The Role of Bacterial Polyhydroalkanoate (PHA) in a Sustainable Future: A Review on the Biological Diversity
Abstract
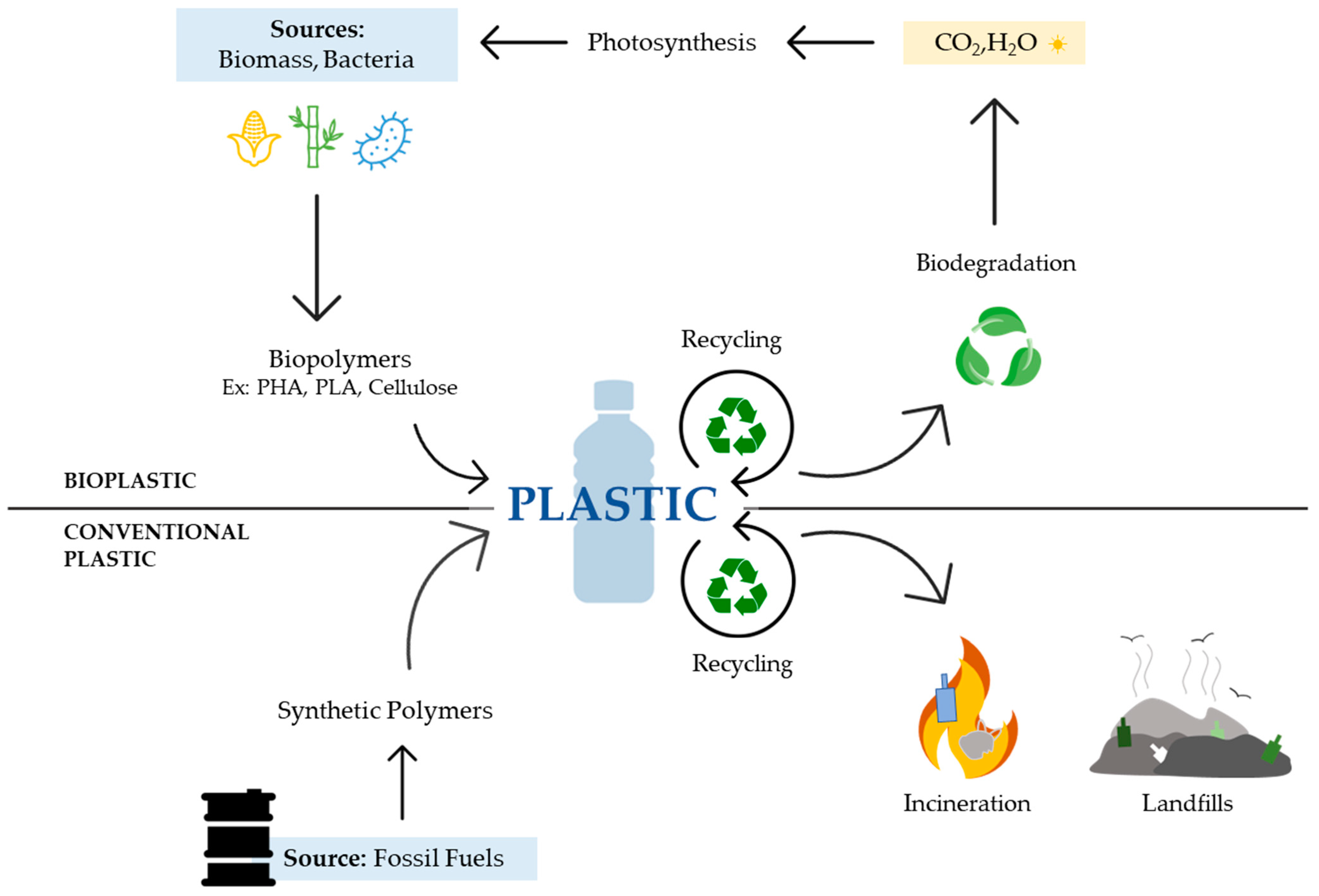
1. Brief Introduction to Plastics
2. COVID-19 Pandemic Increased the Demand for Plastics
3. Biopolymers as Substitutes for Petroleum-Based Plastics
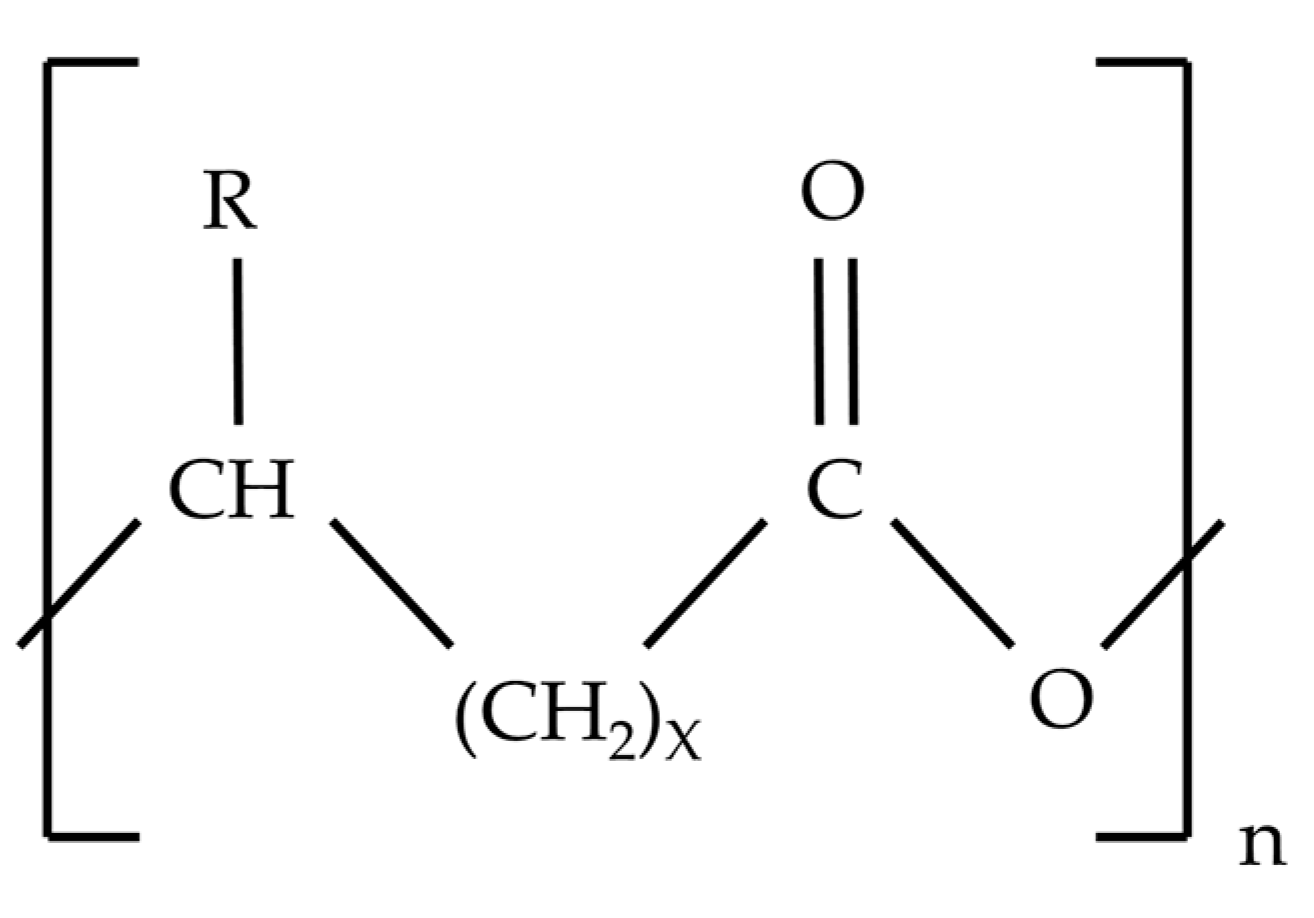
4. Polyhydroxyalkanoates (PHA) and Bacterial Producers
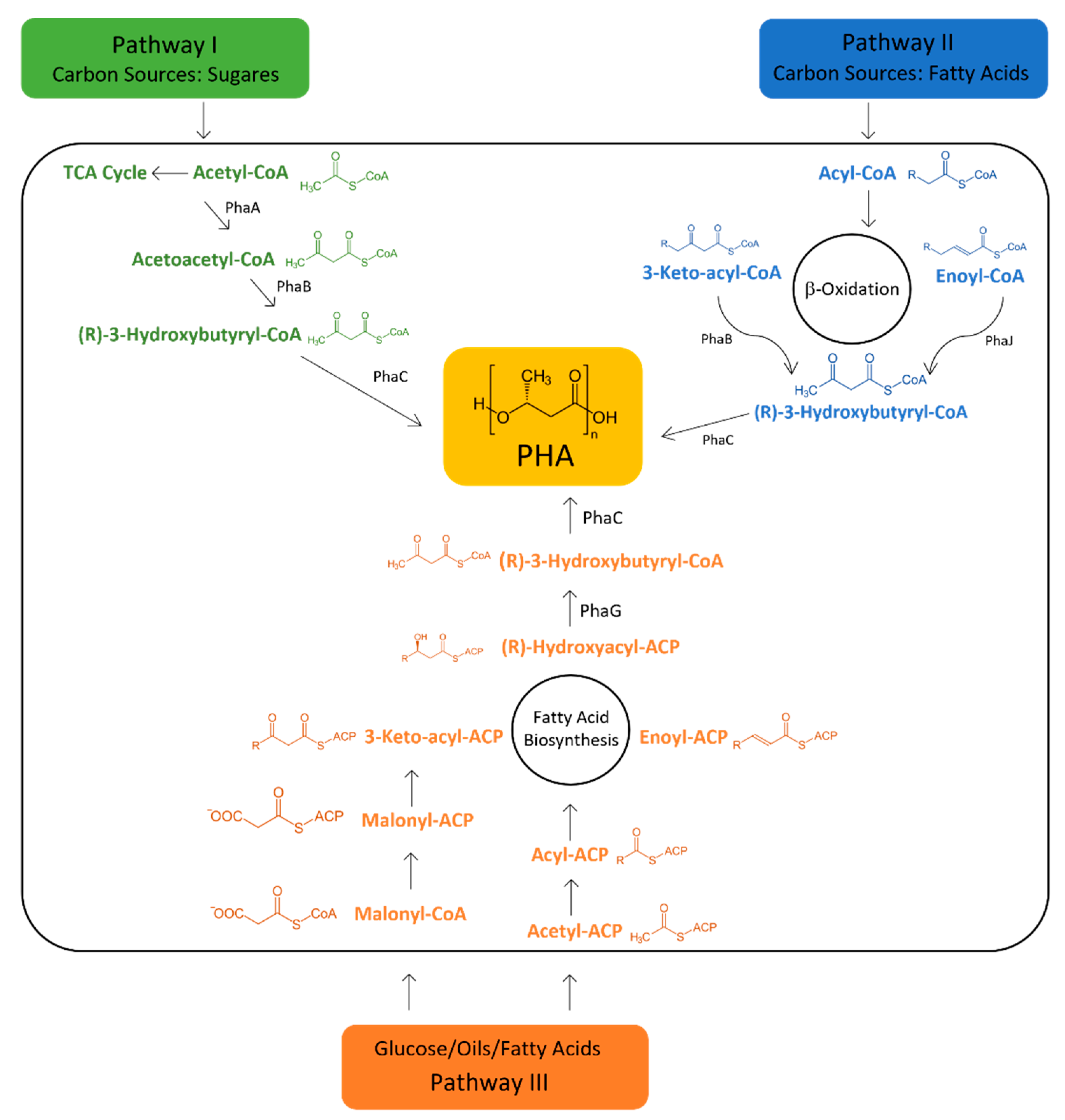
5. Feedstocks for PHA Production
6. Metabolic Pathways Involved in PHA Synthesis
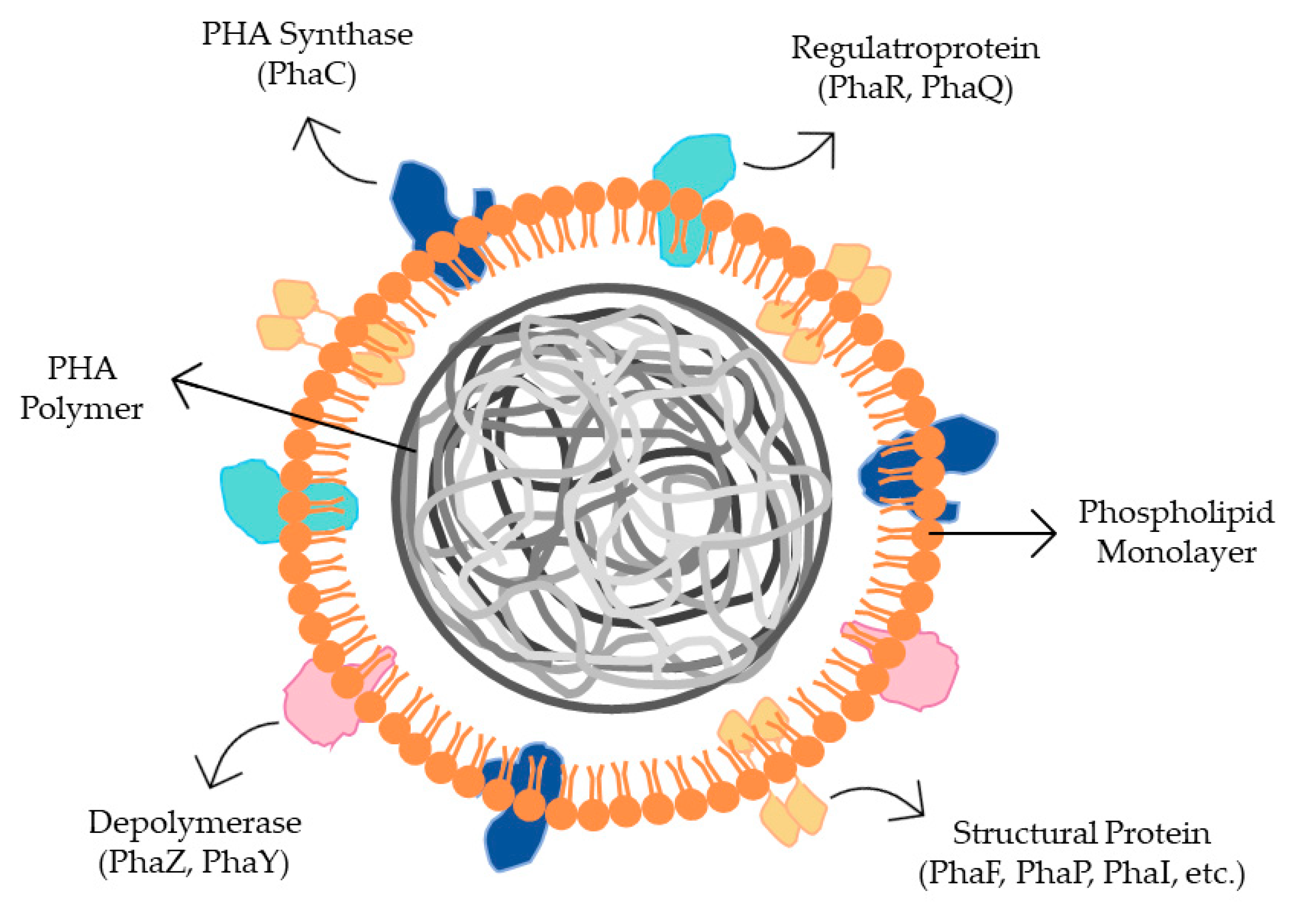
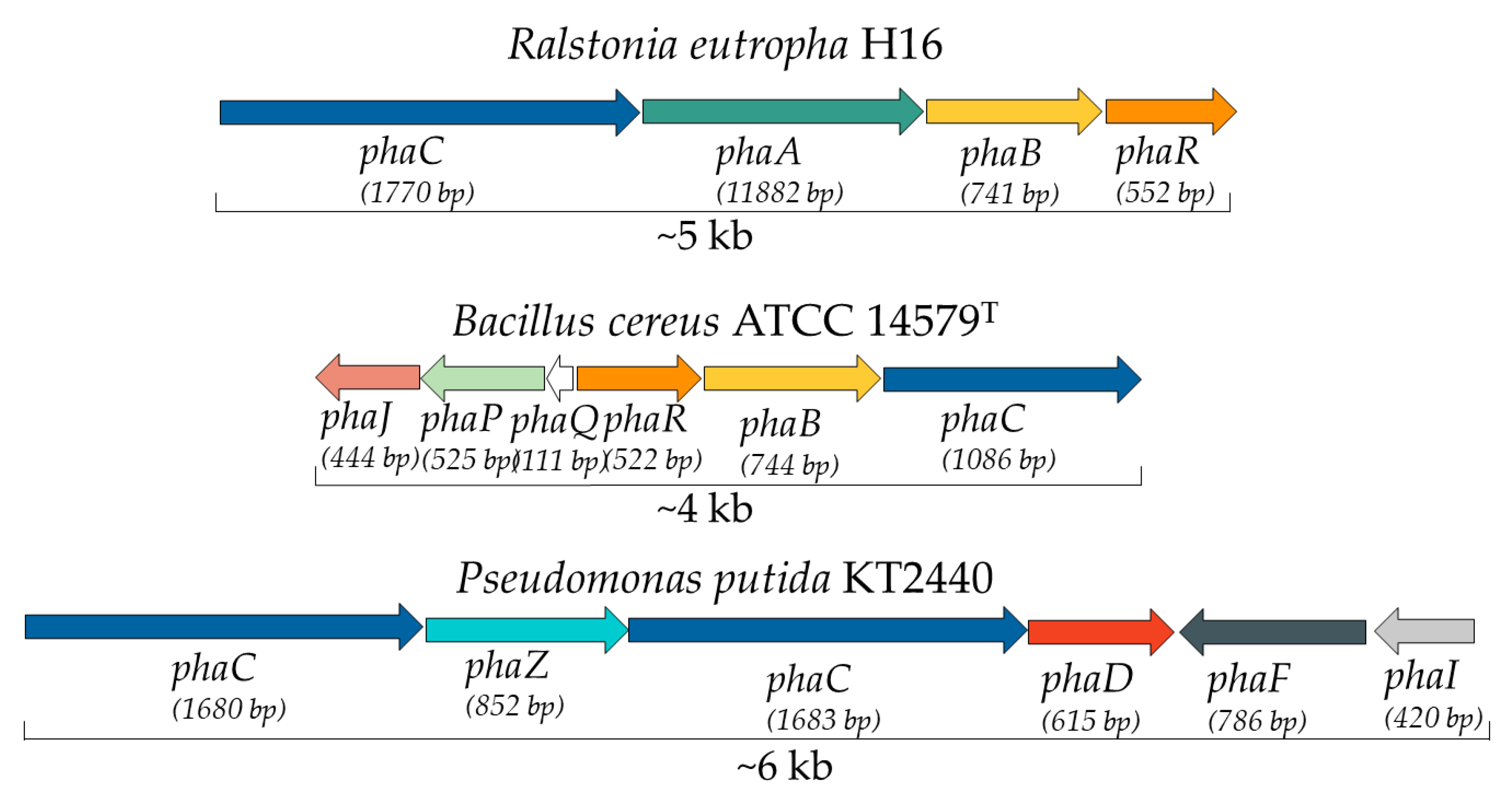
7. Bacterial Genes and Enzymes Involved in PHA Metabolism
8. Strategies for Sustainable Production of PHA
8.1. Upstream Processes in PHA Production
8.2. Downstream Processes
9. Current and Future Applications of PHA-Bacterial Based in Our Daily Life
10. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sidek, I.S.; Draman, S.F.S.; Abdullah, S.R.S.; Anuar, N. Current Development on Bioplastics and Its Future Prospects: An Introductory Review. INWASCON Technol. Mag. 2019, 1, 3–8. [Google Scholar] [CrossRef]
- Baekeland, L.H. The Synthesis, Constitution, and Uses of Bakelite. Ind. Eng. Chem. 1909, 1, 149–161. [Google Scholar] [CrossRef]
- Vu, D.H.; Åkesson, D.; Taherzadeh, M.J.; Ferreira, J.A. Recycling Strategies for Polyhydroxyalkanoate-Based Waste Materials: An Overview. Bioresour. Technol. 2020, 298, 122393. [Google Scholar] [CrossRef]
- Reddy, C.S.K.; Ghai, R.; Rashmi; Kalia, V.C. Polyhydroxyalkanoates: An Overview. Bioresour. Technol. 2003, 87, 137–146. [Google Scholar] [CrossRef]
- UNEP. Plastics: A Roadmap for Sustainability; UNEP: Nairobi, Kenya, 2018; ISBN 9789280737059. [Google Scholar]
- Sakthipriya, N. Plastic Waste Management: A Road Map to Achieve Circular Economy and Recent Innovations in Pyrolysis. Sci. Total Environ. 2022, 809, 151160. [Google Scholar] [CrossRef]
- Krueger, M.C.; Harms, H.; Schlosser, D. Prospects for Microbiological Solutions to Environmental Pollution with Plastics. Appl. Microbiol. Biotechnol. 2015, 99, 8857–8874. [Google Scholar] [CrossRef] [PubMed]
- Melchor-Martínez, E.M.; Macías-Garbett, R.; Alvarado-Ramírez, L.; Araújo, R.G.; Sosa-Hernández, J.E.; Ramírez-Gamboa, D.; Parra-Arroyo, L.; Alvarez, A.G.; Monteverde, R.P.B.; Cazares, K.A.S.; et al. Towards a Circular Economy of Plastics: An Evaluation of the Systematic Transition to a New Generation of Bioplastics. Polymers 2022, 14, 1203. [Google Scholar] [CrossRef] [PubMed]
- North, E.J.; Halden, R.U. Plastics and Environmental Health: The Road Ahead. Rev. Environ. Health 2013, 28, 1–8. [Google Scholar] [CrossRef]
- Kumar, M.; Rathour, R.; Singh, R.; Sun, Y.; Pandey, A.; Gnansounou, E.; Andrew Lin, K.Y.; Tsang, D.C.W.; Thakur, I.S. Bacterial Polyhydroxyalkanoates: Opportunities, Challenges, and Prospects. J. Clean. Prod. 2020, 263, 121500. [Google Scholar] [CrossRef]
- Alimba, C.G.; Faggio, C. Microplastics in the Marine Environment: Current Trends in Environmental Pollution and Mechanisms of Toxicological Profile. Environ. Toxicol. Pharmacol. 2019, 68, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Karbalaei, S.; Hanachi, P.; Walker, T.R.; Cole, M. Occurrence, Sources, Human Health Impacts and Mitigation of Microplastic Pollution. Environ. Sci. Pollut. Res. 2018, 25, 36046–36063. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef] [PubMed]
- Zantis, L.J.; Carroll, E.L.; Nelms, S.E.; Bosker, T. Marine Mammals and Microplastics: A Systematic Review and Call for Standardisation. Environ. Pollut. 2021, 269, 116142. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Song, L.; Li, Y.; Li, C.; Zhang, S. Dietary Intake of Microplastics Impairs Digestive Performance, Induces Hepatic Dysfunction, and Shortens Lifespan in the Annual Fish Nothobranchius guentheri. Biogerontology 2023. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Shi, H.; Li, X.; Gao, C.; Liu, R. Combined Toxicity of Micro/Nanoplastics Loaded with Environmental Pollutants to Organisms and Cells: Role, Effects, and Mechanism. Environ. Int. 2023, 171, 107711. [Google Scholar] [CrossRef]
- Karan, H.; Funk, C.; Grabert, M.; Oey, M.; Hankamer, B. Green Bioplastics as Part of a Circular Bioeconomy. Trends Plant. Sci. 2019, 24, 237–249. [Google Scholar] [CrossRef]
- Favaro, L.; Basaglia, M.; Casella, S. Improving Polyhydroxyalkanoate Production from Inexpensive Carbon Sources by Genetic Approaches: A Review. Biofuels Bioprod. Biorefining 2019, 13, 208–227. [Google Scholar] [CrossRef]
- Meereboer, K.W.; Misra, M.; Mohanty, A.K. Review of Recent Advances in the Biodegradability of Polyhydroxyalkanoate (PHA) Bioplastics and Their Composites. Green Chem. 2020, 22, 5519–5558. [Google Scholar] [CrossRef]
- Parashar, N.; Hait, S. Science of the Total Environment Plastics in the Time of COVID-19 Pandemic: Protector or Polluter? Sci. Total Environ. 2021, 759, 144274. [Google Scholar] [CrossRef]
- Rampal, L.; Liew, B.S. Coronavirus Disease (COVID-19) Pandemic. Med. J. Malays. 2020, 75, 95–97. [Google Scholar]
- Abouzid, M.; El-Sherif, D.M.; al Naggar, Y.; Alshehri, M.M.; Alothman, S.; El-Seedi, H.R.; Trabelsi, R.; Ibrahim, O.M.; Temraz, E.H.; Buimsaedah, A.; et al. Investigating the Current Environmental Situation in the Middle East and North Africa (MENA) Region during the Third Wave of COVID-19 Pandemic: Urban vs. Rural Context. BMC Public Health 2022, 22, 177. [Google Scholar] [CrossRef] [PubMed]
- Vanapalli, K.R.; Sharma, H.B.; Ranjan, V.P.; Samal, B.; Bhattacharya, J.; Dubey, B.K.; Goel, S. Challenges and Strategies for Effective Plastic Waste Management during and Post COVID-19 Pandemic. Sci. Total Environ. 2021, 750, 141514. [Google Scholar] [CrossRef] [PubMed]
- Sarkodie, S.A.; Owusu, P.A. Impact of COVID-19 Pandemic on Waste Management. Environ. Dev. Sustain. 2021, 23, 7951–7960. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.S.; Sharif, S.; Masnoon, A.; Rashid, E. SARS-CoV-2 Pandemic-Induced PPE and Single-Use Plastic Waste Generation Scenario. Waste Manag. Res. 2021, 39, 3–17. [Google Scholar] [CrossRef]
- Dybas, C.L. Surgical Masks on the Beach. Oceanography 2020, 34, 12–14. [Google Scholar]
- Li, C.; Yuan, X.; Sun, Z.; Suvarna, M.; Hu, X.; Wang, X.; Ok, Y.S. Pyrolysis of Waste Surgical Masks into Liquid Fuel and Its Life-Cycle Assessment. Bioresour. Technol. 2022, 346, 126582. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, M.; Li, R. The COVID-19 Pandemic Reshapes the Plastic Pollution Research—A Comparative Analysis of Plastic Pollution Research before and during the Pandemic. Environ. Res. 2022, 208, 112634. [Google Scholar] [CrossRef]
- Gareiou, Z.; Chroni, C.; Kontoleon, K.; el Bachawati, M.; Saba, M.; Martin, R.H.; Zervas, E. Awareness of Citizens for the Single-Use Plastics: Comparison between a High-Income and an Upper-Middle-Income Economy of the Easter Mediterranean Region, Greece and Lebanon. Sustainability 2022, 14, 1912. [Google Scholar] [CrossRef]
- Moradali, M.F.; Rehm, B.H.A. Bacterial Biopolymers: From Pathogenesis to Advanced Materials. Nat. Rev. Microbiol. 2020, 18, 195–210. [Google Scholar] [CrossRef]
- Luengo, J.M.; García, B.; Sandoval, A.; Naharro, G.; Olivera, E.R. Bioplastics from Microorganisms. Curr. Opin. Microbiol. 2003, 6, 251–260. [Google Scholar] [CrossRef]
- Grujić, R.; Vujadinović, D.; Savanović, D. Biopolymers as Food Packaging Materials. In Advances in Applications of Industrial Biomaterials; Springer: Cham, Switzerland, 2017; pp. 139–160. [Google Scholar] [CrossRef]
- Choi, S.Y.; Cho, I.J.; Lee, Y.; Kim, Y.J.; Kim, K.J.; Lee, S.Y. Microbial Polyhydroxyalkanoates and Nonnatural Polyesters. Adv. Mater. 2020, 32, e1907138. [Google Scholar] [CrossRef]
- Raza, Z.A.; Noor, S.; Khalil, S. Recent Developments in the Synthesis of Poly(Hydroxybutyrate) Based Biocomposites. Biotechnol. Prog. 2019, 35, e2855. [Google Scholar] [CrossRef] [PubMed]
- Muneer, F.; Rasul, I.; Azeem, F.; Siddique, M.H.; Zubair, M.; Nadeem, H. Microbial Polyhydroxyalkanoates (PHAs): Efficient Replacement of Synthetic Polymers. J. Polym. Environ. 2020, 28, 2301–2323. [Google Scholar] [CrossRef]
- Kawashima, N.; Yagi, T.; Kojima, K. How Do Bioplastics and Fossil-Based Plastics Play in a Circular Economy? Macromol. Mater. Eng. 2019, 304, 1–14. [Google Scholar] [CrossRef]
- Lemoigne, M. Produit de Deshydratation et de Polymerisation de l’acide β-Oxybutyrique. Bull. Soc. Chim. Biol. 1926, 8, 770–782. [Google Scholar]
- Obruca, S.; Sedlacek, P.; Koller, M. The Underexplored Role of Diverse Stress Factors in Microbial Biopolymer Synthesis. Bioresour. Technol. 2021, 326, 124767. [Google Scholar] [CrossRef]
- Wu, H.; Chen, J.; Chen, G.Q. Engineering the Growth Pattern and Cell Morphology for Enhanced PHB Production by Escherichia coli. Appl. Microbiol. Biotechnol. 2016, 100, 9907–9916. [Google Scholar] [CrossRef]
- Obruca, S.; Sedlacek, P.; Krzyzanek, V.; Mravec, F.; Hrubanova, K.; Samek, O.; Kucera, D.; Benesova, P.; Marova, I. Accumulation of Poly(3-Hydroxybutyrate) Helps Bacterial Cells to Survive Freezing. PLoS ONE 2016, 11, e0157778. [Google Scholar] [CrossRef]
- Reinecke, F.; Steinbüchel, A. Ralstonia eutropha Strain H16 as Model Organism for PHA Metabolism and for Biotechnological Production of Technically Interesting Biopolymers. J. Mol. Microbiol. Biotechnol. 2008, 16, 91–108. [Google Scholar] [CrossRef]
- Mohanrasu, K.; Guru Raj Rao, R.; Dinesh, G.H.; Zhang, K.; Sudhakar, M.; Pugazhendhi, A.; Jeyakanthan, J.; Ponnuchamy, K.; Govarthanan, M.; Arun, A. Production and Characterization of Biodegradable Polyhydroxybutyrate by Micrococcus luteus Isolated from Marine Environment. Int. J. Biol. Macromol. 2021, 186, 125–134. [Google Scholar] [CrossRef]
- Akar, A.; Akkaya, E.U.; Yesiladali, S.K.; Çelikyilmaz, G.; Çokgor, E.U.; Tamerler, C.; Orhon, D.; Çakar, Z.P. Accumulation of Polyhydroxyalkanoates by Microlunatus phosphovorus under Various Growth Conditions under Various Growth Conditions. J. Ind. Microbiol. Biotechnol. 2006, 33, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Haywoodt, G.W.; Anderson, A.J.; Williams, D.R.; Dawes, E.A.; Ewing, D.F. Accumulation of a Containing Primarily 3-Hydroxyvalerate from Simple Carbohydrate Substrates by Rhodococcus sp. NCIMB 40126. Int. J. Biol. Macromol. 1991, 13, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.J.; Williams, D.R.; Dawes, E.A.; Ewing, D.F. Biosynthesis of Poly(3-Hydroxybutyvate-Co-3-Hydroxyvalerate) in Rhodococcus ruber. Can. J. Microbiol. 1995, 41, 4–13. [Google Scholar] [CrossRef]
- Valappil, S.P.; Boccaccini, A.R.; Bucke, C.; Roy, I. Polyhydroxyalkanoates in Gram-Positive Bacteria: Insights from the Genera Bacillus and Streptomyces. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2007, 91, 1–17. [Google Scholar] [CrossRef]
- Tyagi, B.; Takkar, S.; Meena, R.; Thakur, I.S. Production of Polyhydroxybutyrate (PHB) by Parapedobacter sp. ISTM3 Isolated from Mawsmai Cave Utilizing Molasses as Carbon Source. Environ. Technol. Innov. 2021, 24, 101854. [Google Scholar] [CrossRef]
- Simonazzi, M.; Pezzolesi, L.; Galletti, P.; Gualandi, C.; Pistocchi, R.; de Marco, N.; Paganelli, Z.; Samorì, C. Production of Polyhydroxybutyrate by the Cyanobacterium cf. Anabaena sp. Int. J. Biol. Macromol. 2021, 191, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Samantaray, S.; Mallick, N. Impact of Various Stress Conditions on Poly-β-Hydroxybutyrate (PHB) Accumulation in Aulosira fertilissima CCC 444. Curr. Biotechnol. 2015, 4, 366–372. [Google Scholar] [CrossRef]
- Carr, N.G. The Occurrence of Poly-β-Hydroxybutyrate in the Blue-Green Alga, Chlorogloea fritschii. BBA-Biophys. Incl. Photosynth. 1966, 120, 308–310. [Google Scholar] [CrossRef]
- Bhati, R.; Mallick, N. Carbon Dioxide and Poultry Waste Utilization for Production of Polyhydroxyalkanoate Biopolymers by Nostoc muscorum Agardh: A Sustainable Approach. J. Appl. Phycol. 2016, 28, 161–168. [Google Scholar] [CrossRef]
- Vanessa, C.C.; da Silva, C.K.; Ana, L.T.; Jorge, A.V.C.; de Morais, M.G. Polyhydroxybutyrate Production by Spirulina sp. LEB 18 Grown under Different Nutrient Concentrations. Afr. J. Microbiol. Res. 2015, 9, 1586–1594. [Google Scholar] [CrossRef]
- Nishioka, M.; Nakai, K.; Miyake, M.; Asada, Y.; Taya, M. Production of Poly-β-Hydroxybutyrate by Thermophilic Cyanobacterium, Synechococcus sp. MA19, under Phosphate-Limited Conditions. Biotechnol. Lett. 2001, 23, 1095–1099. [Google Scholar] [CrossRef]
- Mendhulkar, V.D.; Shetye, L.A. Synthesis of Biodegradable Polymer Polyhydroxyalkanoate (PHA) in Cyanobacteria Synechococcus Elongates under Mixotrophic Nitrogen- and Phosphate-Mediated Stress Conditions. Ind. Biotechnol. 2017, 13, 85–93. [Google Scholar] [CrossRef]
- Pantazaki, A.A.; Tambaka, M.G.; Langlois, V.; Guerin, P.; Kyriakidis, D.A. Polyhydroxyalkanoate (PHA) Biosynthesis in Thermus thermophilus: Purification and Biochemical Properties of PHA Synthase. Mol. Cell. Biochem. 2003, 254, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Zhang, Y.; Xi, L.; Huo, F.; Zhao, J.; Li, J. Thermophilic Production of Polyhydroxyalkanoates by a Novel Aneurinibacillus Strain Isolated from Gudao Oilfield, China. J. Basic Microbiol. 2015, 55, 1125–1133. [Google Scholar] [CrossRef]
- Obruca, S.; Marova, I.; Melusova, S.; Mravcova, L. Production of Polyhydroxyalkanoates from Cheese Whey Employing Bacillus megaterium CCM 2037. Ann. Microbiol. 2011, 61, 947–953. [Google Scholar] [CrossRef]
- Sinaei, N.; Zare, D.; Azin, M. Production and Characterization of Poly 3-Hydroxybutyrate-Co-3-Hydroxyvalerate in Wheat Starch Wastewater and Its Potential for Nanoparticle Synthesis. Braz. J. Microbiol. 2021, 52, 561–573. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, S.G.; Cho, D.H.; Bhatia, S.K.; Gurav, R.; Yang, S.Y.; Yang, J.; Jeon, J.M.; Yoon, J.J.; Choi, K.Y.; et al. Finding of Novel Lactate Utilizing Bacillus sp. YHY22 and Its Evaluation for Polyhydroxybutyrate (PHB) Production. Int. J. Biol. Macromol. 2022, 201, 653–661. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, H.J.; Kim, S.H.; Suh, M.J.; Cho, J.Y.; Ham, S.; Jeon, J.M.; Yoon, J.J.; Bhatia, S.K.; Gurav, R.; et al. Screening of the Strictly Xylose-Utilizing Bacillus sp. SM01 for Polyhydroxybutyrate and Its Co-Culture with Cupriavidus necator NCIMB 11599 for Enhanced Production of PHB. Int. J. Biol. Macromol. 2021, 181, 410–417. [Google Scholar] [CrossRef]
- Jendrossek, D.; Selchow, O.; Hoppert, M. Poly(3-Hydroxybutyrate) Granules at the Early Stages of Formation Are Localized Close to the Cytoplasmic Membrane in Caryophanon latum. Appl. Environ. Microbiol. 2007, 73, 586–593. [Google Scholar] [CrossRef]
- Giedraityte, G.; Kalediene, L. Purification and Characterization of Polyhydroxybutyrate Produced from Thermophilic Geobacillus sp. AY 946034 Strain. Chemija 2015, 26, 38–45. [Google Scholar]
- Mohapatra, S.; Samantaray, D.P.; Samantaray, S.M.; Mishra, B.B.; Das, S.; Majumdar, S.; Pradhan, S.K.; Rath, S.N.; Rath, C.C.; Akthar, J.; et al. Structural and Thermal Characterization of PHAs Produced by Lysinibacillus sp. through Submerged Fermentation Process. Int. J. Biol. Macromol. 2016, 93, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.L.; Chua, H.; Fu Yu, P.H. Microbial Production of Polyhydroxyalkanoates by Bacteria Isolated from Oil Wastes. Appl. Biochem. Biotechnol. 2000, 84–86, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Lathwal, P.; Nehra, K.; Singh, M.; Rana, J.S. Characterization of Novel and Efficient Poly-3-Hydroxybutyrate (PHB) Producing Bacteria Isolated from Rhizospheric Soils. J. Polym. Environ. 2018, 26, 3437–3450. [Google Scholar] [CrossRef]
- Mercan, N.; Aslim, B.; Yuksekdag, Z.N.; Beyatli, Y. Production of Poly-β-Hydroxybutyrate (PHB) by Some Rhizobium Bacteria. Turk. J. Biol. 2002, 26, 215–219. [Google Scholar]
- Bustamante, D.; Segarra, S.; Tortajada, M.; Ramón, D.; del Cerro, C.; Auxiliadora Prieto, M.; Iglesias, J.R.; Rojas, A. In Silico Prospection of Microorganisms to Produce Polyhydroxyalkanoate from Whey: Caulobacter segnis DSM 29236 as a Suitable Industrial Strain. Microb. Biotechnol. 2019, 12, 487–501. [Google Scholar] [CrossRef]
- Ibrahim, M.H.A.; Lebbe, L.; Willems, A.; Steinbüchel, A. Chelatococcus thermostellatus sp. Nov., a New Thermophile for Bioplastic Synthesis: Comparative Phylogenetic and Physiological Study. AMB Express 2016, 6, 39. [Google Scholar] [CrossRef]
- Lee, S.M.; Cho, D.H.; Jung, H.J.; Kim, B.; Kim, S.H.; Bhatia, S.K.; Gurav, R.; Jeon, J.M.; Yoon, J.J.; Kim, W.; et al. Finding of Novel Polyhydroxybutyrate Producer Loktanella sp. SM43 Capable of Balanced Utilization of Glucose and Xylose from Lignocellulosic Biomass. Int. J. Biol. Macromol. 2022, 208, 809–818. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, P.; Lee, H.S.; Kim, J.H. High Production of Poly-β-Hydroxybutyrate (PHB) from Methylobacterium organophilum under Potassium Limitation. Biotechnol. Lett. 1996, 18, 25–30. [Google Scholar] [CrossRef]
- Orita, I.; Nishikawa, K.; Nakamura, S.; Fukui, T. Biosynthesis of Polyhydroxyalkanoate Copolymers from Methanol by Methylobacterium extorquens AM1 and the Engineered Strains under Cobalt-Deficient Conditions. Appl. Microbiol. Biotechnol. 2014, 98, 3715–3725. [Google Scholar] [CrossRef]
- Wendlandt, K.D.; Jechorek, M.; Helm, J.; Stottmeister, U. Production of PHB with a High Molecular Mass from Methane. Polym. Degrad. Stab. 1998, 59, 191–194. [Google Scholar] [CrossRef]
- Smit, A.M.; Strabala, T.J.; Peng, L.; Rawson, P.; Lloyd-Jones, G.; Jordan, T.W. Proteomic Phenotyping of Novosphingobium nitrogenifigens Reveals a Robust Capacity for Simultaneous Nitrogen Fixation, Polyhydroxyalkanoate Production, and Resistance to Reactive Oxygen Species. Appl. Environ. Microbiol. 2012, 78, 4802–4815. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-K.; Yoon, S.-C.; Nam, J.-D.; Lenz, R.-W. Effect of C/N Ratio on the Production of Poly(3-Hydroxyalkanoates) by the Methylotroph Paracoccus denitrificans. J. Microbiol. Biotechnol. 1997, 7, 391–396. [Google Scholar]
- Suzuki, T.; Yamane, T.; Shimizu, S. Mass Production of Poly-β-Hydroxybutyric Acid by Fed-Batch Culture with Controlled Carbon/Nitrogen Feeding. Appl. Microbiol. Biotechnol. 1986, 24, 370–374. [Google Scholar] [CrossRef]
- Brandl, H.; Gross, R.A.; Lenz, R.W.; Lloyd, R.; Fuller, R.C. The Accumulation of Poly(3-Hydroxyalkanoates) in Rhodobacter sphaeroides. Arch. Microbiol. 1991, 155, 337–340. [Google Scholar] [CrossRef]
- Choi, D.; Chipman, D.C.; Bents, S.C.; Brown, R.C. A Techno-Economic Analysis of Polyhydroxyalkanoate and Hydrogen Production from Syngas Fermentation of Gasified Biomass. Appl. Biochem. Biotechnol. 2010, 160, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Gurav, R.; Kim, B.; Kim, S.; Cho, D.H.; Jung, H.; Kim, Y.G.; Kim, J.S.; Yang, Y.H. Coproduction of Exopolysaccharide and Polyhydroxyalkanoates from Sphingobium yanoikuyae BBL01 Using Biochar Pretreated Plant Biomass Hydrolysate. Bioresour. Technol. 2022, 361, 127753. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Lee, S.Y. Poly(3-Hydroxybutyrate) Production with High Productivity and High Polymer Content by a Fed-Batch Culture of Alcaligenes latus under Nitrogen Limitation. Appl. Environ. Microbiol. 1997, 63, 3703–3706. [Google Scholar] [CrossRef]
- Ng, L.M.; Sudesh, K. Identification of a New Polyhydroxyalkanoate (PHA) Producer Aquitalea sp. USM4 (JCM 19919) and Characterization of Its PHA Synthase. J. Biosci. Bioeng. 2016, 122, 550–557. [Google Scholar] [CrossRef]
- Penloglou, G.; Chatzidoukas, C.; Kiparissides, C. Microbial Production of Polyhydroxybutyrate with Tailor-Made Properties: An Integrated Modelling Approach and Experimental Validation. Biotechnol. Adv. 2012, 30, 329–337. [Google Scholar] [CrossRef]
- Zhu, C.; Nomura, C.T.; Perrotta, J.A.; Stipanovic, A.J.; Nakas, J.P. Production and Characterization of Poly-3-Hydroxybutyrate from Biodiesel-Glycerol by Burkholderia cepacia ATCC 17759. Biotechnol. Prog. 2010, 26, 424–430. [Google Scholar] [CrossRef]
- de Sousa Dias, M.M.; Koller, M.; Puppi, D.; Morelli, A.; Chiellini, F.; Braunegg, G. Fed-Batch Synthesis of Poly(3-Hydroxybutyrate) and Poly(3-Hydroxybutyrate-Co-4-Hydroxybutyrate) from Sucrose and 4-Hydroxybutyrate Precursors by Burkholderia sacchari Strain DSM 17165. Bioengineering 2017, 4, 36. [Google Scholar] [CrossRef]
- Mothes, G.; Ackermann, J.U. Synthesis of Poly(3-Hydroxybutyrate-Co-4-Hydroxybutyrate) with a Target Mole Fraction of 4-Hydroxybutyric Acid Units by Two-Stage Continuous Cultivation of Delftia acidovorans P4a. Eng. Life Sci. 2005, 5, 58–62. [Google Scholar] [CrossRef]
- Choi, M.H.; Yoon, S.C.; Lenz, R.W. Production of Poly(3-Hydroxybutyric Acid-Co-4-Hydroxybutyric Acid) and Poly(4-Hydroxybutyric Acid) without Subsequent Degradation by Hydrogenophaga pseudoflava. Appl. Environ. Microbiol. 1999, 65, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.H.; Lee, H.J.; Rho, J.K.; Yoon, S.C.; Nam, J.D.; Lim, D.; Lenz, R.W. Biosynthesis and Local Sequence Specific Degradation of Poly(3-Hydroxyvalerate-Co-4-Hydroxybutyrate) in Hydrogenophaga pseudoflava. Biomacromolecules 2003, 4, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Singhal, A.; Verma, P.K.; Thakur, I.S. Production and Characterization of Polyhydroxyalkanoate from Lignin Derivatives by Pandoraea sp. ISTKB. ACS Omega 2017, 2, 9156–9163. [Google Scholar] [CrossRef]
- Kim, B.S.; Lee, S.C.; Lee, S.Y.; Chang, H.N.; Chang, Y.K.; Woo, S.I. Production of Poly(3-Hydroxybutyric Acid) by Fed-Batch Culture of Alcaligenes eutrophus with Glucose Concentration Control. Biotechnol. Bioeng. 1994, 43, 892–898. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, R.; Cai, J.Y.; Liu, Z.; Zheng, Y.; Wang, H.; Li, Q.; He, N. Biosynthesis and Thermal Properties of PHBV Produced from Levulinic Acid by Ralstonia eutropha. PLoS ONE 2013, 8, e60318. [Google Scholar] [CrossRef] [PubMed]
- Kourilova, X.; Pernicova, I.; Sedlar, K.; Musilova, J.; Sedlacek, P.; Kalina, M.; Koller, M.; Obruca, S. Production of Polyhydroxyalkanoates (PHA) by a Thermophilic Strain of Schlegelella thermodepolymerans from Xylose Rich Substrates. Bioresour. Technol. 2020, 315, 123885. [Google Scholar] [CrossRef] [PubMed]
- Hai, T.; Lange, D.; Rabus, R.; Steinbüchel, A. Polyhydroxyalkanoate (PHA) Accumulation in Sulfate-Reducing Bacteria and Identification of a Class III PHA Synthase (PhaEC) in Desulfococcus multivorans. Appl. Environ. Microbiol. 2004, 70, 4440–4448. [Google Scholar] [CrossRef]
- Sabapathy, P.C.; Devaraj, S.; Parthiban, A.; Kathirvel, P. Bioprocess Optimization of PHB Homopolymer and Copolymer P3 (HB-Co-HV) by Acinetobacter junii BP25 Utilizing Rice Mill Effluent as Sustainable Substrate. Environ. Technol. 2018, 39, 1430–1441. [Google Scholar] [CrossRef]
- Chen, G.Q.; Zhang, G.; Park, S.J.; Lee, S.Y. Industrial Scale Production of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate). Appl. Microbiol. Biotechnol. 2001, 57, 50–55. [Google Scholar] [CrossRef]
- Zhang, H.F.; Ma, L.; Wang, Z.H.; Chen, G.Q. Biosynthesis and Characterization of 3-Hydroxyalkanoate Terpolyesters with Adjustable Properties by Aeromonas hydrophila. Biotechnol. Bioeng. 2009, 104, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.Y.; Hung, J.Y.; Shiau, T.J.; Wei, Y.H. Exploring Two-Stage Fermentation Strategy of Polyhydroxyalkanoate Production Using Aeromonas hydrophila. Biochem. Eng. J. 2013, 78, 80–84. [Google Scholar] [CrossRef]
- Page, W.J.; Cornish, A. Growth of Azotobacter vinelandii UWD in Fish Peptone Medium and Simplified Extraction of Poly-β-Hydroxybutyrate. Appl. Environ. Microbiol. 1993, 59, 4236–4244. [Google Scholar] [CrossRef] [PubMed]
- Bonartsev, A.P.; Yakovlev, S.G.; Zharkova, I.I.; Boskhomdzhiev, A.P.; Bagrov, D.V.; Myshkina, V.L.; Makhina, T.K.; Kharitonova, E.P.; Samsonova, O.V.; Feofanov, A.V.; et al. Cell Attachment on Poly(3-Hydroxybutyrate)-Poly(Ethylene Glycol) Copolymer Produced by Azotobacter chroococcum 7B. BMC Biochem. 2013, 14, 1. [Google Scholar] [CrossRef]
- Quillaguamán, J.; Doan-Van, T.; Guzmán, H.; Guzmán, D.; Martín, J.; Everest, A.; Hatti-Kaul, R. Poly(3-Hydroxybutyrate) Production by Halomonas boliviensis in Fed-Batch Culture. Appl. Microbiol. Biotechnol. 2008, 78, 227–232. [Google Scholar] [CrossRef]
- Jung, H.J.; Kim, S.H.; Cho, D.H.; Kim, B.C.; Bhatia, S.K.; Lee, J.; Jeon, J.M.; Yoon, J.J.; Yang, Y.H. Finding of Novel Galactose Utilizing Halomonas sp. YK44 for Polyhydroxybutyrate (PHB) Production. Polymers 2022, 14, 5407. [Google Scholar] [CrossRef] [PubMed]
- El-malek, F.A.; Farag, A.; Omar, S.; Khairy, H. Polyhydroxyalkanoates (PHA) from Halomonas pacifica ASL10 and Halomonas salifodiane ASL11 Isolated from Mariout Salt Lakes. Int. J. Biol. Macromol. 2020, 161, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Preusting, H.; Hazenberg, W.; Witholt, B. Continuous Production of Poly(3-Hydroxyalkanoates) by Pseudomonas oleovorans in a High-Cell-Density, Two-Liquid-Phase Chemostat. Enzyme Microb. Technol. 1993, 15, 311–316. [Google Scholar] [CrossRef]
- Silva-Queiroz, S.R.; Silva, L.F.; Pradella, J.G.C.; Pereira, E.M.; Gomez, J.G.C. PHAMCL Biosynthesis Systems in Pseudomonas aeruginosa and Pseudomonas putida Strains Show Differences on Monomer Specificities. J. Biotechnol. 2009, 143, 111–118. [Google Scholar] [CrossRef]
- Munoz, L.E.A.; Riley, M.R. Utilization of Cellulosic Waste from Tequila Bagasse and Production of Polyhydroxyalkanoate (PHA) Bioplastics by Saccharophagus degradans. Biotechnol. Bioeng. 2008, 100, 882–888. [Google Scholar] [CrossRef] [PubMed]
- van Thuoc, D.; My, D.N.; Loan, T.T.; Sudesh, K. Utilization of Waste Fish Oil and Glycerol as Carbon Sources for Polyhydroxyalkanoate Production by Salinivibrio sp. M318. Int. J. Biol. Macromol. 2019, 141, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Gupta, A.; Thakur, I.S. Carbon Dioxide Sequestration by Chemolithotrophic Oleaginous Bacteria for Production and Optimization of Polyhydroxyalkanoate. Bioresour. Technol. 2016, 213, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.C.; Chen, C.C.; Choi, M.H.; Kung, S.S.; Wei, Y.H. Production of Poly-β-Hydroxybutyrate (PHB) by Vibrio spp. Isolated from Marine Environment. J. Biotechnol. 2007, 132, 259–263. [Google Scholar] [CrossRef]
- Ibrahim, M.H.A.; Steinbüchel, A. Poly(3-Hydroxybutyrate) Production from Glycerol by Zobellella denitrificans MW1 via High-Cell-Density Fed-Batch Fermentation and Simplified Solvent Extraction. Appl. Environ. Microbiol. 2009, 75, 6222–6231. [Google Scholar] [CrossRef]
- Haddadi, M.H.; Asadolahi, R.; Negahdari, B. The Bioextraction of Bioplastics with Focus on Polyhydroxybutyrate: A Review. Int. J. Environ. Sci. Technol. 2019, 16, 3935–3948. [Google Scholar] [CrossRef]
- Ganapathy, K.; Ramasamy, R.; Dhinakarasamy, I. Polyhydroxybutyrate Production from Marine Source and Its Application. Int. J. Biol. Macromol. 2018, 111, 102–108. [Google Scholar] [CrossRef]
- Mozejko-Ciesielska, J.; Szacherska, K.; Marciniak, P. Pseudomonas Species as Producers of Eco-Friendly Polyhydroxyalkanoates. J. Polym. Environ. 2019, 27, 1151–1166. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Z.; Tsui, T.H.; Loh, K.C.; Dai, Y.; Tong, Y.W. A Review on Enhancing Cupriavidus necator Fermentation for Poly(3-Hydroxybutyrate) (PHB) Production From Low-Cost Carbon Sources. Front. Bioeng. Biotechnol. 2022, 10, 946085. [Google Scholar] [CrossRef]
- Sehgal, R.; Gupta, R. Polyhydroxyalkanoate and Its Efficient Production: An Eco-Friendly Approach towards Development. 3 Biotech. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, S.M.; Park, S.L.; Choi, T.R.; Song, H.S.; Kim, H.J.; Bhatia, S.K.; Gurav, R.; Kim, Y.G.; Kim, J.H.; et al. Tung Oil-Based Production of High 3-Hydroxyhexanoate-Containing Terpolymer Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate-Co-3-Hydroxyhexanoate) Using Engineered Ralstonia eutropha. Polymers 2021, 13, 1084. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Hill, D.J.; Kowalczuk, M.; Johnston, B.; Adamus, G.; Irorere, V.; Radecka, I. Carbon Sources for Polyhydroxyalkanoates and an Integrated Biorefinery. Int. J. Mol. Sci. 2016, 17, 1157. [Google Scholar] [CrossRef] [PubMed]
- Yañez, L.; Conejeros, R.; Vergara-Fernández, A.; Scott, F. Beyond Intracellular Accumulation of Polyhydroxyalkanoates: Chiral Hydroxyalkanoic Acids and Polymer Secretion. Front. Bioeng. Biotechnol. 2020, 8, 248. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Otari, S.V.; Jeon, J.M.; Gurav, R.; Choi, Y.K.; Bhatia, R.K.; Pugazhendhi, A.; Kumar, V.; Rajesh Banu, J.; Yoon, J.J.; et al. Biowaste-to-Bioplastic (Polyhydroxyalkanoates): Conversion Technologies, Strategies, Challenges, and Perspective. Bioresour. Technol. 2021, 326, 124733. [Google Scholar] [CrossRef]
- Li, M.; Wilkins, M.R. Recent Advances in Polyhydroxyalkanoate Production: Feedstocks, Strains and Process Developments. Int. J. Biol. Macromol. 2020, 156, 691–703. [Google Scholar] [CrossRef]
- Argiz, L.; González-Cabaleiro, R.; Val del Río, Á.; González-López, J.; Mosquera-Corral, A. A Novel Strategy for Triacylglycerides and Polyhydroxyalkanoates Production Using Waste Lipids. Sci. Total Environ. 2021, 763, 142944. [Google Scholar] [CrossRef] [PubMed]
- Vu, D.H.; Mahboubi, A.; Root, A.; Heinmaa, I.; Taherzadeh, M.J.; Åkesson, D. Thorough Investigation of the Effects of Cultivation Factors on Polyhydroalkanoates (PHAs) Production by Cupriavidus necator from Food Waste-Derived Volatile Fatty Acids. Fermentation 2022, 8, 605. [Google Scholar] [CrossRef]
- Wen, Q.; Liu, B.; Li, F.; Chen, Z. Substrate Strategy Optimization for Polyhydroxyalkanoates Producing Culture Enrichment from Crude Glycerol. Bioresour. Technol. 2020, 311, 123516. [Google Scholar] [CrossRef]
- Suriyamongkol, P.; Weselake, R.; Narine, S.; Moloney, M.; Shah, S. Biotechnological Approaches for the Production of Polyhydroxyalkanoates in Microorganisms and Plants—A Review. Biotechnol. Adv. 2007, 25, 148–175. [Google Scholar] [CrossRef]
- Mezzina, M.P.; Manoli, M.T.; Prieto, M.A.; Nikel, P.I. Engineering Native and Synthetic Pathways in Pseudomonas putida for the Production of Tailored Polyhydroxyalkanoates. Biotechnol. J. 2020, 16, e2000165. [Google Scholar] [CrossRef]
- Manoli, M.-T.; Nogales, J.; Prieto, A. Synthetic Control of Metabolic States in Pseudomonas putida by Tuning Polyhydroxyalkanoate Cycle. Mbio 2022, 13, e0179421. [Google Scholar] [CrossRef] [PubMed]
- Ciobotǎ, V.; Burkhardt, E.M.; Schumacher, W.; Rösch, P.; Küsel, K.; Popp, J. The Influence of Intracellular Storage Material on Bacterial Identification by Means of Raman Spectroscopy. Anal. Bioanal. Chem. 2010, 397, 2929–2937. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, Y.; Wu, Q.; Wang, Y.; Chen, G.Q. Synthetic Biology and Genome-Editing Tools for Improving PHA Metabolic Engineering. Trends Biotechnol. 2020, 38, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Chek, M.F.; Hiroe, A.; Hakoshima, T.; Sudesh, K.; Taguchi, S. PHA Synthase (PhaC): Interpreting the Functions of Bioplastic-Producing Enzyme from a Structural Perspective. Appl. Microbiol. Biotechnol. 2019, 103, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Rehm, B.H.A.; Steinbüchel, A. Biochemical and Genetic Analysis of PHA Synthases and Other Proteins Required for PHA Synthesis. Int. J. Biol. Macromol. 1999, 25, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Kutralam-Muniasamy, G.; Corona-Hernandez, J.; Narayanasamy, R.K.; Marsch, R.; Pérez-Guevara, F. Phylogenetic Diversification and Developmental Implications of Poly-(R)-3-Hydroxyalkanoate Gene Cluster Assembly in Prokaryotes. FEMS Microbiol. Lett. 2017, 364, 1–28. [Google Scholar] [CrossRef]
- Yashavanth, P.R.; Das, M.; Maiti, S.K. Recent Progress and Challenges in Cyanobacterial Autotrophic Production of Polyhydroxybutyrate (PHB), a Bioplastic. J. Environ. Chem. Eng. 2021, 9, 105379. [Google Scholar] [CrossRef]
- Chmelová, D.; Legerská, B.; Ondrejovič, M.; Miertuš, S. Optimization of Propagation Medium for Enhanced Polyhydroxyalkanoate Production by Pseudomonas oleovorans. Fermentation 2022, 8, 16. [Google Scholar] [CrossRef]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, Production, Recent Developments and Applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Shetye, L.; Mendhulkar, V.D. Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Synthesis in Nostoc muscorum from Biodiesel Industry Waste: A Sustainable Model of Bioplastic Production. J. Appl. Phycol. 2022, 34, 1377–1387. [Google Scholar] [CrossRef]
- Samorì, C.; Abbondanzi, F.; Galletti, P.; Giorgini, L.; Mazzocchetti, L.; Torri, C.; Tagliavini, E. Extraction of Polyhydroxyalkanoates from Mixed Microbial Cultures: Impact on Polymer Quality and Recovery. Bioresour. Technol. 2015, 189, 195–202. [Google Scholar] [CrossRef]
- Shen, M.Y.; Chu, C.Y.; Sawatdeenarunat, C.; Bhuyar, P. Production, Downstream Processing, and Characterization of Polyhydroxyalkanoates (PHAs) Boosted by Pyruvate Supplement Using Mixed Microbial Culture (MMC) and Organic Wastewater. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Vermeer, C.M.; Bons, L.J.; Kleerebezem, R. Production of a Newly Discovered PHA Family Member with an Isobutyrate-Fed Enrichment Culture. Appl. Microbiol. Biotechnol. 2022, 106, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Li, Z.; Liu, L.; Zhao, D.; Zhang, X.; Bi, C. Genome Editing of Ralstonia eutropha Using an Electroporation-Based CRISPR-Cas9 Technique. Biotechnol. Biofuels 2018, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.R.; Yang, S.Y.; Moon, Y.M.; Choi, T.R.; Song, H.S.; Bhatia, S.K.; Gurav, R.; Kim, E.J.; Kim, B.G.; Yang, Y.H. Construction of Efficient Platform Escherichia coli Strains for Polyhydroxyalkanoate Production by Engineering Branched Pathway. Polymers 2019, 11, 509. [Google Scholar] [CrossRef]
- Goto, S.; Miyahara, Y.; Taguchi, S.; Tsuge, T.; Hiroe, A. Enhanced Production of (R)-3-Hydroxybutyrate Oligomers by Coexpression of Molecular Chaperones in Recombinant Escherichia coli Harboring a Polyhydroxyalkanoate Synthase Derived from Bacillus cereus YB-4. Microorganisms 2022, 10, 458. [Google Scholar] [CrossRef]
- Sakurai, T.; Mizuno, S.; Miyahara, Y.; Hiroe, A.; Taguchi, S.; Tsuge, T. Optimization of Culture Conditions for Secretory Production of 3-Hydroxybutyrate Oligomers Using Recombinant Escherichia coli. Front. Bioeng. Biotechnol. 2022, 10, 829134. [Google Scholar] [CrossRef]
- Lorini, L.; Martinelli, A.; Capuani, G.; Frison, N.; Reis, M.; Sommer Ferreira, B.; Villano, M.; Majone, M.; Valentino, F. Characterization of Polyhydroxyalkanoates Produced at Pilot Scale From Different Organic Wastes. Front. Bioeng. Biotechnol. 2021, 9, 628719. [Google Scholar] [CrossRef]
- Tamis, J.; Mulders, M.; Dijkman, H.; Rozendal, R.; van Loosdrecht, M.C.M.; Kleerebezem, R. Pilot-Scale Polyhydroxyalkanoate Production from Paper Mill Wastewater: Process Characteristics and Identification of Bottlenecks for Full-Scale Implementation. J. Environ. Eng. 2018, 144, 04018107. [Google Scholar] [CrossRef]
- Madkour, M.H.; Heinrich, D.; Alghamdi, M.A.; Shabbaj, I.I.; Steinbüchel, A. PHA Recovery from Biomass. Biomacromolecules 2013, 14, 2963–2972. [Google Scholar] [CrossRef]
- Mongili, B.; Abdel Azim, A.; Fraterrigo Garofalo, S.; Batuecas, E.; Re, A.; Bocchini, S.; Fino, D. Novel Insights in Dimethyl Carbonate-Based Extraction of Polyhydroxybutyrate (PHB). Biotechnol. Biofuels 2021, 14, 13. [Google Scholar] [CrossRef]
- Pérez-Rivero, C.; López-Gómez, J.P.; Roy, I. A Sustainable Approach for the Downstream Processing of Bacterial Polyhydroxyalkanoates: State-of-the-Art and Latest Developments. Biochem. Eng. J. 2019, 150, 107283. [Google Scholar] [CrossRef]
- Quines, L.K.M.; Schmidt, M.; Zanfonato, K.; Schmidell, W.; Aragão, G.M.F. Methods of Extraction of Polyhydroxyalkanoates from Bacterial Biomass. Quim Nova 2015, 38, 1207–1218. [Google Scholar] [CrossRef]
- Kurian, N.S.; Das, B. Comparative Analysis of Various Extraction Processes Based on Economy, Eco-Friendly, Purity and Recovery of Polyhydroxyalkanoate: A Review. Int. J. Biol. Macromol. 2021, 183, 1881–1890. [Google Scholar] [CrossRef]
- Samorì, C.; Basaglia, M.; Casella, S.; Favaro, L.; Galletti, P.; Giorgini, L.; Marchi, D.; Mazzocchetti, L.; Torri, C.; Tagliavini, E. Dimethyl Carbonate and Switchable Anionic Surfactants: Two Effective Tools for the Extraction of Polyhydroxyalkanoates from Microbial Biomass. Green Chem. 2015, 17, 1047–1056. [Google Scholar] [CrossRef]
- Tsang, Y.F.; Kumar, V.; Samadar, P.; Yang, Y.; Lee, J.; Ok, Y.S.; Song, H.; Kim, K.H.; Kwon, E.E.; Jeon, Y.J. Production of Bioplastic through Food Waste Valorization. Environ. Int. 2019, 127, 625–644. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Mukherjee, A. A New Wave of Industrialization of PHA Biopolyesters. Bioengineering 2022, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Girdhar, M.; Kumar, R.; Chaturvedi, H.S.; Vadhel, A.; Solanki, P.R.; Kumar, A.; Kumar, D.; Mamidi, N. Polyhydroxybutyrate-Based Nanocomposites for Bone Tissue Engineering. Pharmaceuticals 2021, 14, 1163. [Google Scholar] [CrossRef] [PubMed]
- Raza, Z.A.; Khalil, S.; Majeed, M.I.; Sarwar, T. Aminolysis of Poly(Hydroxybutyrate)-Based Multicomponent Films for the Impregnation of Bovine Serum Albumin. Polym. Bull. 2022, 80, 2019–2043. [Google Scholar] [CrossRef]
- Fedorov, M.B.; Vikhoreva, G.A.; Kil’deeva, N.R.; Kechek’Yan, A.S.; Gerasimov, V.I.; Gal’braikh, L.S. Structural Changes in Films and Properties of Surgical Sutures with Polyhydroxybutyrate Coating. Fibre Chem. 2008, 40, 118–122. [Google Scholar] [CrossRef]
- Salahuddin, N.; Gaber, M.; Mousa, M.; Abdelwahab, M.A. Dual Drug Delivery System Based on Biodegradable Modified Poly(3-Hydroxybutyrate)-NiO Nanocomposite and Sequential Release of Drugs. Polym. Bull. 2022, 79, 10969–10990. [Google Scholar] [CrossRef]
- Kang, J.; Yun, S. il Chitosan-Reinforced PHB Hydrogel and Aerogel Monoliths Fabricated by Phase Separation with the Solvent-Exchange Method. Carbohydr. Polym. 2022, 284, 119184. [Google Scholar] [CrossRef] [PubMed]
- Pavelková, R.; Plachá, M.; Tarageľ, M.; Bendová, A.; Matoušková, P.; Márová, I. Application of Phb-Liposome Particles and Nanofibers in Cosmetics. In Proceedings of the NANOCON Conference Proceedings—International Conference on Nanomaterials 2020, Brno, Czech Republic, 16–18 October 2020; pp. 514–519. [Google Scholar] [CrossRef]
- Guimarães, T.C.; Araújo, E.S.; Hernández-Macedo, M.L.; López, J.A. Polyhydroxyalkanoates: Biosynthesis from Alternative Carbon Sources and Analytic Methods: A Short Review. J. Polym. Environ. 2022, 30, 2669–2684. [Google Scholar] [CrossRef]
| Phylum (Class) | Genus | Substrate | PHA Type | PHA Yield (% dcw) | Reference |
|---|---|---|---|---|---|
| Actinobacteria (Actinobacteria) | Micrococcus | Glucose | PHB | 56.59 | [42] |
| Microlunatus | Glucose | PHB | 20–30 | [43] | |
| Nocardia | Acetate, Succinate | PHB-co-HV | 20 | [44] | |
| Rhodococcus | Glucose | PHB-co-HV | 40 | [45] | |
| Streptomyces | Glucose | PHB | 1.2–88 | [46] | |
| Bacteroidetes (Sphingobacteriia) | Parapedobacter | Molasses | PHB | 50.24 | [47] |
| Cyanobacteria (Cyanophyceae) | Anabaena | Sodium acetate | PHB | 40 | [48] |
| Aulosira | Glucose | PHB | 48.7 | [49] | |
| Chlorogloea | Acetate | PHB | 10.0 | [50] | |
| Nostoc | Conditioning of effluent gases | PHB-co-HV | 65 | [51] | |
| Spirulina | Zarrouk medium | PHB | 30.7 | [52] | |
| Synechococcus | CO2 | PHB | 62 | [53] | |
| Sucrose | PHB | 17.15 | [54] | ||
| Deinococcus-Thermus (Deinococci) | Thermus | Sodium gluconate, sodium octanoate | mcl-PHA | 35–40 | [55] |
| Firmicutes (Bacilli) | Aneurinibacillus | Glucose | PHB-co-HV | 10–15 | [56] |
| Bacillus | Cheese whey | PHB | 51.57 | [57] | |
| Wheat starch wastewater | PHB-co-HV | 59.5 | [58] | ||
| Lactate | PHB | 64.7 | [59] | ||
| Xylose | PHB | 62 | [60] | ||
| Caryophanon | Glucose | PHA | - | [61] | |
| Geobacillus | Glucose | PHB | 68.9 | [62] | |
| Lysinibacillus | Glucose | P(3HB-co-3HDD-co-3HTD) | - | [63] | |
| Staphylococcus | Hydrocarbons | PHB | 15.2 | [64] | |
| Firmicutes (Clostridia) | Clostridium | - | PHA | 26.75 | [65] |
| Proteobacteria (Alphaproacteobteria) | Bradyrhizobium | YEM medium | PHB | 13.95 | [66] |
| Caulobacter | Whey | PHB | 31.5 | [67] | |
| Chelatococcus | Glucose | PHB | 44.5 | [68] | |
| Loktanela | lignocellulosic biomass | PHB | 78.3 | [69] | |
| Methylobacterium | Methanol | PHB | 52–56 | [70] | |
| Methylobacterium Methylocystis | Methanol | PHB-co-HV | 8.4 | [71] | |
| Methane | PHB | 51 | [72] | ||
| Novosphingobium | Glucose | PHB | 80 | [73] | |
| Paracoccus | n-hexanoic and n-octanoic | PHB-co-HV | 49–61 | [74] | |
| Protomonas | Methanol | PHB | 64 | [75] | |
| Rhizobium | L-Cysteine, L-Glycine, Arabinose, Glucose, Sucrose | PHB | 5.5–70.0 | [66] | |
| Rhodobacter | Acetate | Scl-PHA | 50.8 | [76] | |
| Rhodospirillum | VFAs | PHB-co-HV | - | [77] | |
| Sphingobium | Biomass hydrolysate | PHA | 40.8 | [78] | |
| Proteobacteria (Betaproteobacteria) | Alcaligenes | Sucrose | PHB | 88 | [79] |
| Aquitalea | Hydrocarbons | PHB | 25–27 | [80] | |
| Azohydromonas | Sucrose | PHB | 95 | [81] | |
| Burkholderia | Glycerol | PHB | 81.9 | [82] | |
| Sucrose + precursor GBL | P(3HB-co-4HB) | 71.5 | [83] | ||
| Delftia | Acetic acid + precursor GBL | P(3HB-co-4HB) | 52.0–60.0 | [84] | |
| Hydrogenophaga | g-butyrolactone | P(3HB-co-4HB) | 8.0 | [85] | |
| Hydrogenophaga Pandoraea | Lactones | P(3HV-co-4HB) | 18.0–26.1 | [86] | |
| Kraft lignin | scl-PHA | 60.0 | [87] | ||
| Ralstonia | Glucose | PHB | 76.0 | [88] | |
| Ralstonia Schlegelella | Glucose + Levulinic acid | PHB-co-HV | 81.2 | [89] | |
| Xylose + HV precursors | PHB-co-HV | 35.5–68.9 | [90] | ||
| Proteobacteria (Deltaproteobacteria) | Desulfonema | Benzoate | PHB | 5.4–88.0 | [91] |
| Proteobacteria (Gammaproteobacteria) | Acinetobacter | Rice mill effluent | PHB | 94.3 | [92] |
| Acinetobacter Aeromonas | Rice mill effluent + VA | PHB-co-HV | 85.9 | ||
| Glucose + lauric acid | P(3HB-co-3HHx) | 50 | [93] | ||
| Aeromonas Azotobacter | Lauric acid and valeric acid | P(3HB-co-3HV-3HHx) | 71 | [94] | |
| Coconut oil | PHB | 49.6 | [95] | ||
| Glucose | PHB | 85 | [96] | ||
| Azotobacter | Sucrose + valeric acid + sodium citrate | PHB-co-HV | 68.1 | [97] | |
| Azotobacter | Sucrose + PEG 300 | PHB-PEG | 34.2 | [97] | |
| Halomonas | Glucose | PHB | 81 | [98] | |
| Galactose | PHB | 78.1 | [99] | ||
| Sucrose | PHB-co-HV | 80.1 | [100] | ||
| Klebsiella | - | PHA | [65] | ||
| Pseudomonas | Hydrocarbons | mcl-PHA | 25 | [101] | |
| Pseudomonas Saccharophagus | Oils/Glycerol | mcl-PHA | 8.0–61.8 | [102] | |
| Cellulosic Waste | PHA | - | [103] | ||
| Salinivibrio | Waste Fish Oil + Glycerol | PHB | 51.7 | [104] | |
| Serratia | Bicarbonate and glucose | PHV | - | [105] | |
| Vibrio | Acetate, glycerol, succinate, glucose and sucrose | PHB | 1.0–45.5 | [106] | |
| Zobellella | Glycerol | PHB | 66.9–87.0 | [107] |
| Critical Points | Opportunities | Benefits |
|---|---|---|
| Feedstocks | Alternative carbon sources | Cost reduction Waste management |
| Reduced diversity of producers | Assess microbial diversity (Biological Resource Centres) | New more productive pathways |
| Low microbial production | Microbial mixed cultures Microbial cell factories | Higher productivity |
| Bioindustry | New industrial production strategies Greener downstream processes | More efficient processes Reduction of environmental impact |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicente, D.; Proença, D.N.; Morais, P.V. The Role of Bacterial Polyhydroalkanoate (PHA) in a Sustainable Future: A Review on the Biological Diversity. Int. J. Environ. Res. Public Health 2023, 20, 2959. https://doi.org/10.3390/ijerph20042959
Vicente D, Proença DN, Morais PV. The Role of Bacterial Polyhydroalkanoate (PHA) in a Sustainable Future: A Review on the Biological Diversity. International Journal of Environmental Research and Public Health. 2023; 20(4):2959. https://doi.org/10.3390/ijerph20042959
Chicago/Turabian StyleVicente, Diogo, Diogo Neves Proença, and Paula V. Morais. 2023. "The Role of Bacterial Polyhydroalkanoate (PHA) in a Sustainable Future: A Review on the Biological Diversity" International Journal of Environmental Research and Public Health 20, no. 4: 2959. https://doi.org/10.3390/ijerph20042959
APA StyleVicente, D., Proença, D. N., & Morais, P. V. (2023). The Role of Bacterial Polyhydroalkanoate (PHA) in a Sustainable Future: A Review on the Biological Diversity. International Journal of Environmental Research and Public Health, 20(4), 2959. https://doi.org/10.3390/ijerph20042959






