2D/2D Phosphorus-Doped g-C3N4/Bi2WO6 Direct Z-Scheme Heterojunction Photocatalytic System for Tetracycline Hydrochloride (TC-HCl) Degradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of P-Doped g-C3N4
2.3. Preparation of P-Doped g-C3N4/Bi2WO6
2.4. Characterization of the Catalysts
2.5. Evaluation of Photocatalytic Performance
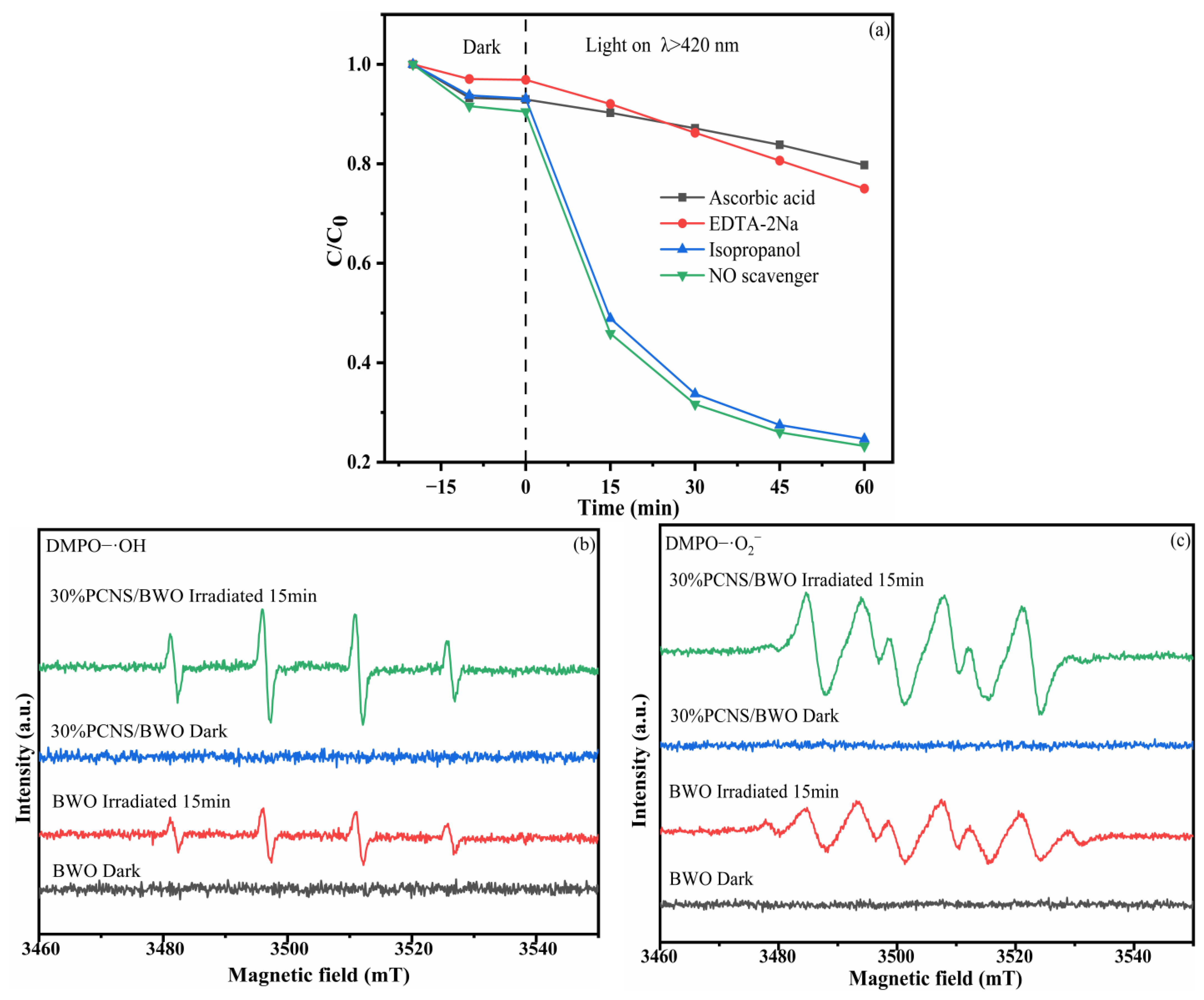
2.6. Detection of Active Substances
3. Results and Discussion
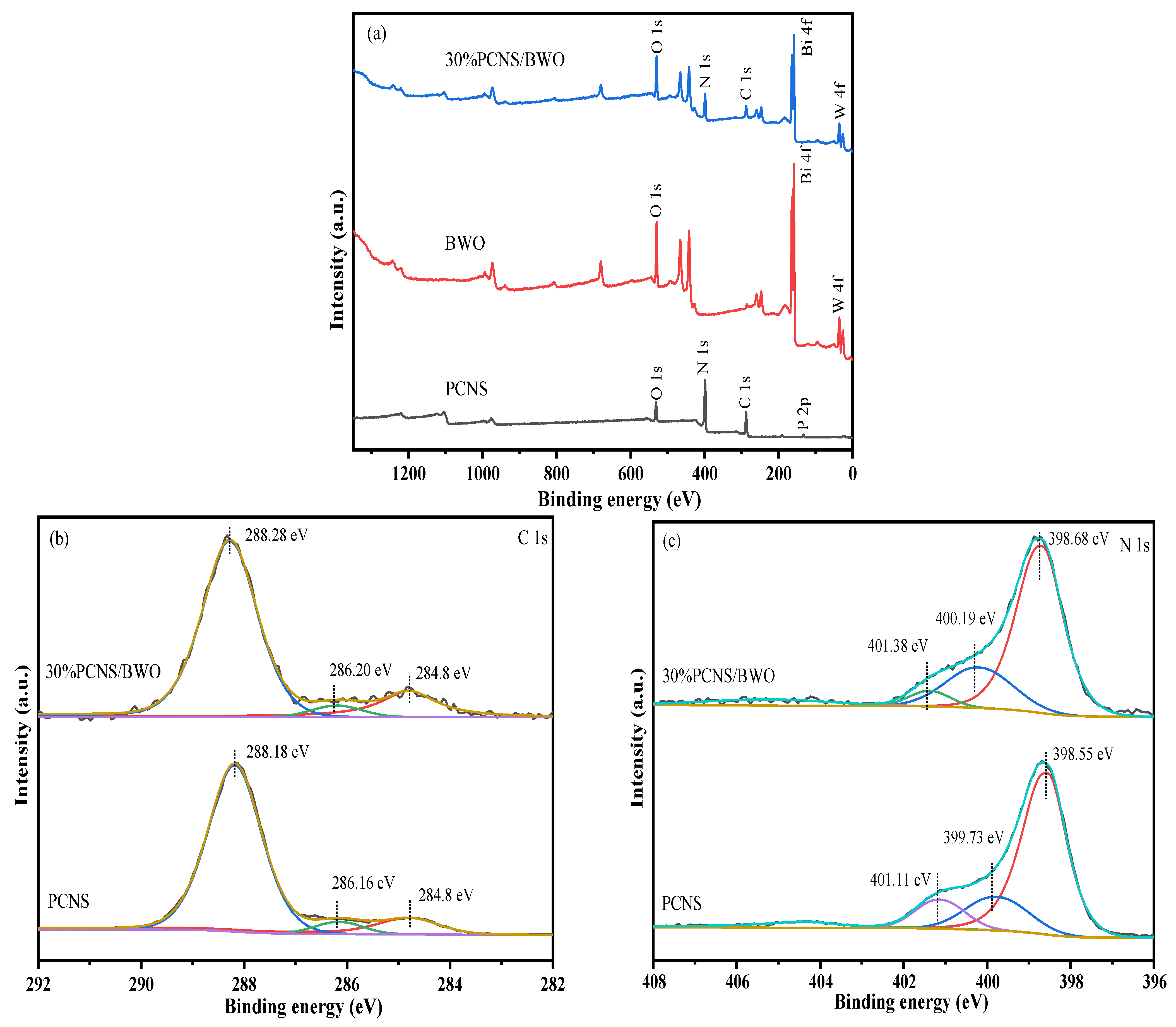
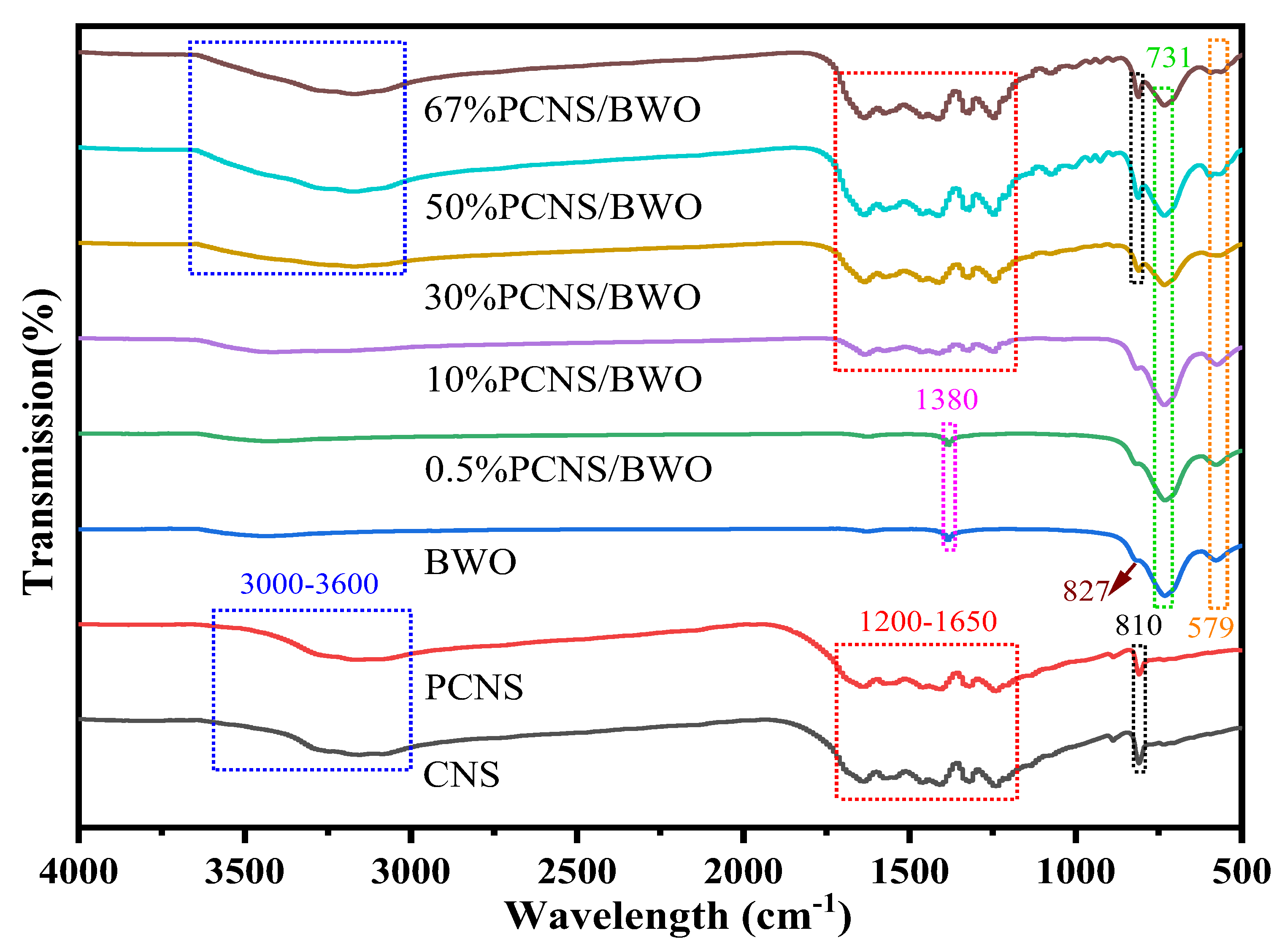
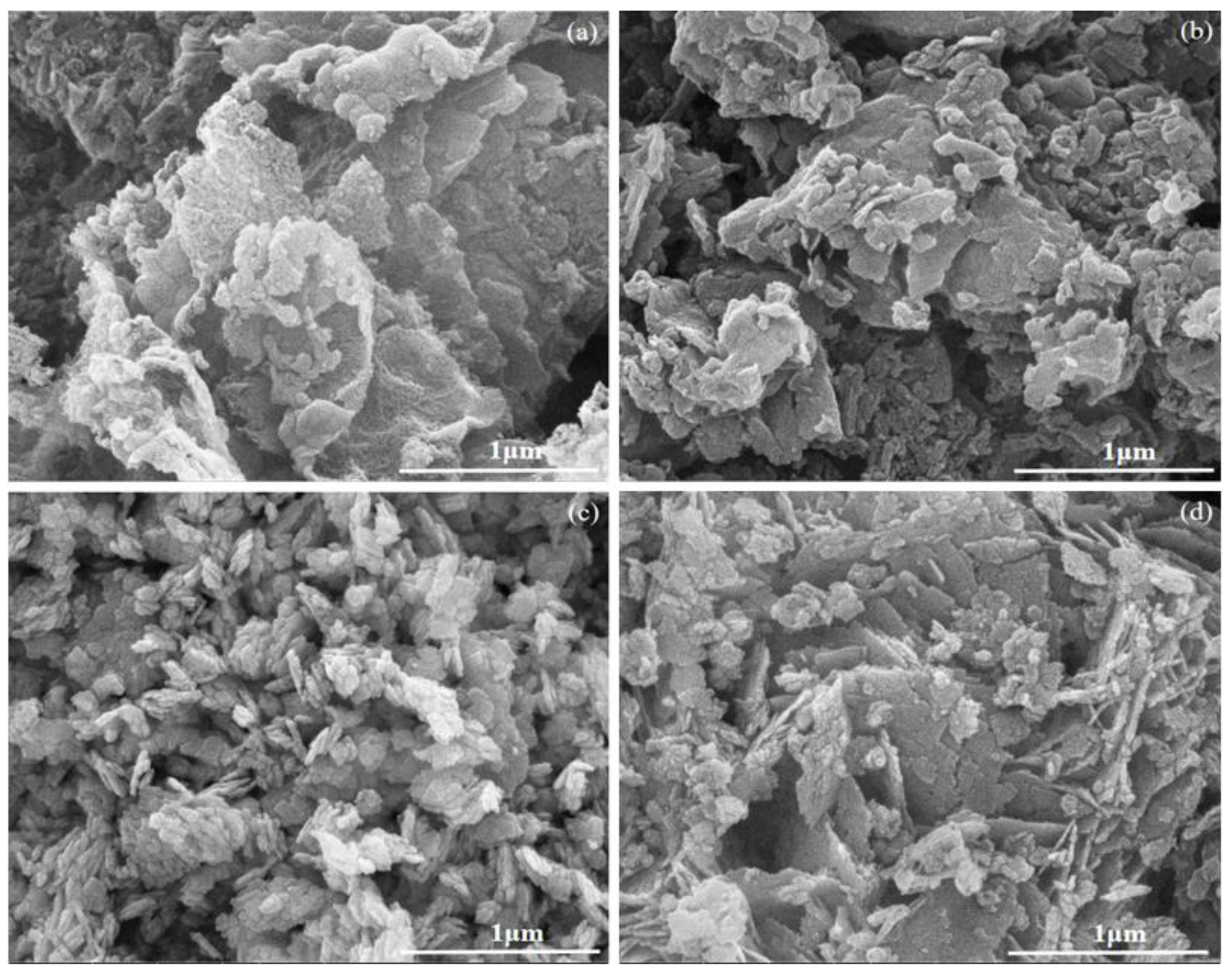
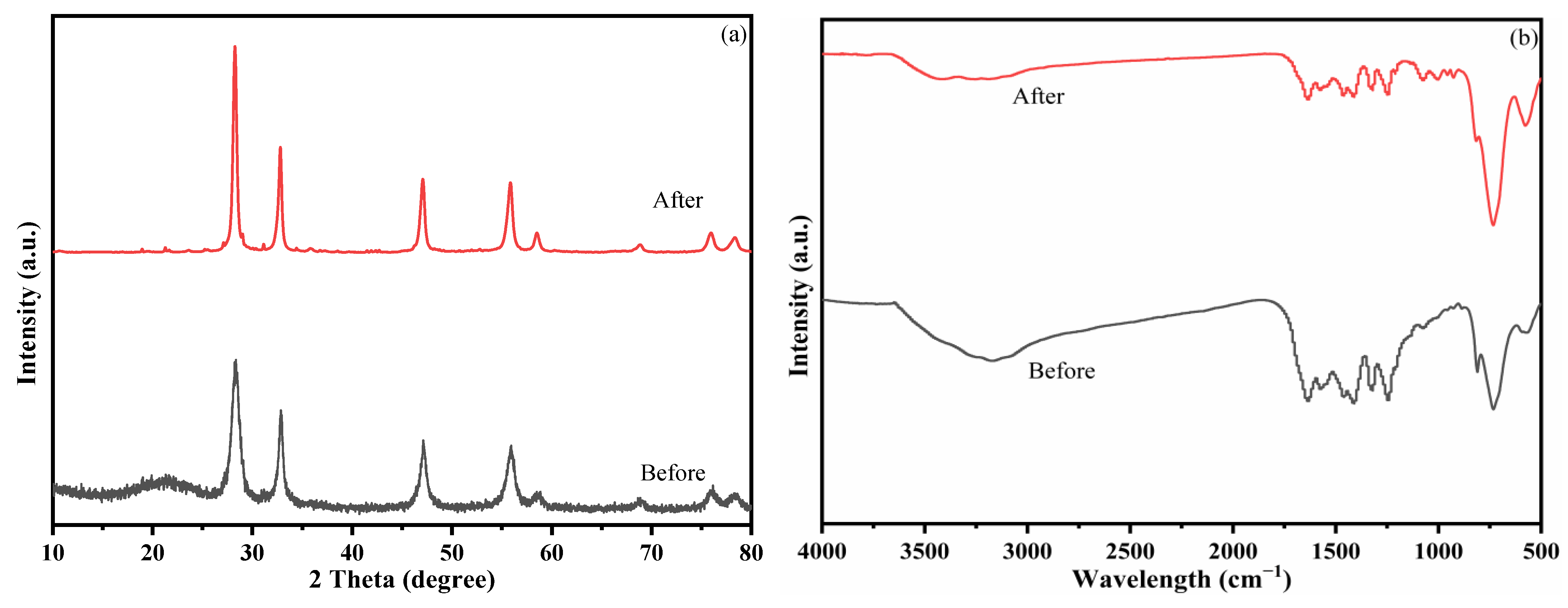
3.1. Characterization
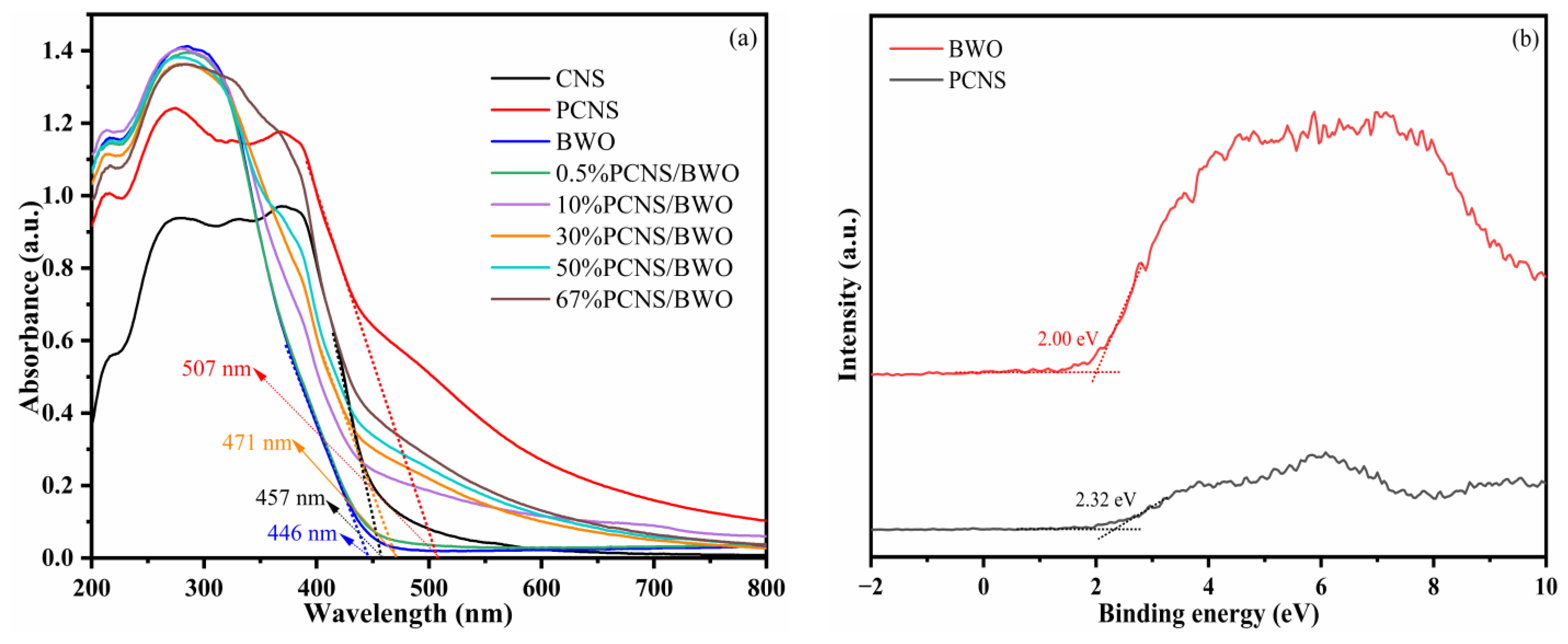
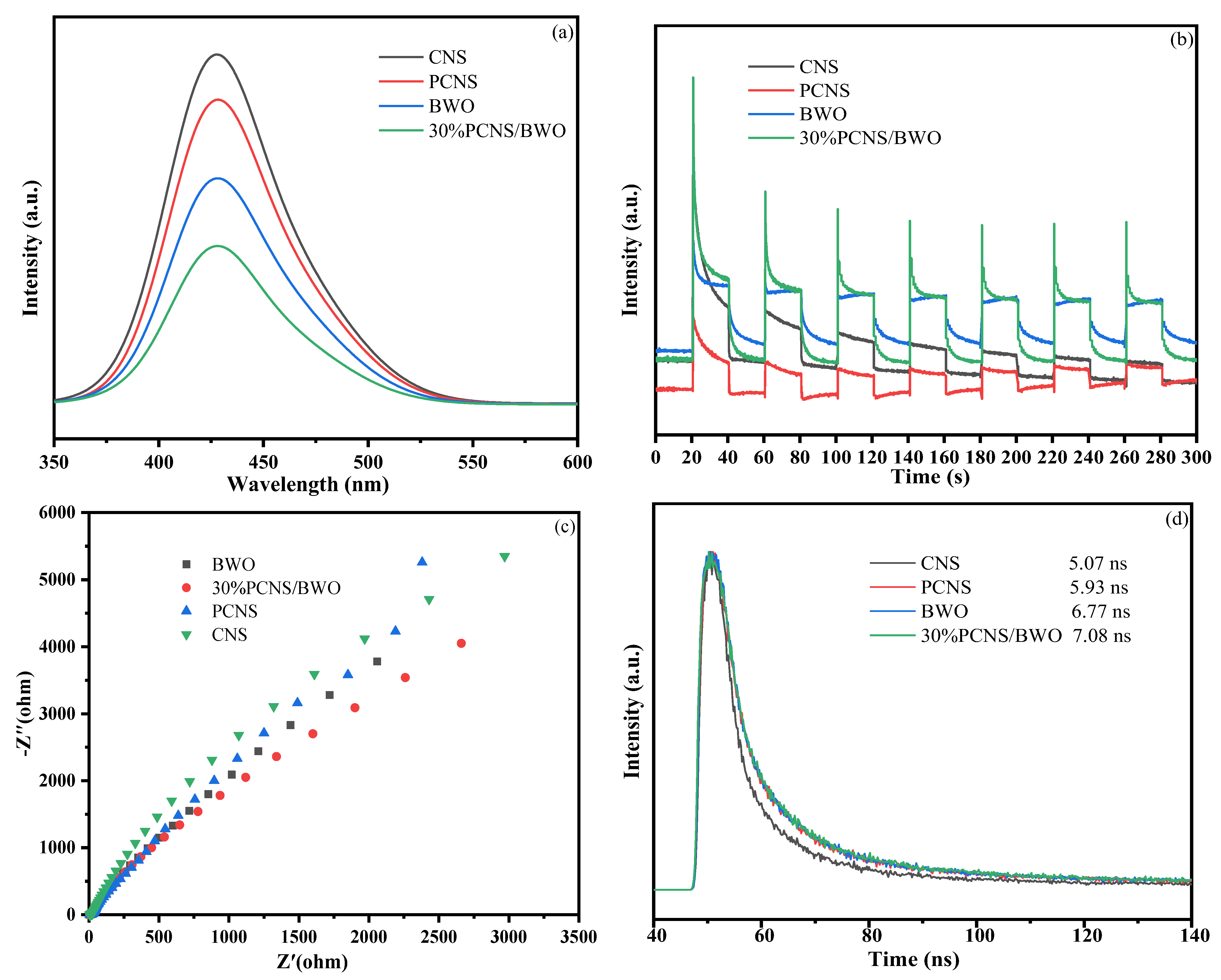
3.2. Optical Properties
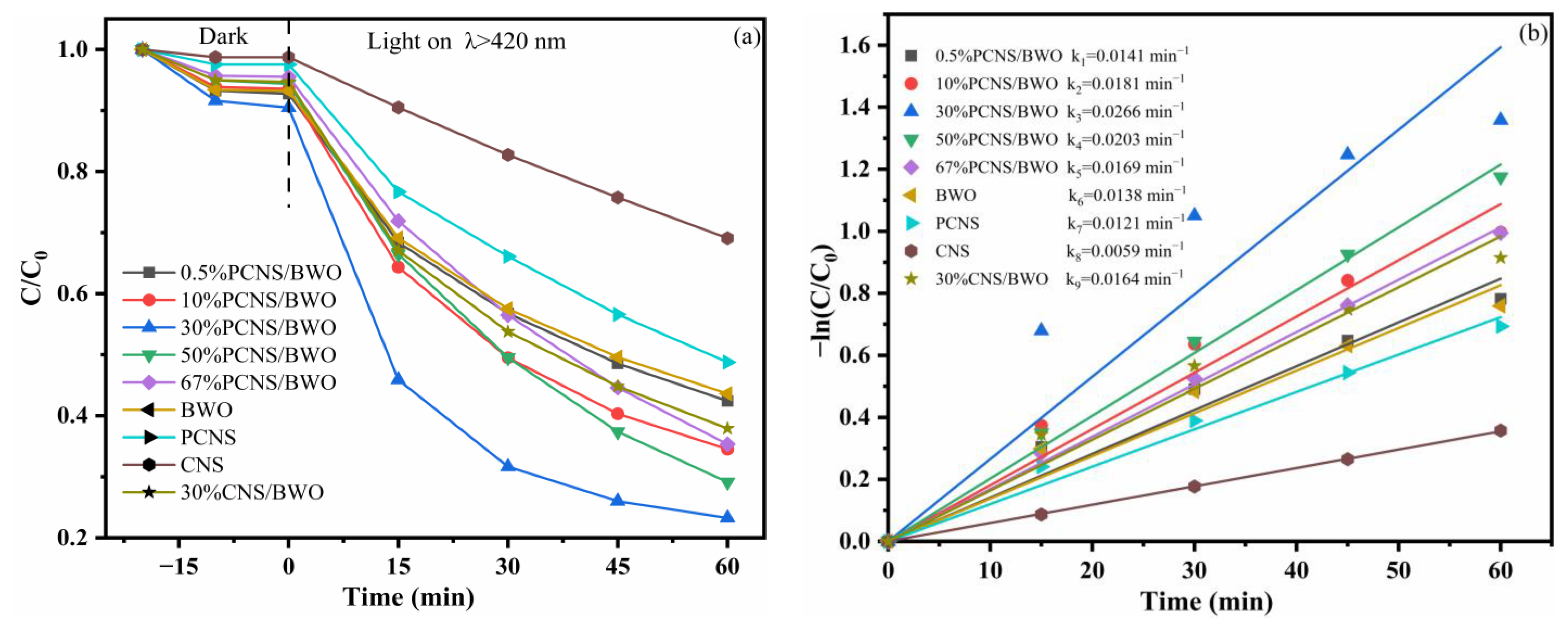
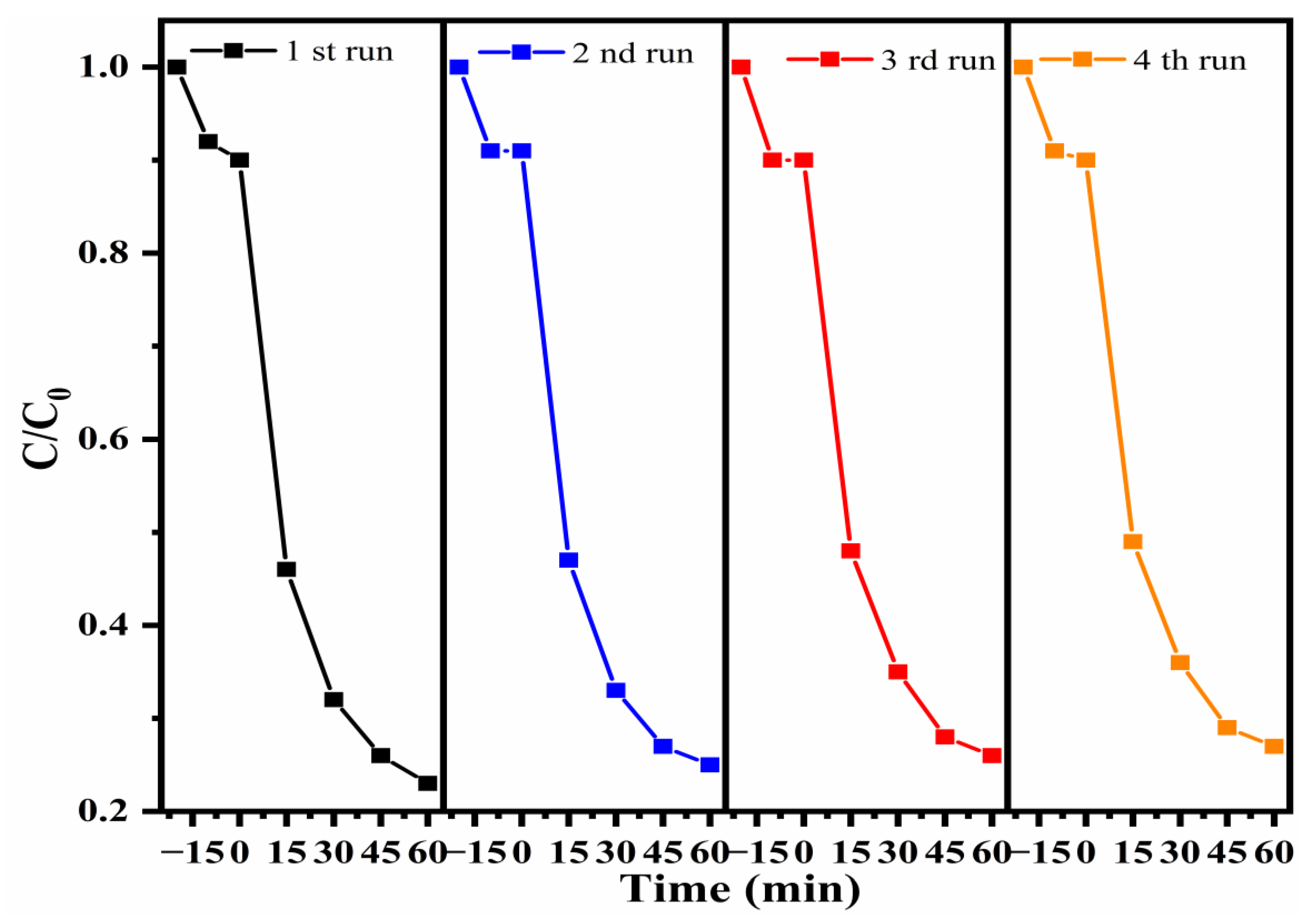
3.3. Study of Photocatalytic Performance
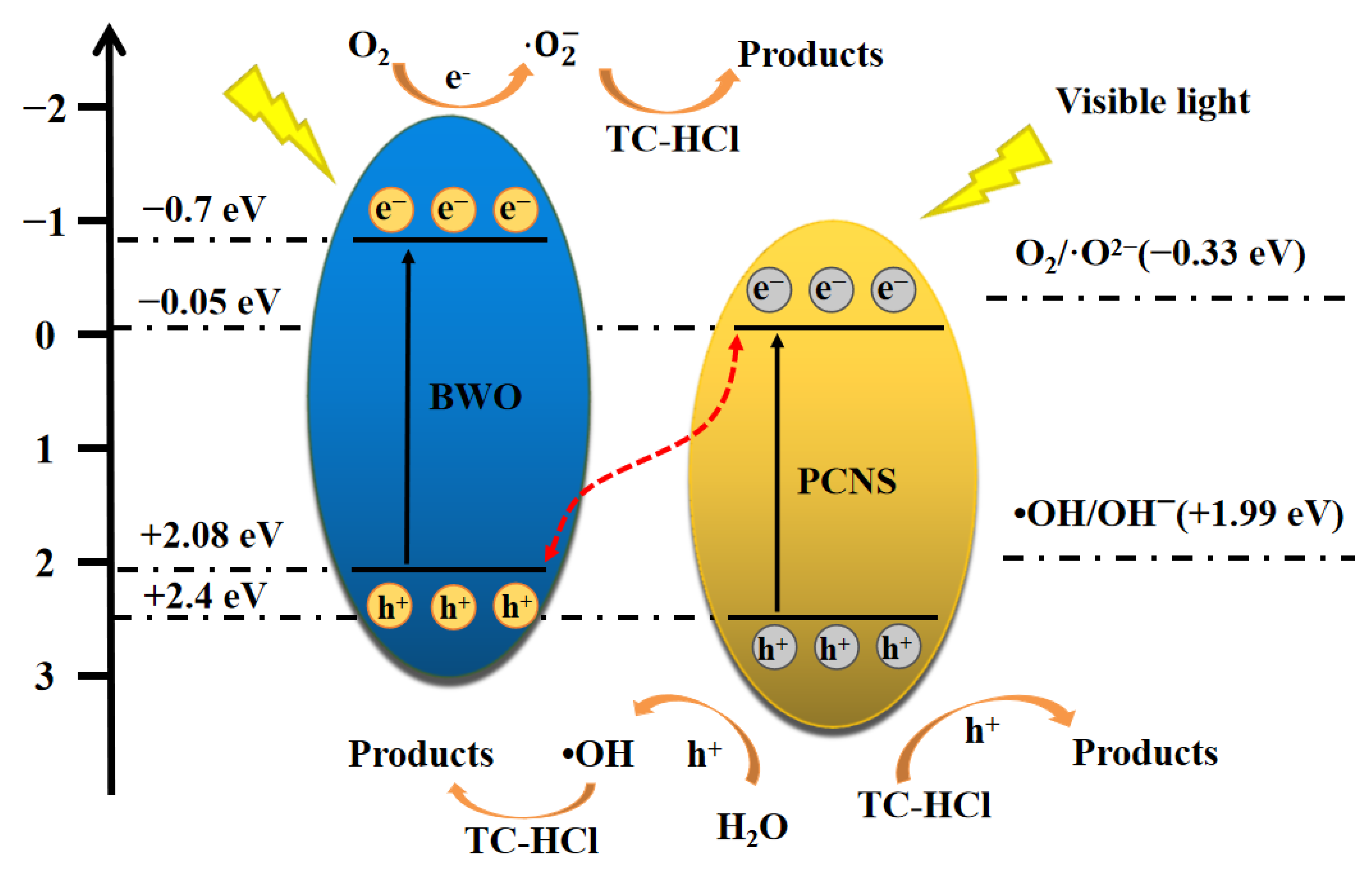
3.4. Mechanism and Pathway of Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, Z.P.; Zhang, L.; Ma, X.; Zhang, Y.H.; Shi, F.N. Design of Z-scheme g-C3N4/BC/Bi25FeO40 photocatalyst with unique electron transfer channels for efficient degradation of tetracycline hydrochloride waste. Chemosphere 2022, 289, 133262. [Google Scholar] [CrossRef] [PubMed]
- ZZhang, Z.; Chen, Y.; Wang, P.; Wang, Z.; Zuo, C.; Chen, W.Q.; Ao, T.Q. Facile fabrication of N-doped hierarchical porous carbons derived from soft-templated ZIF-8 for enhanced adsorptive removal of tetracycline hydrochloride from water. J. Hazard. Mater. 2022, 423, 127103. [Google Scholar] [CrossRef] [PubMed]
- YYuan, L.J.; Wang, Z.H.; Gu, F.B. Efficient degradation of tetracycline hydrochloride by direct Z-scheme HKUST-1@ m-BiVO4 catalysts with self-produced H2O2 under both dark and light. J. Environ. Chem. Eng. 2022, 10, 107964. [Google Scholar] [CrossRef]
- LLiu, K.Y.; Zheng, F.D.; Xiao, Y.; Fang, J.Y.; Zhao, C.F.; Zhao, N.; Zhao, C.M.; Zhang, W.H.; Zhang, W.X.; Qiu, R.L. High Fe utilization efficiency and low toxicity of Fe3C@ Fe0 loaded biochar for removing of tetracycline hydrochloride in wastewater. J. Clean. Prod. 2022, 353, 131630. [Google Scholar] [CrossRef]
- XXie, J.L.; Luo, X.; Chen, L.; Gong, X.B.; Zhang, L.R.; Tian, J. ZIF-8 derived boron, nitrogen co-doped porous carbon as metal-free peroxymonosulfate activator for tetracycline hydrochloride degradation: Performance, mechanism and biotoxicity. Chem. Eng. J. 2022, 440, 135760. [Google Scholar] [CrossRef]
- LLi, Y.C.; Yu, B.; Hu, Z.Q.; Wang, H. Construction of direct Z-scheme SnS2@ ZnIn2S4@ kaolinite heterostructure photocatalyst for efficient photocatalytic degradation of tetracycline hydrochloride. Chem. Eng. J. 2022, 429, 132105. [Google Scholar] [CrossRef]
- GGu, W.X.; Li, Q.; Zhu, H.Y.; Zou, L.Y. Facile interface engineering of hierarchical flower spherical-like Bi-metal–organic framework microsphere/Bi2MoO6 heterostructure for high-performance visible–light photocatalytic tetracycline hydrochloride degradation. J. Colloid Interf. Sci. 2022, 606, 1998–2010. [Google Scholar] [CrossRef]
- SShi, Z.; Zhang, Y.; Shen, X.F.; Duoerkun, G.; Zhu, B.; Zhang, L.S.; Li, M.Q.; Chen, Z.G. Fabrication of g-C3N4/BiOBr heterojunctions on carbon fibers as weaveable photocatalyst for degrading tetracycline hydrochloride under visible light. Chem. Eng. J. 2020, 386, 124010. [Google Scholar] [CrossRef]
- OOrimolade, B.O.; Idris, A.O.; Feleni, U.; Mamba, B. Recent advances in degradation of pharmaceuticals using Bi2WO6 mediated photocatalysis—A comprehensive review. Environ. Pollut. 2021, 289, 117891. [Google Scholar] [CrossRef]
- ZZhu, Z.; Wan, S.P.; Zhao, Y.X.; Gu, Y.X.; Wang, Y.B.; Qin, Y.; Zhang, Z.H.; Ge, X.L.; Zhong, Q.; Bu, Y.F. Recent advances in bismuth-based multimetal oxide photocatalysts for hydrogen production from water splitting: Competitiveness, challenges, and future perspectives. Mater. Rep. Energy 2021, 1, 100019. [Google Scholar]
- Zhu, D.D.; Zhou, Q.X. Novel Bi2WO6 modified by N-doped graphitic carbon nitride photocatalyst for efficient photocatalytic degradation of phenol under visible light. Appl. Catal. B Environ. 2020, 268, 118426. [Google Scholar] [CrossRef]
- Chen, Y.L.; Zhang, F.F.; Guan, S.Y.; Shi, W.; Wang, X.Y.; Huang, C.T.; Chen, Q. Visible light degradation of tetracycline by hierarchical nanoflower structured fluorine-doped Bi2WO6. Mat. Sci. Semicon. Proc. 2022, 140, 106385. [Google Scholar] [CrossRef]
- Kang, Z.H.; Qin, N.; Lin, E.Z.; Wu, J.; Yuan, B.W.; Bao, D.H. Effect of Bi2WO6 nanosheets on the ultrasonic degradation of organic dyes: Roles of adsorption and piezocatalysis. J. Clean. Prod. 2020, 261, 121125. [Google Scholar] [CrossRef]
- Yang, A.M.; Han, Y.; Li, S.S.; Xing, H.W.; Pan, Y.H.; Liu, W.X. Synthesis and comparison of photocatalytic properties for Bi2WO6 nanofibers and hierarchical microspheres. J. Alloy. Compd. 2017, 695, 915–921. [Google Scholar] [CrossRef]
- Wang, J.W.; Zhang, W.K.; Zhang, X.B.; Wang, F.M.; Yang, Y.Q.; Lv, G.J. Enhanced photocatalytic ability and easy retrievable photocatalysts of Bi2WO6 quantum dots decorated magnetic carbon nano-onions. J. Alloy. Compd. 2020, 826, 154217. [Google Scholar] [CrossRef]
- Chen, T.; Liu, L.Z.; Hu, C.; Huang, H.W. Recent advances on Bi2WO6-based photocatalysts for environmental and energy applications. Chin. J. Cata. 2021, 42, 1413–1438. [Google Scholar] [CrossRef]
- Ahmad, I.; Shukrullah, S.; Naz, M.; Ahmad, M.; Ahmed, E.; Liu, Y.; Hussain, A.; Iqbal, S.; Ullah, S. Recent advances and challenges in 2D/2D heterojunction photocatalysts for solar fuels applications. Adv. Colloid. Interfac. 2022, 304, 102661. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Sun, H.G.; Tian, Z.X.; Zhao, Z.X.; Li, P. Z-scheme 2D/2D WS2/Bi2WO6 heterostructures with enhanced photocatalytic performance. Appl. Catal. A Gen. 2022, 631, 118485. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, K.L.; Han, X.X.; Zhao, Q.; Zhang, Y.Y.; Qi, M.; Wang, D.J.; Fu, F. 2D/2D type-II Cu2ZnSnS4/Bi2WO6 heterojunctions to promote visible-light-driven photo-Fenton catalytic activity. Chin. J. Cata. 2020, 41, 503–513. [Google Scholar] [CrossRef]
- Guan, Z.L.; Li, X.M.; Wu, Y.; Chen, Z.; Huang, X.D.; Wang, D.B.; Yang, Q.; Liu, J.L.; Tian, S.H.; Chen, X.Y.; et al. AgBr nanoparticles decorated 2D/2D GO/Bi2WO6 photocatalyst with enhanced photocatalytic performance for the removal of tetracycline hydrochloride. Chem. Eng. J. 2021, 410, 128283. [Google Scholar] [CrossRef]
- Yuan, L.; Weng, B.; Colmenares, J.C.; Sun, Y.G.; Xu, Y.J. Multichannel charge transfer and mechanistic insight in metal decorated 2D–2D Bi2WO6–TiO2 cascade with enhanced photocatalytic performance. Small 2017, 13, 1702253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, P.F.; Yang, L.; Zhou, B.H.; Pan, J. Electrostatic self-assembly of 2D/2D Bi2WO6/ZnIn2S4 heterojunction with enhanced photocatalytic degradation of tetracycline hydrochloride. J. Solid. State. Chem. 2022, 314, 123408. [Google Scholar] [CrossRef]
- Khedr, T.M.; Wang, K.; Kowalski, D.; El-Sheikh, S.M.; Abdeldayem, H.M.; Ohtani, B.; Kowalska, E. Bi2WO6-based Z-scheme photocatalysts: Principles, mechanisms and photocatalytic applications. J. Environ. Chem. Eng. 2022, 10, 107838. [Google Scholar] [CrossRef]
- Zeng, Y.S.; Yin, Q.; Liu, Z.; Dong, H. Attapulgite-interpenetrated g-C3N4/Bi2WO6 quantum-dots Z-scheme heterojunction for 2-mercaptobenzothiazole degradation with mechanism insight. Chem. Eng. J. 2022, 435, 134918. [Google Scholar] [CrossRef]
- Long, G.Y.; Ding, J.F.; Xie, L.H.; Sun, R.Z.; Chen, M.X.; Zhou, Y.F.; Huang, X.Y.; Han, G.R.; Li, Y.J.; Zhao, W.R. Fabrication of mediator-free g-C3N4/Bi2WO6 Z-scheme with enhanced photocatalytic reduction dechlorination performance of 2, 4-DCP. Appl. Surf. Sci. 2018, 455, 1010–1018. [Google Scholar] [CrossRef]
- Zhang, M.J.; Zhang, Y.; Tang, L.; Zeng, G.M.; Wang, J.J.; Zhu, Y.; Feng, C.Y.; Deng, Y.C.; He, W.Z. Ultrathin Bi2WO6 nanosheets loaded g-C3N4 quantum dots: A direct Z-scheme photocatalyst with enhanced photocatalytic activity towards degradation of organic pollutants under wide spectrum light irradiation. J. Colloid Interf. Sci. 2019, 539, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, S.; Sahoo, D.P.; Parida, K. Recent advances in anion doped g-C3N4 photocatalysts: A review. Carbon 2021, 172, 682–711. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, H.L.; Yao, W.Q.; Chen, K.; Chen, D.M. One step synthesis of P-doped g-C3N4 with the enhanced visible light photocatalytic activity. Appl. Surf. Sci. 2018, 430, 309–315. [Google Scholar] [CrossRef]
- Zhang, L.G.; Chen, X.F.; Guan, J.; Jiang, Y.J.; Hou, T.G.; Mu, X.D. Facile synthesis of phosphorus doped graphitic carbon nitride polymers with enhanced visible-light photocatalytic activity. Mater. Res. Bull. 2013, 48, 3485–3491. [Google Scholar] [CrossRef]
- Uddin, A.; Rauf, A.; Wu, T.; Khan, R.; Yu, Y.L.; Tan, L.; Jiang, F.; Chen, H. In2O3/oxygen doped g-C3N4 towards photocatalytic BPA degradation: Balance of oxygen between metal oxides and doped g-C3N4. J. Colloid Interf. Sci. 2021, 602, 261–273. [Google Scholar] [CrossRef]
- Jia, J.K.; Zhang, X.R.; Jiang, C.Y.; Huang, W.X.; Wang, Y.P. Visible-light-driven nitrogen-doped carbon quantum dots decorated g-C3N4/Bi2WO6 Z-scheme composite with enhanced photocatalytic activity and mechanism insight. J. Alloy. Compd. 2020, 835, 155180. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, J.S.; Zhao, L.; Wu, B.Y.; Zhang, B.L.; Zhang, P.P.; Zhang, S.G.; Dong, C.F. Boosting exciton dissociation and charge transfer in P-doped 2D porous g-C3N4 for enhanced H2 production and molecular oxygen activation. Ceram. Int. 2022, 48, 4031–4046. [Google Scholar] [CrossRef]
- Su, C.Y.; Zhou, Y.Z.; Zhang, L.L.; Yu, X.H.; Gao, S.; Sun, X.J.; Cheng, C.; Liu, Q.Q.; Yang, J. Enhanced n→π* electron transition of porous P-doped g-C3N4 nanosheets for improved photocatalytic H2 evolution performance. Ceram. Int. 2020, 46, 8444–8451. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chai, H.; Xu, P.; Wang, P.; Wang, X.J.; Shen, T.Y.; Zheng, Q.Z.; Zhang, G.S. One-step synthesis of a 3D/2D Bi2WO6/g-C3N4 heterojunction for effective photocatalytic degradation of atrazine: Kinetics, degradation mechanisms and ecotoxicity. Sep. Purif. Technol. 2022, 288, 120609. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.J.; Tang, L.; Wang, J.J.; Zhu, Y.; Feng, C.Y.; Deng, Y.C.; He, W.Z.; Hu, Y. Platinum like cocatalysts tungsten carbide loaded hollow tubular g-C3N4 achieving effective space separation of carriers to degrade antibiotics. Chem. Eng. J. 2020, 391, 123487. [Google Scholar] [CrossRef]
- Du, F.Y.; Lai, Z.; Tang, H.Y.; Wang, H.Y.; Zhao, C.X. Construction of dual Z-scheme Bi2WO6/g-C3N4/black phosphorus quantum dots composites for effective bisphenol A degradation. J. Environ. Sci. 2023, 124, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.Y.; Xue, W.H.; Dong, S.; Liu, E.Z.; Li, H.; Xu, K.Z. Construction of S-scheme Bi2WO6/g-C3N4 heterostructure nanosheets with enhanced visible-light photocatalytic degradation for ammonium dinitramide. J. Hazard. Mater. 2021, 412, 125217. [Google Scholar] [CrossRef]
- Chen, F.; Li, D.; Luo, B.F.; Chen, M.; Shi, W.D. Two-dimensional heterojunction photocatalysts constructed by graphite-like C3N4 and Bi2WO6 nanosheets: Enhanced photocatalytic activities for water purification. J. Alloy. Compd. 2017, 694, 193–200. [Google Scholar] [CrossRef]
- Guo, W.; Fan, K.; Zhang, J.J.; Xu, C.J. 2D/2D Z-scheme Bi2WO6/Porous-g-C3N4 with synergy of adsorption and visible-light-driven photodegradation. Appl. Surf. Sci. 2018, 447, 125–134. [Google Scholar] [CrossRef]
- Wang, J.J.; Tang, L.; Zeng, G.M.; Deng, Y.C.; Liu, Y.N.; Wang, L.L.; Zhou, Y.Y.; Guo, Z.; Wang, J.J.; Zhang, C. Atomic scale g-C3N4/Bi2WO6 2D/2D heterojunction with enhanced photocatalytic degradation of ibuprofen under visible light irradiation. Appl. Catal. B Environ. 2017, 209, 285–294. [Google Scholar] [CrossRef]
- Wang, Y.N.; Zhang, C.; Zeng, Y.Q.; Cai, W.; Wan, S.P.; Li, Z.Y.; Zhang, S.L.; Zhong, Q. Ag and MOFs-derived hollow Co3O4 decorated in the 3D g-C3N4 for creating dual transferring channels of electrons and holes to boost CO2 photoreduction performance. J. Colloid Interface Sci. 2022, 609, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Zhang, Q.; Zou, Y.Z.; Cao, Y.H.; Cui, W.; Dong, F.; Zhou, Y. Ti3C2 MXene modified g-C3N4 with enhanced visible-light photocatalytic performance for NO purification. J. Colloid Interface Sci. 2020, 575, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.J.; Jing, Z.Y.; Chen, X.M.; Wang, Z.X.; Ren, H.F.; Huang, L.H. Preparation and photocatalytic performance study of dual Z-scheme Bi2Zr2O7/g-C3N4/Ag3PO4 for removal of antibiotics by visible-light. J. Environ. Sci. 2023, 125, 349–361. [Google Scholar] [CrossRef]
- Dai, Z.R.; Lian, J.J.; Sun, Y.S.; Li, L.; Zhang, H.; Hu, N.; Ding, D.X. Fabrication of g-C3N4/Sn3O4/Ni electrode for highly efficient photoelectrocatalytic reduction of U (VI). Chem. Eng. J. 2022, 433, 133766. [Google Scholar] [CrossRef]
- Jiang, L.B.; Yuan, X.Z.; Zeng, G.M.; Liang, J.; Wu, Z.B.; Yu, H.B.; Mo, D.; Wang, H.; Xiao, Z.H.; Zhou, C.Y. Nitrogen self-doped g-C3N4 nanosheets with tunable band structures for enhanced photocatalytic tetracycline degradation. J. Colloid Interface Sci. 2019, 536, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.X.; Li, D.G.; Liu, Y.; Li, R.B.; Chen, P.; Liu, H.J.; Lv, W.Y.; Liu, G.G.; Feng, Y.P. Ultrathin Ag2WO4-coated P-doped g-C3N4 nanosheets with remarkable photocatalytic performance for indomethacin degradation. J. Hazard. Mater. 2020, 392, 122355. [Google Scholar] [CrossRef]
- Qiao, M.; Fu, L.J.; Barcelo, D. Removal of polycyclic aromatic hydrocarbons by g-C3N4 nanosheets under visible light irradiation and effect of typical co-existence substances in river water. Process Saf. Environ. Prot. 2022, 159, 376–381. [Google Scholar] [CrossRef]
- Zhao, T.; Zheng, M.; Fu, C.X.; Li, G.R.; Xiong, Y.; Qiu, W.H.; Zhang, T.; Zhang, J.; Zheng, C.M. Effect of low-level H2O2 and Fe (II) on the UV treatment of tetracycline antibiotics and the toxicity of reaction solutions to zebrafish embryos. Chem. Eng. J. 2020, 394, 125021. [Google Scholar] [CrossRef]
- Sun, Q.; Zhao, Y.; Zhang, J.; Sheng, J. Efficient degradation of antibiotics over Co (II)-doped Bi2MoO6 nanohybrid via the synergy of peroxymonosulfate activation and photocatalytic reaction under visible irradiation. Chemosphere 2022, 302, 134807. [Google Scholar] [CrossRef]
- Du, C.Y.; Zhang, Z.; Tan, S.Y.; Yu, G.L.; Chen, H.; Zhou, L.; Yu, L.; Su, Y.H.; Zhang, Y.; Deng, F.F.; et al. Construction of Z-scheme g-C3N4/MnO2/GO ternary photocatalyst with enhanced photodegradation ability of tetracycline hydrochloride under visible light radiation. Environ. Res. 2021, 200, 111427. [Google Scholar] [CrossRef]
- Wang, C.L.; Sun, X.J.; Zhang, M.X.; Wang, Y.B.; Tan, Z.H.; Li, J.; Xi, B.D. Ultrasound-assisted room-temperature in situ precipitation synthesis of BC doped Bi4O5Br2 for enhanced photocatalytic activity in pollutants degradation under visible light. J. Alloy. Compd. 2021, 889, 161609. [Google Scholar] [CrossRef]
- He, X.; Lei, L.; Wen, J.L.; Zhao, Y.F.; Cui, L.Z.; Wu, G.P. One-pot synthesis of C-doping and defects co-modified g-C3N4 for enhanced visible-light photocatalytic degradation of bisphenol A. J. Environ. Chem. Eng. 2022, 10, 106911. [Google Scholar] [CrossRef]
- Wang, T.T.; Zheng, J.Y.; Cai, J.J.; Liu, Q.Q.; Zhang, X.X. Visible-light-driven photocatalytic degradation of dye and antibiotics by activated biochar composited with K+ doped g-C3N4: Effects, mechanisms, actual wastewater treatment and disinfection. Sci. Total Environ. 2022, 839, 155955. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kanth, S.K.; Xu, S.S.; Han, N.; Zhu, L.; Fan, L.L.; Liu, C.; Zhang, Q.F. Visible light driven g-C3N4/Bi4NbO8X (XCl, Br) heterojunction photocatalyst for the degradation of organic pollutants. J. Alloy. Compd. 2022, 895, 162576. [Google Scholar] [CrossRef]
- Huang, K.L.; Li, C.H.; Wang, L.; Wang, W.T.; Meng, X.C. Layered Ti3C2 MXene and silver co-modified g-C3N4 with enhanced visible light-driven photocatalytic activity. Chem. Eng. J. 2021, 425, 131493. [Google Scholar] [CrossRef]
- Deng, Y.C.; Tang, L.; Zeng, G.M.; Wang, J.J.; Zhou, Y.Y.; Wang, J.J.; Tang, J.; Wang, L.L.; Feng, C.Y. Facile fabrication of mediator-free Z-scheme photocatalyst of phosphorous-doped ultrathin graphitic carbon nitride nanosheets and bismuth vanadate composites with enhanced tetracycline degradation under visible light. J. Colloid Interface Sci. 2018, 509, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.C.; Ou, R.P.; Yu, H.B.; Zhao, Y.H.; Lu, Y.; Huo, M.X.; Huo, H.L.; Wang, X.Z. Engineering of g-C3N4 nanoparticles/WO3 hollow microspheres photocatalyst with Z-scheme heterostructure for boosting tetracycline hydrochloride degradation. Sep. Purif. Technol. 2021, 255, 117646. [Google Scholar] [CrossRef]
- Yang, Y.J.; Bian, Z.Y.; Zhang, L.; Wang, H. Bi@BiOx(OH)y modified oxidized g-C3N4 photocatalytic removal of tetracycline hydrochloride with highly effective oxygen activation. J. Hazard. Mater. 2022, 427, 127866. [Google Scholar] [CrossRef]
- Chen, Y.W.; Cui, K.P.; Cui, M.S.; Liu, T.; Chen, X.; Chen, Y.H.; Nie, X.B.; Xu, Z.J.; Li, C.X. Insight into the degradation of tetracycline hydrochloride by non-radical-dominated peroxymonosulfate activation with hollow shell-core Co@ NC: Role of cobalt species. Sep. Purif. Technol. 2022, 289, 120662. [Google Scholar] [CrossRef]
- Zheng, J.F.; Li, L.; Dai, Z.Y.; Tian, Y.L.; Fang, T.; Xin, S.T.; Zhu, B.C.; Liu, Z.J.; Nie, L.H. A novel Fenton-like catalyst of Ag3PO4/g-C3N4: Its performance and mechanism for tetracycline hydrochloride degradation in dark. Appl. Surf. Sci. 2022, 571, 151305. [Google Scholar] [CrossRef]
- Chen, Q.L.; Yang, W.L.; Zhu, J.J.; Fu, L.C.; Li, D.Y.; Zhou, L.P. Enhanced visible light photocatalytic activity of g-C3N4 decorated ZrO2-x nanotubes heterostructure for degradation of tetracycline hydrochloride. J. Hazard. Mater. 2020, 384, 121275. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Xue, P.; Wang, R.M.; Liu, L.; Liu, E.Z.; Bai, X.; Fan, J.; Hu, X.Y. Synergistic effects in simultaneous photocatalytic removal of Cr (VI) and tetracycline hydrochloride by Z-scheme Co3O4/Ag/Bi2WO6 heterojunction. Appl. Surf. Sci. 2019, 483, 677–687. [Google Scholar] [CrossRef]
- Hu, P.B.; Yao, C.F.; Yang, L.; Xin, Y.M.; Miao, Y.Q. Boosted photodegradation of tetracycline hydrochloride over Z-scheme MIL-88B (Fe)/Bi2WO6 composites under visible light. Colloids Surf. Physicochem. Eng. Aspects 2021, 627, 127248. [Google Scholar] [CrossRef]
- Yin, S.; Chen, Y.; Gao, C.; Hu, Q.S.; Li, M.; Ding, Y.; Di, J.; Xia, J.X.; Li, H.M. In-situ preparation of MIL-125(Ti)/Bi2WO6 photocatalyst with accelerating charge carriers for the photodegradation of tetracycline hydrochloride. J. Photochem. Photobiol. A Chem. 2020, 387, 112149. [Google Scholar] [CrossRef]
- Peng, R.F.; Kang, Y.X.; Deng, X.H.; Zhang, X.M.; Xie, F.; Wang, H.Y.; Liu, W.W. Topotactic transformed face-to-face heterojunction of BiOCl/Bi2WO6 for improved tetracycline photodegradation. J. Environ. Chem. Eng. 2021, 9, 106750. [Google Scholar] [CrossRef]
- Ren, X.Z.; Wu, K.; Qin, Z.G.; Zhao, X.C.; Yang, H. The construction of type II heterojunction of Bi2WO6/BiOBr photocatalyst with improved photocatalytic performance. J. Alloy. Compd. 2019, 788, 102–109. [Google Scholar] [CrossRef]
- Cui, C.; Guo, R.H.; Xiao, H.Y.; Ren, E.H.; Song, Q.S.; Xiang, C.; Lai, X.X.; Lan, J.W.; Jiang, S.X. Bi2WO6/Nb2CTx MXene hybrid nanosheets with enhanced visible-light-driven photocatalytic activity for organic pollutants degradation. Appl. Surf. Sci. 2020, 505, 144595. [Google Scholar] [CrossRef]
- Lu, X.Y.; Che, W.J.; Hu, X.F.; Wang, Y.; Zhang, A.T.; Deng, F.; Luo, S.L.; Dionysiou, D.D. The facile fabrication of novel visible-light-driven Z-scheme CuInS2/Bi2WO6 heterojunction with intimate interface contact by in situ hydrothermal growth strategy for extraordinary photocatalytic performance. Chem. Eng. J. 2019, 356, 819–829. [Google Scholar] [CrossRef]
- Li, S.J.; Chen, J.L.; Hu, S.W.; Wang, H.L.; Jiang, W.; Chen, X.B. Facile construction of novel Bi2WO6/Ta3N5 Z-scheme heterojunction nanofibers for efficient degradation of harmful pharmaceutical pollutants. Chem. Eng. J. 2020, 402, 126165. [Google Scholar] [CrossRef]
- Xue, W.J.; Huang, D.L.; Li, J.; Zeng, G.M.; Deng, R.; Yang, Y.; Chen, S.; Li, Z.H.; Gong, X.M.; Li, B. Assembly of AgI nanoparticles and ultrathin g-C3N4 nanosheets codecorated Bi2WO6 direct dual Z-scheme photocatalyst: An efficient, sustainable and heterogeneous catalyst with enhanced photocatalytic performance. Chem. Eng. J. 2019, 373, 1144–1157. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, X.; Sun, X.; Li, D.; Xie, W.; Mao, Y.; Liu, Z.; Liu, Z. 2D/2D Phosphorus-Doped g-C3N4/Bi2WO6 Direct Z-Scheme Heterojunction Photocatalytic System for Tetracycline Hydrochloride (TC-HCl) Degradation. Int. J. Environ. Res. Public Health 2022, 19, 14935. https://doi.org/10.3390/ijerph192214935
Yin X, Sun X, Li D, Xie W, Mao Y, Liu Z, Liu Z. 2D/2D Phosphorus-Doped g-C3N4/Bi2WO6 Direct Z-Scheme Heterojunction Photocatalytic System for Tetracycline Hydrochloride (TC-HCl) Degradation. International Journal of Environmental Research and Public Health. 2022; 19(22):14935. https://doi.org/10.3390/ijerph192214935
Chicago/Turabian StyleYin, Xudong, Xiaojie Sun, Dehao Li, Wenyu Xie, Yufeng Mao, Zhenghui Liu, and Zhisen Liu. 2022. "2D/2D Phosphorus-Doped g-C3N4/Bi2WO6 Direct Z-Scheme Heterojunction Photocatalytic System for Tetracycline Hydrochloride (TC-HCl) Degradation" International Journal of Environmental Research and Public Health 19, no. 22: 14935. https://doi.org/10.3390/ijerph192214935
APA StyleYin, X., Sun, X., Li, D., Xie, W., Mao, Y., Liu, Z., & Liu, Z. (2022). 2D/2D Phosphorus-Doped g-C3N4/Bi2WO6 Direct Z-Scheme Heterojunction Photocatalytic System for Tetracycline Hydrochloride (TC-HCl) Degradation. International Journal of Environmental Research and Public Health, 19(22), 14935. https://doi.org/10.3390/ijerph192214935






