignancy in Patients with Cushing’s Syndrome. Bull. Exp. Biol. Med. 2019, 167, 676–680. [Google Scholar] [CrossRef]
- Tsochatzis, E.; Lopes, J.A.; Gika, H.; Theodoridis, G. Polystyrene Biodegradation by Tenebrio molitor Larvae: Identification of Generated Substances Using a GC-MS Untargeted Screening Method. Polymers 2021, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Marcocci, L.; Suzuki, Y.J. Metabolomics Studies to Assess Biological Functions of Vitamin E Nicotinate. Antioxidants 2019, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Kundu, M.; Jana, M.; Mishra, R.K.; Yung, Y.; Luan, C.H.; Gonzalez, F.J.; Pahan, K. Identification and characterization of PPARα ligands in the hippocampus. Nat. Chem. Biol. 2016, 12, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Lambert, D.M.; Vandevoorde, S.; Diependaele, G.; Govaerts, S.J.; Robert, A.R. Anticonvulsant activity of N-palmitoylethanolamide, a putative endocannabinoid, in mice. Epilepsia 2001, 42, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Aneetha, H.; O’Dell, D.K.; Tan, B.; Walker, J.M.; Hurley, T.D. Alcohol dehydrogenase-catalyzed in vitro oxidation of anandamide to N-arachidonoyl glycine, a lipid mediator: Synthesis of N-acyl glycinals. Bioorg. Med. Chem. Lett. 2009, 19, 237–241. [Google Scholar] [CrossRef]
- Farrell, E.K.; Chen, Y.; Barazanji, M.; Jeffries, K.A.; Cameroamortegui, F.; Merkler, D.J. Primary fatty acid amide metabolism: Conversion of fatty acids and an ethanolamine in N18TG2 and SCP cells 1. J. Lipid Res. 2012, 53, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.J.; Pan, X.L.; Abbas, H.M.K.; Li, F.F.; Dong, W.B. Metabolites contributing to Rhizoctonia solani AG-1-IA maturation and sclerotial differentiation revealed by UPLC-QTOF-MS metabolomics. PLoS ONE 2017, 12, e0177464. [Google Scholar] [CrossRef]
- Li, S.S.; Liu, Y.; Li, H.; Wang, L.P.; Xue, L.F.; Yin, G.S.; Wu, X.S. Identification of psoriasis vulgaris biomarkers in human plasma by non-targeted metabolomics based on UPLC-Q-TOF/MS. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3940–3950. [Google Scholar] [CrossRef]
- Ezzili, C.; Otrubova, K.; Boger, D.L. Fatty acid amide signaling molecules. Bioorg. Med. Chem. Lett. 2010, 20, 5959–5968. [Google Scholar] [CrossRef]
- Yamamoto, S.; Takehara, M.; Ushimaru, M. Inhibitory action of linoleamide and oleamide toward sarco/endoplasmic reticulum Ca2+-ATPase. BBA-Gen Subjects. 2017, 1861, 3399–3405. [Google Scholar] [CrossRef]
- Schubert, C.; Zedler, S.; Strecker, A.; Unden, G. L-Aspartate as a high-quality nitrogen source in Escherichia coli: Regulation of L-aspartase by the nitrogen regulatory system and interaction of L-aspartase with GlnB. Mol. Microbiol. 2021, 115, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.Y.; Yang, D.X.; Jiang, M.; Zheng, J.; Peng, B. L-aspartic acid promotes fish survival against Vibrio alginolyticus infection through nitric oxide-induced phagocytosis. Fish Shellfish. Immunol. 2020, 97, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Friedrichsen, G.M.; Jakobsen, P.; Taub, M.; Begtrup, M. Application of enzymatically stable dipeptides for enhancement of intestinal permeability. Synthesis and In vitro evaluation of dipeptide-Coupled compounds. Bioorg. Med. Chem. 2001, 9, 2625–2632. [Google Scholar] [CrossRef] [PubMed]
- Ferraiuolo, R.-M.; Meister, D.; Leckie, D.; Dashti, M.; Franke, J.; Porter, L.A.; Trant, J.F. Neuro- and hepatic toxicological profile of (S)-2,4-diaminobutanoic acid in embryonic, adolescent and adult zebrafish. J. Appl. Toxicol. 2019, 39, 1568–1577. [Google Scholar] [CrossRef]
- Sage, A.T.; Walter, L.A.; Shi, Y.; Khan, M.I.; Kaneto, H.; Capretta, A.; Werstuck, G.H. Hexosamine biosynthesis pathway flux promotes endoplasmic reticulum stress, lipid accumulation, and inflammatory gene expression in hepatic cells. J. Physiol. Endocrinol. Metab. 2009, 298, E499–E511. [Google Scholar] [CrossRef]
- Zachara, N.E.; Hart, G.W. Cell signaling, the essential role of O-GlcNAc! Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2006, 1761, 599–617. [Google Scholar] [CrossRef]
- Oikari, S.; Kettunen, T.; Tiainen, S.; Häyrinen, J.; Masarwah, A.; Sudah, M.; Sutela, A.; Vanninen, R.; Tammi, M.; Auvinen, P. UDP-sugar accumulation drives hyaluronan synthesis in breast cancer. Matrix Biol. 2018, 67, 63–74. [Google Scholar] [CrossRef]
- Eom, H.J.; Park, W. Inhibitory effect of taurine on biofilm formation during alkane degradation in Acinetobacter oleivorans DR1. Microb.Ecol. 2017, 74, 821–831. [Google Scholar] [CrossRef]
- Kim, K.R.; Owens, G.; Kwon, S.I.; So, K.H.; Lee, D.B.; Ok, Y.S. Occurrence and environmental fate of veterinary antibiotics in the terrestrial environment. Water Air.Soil Pollut. 2011, 214, 163–174. [Google Scholar] [CrossRef]
- Tan, H.; Kong, D.L.; Li, Q.Q.; Zhou, Y.Q.; Jiang, X.; Wang, Z.Y.; Parales, R.E.; Ruan, Z.Y. Metabolomics reveals the mechanism of tetracycline biodegradation by a Sphingobacterium mizutaii S121. Environ. Pollut. 2022, 305, 119299. [Google Scholar] [CrossRef]
- Tripathi, M.; Pandey, M.B.; Jha, R.N.; Pandey, V.B.; Tripathi, P.N.; Singh, J.P. Cyclopeptide alkaloids from Zizyphus jujuba. Fitoterapia 2001, 72, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.Y.; Mu, P.Q.; Wen, J.K.; Sun, Y.; Chen, Q.M.; Deng, Y.Q. Detoxification of trichothecene mycotoxins by a novel bacterium, Eggerthella sp. DII-9. Food Chem. Toxicol. 2018, 112, 310–319. [Google Scholar] [CrossRef]
- Gosset, G. Engineering of Microorganisms for the Production of Chemicals and Biofuels from Renewable Resources; Springer International Publishing: Cham, Switzerland, 2017; pp. 93–123. [Google Scholar] [CrossRef]
- Vimala, A.; Harinarayanan, R. Transketolase activity modulates glycerol-3-phosphate levels in Escherichia coli. Mol. Microbiol. 2016, 100, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Hestad, K.; Alexander, J.; Rootwelt, H.; Aaseth, J.O. The role of tryptophan dysmetabolism and quinolinic acid in depressive and neurodegenerative diseases. Biomolecules 2022, 12, 998. [Google Scholar] [CrossRef]
- Bansal, Y.; Singh, R.; Parhar, I.; Kuhad, A.; Soga, T. Quinolinic Acid and Nuclear Factor Erythroid 2-Related Factor 2 in Depression: Role in Neuroprogression. Front. Pharmacol. 2019, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Verdonk, F.; Petit, A.-C.; Abdel-Ahad, P.; Vinckier, F.; Jouvion, G.; de Maricourt, P.; De Medeiros, G.F.; Danckaert, A.; Van Steenwinckel, J.; Blatzer, M.; et al. Microglial production of quinolinic acid as a target and a marker of the antidepressant effect of ketamine. Brain Behav. Immun. 2019, 81, 361–373. [Google Scholar] [CrossRef] [PubMed]
| Experiment Name | Sample Name | Experiment Content | Influence Factor |
|---|---|---|---|
| Effect of time on ABM degradation | Blank group | MSM medium + 1 mg/L ABM + 1 g/L glucose, 3 parallel | Treatment 0 day, 2 days, 4 days, and 7 days |
| Experimental groups | MSM medium + 1 mg/L ABM + 1 g/L B. sp LM24 + 1 g/L glucose, 3 parallel | Treatment 0 day, 2 days, 4 days, and 7 days | |
| Effect of substrate concentration on ABM degradation | Blank group | MSM medium + 1 g/L glucose, 3 paralle, treatment 4 day | Add 0.5 mg/L, 1 mg/L, and 2 mg/L ABM |
| Experimental groups | MSM medium + 1 g/L B. sp LM24 + 1 g/L glucose, 3 parallel, treatment 4 day | Add 0.5 mg/L, 1 mg/L, and 2 mg/L ABM | |
| Effect of glucose concentration on ABM degradation | Blank group | MSM medium + 1 mg/L ABM, 3 paralle, treatment 4 day | Add 0, 0.5 g/L, 1 g/L, 2 g/L, and 4 g/L glucose |
| Experimental groups | MSM medium + 1 mg/L ABM + 1 g/L B. sp LM24, 3 parallel, treatment 4 day | Add 0, 0.5 g/L, 1 g/L, 2 g/L, and 4 g/L glucose | |
| Effect of bacteria concentration on ABM degradation | Blank group | MSM medium + 1 mg/L ABM + 1 g/L glucose, 3 paralle, treatment 4 days | \ |
| Experimental groups | MSM medium + 1 mg/L ABM + 1 g/L glucose, 3 paralle, treatment 4 days | Add 0.5 g/L, 1 g/L, 2 g/L, and 4 g/L B. sp LM24 | |
| Detection of extracellular degradation products | Control group | MSM medium + 1 mg/L ABM + 1 g/L glucose, 3 paralle | Treatment 2 days and 4 days |
| Experimental groups | MSM medium + 1 mg/L ABM + 1 g/L B. sp LM24 + 1 g/L glucose, 3 parallel | Treatment 2 days and 4 days | |
| Metabolic experiments | Control group | MSM medium + 1 g/L B. sp LM24 + 1 g/L glucose, 2 paralle | Treatment 24 h |
| Experimental groups | MSM medium + 1 g/L B. sp LM24 + 1 g/L glucose, 2 parallel | Add 0.5 mg/L and 1 mg/L ABM; treatment 24 h | |
| Control group | MSM medium + 1 g/L B. sp LM24 + 1 g/L glucose, 2 paralle | Treatment 48 h | |
| Experimental groups | MSM medium + 1 g/L B. sp LM24 + 1 g/L glucose, 2 parallel | Add 0.5 mg/L and 1 mg/L ABM; treatment 48 h |
| S/N | Name of Metabolite | Log2FC Value | Metabolite Classification |
|---|---|---|---|
| Up-regulated metabolites | |||
| 1 | Hydroxyanigorufone | 2.45 | Benzene compounds |
| 2 | 16-Oxoandrostenediol | 9.77 | Lipids and lipid molecules |
| 3 | PA (0:0/16:0) | 1.57 | |
| 4 | 5S-HETE di-endoperoxide | 1.32 | |
| 5 | Glycerol 3-phosphate | 0.96 | |
| 6 | 4-thiodimethylarsenobutanoic acid | 0.95 | |
| 7 | Palmitic amide | 0.20 | |
| 8 | Uridine diphosphate-N-acetylgalactosamine | 2.17 | Nucleosides, nucleotides, and analogues |
| 9 | Uridine diphosphate-N-acetylglucosamine | 1.84 | |
| 10 | Parathion | 2.70 | Organic acids and their derivatives |
| 11 | Glutamylalanine | 1.36 | |
| 12 | L-Aspartic Acid | 1.30 | |
| 13 | Octadecanamide | 0.23 | |
| 14 | D-Erythroascorbic acid 1′-a-D-xylopyranoside | 1.41 | Organic oxygen compounds |
| 15 | Propaphos | 29.86 | Unclassified |
| 16 | N2-Succinylglutamic acid | 2.87 | |
| 17 | N-Succinyl-L-glutamate | 2.74 | |
| 18 | ensulizole | 1.91 | |
| 19 | 6alpha-Fluoroprednisolone | 1.80 | |
| 20 | 2-Iodophenol methyl ether | 1.47 | |
| 21 | 3-Hydroxypromazine glucuronide | 1.30 | |
| 22 | 4-Hydroxy-3-nitrosobenzamide | 0.94 | |
| Down-regulated metabolites | |||
| 1 | Piperolactam D | −3.12 | Alkaloids and their derivatives |
| 2 | chondroitin sulfate E (GalNAc4,6diS-GlcA), precursor 5a | −0.66 | Benzene compounds |
| 3 | PS(O-18:0/14:0) | −3.08 | Lipids and lipid molecules |
| 4 | 17-phenyl-18,19,20-trinor-prostaglandin E2 | −3.01 | |
| 5 | (3R, 7R)-1,3,7-Octanetriol | −0.93 | |
| 6 | PA(16:0/18:1(11Z)) | −0.89 | |
| 7 | 6b-Angeloyl-3b,8b,9b-trihydroxy-7(11)-eremophilen-12,8-olide | −0.49 | |
| 8 | all-trans-retinyl oleate | −0.39 | |
| 9 | PA(18:0/18:2(9Z,12Z)) | −0.28 | |
| 10 | octadec-11Z-enol | −0.26 | |
| 11 | 13Z-Octadecen-1-ol | −0.25 | |
| 12 | N-stearoyl valine | −0.15 | |
| 13 | N-(2′-(4-benzenesulfonamide)-ethyl) arachidonoyl amine | −0.13 | |
| 14 | Carboprost Tromethamine | −0.13 | |
| 15 | Glycineamideribotide | −2.77 | Nucleosides, nucleotides, and analogues |
| 16 | L-2,4-diaminobutyric acid | −0.27 | Organic acids and their derivatives |
| 17 | 6-Hydroxypseudooxynicotine | −3.29 | Organic oxygen compounds |
| 18 | 4-[2,2′-Bithiophen-5-yl]-3-butyn-1-ol | −3.25 | Organic heterocyclic compounds |
| 19 | Enprofylline | −0.54 | |
| 20 | 3-Methylpyrrolo[1,2-a]pyrazine | −0.41 | |
| 21 | 1-Isothiocyanato-8-(methylthio)octane | −0.80 | Organosulfur compounds |
| 22 | Porson | −0.58 | Phenylpropionate and polyketones |
| 23 | Phenylbutazone glucuronide | −7.18 | Unclassified |
| 24 | Talampicillin | −3.84 | |
| 25 | Benfuresate | −3.25 | |
| 26 | 5-(3,4-Dihydroxyphenyl)-5-ethylbarbituric acid | −3.04 | |
| 27 | Fluprostenol methyl ester | −2.91 | |
| 28 | Atraton | −0.50 | |
| 29 | N,N-Dimethyl-1,4-phenylenediamine | −0.42 | |
| 30 | 2,4,6-Triaminotoluene | −0.40 | |
| 31 | 2-Imino-4-methylpiperidine | −0.38 | |
| 32 | Azoprocarbazine | −0.16 | |
| 33 | GW 4869 | −0.14 | |
| S/N | Name of Metabolite | Log2FC Value | Metabolite Classification |
|---|---|---|---|
| Up-regulated metabolites | |||
| 1 | (±)-1-(4-Methylphenyl)ethanol | 1.55 | Benzene compounds |
| 2 | 2,5-Dimethylbenzaldehyde | 1.49 | |
| 3 | Propylene glycol mono- and diesters of fats and fatty acids | 2.65 | Lipids and lipid molecules |
| 4 | Cer(d18:0/22:0(2OH)) | 2.15 | |
| 5 | Lucidenolactone | 1.94 | |
| 6 | Cer(t20:0/18:0) | 1.89 | |
| 7 | Cer(d18:1/20:0) | 1.79 | |
| 8 | Cer(d18:0/18:0) | 1.51 | |
| 9 | Cer(d20:0/18:0) | 1.41 | |
| 10 | N,N-dimethyl-Safingol | 1.28 | |
| 11 | Toxin T2 tetrol | 1.05 | |
| 12 | PE-Cer(d14:2(4E,6E)/18:0(2OH)) | 0.76 | |
| 13 | Eplerenone | 0.46 | |
| 14 | PA(P-16:0/13:0) | 0.24 | |
| 15 | Geranylcitronellol | 0.24 | |
| 16 | Argenonic acid | 0.20 | |
| 17 | Jubanine C | 2.44 | Organic acids and their derivatives |
| 18 | 3-Methylcyclopentadecanone | 1.03 | Organic oxygen compounds |
| 19 | Austalide L | 1.65 | Phenylpropionate and polyketones |
| 20 | Imazaquin | 2.39 | Unclassified |
| 21 | Sesamex | 1.08 | |
| 22 | 24-vinyloxy-cholest-5,23Z-dien-3beta-ol | 0.21 | |
| 23 | 1α,25-Dihydroxy-2Z-ethylidene-19-norvitamin D3 | 0.20 | |
| Down-regulated metabolites | |||
| 1 | D-Maltose | −2.05 | Organic oxygen compounds |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Xie, Q.; Ye, J.; Wang, R.; Yin, X.; Xie, W.; Li, D. Metabolic Mechanism of Bacillus sp. LM24 under Abamectin Stress. Int. J. Environ. Res. Public Health 2023, 20, 3068. https://doi.org/10.3390/ijerph20043068
Zhu Y, Xie Q, Ye J, Wang R, Yin X, Xie W, Li D. Metabolic Mechanism of Bacillus sp. LM24 under Abamectin Stress. International Journal of Environmental Research and Public Health. 2023; 20(4):3068. https://doi.org/10.3390/ijerph20043068
Chicago/Turabian StyleZhu, Yueping, Qilai Xie, Jinshao Ye, Ruzhen Wang, Xudong Yin, Wenyu Xie, and Dehao Li. 2023. "Metabolic Mechanism of Bacillus sp. LM24 under Abamectin Stress" International Journal of Environmental Research and Public Health 20, no. 4: 3068. https://doi.org/10.3390/ijerph20043068
APA StyleZhu, Y., Xie, Q., Ye, J., Wang, R., Yin, X., Xie, W., & Li, D. (2023). Metabolic Mechanism of Bacillus sp. LM24 under Abamectin Stress. International Journal of Environmental Research and Public Health, 20(4), 3068. https://doi.org/10.3390/ijerph20043068





