Seaweed Derived Lipids Are a Potential Anti-Inflammatory Agent: A Review
Abstract
1. Introduction
2. Methods
3. Definition and Classification of Seaweeds
4. Seaweeds in Human Diet
5. Seaweed Lipids and Fatty Acids Composition
| SFA | C16:0 | C18:0 | MUFA | C16:1 | C18:1 | PUFA | C18:2 (LA) | C18:3 (ALA) | C20:4 (ARA) | C20:5 (EPA) | C22:6 (DHA) | n-6/ n-3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHLOROPHYTA | 46.87 ± 17.78 | 31.17 ± 11.55 | 5.90 ± 9.68 | 16.90 ± 8.05 | 4.02 ± 3.26 | 10.24 ± 5.93 | 27.56 ± 15.05 | 7.92 ± 6.65 | 5.26 ± 4.91 | 2.16 ± 2.35 | 1.82 ± 1.56 | 3.56 ± 7.76 | 1.02 ± 1.32 |
| Caulerpaceae | 49.26 ± 22.51 | 33.67 ± 10.18 | 5.25 ± 5.70 | 19.67 ± 13.45 | 5.72 ± 2.17 | 17.35 ± 9.42 | 18.86 ± 7.54 | 5.99 ± 2.80 | 7.50 ± 2.47 | 1.49 ± 1.17 | 1.59 ± 0.90 | n.d.a | 1.14 ± 0.89 |
| Caulerpa lentillifera [78] | 29.80 ± 1.65 | 33.69 ± 0.64 | 13.57 ± 0.91 | 9.08 ± 2.75 | n.d.a | n.d.a | 13.06 ± 0.32 | n.d.a | n.d.a | 2.84 ± 0.53 | n.d.a | n.d.a | 0.79 ± 0.05 |
| Caulerpa racemosa [79,80] | 64.68 ± 33.07 | 39.16 ± 16.35 | 3.40 ± 0.14 | 18.45 ± 22.82 | 3.97 ± 0.74 | n.d.a | 15.61 ± 8.47 | 6.34 ± 3.75 | 4.89 ± 3.25 | n.d.a | n.d.a | n.d.a | 1.84 ± 1.23 |
| Cladophoraceae | 47.90 ± 10.79 | 28.49 ± 6.32 | 0.82 ± 0.40 | 21.49 ± 6.78 | 5.62 ± 5.07 | 12.55 ± 4.02 | 23.77 ± 8.90 | 17.69 ± 11.74 | 5.20 ± 4.84 | 0.89 ± 0.56 | n.d.a | n.d.a | n.d.a |
| Chaetomorpha linum [37,81] | n.d.a | 27.95 ± 7.00 | n.d.a | n.d.a | 2.50 ± 2.54 | 13.05 ± 6.15 | n.d.a | 15.35 ± 18.73 | 2.55 ± 2.19 | 0.65 ± 0.77 | 0.95 ± 0.49 | 1.15 ± 1.06 | 2.73 ± 1.37 |
| Cladophora albida [82] | 50.03 ± 0.56 | 33.04 ± 0.52 | 1.28 ± 0.19 | 27.73 ± 0.11 | 13.90 ± 0.09 | 12.51 ± 0.02 | 22.24 ± 0.24 | 15.54 ± 0.22 | n.d.a | 1.37 ± 0.07 | 2.02 ± 0.05 | 0.86 ± 0.03 | 6.73 |
| Cladophora glomerata [36] | 14.10 ± 2.31 | 44.28 ± 0.81 | 0.92 ± 0.05 | 62.52 ± 11.48 | 0.37 ± 0.02 | 20.71 ± 1.65 | 23.37 ± 4.60 | 8.19 ± 0.82 | 1.12 ± 0.12 | 1.43 ± 0.04 | n.d.a | n.d.a | n.d.a |
| Cladophora Rupestris [83] | 40.80 ± 0.50 | 31.80 ± 2.80 | n.d.a | 27.30 ± 1.00 | 8.30 ± 1.10 | 10.50 ± 2.10 | 21.50 ± 3.10 | 7.40 ± 0.80 | 5.20 ± 1.70 | n.d.a | 2.50 ± 0.60 | n.d.a | 0.50 ± 0.00 |
| Rhizoclonium riparium [37] | 34.40 ± 0.10 | 20.30 ± 0.20 | 0.40 ± 0.00 | 23.00 ± 0.2 | 6.40 ± 0.00 | 15.70 ± 0.20 | 33.10 ± 0.40 | n.d.a | 10.50 ± 0.00 | 0.90 ± 0.00 | 2.70 ± 0.00 | 0.40 ± 0.00 | 1.70 ± 0.10 |
| Codiaceae | 46.28 ± 22.74 | 30.35 ± 9.43 | 1.87 ± 0.63 | 16.04 ± 1.06 | 5.55 ± 0.20 | 8.75 ± 6.41 | 37.67 ± 21.67 | 6.19 ± 3.49 | 2.40 ± 0.42 | 3.95 ± 0.77 | 4.69 ± 4.53 | n.d.a | 1.12 ± 1.27 |
| Codium fragile [82] | 62.37 ± 1.50 | 40.73 ± 0.83 | 1.51 ± 0.06 | 15.29 ± 0.97 | 5.41 ± 0.17 | 0.40 ± 0.94 | 22.34 ± 0.55 | 9.21 ± 0.32 | n.d.a | 3.41 ± 0.20 | 1.48 ± 0.17 | n.d.a | 2.02 |
| Codium isthmocladum [84] | n.d.a | 28.03 ± 2.67 | 1.50 ± 0.12 | n.d.a | n.d.a | 13.66 ± 1.35 | n.d.a | 2.36 ± 0.18 | 2.10 ± 0.19 | n.d.a | n.d.a | n.d.a | n.d.a |
| Codium tomentosum [85] | 30.20 ± 1.60 | 22.30 ± 1.20 | 2.60 ± 0.60 | 16.80 ± 0.30 | 4.90 ± 0.20 | 11.10 ± 0.40 | 53.00 ± 1.40 | 3.40 ± 0.10 | 14.00 ± 0.60 | n.d.a | 7.90 ± 0.80 | n.d.a | 0.22 |
| Ulvaceae | 46.90 ± 19.28 | 34.26 ± 12.36 | 7.98 ± 12.18 | 14.91 ± 6.93 | 3.22 ± 2.86 | 9.85 ± 4.59 | 28.15 ± 16.35 | 7.28 ± 4.83 | 5.65 ± 5.62 | 2.50 ± 2.79 | 1.41 ± 0.82 | 1.37 ± 1.34 | 0.62 ± 0.40 |
| Enteromorpha compressa [81] | n.d.a | 23.10 | n.d.a | n.d.a | 1.10 | 6.30 | 53.90 | 3.80 | 21.90 | 0.50 | 1.40 | n.d.a | n.d.a |
| Enteromorpha intestinalis [35,83] | 42.80 ± 25.17 | 31.05 ± 11.10 | n.d.a | 23.60 ± 1.69 | 4.85 ± 4.31 | 12.35 ± 4.03 | 25.95 ± 15.76 | 7.10 ± 1.83 | 7.85 ± 9.26 | n.d.a | 0.55 ± 0.35 | n.d.a | 0.35 ± 0.21 |
| Ulva prolifera [37] | 38.10 ± 0.20 | 21.00 ± 0.20 | 0.20 ± 0.00 | 14.90 ± 0.10 | 1.60 ± 0.10 | 11.50 ± 0.20 | 39.00 ± 0.5 | 22.00 ± 0.80 | 0.20 ± 0.10 | 1.70 ± 0.00 | 2.20 ± 0.10 | 0.20 ± 0.00 | 0.60 ± 0.00 |
| Ulva armoricana [69] | 46.50 ± 0.10 | 42.00 ± 0.20 | 1.00 ± 0.10 | 24.30 ± 0.10 | n.d.a | 17.30 ± 0.10 | 29.20 ± 0.10 | 8.40 ± 0.10 | 0.50 ± 0.10 | Trace | Trace | n.d.a | 0.10 ± 0.10 |
| Ulva Australia [78] | 25.67 ± 0.47 | 24.37 ± 0.35 | 35.41 ± 0.44 | 3.45 ± 0.24 | n.d.a | n.d.a | 36.23 ± 0.82 | n.d.a | n.d.a | 4.23 ± 0.08 | n.d.a | n.d.a | 0.25 ± 0.01 |
| Ulva chaugulii [86] | 51.40 ± 0.11 | 44.60 ± 0.20 | 1.10 ± 0.05 | 19.40 ± 0.10 | 6.40 ± 0.03 | 8.90 ± 0.03 | 29.20 ± 0.10 | 7.00 ± 0.10 | 11.00 ± 0.04 | 0.70 ± 0.01 | 1.20 ± 0.01 | 1.10 ± 0.10 | 0.50 ± 0.00 |
| Ulva fasciata [80,87] | 70.41 ± 2.38 | 47.32 ± 17.79 | 3.74 ± 0.76 | 12.23 ± 0.65 | n.d.a | 8.83 ± 4.15 | 9.97 ± 0.74 | 4.55 ± 1.05 | 4.82 ± 1.15 | n.d.a | n.d.a | n.d.a | n.d.a |
| Ulva intestinalis [37,78] | 31.28 ± 9.77 | 21.28 ± 2.80 | 18.45 ± 25.67 | 10.42 ± 8.73 | n.d.a | n.d.a | 32.58 ± 9.30 | n.d.a | n.d.a | n.d.a | n.d.a | n.d.a | 0.87 ± 0.74 |
| Ulva lactuca [35,37,79,81,83,88] | 54.95 ± 26.77 | 33.39 ± 12.87 | 2.21 ± 1.32 | 15.45 ± 7.71 | 3.31 ± 3.64 | 8.88 ± 4.49 | 27.62 ± 19.64 | 5.54 ± 3.57 | 5.64 ± 6.03 | 2.50 ± 4.03 | 1.69 ± 1.12 | 0.65 ± 0.63 | 0.77 ± 0.48 |
| Ulva ohnoi [86] | 54.10 ± 0.60 | 41.30 ± 0.40 | 1.70 ± 0.17 | 11.50 ± 0.90 | 3.90 ± 0.04 | 0.70 ± 0.10 | 2.60 ± 0.03 | 11.60 ± 0.10 | 0.90 ± 0.06 | 0.70 ± 0.02 | 1.50 ± 0.04 | 2.30 ± 0.10 | 0.70 ± 0.00 |
| Ulva reticulata [78] | 43.01 ± 0.46 | 44.66 ± 0.38 | 23.96 ± 0.38 | 6.88 ± 0.42 | n.d.a | n.d.a | 25.75 ± 0.52 | n.d.a | n.d.a | 6.01 ± 0.63 | n.d.a | n.d.a | 0.11 ± 0.02 |
| Ulva rigida [85] | 24.10 ± 1.40 | 20.20 ± 0.40 | 2.90 ± 1.00 | 13.00 ± 0.30 | 1.30 ± 0.10 | 9.50 ± 0.30 | 62.90 ± 1.10 | 1.50 ± 0.10 | 10.90 ± 0.40 | 1.20 ± 0.10 | 1.40 ± 0.10 | 4.10 ± 0.10 | 0.33 |
| Ulva tepida [86] | 44.50 ± 0.80 | 35.70 ± 0.60 | 1.30 ± 0.13 | 20.70 ± 0.29 | 1.40 ± 0.02 | 15.70 ± 0.10 | 35.40 ± 0.50 | 8.60 ± 0.10 | 13.40 ± 0.20 | 0.40 ± 0.10 | 0.80 ± 0.10 | 1.80 ± 0.10 | 0.50 ± 0.00 |
| OCHROPHYTA | 38.00 ± 13.31 | 25.77 ± 10.22 | 8.19 ± 12.98 | 21.36 ± 7.77 | 3.89 ± 3.70 | 15.25 ± 8.64 | 36.87 ± 15.84 | 6.21 ± 3.84 | 4.41 ± 2.70 | 11.61 ± 6.94 | 7.80 ± 6.92 | 2.15 ± 4.18 | 1.28 ± 0.83 |
| Acinetosporaceae | |||||||||||||
| Feldmannia indica [86] | 61.30 ± 0.10 | 49.00 ± 0.20 | 1.90 ± 0.03 | 21.10 ± 0.04 | 4.40 ± 0.10 | 16.00 ± 0.04 | 17.50 ± 0.10 | 3.70 ± 0.01 | 5.10 ± 0.02 | 4.00 ± 0.02 | 1.50 ± 0.02 | 0.1 ± 0.00 | 1.0 ± 0.00 |
| Agaraceae | |||||||||||||
| Costaria costata [78] | 37.46 ± 2.02 | 23.88 ± 1.12 | 30.84 ± 1.13 | 15.92 ± 1.46 | n.d.a | n.d.a | 36.59 ± 2.62 | n.d.a | n.d.a | 22.00 ± 1.92 | n.d.a | n.d.a | 1.40 ± 0.18 |
| Alariaceae | 28.67 ± 10.17 | 20.25 ± 7.03 | 10.49 ± 18.54 | 15.75 ± 8.92 | 0.88 ± 0.55 | 13.65 ± 9.24 | 54.94 ± 17.93 | 6.43 ± 2.40 | 5.17 ± 3.49 | 10.70 ± 5.30 | 11.93 ± 4.34 | n.d.a | 0.677 ± 0.23 |
| Alaria esculenta [83] | 37.4 ± 0.4 | 26.9 ± 0.4 | 1.7 ± 0.0 | 25.4 ± 0.5 | 1.5 ± 0.0 | 23.9 ± 0.5 | 33.2 ± 0.2 | 8.2 ± 0.2 | 5.1 ± 0.0 | 4.6 ± 0.0 | 7.1 ± 0.1 | n.d.a | 0.6 ± 0.0 |
| Alaria marginata [81] | n.d.a | 14.9 | 1.1 | n.d.a | 1.8 | 11.1 | 64.7 | 3.7 | 9.4 | 14.9 | 15.5 | n.d.a | n.d.a |
| Undaria pinnatifida [78,89] | 24.31 ± 9.63 | 19.61 ± 8.64 | 19.58 ± 26.48 | 10.93 ± 4.43 | 1.66 ± 0.06 | n.d.a | 60.94 ± 18.03 | n.d.a | n.d.a | n.d.a | n.d.a | 8.55 ± 0.37 | 0.84 ± 0.22 |
| Chordariaceae | |||||||||||||
| Myriogloea sciurus [90] * | 65.50 | 46.30 | 3.80 | 26.70 | 6.50 | 19.70 | 7.80 | 3.70 | 1.90 | 1.30 | 0.90 | 0.00 | 1.80 |
| Cladostephaceae | |||||||||||||
| Cladostephus spongiosus [82] | 31.74 ± 0.35 | 21.33 ± 0.35 | 1.15 ± 0.03 | 12.15 ± 0.33 | 5.72 ± 0.28 | 6.43 ± 0.18 | 56.11 ± 0.35 | 23.14 ± 0.26 | 3.10 ± 0.03 | 16.43 ± 0.13 | 11.46 ± 0.10 | n.d.a | 3.89 |
| Dictyotaceae | 38.53 ± 13.50 | 24.83 ± 9.14 | 1.87 ± 0.92 | 21.01 ± 4.75 | 8.32 ± 6.03 | 12.21 ± 5.04 | 24.63 ± 11.11 | 5.34 ± 3.49 | 2.27 ± 0.90 | 9.31 ± 6.57 | 5.31 ± 4.08 | 0.42 ± 0.36 | 1.51 ± 1.07 |
| Dictyopteris jolyana [84] | n.d.a | 21.05 ± 1.05 | 0.91 ± 0.05 | n.d.a | n.d.a | 17.67 ± 0.90 | n.d.a | 7.89 ± 0.40 | 2.69 ± 0.13 | n.d.a | n.d.a | n.d.a | n.d.a |
| Dictyota dichotoma [36] | 25.98 ± 2.52 | 13.36 ± 1.56 | 3.52 ± 0.16 | 14.53 ± 1.21 | 3.71 ± 0.36 | 10.82 ± 0.85 | 18.26 ± 1.36 | 1.85 ± 0.13 | 1.68 ± 0.36 | 7.54 ± 0.60 | 4.77 ± 0.21 | n.d.a | 0.70 |
| Dictyota dichotoma [90] | 45.98 ± 0.47 | 24.75 ± 0.32 | 2.85 ± 0.08 | 24.28 ± 0.13 | 15.49 ± 0.09 | 8.49 ± 0.13 | 29.74 ± 0.67 | 5.55 ± 0.02 | 2.63 ± 0.20 | 11.46 ± 0.59 | 6.57 ± 0.22 | n.d.a | 3.52 |
| Dictyota spiralis [90] | 40.20 ± 0.31 | 21.69 ± 0.22 | 2.43 ± 0.04 | 29.34 ± 0.14 | 19.58 ± 0.12 | 9.47 ± 0.10 | 30.46 ± 0.28 | 6.05 ± 0.10 | 3.38 ± 0.05 | 18.40 ± 0.21 | n.d.a | n.d.a | n.d.a |
| Padina boergesenii [86] | 68.00 ± 0.40 | 49.20 ± 0.30 | 2.30 ± 0.10 | 20.50 ± 0.80 | 3.20 ± 0.30 | 16.80 ± 0.80 | 11.40 ± 0.50 | 3.30 ± 0.10 | 2.20 ± 0.1 | 2.0 ± 0.1 | 0.3 ± 0.02 | n.d.a | 1.4 ± 0.03 |
| Padina pavonia [36,91] | 31.88 ± 1.17 | 21.45 ± 4.24 | 2.10 ± 0.61 | 21.86 ± 3.74 | 6.33 ± 1.16 | 14.60 ± 1.27 | 21.30 ± 2.66 | 6.75 ± 7.33 | 2.04 ± 2.24 | 7.56 ± 1.53 | 3.98 ± 0.23 | 0.28 ± 0.08 | 1.07 ± 0.51 |
| Spatoglossum schroederi [84] | n.d.a | 30.44 ± 1.19 | 1.16 ± 0.25 | n.d.a | n.d.a | 18.26 ± 0.86 | n.d.a | 3.93 ± 0.18 | 2.01 ± 0.06 | n.d.a | n.d.a | n.d.a | n.d.a |
| Stypopodium schimperi [91] | 28.86 * | 21.88 ± 0.05 | 0.53 ± 0.05 | 18.36 * | 3.82 ± 0.02 | 13.9 ± 0.19 | 17.45 * | 1.26 ± 0.01 | 2.40 ± 0.02 | 1.33 ± 0.03 | 4.07 ± 0.1 | 0.15 ± 0.0 | 0.53 |
| Taonia atomaria [82] | 35.47 ± 1.02 | 25.41 ± 0.97 | 1.04 ± 0.21 | 17.34 ± 0.61 | 8.09 ± 0.10 | 8.09 ± 0.71 | 47.19 ± 0.65 | 10.08 ± 0.32 | 1.71 ± 0.08 | 18.64 ± 0.11 | 13.55 ± 0.55 | 0.84 ± 0.03 | 2.28 |
| Zonaria tournefortii [84] | n.d.a | 22.49 ± 1.17 | 1.69 ± 0.09 | n.d.a | n.d.a | 1.69 ± 0.09 | n.d.a | 5.40 ± 0.39 | n.d.a | n.d.a | n.d.a | n.d.a | n.d.a |
| Fucaceae | 25.14 ± 2.87 | 13.46 ± 3.44 | 1.45 ± 1.14 | 33.21 ± 11.11 | 1.45 ± 0.47 | 28.91 ± 11.90 | 42.02 ± 10.09 | 8.58 ± 2.45 | 4.40 ± 2.70 | 12.56 ± 4.49 | 7.68 ± 3.22 | n.d.a | 1.84 ± 0.70 |
| Ascophyllum nodosum [53] | 25.14 ± 0.49 | 13.42 ± 0.46 | 0.76 ± 0.01 | 31.15 ± 0.23 | 2.24 ± 0.01 | 27.83 ± 0.26 | 43.47 ± 0.54 | 7.47 ± 0.12 | 4.45 ± 0.03 | 17.25 ± 0.26 | 7.24 ± 0.08 | 0.00 ± 0.00 | 2.62 ± 0.01 |
| Fucus distichus [81] * | n.d.a | 19.60 | 0.80 | n.d.a | 2.20 | 16.70 | 49.10 | 7.70 | 7.90 | 14.70 | 10.90 | n.d.a | n.d.a |
| Fucus vesiculosus [53,83,84,85] | 26.25 ± 2.64 | 11.65 ± 0.35 | 1.75 ± 1.61 | 30.91 ± 14.02 | 1.09 ± 0.09 | 29.38 ± 14.43 | 41.86 ± 14.01 | 7.64 ± 1.08 | 3.69 ± 3.50 | 11.63 ± 5.98 | 7.98 ± 3.71 | n.d.a | 1.64 ± 0.39 |
| Himanthaliaceae | |||||||||||||
| Himanthalia elongata [92] | 39.06 ± 2.11 | 32.53 ± 1.61 | 0.68 ± 0.15 | 22.75 ± 2.26 | 2.79 ± 0.25 | 19.69 ± 2.01 | 38.16 ± 7.84 | 4.39 ± 0.34 | 8.79 ± 0.71 | 10.69 ± 1.30 | 5.50 ± 1.78 | n.d.a | 0.81 |
| Hormosiraceae | |||||||||||||
| Hormosira banksii [90] * | 40.60 | 27.50 | 1.50 | 24.60 | 3.70 | 18.70 | 34.80 | 5.50 | 7.40 | 13.20 | 5.80 | 0.00 | 1.50 |
| Laminariaceae | 36.16 ± 9.97 | 23.53 ± 5.17 | 5.93 ± 12.35 | 21.65 ± 7.89 | 3.26 ± 3.92 | 21.24 ± 9.70 | 38.91 ± 11.58 | 6.03 ± 2.46 | 4.72 ± 2.09 | 11.07 ± 6.32 | 8.04 ± 3.60 | n.d.a | 1.06 ± 0.96 |
| Ecklonia radiate [90] * | 50.70 | 27.90 | 1.80 | 33.00 | 11.40 | 20.20 | 16.30 | 3.00 | 1.80 | 8.70 | 2.30 | 0.00 | 3.00 |
| Hedophyllum sessile [81] * | n.d.a | 20.10 | 2.50 | n.d.a | 1.20 | 40.70 | 27.10 | 6.50 | 3.30 | 9.70 | 3.10 | n.d.a | n.d.a |
| Laminaria dentigera [81] * | n.d.a | 29.60 | 2.40 | n.d.a | 2.30 | 20.00 | 40.20 | 5.10 | 4.70 | 9.80 | 10.40 | n.d.a | n.d.a |
| Laminaria digitata [83,93] | 36.03 ± 6.41 | 25.79 ± 5.78 | 1.82 ± 1.44 | 16.50 ± 3.10 | 3.12 ± 3.13 | 13.38 ± 6.24 | 44.15 ± 2.04 | 7.24 ± 3.19 | 6.79 ± 1.00 | 7.55 ± 0.78 | 11.56 ± 0.22 | n.d.a | n.d.a |
| Laminaria hyperborea [83] | 33.70 ± 0.70 | 23.30 ± 0.40 | 1.30 ± 0.50 | 26.50 ± 4.20 | n.d.a | 26.50 ± 4.20 | 34.20 ± 3.20 | 5.00 ± 0.20 | 3.80 ± 0.40 | 7.60 ± 0.80 | 9.50 ± 1.00 | n.d.a | 0.60 ± 0.00 |
| Macrocystis integrifolia [81] * | n.d.a | 16.40 | 1.10 | n.d.a | 2.60 | 12.20 | 52.50 | 4.30 | 7.50 | 14.70 | 8.70 | n.d.a | n.d.a |
| Postelsia palmaeformis [81] * | n.d.a | 26.10 | 2.20 | n.d.a | 1.20 | 23.60 | 40.30 | 9.90 | 6.50 | 8.20 | 7.20 | n.d.a | n.d.a |
| Saccharina japonica [78] | 24.35 ± 1.18 | 16.79 ± 0.75 | 38.83 ± 0.79 | 15.76 ± 0.64 | n.d.a | n.d.a | 51.28 ± 0.61 | n.d.a | n.d.a | 26.93 ± 0.18 | n.d.a | n.d.a | 0.65 ± 0.09 |
| Lessoniaceae | n.d.a | 24.37 ± 3.55 | n.d.a | n.d.a | n.d.a | 16.62 ± 3.33 | n.d.a | 4.58 ± 1.50 | n.d.a | 8.41 ± 7.23 | 6.20 ± 3.80 | n.d.a | 0.52 ± 0.0 |
| Ecklonia kurome [94] | n.d.a | 24.43 ± 0.64 | n.d.a | n.d.a | 6.93 ± 0.17 | 16.07 ± 0.14 | n.d.a | 4.74 ± 0.19 | 4.62 ± 0.38 | 1.03 ± 0.18 | 6.40 ± 0.26 | 1.03 ± 0.18 | 0.52 |
| Egregia menziesii [81] * | n.d.a | 20.80 | 1.60 | n.d.a | 1.50 | 13.60 | 54.90 | 6.00 | 9.30 | 15.50 | 9.90 | n.d.a | n.d.a |
| Monodopsidaceae | |||||||||||||
| Nannochloropsis oceanica [95] | 33.00 ± 5.10 | 22.60 ± 2.00 | 6.30 ± 3.20 | 27.40 ± 0.80 | 0.10 ± 0.00 | 0.50 ± 0.10 | 39.70 ± 3.40 | 4.10 ± 0.30 | 3.20 ± 0.30 | 5.30 ± 0.60 | 30.80 ± 2.40 | n.d.a | 0.3 ± 0.0 |
| Phyllariaceae | |||||||||||||
| Saccorhiza polyschides [92] | 48.54 ± 0.85 | 42.14 ± 0.58 | 0.65 ± 0.02 | 29.74 ± 0.92 | 9.06 ± 0.40 | 15.01 ± 0.27 | 21.70 ± 0.80 | 2.84 ± 0.08 | 3.36 ± 0.09 | 6.11 ± 0.33 | 3.01 ± 0.11 | n.d.a_ | 0.71 |
| Ralfsiaceae | |||||||||||||
| Analipus japonicus [81] * | n.d.a | 19.90 | 0.70 | n.d.a | 0.70 | 10.90 | 59.10 | 8.40 | 8.10 | 14.90 | 13.20 | n.d.a | n.d.a |
| Sargassaceae | 39.30 ± 13.69 | 28.11 ± 10.45 | 16.24 ± 15.37 | 18.40 ± 5.00 | 3.46 ± 2.42 | 10.98 ± 3.99 | 39.18 ± 16.51 | 5.74 ± 2.05 | 5.48 ± 3.14 | 14.78 ± 7.50 | 8.19 ± 9.60 | 3.22 ± 5.28 | 1.33 ± 0.69 |
| Bifurcaria bifurcata [53,68] | 38.85 ± 13.06 | 23.71 ± 8.99 | 6.08 ± 6.13 | 21.35 ± 5.88 | 2.67 ± 0.23 | 12.36 ± 0.35 | 41.81 ± 7.20 | 2.57 ± 0.92 | 2.13 ± 2.41 | 14.76 ± 0.68 | 4.40 ± 0.44 | 11.10 ± 1.13 | 1.31 ± 0.14 |
| Cystoseira Hakodatensi [94] | n.d.a | 18.49 ± 0.30 | n.d.a | n.d.a | 0.63 ± 0.08 | 11.08 ± 0.24 | n.d.a | 6.95 ± 0.15 | 6.87 ± 0.18 | 16.59 ± 0.11 | 12.96 ± 0.18 | n.d.a | 1.32 |
| Cystoseria osmundacea [81] * | n.d.a | 22.5 | 0.8 | n.d.a | 2.6 | 11.8 | 54.1 | 5.8 | 10.6 | 19.1 | 5.5 | n.d.a | n.d.a |
| Hizikia fusiforme [89] | 28.10 ± 4.30 | 26.80 ± 3.84 | 0.76 ± 0.31 | 13.40 ± 6.40 | 0.15 ± 0.07 | 7.68 ± 4.22 | 57.0 ± 11.6 | 3.56 ± 1.45 | 0.56 ± 0.21 | 5.30 ± 1.98 | 42.4 ± 11.88 | n.d.a | 0.31 ± 0.10 |
| Sargassum aquifolium [79,86] | 61.21 ± 0.52 | 49.12 ± 0.51 | 3.23 ± 0.01 | 26.01 ± 0.30 | 4.63 ± 0.11 | 19.80 ± 0.21 | 12.91 ± 0.33 | 3.30 ± 0.11 | 1.72 ± 0.04 | 4.31 ± 0.10 | 0.90 ± 0.12 | n.d.a | 2.9 ± 0.03 |
| Sargassum crassifolium [94] | n.d.a | 25.14 ± 2.90 | n.d.a | n.d.a | 2.64 ± 0.38 | 11.17 ± 0.41 | n.d.a | 6.71 ± 0.36 | 5.36 ± 0.33 | 8.46 ± 0.19 | 1.79 ± 0.57 | n.d.a | 1005 |
| Sargassum fusiforme [78] | 27.62 ± 0.58 | 24.80 ± 0.52 | 27.01 ± 0.43 | 11.95 ± 0.22 | n.d.a | n.d.a | 47.32 ± 1.08 | n.d.a | n.d.a | 30.38 ± 0.45 | n.d.a | n.d.a | 0.57 ± 0.01 |
| Sargassum horneri [78,94] | 26.98 ± 0.00 | 25.24 ± 1.91 | 28.90 ± 2.33 | 14.24 ± 0.00 | n.d.a | n.d.a | 49.00 ± 0.00 | n.d.a | n.d.a | 23.04 ± 0.52 | n.d.a | n.d.a | 0.99 ± 0.30 |
| Sargassum Ilicifolium [85] | 55.20 ± 3.33 | 46.10 ± 2.80 | 1.90 ± 0.46 | 27.50 ± 2.58 | 8.20 ± 0.64 | 18.80 ± 2.16 | 17.40 ± 0.68 | 7.90 ± 0.42 | 5.90 ± 0.72 | 1.00 ± 0.12 | 1.90 ± 0.22 | 0.2 ± 0.11 | 1.1 |
| Sargassum muticum [68] | 32.62 ± 0.92 | 24.18 ± 0.48 | 3.29 ± 0.33 | 16.36 ± 0.49 | 2.34 ± 0.43 | 7.91 ± 0.14 | 51.02 ± 4.79 | 5.61 ± 0.26 | 7.07 ± 0.07 | 12.53 ± 0.72 | 9.71 ± 0.34 | n.d.a | 0.74 ± 0.03 |
| Sargassum oligocystum [85] | 61.63 ± 6.17 | 41.56 ± 3.27 | 2.86 ± 0.32 | 17.71 ± 1.12 | n.d.a | 17.71 ± 1.12 | 10.13 ± 0.79 | 6.33 ± 0.23 | 3.80 ± 0.56 | n.d.a | n.d.a | n.d.a | n.d.a |
| Sargassum pallidum [96] | 27.30 ± 1.00 | 21.80 ± 0.90 | 0.30 ± 0.00 | 17.20 ± 0.70 | 7.00 ± 0.30 | 7.80 ± 0.40 | 55.50 ± 2.90 | 10.90 ± 0.70 | 1.20 ± 0.0 | 17.10 ± 0.80 | 5.40 ± 0.20 | 0.1 ± 0.0 | 1.94 |
| Sargassum siliquastrum [94] | n.d.a | 4.78 ± 0.90 | n.d.a | n.d.a | 1.68 ± 0.19 | 8.62 ± 0.18 | n.d.a | 5.51 ± 0.14 | 9.48 ± 0.23 | 16.95 ± 0.07 | 8.82 ± 0.30 | n.d.a | 1.05 |
| Sargassum vulgare [82] | 42.34 ± 0.28 | 31.23 ± 0.24 | 1.62 ± 0.11 | 19.03 ± 0.14 | 8.61 ± 0.11 | 7.40 ± 0.06 | 38.63 ± 0.32 | 7.59 ± 0.02 | n.d.a | 18.64 ± 0.04 | 8.60 ± 0.12 | 1.50 ± 0.07 | 2.82 |
| Turbinaria ornate [94] | n.d.a | 30.24 ± 0.79 | n.d.a | n.d.a | 3.54 ± 0.13 | 9.53 ± 0.19 | n.d.a | 5.66 ± 0.20 | 8.27 ± 0.50 | 15.06 ± 0.43 | 4.21 ± 0.20 | n.d.a | 0.95 |
| Scytosiphonaceae | 44.74 ± 21.01 | 33.26 ± 15.79 | 2.60 ± 1.62 | 20.34 ± 9.09 | 2.53 ± 0.90 | 17.59 ± 8.30 | 19.95 ± 10.44 | 2.62 ± 0.45 | 1.68 ± 0.47 | 3.66 ± 3.37 | 3.90 ± 2.41 | 6.27 ± 7.01 | 0.89 ± 0.53 |
| Colpomenia sinuosa [36,78] | 39.89 ± 16.98 | 29.37 ± 12.20 | 1.90 ± 2.41 | 23.27 ± 13.61 | 3.08 ± 0.87 | 19.89 ± 12.31 | 21.91 ± 9.36 | 2.89 ± 0.28 | 1.55 ± 0.34 | 3.48 ± 4.36 | 5.18 ± 0.31 | 6.27 ± 8.59 | 0.74 ± 0.36 |
| Seirococcaceae | |||||||||||||
| Phyllospora comosa [90] * | 42.00 | 25.40 | 1.70 | 20.80 | 6.10 | 13.70 | 37.20 | 7.00 | 3.90 | 21.10 | 3.90 | 0.00 | 3.80 |
| Stypocaulaceae | |||||||||||||
| Halopteris scoparia [82] | 34.89 ± 0.51 | 24.36 ± 0.45 | 1.92 ± 0.10 | 14.09 ± 0.28 | 5.47 ± 0.09 | 8.23 ± 0.34 | 51.01 ± 0.98 | 20.35 ± 0.14 | n.d.a | 13.96 ± 0.36 | 14.39 ± 0.25 | 0.99 ± 0.86 | 2.32 |
| RHODOPHYTA | 45.29 ± 15.54 | 35.23 ± 14.64 | 4.36 ± 4.94 | 16.86 ± 8.51 | 4.56 ± 4.87 | 7.50 ± 4.81 | 31.29 ± 17.70 | 1.90 ± 1.27 | 1.98 ± 3.11 | 10.88 ± 10.98 | 23.33 ± 16.63 | 6.91 ± 16.76 | 1.68 ± 3.14 |
| Alsidieae | |||||||||||||
| Alsidium seaforthii [84] | n.d.a | 21.65 ± 0.47 | 2.63 ± 0.20 | n.d.a | n.d.a | 10.40 ± 0.87 | n.d.a | 4.68 ± 1.05 | n.d.a | n.d.a | n.d.a | n.d.a | n.d.a |
| Bangiaceae | n.d.a | n.d.a | 3.87 ± 1.30 | n.d.a | 10.75 ± 9.25 | 4.37 ± 2.78 | n.d.a | n.d.a | n.d.a | 6.13 ± 6.25 | 28.92 ± 12.19 | n.d.a | n.d.a |
| Porphyra columbina [97] | n.d.a | 21.55 ± 0.70 | 3.55 ± 0.15 | n.d.a | 3.13 ± 0.27 | 8.50 ± 0.54 | n.d.a | 3.41 ± 0.09 | 11.40 ± 1.15 | 15.52 + 0.08 | 28.36 + 0.33 | n.d.a | n.d.a |
| Porphyra dioica [85,98] | 34.32 ± 4.49 | 25.71 ± 3.40 | 3.53 ± 1.93 | 15.47 ± 9.94 | 10.78 ± 11.90 | 2.85 ± 0.63 | 48.78 ± 12.42 | 1.67 ± 0.04 | 1.95 ± 0.07 | 3.16 ± 0.19 | 33.42 ± 18.27 | n.d.a | 1.22 ± 1.49 |
| Bonnemaisoniaceae | |||||||||||||
| Asparagopsis armata [82] | 81.3 ± 0.56 | 53.21 ± 0.52 | 2.81 ± 0.16 | 13.99 ± 0.94 | 4.87 ± 0.92 | 9.12 ± 0.31 | 4.70 ± 0.38 | n.d.a | n.d.a | 1.79 ± 0.34 | 2.90 ± 0.15 | n.d.a | 0.62 |
| Callithamniaceae | |||||||||||||
| Callitkamnion corymbosum [99] | 34.50 * | 25.90 ± 0.20 | 2.20 ± 0.10 | 35.80 * | 3.40 ± 0.10 | 6.00 ± 0.20 | 29.10 * | 2.60 ± 0.10 | 3.50 ± 0.20 | 12.30 ± 0.30 | 43.40 ± 0.80 | n.d.a | n.d.a |
| Ceramiaceae | 49.16 ± 4.27 | 36.29 ± 4.75 | 1.37 ± 0.06 | 19.09 ± 0.46 | 12.42 ± 0.61 | 5.48 ± 0.61 | 21.95 ± 18.73 | 1.41 ± 0.32 | 1.52 ± 1.41 | 4.43 ± 0.93 | 14.12 ± 18.6 | 0.69 ± 0.15 | 1.57 ± 1.81 |
| Spyridia filamentosa [91] | 52.18 * | 39.65 ± 0.83 | 1.41 ± 0.06 | 19.53 * | 12.09 ± 0.3 | 5.04 ± 0.03 | 8.70 * | 1.18 ± 0.07 | 0.52 ± 0.06 | 5.09 ± 0.82 | 0.99 ± 0.14 | 0.69 ± 0.15 | 2.85 |
| Bornetia secundiflora [82] | 46.14 ± 0.82 | 32.93 ± 0.75 | 1.33 ± 0.22 | 18.66 ± 0.46 | 12.75 ± 0.26 | 5.91 ± 0.45 | 35.20 ± 0.66 | 1.64 ± 0.10 | 2.53 ± 0.02 | 3.78 ± 0.10 | 27.26 ± 0.64 | n.d.a | 0.29 |
| Corallinaceae | |||||||||||||
| Jania rubens [91] * | 40.08 * | 29.85 ± 3.15 | 0.75 ± 0.08 | 12.52 * | 5.15 ± 0.34 | 4.36 ± 0.18 | 34.14 * | 1.05 ± 0.03 | 0.10 ± 0.04 | 4.43 ± 0.07 | 24.47 ± 0.94 | 0.53 ± 0.05 | 0.24 |
| Dasyaceae | |||||||||||||
| Dasya rigidula [91] | 60.43 * | 45.15 ± 0.22 | 3.20 ± 0.36 | 23.43 * | 10.59 ± 0.13 | 11.93 ± 0.84 | 8.57 * | 2.02 ± 0.15 | 0.34 ± 0.03 | 1.85 ± 0.17 | 4.36 ± 0.35 | n.d.a | 0.82 |
| Delesseriaceae | n.d.a | 34.40 ± 7.07 | n.d.a | n.d.a | 4.10 ± 3.67 | 12.75 ± 10.11 | n.d.a | 1.60 ± 1.41 | 1.15 ± 1.34 | 14.85 ± 6.43 | 19.15 ± 18.87 | n.d.a | n.d.a |
| Apoglossum ruscifofium [99] | 21.1 * | 39.40 ± 0.40 | n.d.a | 29.9 * | 6.70 ± 0.30 | 19.90 ± 0.40 | 42.4 * | 2.60 ± 0.l0 | 2.10 ± 0.30 | 10.30 ± 0.50 | 5.80 ± 0.30 | n.d.a | n.d.a |
| Cryptopleura violaceae [81] * | n.d.a | 29.40 | 0.90 | n.d.a | 1.50 | 4.40 | n.d.a | 0.60 | 0.50 | 19.40 | 32.50 | n.d.a | n.d.a |
| Endocladiaceae | |||||||||||||
| Gloiopeltis furcata [78] | 26.97 ± 1.45 | 23.34 ± 1.31 | 19.36 ± 0.11 | 18.05 ± 0.23 | n.d.a | n.d.a | 45.33 ± 2.36 | n.d.a | n.d.a | 44.89 ± 2.24 | n.d.a | n.d.a | 0.19 ± 0.00 |
| Florideophyceae | |||||||||||||
| Ceramium strictum [99] | 34.80 * | 24.00 ± 0.60 | 3.30 ± 0.20 | 30.30 * | 7.30 ± 0.30 | 18.30 ± 0.20 | 34.90 * | 2.00 ± 0.10 | 15.10 ± 0.30 | 3.90 ± 0.10 | n.d.a | n.d.a | n.d.a |
| Halymeniaceae | n.d.a | 27.93 ± 5.50 | 2.44 ± 2.59 | n.d.a | 0.92 ± 0.56 | 8.04 ± 1.21 | n.d.a | 1.89 ± 1.81 | 1.13 ± 1.08 | 18.70 ± 5.33 | 26.95 ± 5.71 | n.d.a | n.d.a |
| Grateloupia turuturu [100] | 37.49 ± 5.91 | 22.03 ± 0.98 | 5.44 ± 0.66 | 16.66 ± 1.29 | 1.58 ± 0.14 | 7.44 ± 0.24 | 49.51 ± 6.18 | 3.99 ± 0.28 | 2.13 ± 0.18 | 12.91 ± 0.56 | 20.86 ± 0.56 | n.d.a | n.d.a |
| Halymenia brasiliana [84] | n.d.a | 35.32 ± 1.08 | n.d.a | n.d.a | n.d.a | 6.62 ± 0.34 | n.d.a | n.d.a | 2.01 ± 1.74 | n.d.a | n.d.a | n.d.a | n.d.a |
| Prionitis lanceolata [81] * | n.d.a | 27.70 | 1.00 | n.d.a | 0.60 | 6.30 | n.d.a | 1.00 | 1.10 | 19.80 | 32.20 | n.d.a | n.d.a |
| Prionitis linearis [81] * | n.d.a | 26.70 | 0.90 | n.d.a | 0.60 | 6.20 | n.d.a | 0.70 | 0.60 | 23.40 | 27.80 | n.d.a | n.d.a |
| Hypneaceae | 65.76 ± 2.32 | 40.43 ± 27.39 | 3.35 ± 1.77 | 16.00 ± 10.74 | 8.50 ± 0.14 | 5.95 ± 3.47 | 6.97 ± 3.47 | 3.27 ± 3.15 | 0.89 ± 0.83 | 5.4 ± 0.42 | 1.00 ± 0.04 | 0.70 ± 0.13 | 2.70 |
| Hypnea musciformis [35] | 64.12 ± 20.82 | 21.06 ± 3.88 | 4.60 ± 0.58 | 8.41 ± 1.64 | n.d.a | 8.41 ± 1.64 | 4.75 ± 3.84 | 3.27 ± 2.43 | 1.48 ± 1.41 | n.d.a | n.d.a | n.d.a | n.d.a |
| Hypnea valentiae [35] | 67.40 ± 0.31 | 59.80 ± 1.09 | 2.10 ± 0.37 | 23.60 ± 0.68 | 8.50 ± 0.14 | 3.50 ± 0.28 | 9.20 ± 0.80 | n.d.a | 0.30 ± 0.12 | 5.4 ± 0.42 | 1.00 ± 0.04 | 0.70 ± 0.13 | 2.70 |
| Gigartinaceae | 38.33 ± 6.48 | 31.89 ± 5.27 | 7.04 ± 6.95 | 11.06 ± 3.01 | 0.56 ± 0.25 | 9.55 ± 3.62 | 43.84 ± 12.60 | 1.32 ± 0.45 | 0.37 ± 0.30 | 21.60 ± 14.97 | 38.69 ± 6.10 | n.d.a | 0.67 ± 0.12 |
| Chondrus crispus [98] | 32.44 ± 0.90 | 27.33 ± 0.50 | 2.71 ± 0.05 | 8.82 ± 0.24 | 0.58 ± 0.08 | 6.06 ± 0.03 | 56.42 ± 1.29 | 1.58 ± 0.05 | 0.72 ± 0.01 | 19.85 ± 0.82 | 33.47 ± 0.21 | n.d.a | 0.66 ± 0.03 |
| Chondrus yendoi [78] | 37.28 ± 0.62 | 33.12 ± 0.48 | 12.60 ± 0.23 | 9.89 ± 0.19 | n.d.a | n.d.a | 43.91 ± 0.85 | n.d.a | n.d.a | 42.35 ± 0.89 | n.d.a | n.d.a | 0.81 ± 0.03 |
| Gigartina harveyana [81] * | n.d.a | 28.60 | 1.70 | n.d.a | 0.80 | 12.50 | n.d.a | 1.60 | 0.60 | 10.40 | 37.20 | n.d.a | n.d.a |
| Iridaea cordata [81] * | n.d.a | 29.90 | 1.80 | n.d.a | 0.30 | 8.50 | n.d.a | 0.80 | 0.50 | 5.30 | 45.40 | n.d.a | n.d.a |
| Mazzaella japonica [78] | 45.29 ± 0.51 | 40.50 ± 0.38 | 16.42 ± 0.05 | 14.49 ± 0.61 | n.d.a | n.d.a | 31.21 ± 0.72 | n.d.a | n.d.a | 30.12 ± 0.29 | n.d.a | n.d.a | 0.56 ± 0.01 |
| Gracilariaceae | 44.18 ± 21.02 | 47.83 ± 26.16 | 3.72 ± 2.97 | 19.41 ± 9.98 | 2.31 ± 2.34 | 5.79 ± 5.67 | 28.44 ± 18.25 | 1.00 ± 0.78 | 2.29 ± 2.82 | 8.45 ± 12.01 | 3.29 ± 2.02 | n.d.a | 2.38 ± 2.71 |
| Gracilaria changii [101] | 7.53 ± 1.72 | 4.28 ± 0.58 | 1.82 ± 0.27 | 38.30 ± 7.20 | 0.28 ± 0.04 | 0.15 ± 0.07 | 51.20 ± 6.78 | 0.59 ± 0.08 | 0.17 ± 0.02 | 0.26 ± 0.02 | n.d.a | 48.36 ± 6.76 | 0.02 ± 0.00 |
| Gracilaria corticata [35] | 58.7 ± 3.75 | 43.6 ± 3.14 | 2.2 ± 0.72 | 22.0 ± 3.65 | 6.9 ± 0.40 | 13.8 ± 3.30 | 19.1 ± 2.86 | n.d.a | 7.7 ± 1.88 | 5.2 ± 0.88 | n.d.a | n.d.a | 1.4 |
| Gracilaria domingensis [84] | n.d.a | 68.16 ± 1.15 | n.d.a | n.d.a | n.d.a | 8.38 ± 0.10 | n.d.a | n.d.a | n.d.a | n.d.a | n.d.a | n.d.a | n.d.a |
| Gracilaria edulis [102] | n.d.a | 84.60 | 1.24 | n.d.a | 0.38 | 0.71 | n.d.a | 0.16 | n.d.a | 0.67 | n.d.a | n.d.a | n.d.a |
| Gracilaria folifera [102] | n.d.a | 81.28 | 1.51 | n.d.a | 0.47 | 1.00 | n.d.a | 0.21 | 0.10 | 0.57 | n.d.a | n.d.a | n.d.a |
| Gracilaria gracilis [85] | 34.9 ± 0.9 | 27.1 ± 1.2 | 4.6 ± 0.8 | 12.5 ± 0.7 | 2.8 ± 0.8 | 9.7 ± 0.4 | 52.6 ± 1.4 | 2 ± 0.4 | 2.7 ± 0.2 | 35.4 ± 1.5 | 5.5 ± 0.2 | n.d.a | 2.47 |
| Gracilaria salicornia [88] | 48.92 ± 6.83 | 33.39 ± 8.86 | 3.04 ± 0.66 | 16.36 ± 1.54 | 2.46 ± 0.12 | 11.72 ± 2.01 | 17.30 ± 1.18 | 1.45 ± 0.38 | 1.65 ± 0.04 | 8.05 ± 1.98 | 1.53 ± 0.27 | n.d.a | 1.2 |
| Gracilariopsis longissima [78] | 47.78 ± 2.36 | 46.44 ± 0.91 | 10.16 ± 0.50 | 11.18 ± 1.73 | n.d.a | n.d.a | 13.90 ± 0.64 | n.d.a | n.d.a | 15.21 ± 0.69 | n.d.a | n.d.a | 7.69 ± 1.58 |
| Gracilariopsis longissima [103] | 67.30 ± 3.10 | 41.67 ± 1.81 | 5.21 ± 0.31 | 16.16 ± 1.15 | 2.91 ± 0.10 | 0.93 ± 0.01 | 16.54 ± 1.24 | 1.60 ± 0.20 | 1.46 ± 0.00 | 2.26 ± 0.11 | 2.84 ± 0.33 | 1.85 ± 0.21 | 1.50 |
| Palmariaceae | |||||||||||||
| Palmaria palmata [83,85,99,104] | 44.12 ± 2.06 | 30.25 ± 5.22 | 5.37 ± 6.17 | 6.32 ± 2.06 | 1.68 ± 0.30 | 3.87 ± 1.15 | 44.86 ± 8.79 | 1.09 ± 0.70 | n.d.a | 0.92 ± 0.23 | 41.17 ± 9.10 | n.d.a | 0.045 ± 0.06 |
| Plocamiaceae | 74.10 ± 4.10 | 17.20 ± 17.68 | 0.65 ± 0.35 | 4.30 ± 0.10 | 1.25 ± 1.63 | 1.05 ± 1.06 | 20.40 ± 4.40 | 0.35 ± 0.35 | 0.50 | 4.40 ± 5.37 | 19.00 ± 25.74 | n.d.a | 0.70 ± 0.10 |
| Plocamium brasiliense [105] | 74.10 ± 4.10 | 4.70 ± 0.10 | 0.40 ± 0.00 | 4.30 ± 0.10 | 0.10 ± 0.00 | 0.30 ± 0.00 | 20.40 ± 4.40 | 0.10 ± 0.00 | n.d.a | 0.60 ± 0.30 | 0.80 ± 0.10 | n.d.a | 0.70 ± 0.10 |
| Plocamium violaceum [81] * | n.d.a | 29.70 | 0.90 | n.d.a | 2.40 | 1.80 | n.d.a | 0.60 | 0.50 | 8.20 | 37.20 | n.d.a | n.d.a |
| Pterocladiaceae | |||||||||||||
| Pterocladiella capillacea [82] | 60.62 ± 0.65 | 47.94 ± 0.64 | 2.21 ± 0.04 | 8.45 ± 0.10 | 3.15 ± 0.09 | 5.30 ± 0.01 | 30.94 ± 0.40 | 2.27 ± 0.05 | 1.94 ± 0.10 | 10.33 ± 0.09 | 15.26 ± 0.13 | n.d.a | 0.60 |
| Rhizophyllidaceae | |||||||||||||
| Ochtodes secundiramea [105] | 66.10 ± 1.30 | 6.10 ± 0.10 | 0.40 ± 0.10 | 21.50 ± 0.10 | 0.10 ± 0.00 | 2.50 ± 0.10 | 8.30 ± 0.10 | 0.20 ± 0.00 | n.d.a | n.d.a | 0.80 ± 0.00 | n.d.a | 4.00 ± 0.0 |
| Rhodomelaceae | 45.66 ± 18.37 | 32.95 ± 8.64 | 4.65 ± 6.88 | 17.81 ± 4.97 | 4.94 ± 3.91 | 9.54 ± 2.77 | 27.64 ± 17.97 | 3.15 ± 1.41 | 1.02 ± 1.83 | 11.45 ± 5.18 | 22.07 ± 12.34 | 2.44 ± 0.23 | 0.52 ± 0.19 |
| Acanthophora spicifera [102] | n.d.a | 40.27 | 1.43 | n.d.a | 1.43 | 10.21 | n.d.a | 1.37 | 0.79 | 10.19 | 6.18 | n.d.a | n.d.a |
| Alsidium triquetrum [84] | n.d.a | 27.36 ± 0.33 | n.d.a | n.d.a | n.d.a | 7.50 ± 0.31 | n.d.a | 3.24 ± 0.10 | n.d.a | n.d.a | n.d.a | n.d.a | |
| Chondria crassicaulis [78] | 36.88 ± 1.23 | 38.41 ± 1.54 | 19.93 ± 1.20 | 20.49 ± 0.88 | n.d.a | n.d.a | 28.15 ± 1.37 | n.d.a | n.d.a | 18.50 ± 0.69 | n.d.a | n.d.a | 0.42 ± 0.03 |
| Laurencia intermedia [88] | 71.04 ± 0.49 | 46.88 ± 0.37 | 2.90 ± 0.07 | 11.22 ± 0.13 | n.d.a | 11.22 ± 0.13 | 3.07 ± 0.06 | 2.70 ± 0.19 | 0.37 ± 0.15 | n.d.a | n.d.a | n.d.a | n.d.a |
| Laurencia papillosa [91] | 46.14 | 35.27 ± 0.79 | 1.96 ± 0.07 | 16.93 | 8.64 ± 0.11 | 8.29 ± 0.22 | 33.57 | 5.04 ± 0.15 | 0.31 ± 0.01 | 8.17 ± 0.1 | 18.42 ± 0.83 | 2.44 ± 0.23 | 0.75 |
| Odonthalia floccose [81] * | n.d.a | 28.60 | 0.80 | n.d.a | 1.70 | 5.30 | n.d.a | 1.40 | 0.40 | 14.80 | 31.60 | n.d.a | n.d.a |
| Osmundaria obtusiloba [84] | n.d.a | 21.27 ± 2.50 | 4.67 ± 1.05 | n.d.a | n.d.a | 9.82 ± 1.69 | n.d.a | 4.21 ± 1.41 | 5.86 ± 0.48 | n.d.a | n.d.a | n.d.a | n.d.a |
| Vertebrata lanosa [83] | 28.60 ± 0.20 | 25.60 ± 0.10 | 0.90 ± 0.10 | 22.60 ± 0.80 | 8.00 ± 0.00 | 5.60 ± 1.00 | 45.80 ± 0.90 | 4.10 ± 0.10 | 0.80 ± 0.10 | 5.60 ± 0.20 | 32.10 ± 1.00 | n.d.a | 0.40 ± 0.00 |
| Rhodymeniaceae | |||||||||||||
| Botryocladia occidentalis [84] | n.d.a | 53.58 ± 0.78 | 1.11 ± 1.93 | n.d.a | n.d.a | 20.09 ± 0.63 | n.d.a | n.d.a | n.d.a | n.d.a | n.d.a | n.d.a | n.d.a |
| Solieriaceae | 57.80 ± 3.66 | 38.20 ± 10.26 | 9.63 ± 7.20 | 16.11 ± 10.77 | 5.05 ± 6.85 | 3.44 ± 1.73 | 13.18 ± 9.72 | 1.23 ± 1.46 | 0.29 ± 0.27 | 15.20 ± 7.63 | 2.95 ± 2.89 | n.d.a | 8.80 ± 9.75 |
| Eucheuma denticulatum [88] | 53.72 ± 1.55 | 43.22 ± 17.76 | 2.30 ± 1.06 | 4.83 ± 3.43 | n.d.a | 4.83 ± 3.43 | 2.75 ± 2.10 | 2.27 ± 1.50 | 0.49 ± 0.18 | n.d.a | n.d.a | n.d.a | n.d.a |
| Solieria chordalis [75] | 58.90 ± 0.10 | 45.00 ± 0.20 | 9.90 ± 0.10 | 26.30 ± 0.10 | 0.20 ± 0.10 | 4.00 ± 0.10 | 14.80 ± 0.10 | n.d.a | n.d.a | 9.80 ± 0.10 | 5.00 ± 0.10 | n.d.a | 1.90 ± 0.10 |
| Solieria robusta [91] * | 60.80 | 26.40 | 16.70 | 17.20 | 9.90 | 1.50 | 22.00 | 0.20 | 0.10 | 20.60 | 0.90 | 0.00 | 15.70 |
| Spyridiaceae | |||||||||||||
| Spyridia clavata [84] | n.d.a | 35.50 ± 3.81 | 2.45 ± 2.27 | n.d.a | n.d.a | 13.99 ± 1.45 | n.d.a | n.d.a | n.d.a | n.d.a | n.d.a | n.d.a | n.d.a |
6. The Anti-Inflammatory Activity of Seaweed Lipids
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, A.U. An overview of inflammation: Mechanism and consequences. Front. Biol. 2011, 6, 274–281. [Google Scholar] [CrossRef]
- Kotas, M.E.; Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 2015, 160, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Schönbein, G.W. Analysis of inflammation. Annu. Rev. Biomed. Eng. 2006, 8, 93–151. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Friedman, E.; Shorey, C. Inflammation in multimorbidity and disability: An integrative review. Health Psychol. 2019, 38, 791. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Sochocka, M.; Donskow-Łysoniewska, K.; Diniz, B.S.; Kurpas, D.; Brzozowska, E.; Leszek, J. The Gut Microbiome Alterations and Inflammation-Driven Pathogenesis of Alzheimer’s Disease—A Critical Review. Mol. Neurobiol. 2019, 56, 1841–1851. [Google Scholar] [CrossRef]
- Akbari, M.; Hassan-Zadeh, V. IL-6 signalling pathways and the development of type 2 diabetes. Inflammopharmacology 2018, 26, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Kataria, Y.; Ellervik, C.; Mandrup-Poulsen, T. Treatment of type 2 diabetes by targeting interleukin-1: A meta-analysis of 2921 patients. Semin. Immunopathol. 2019, 41, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Elimam, H.; Abdulla, A.M.; Taha, I.M. Inflammatory markers and control of type 2 diabetes mellitus. Diabetes Metab. Syndr. 2019, 13, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Sethwala, A.M.; Goh, I.; Amerena, J.V. Combating Inflammation in Cardiovascular Disease. Heart Lung Circ. 2021, 30, 197–206. [Google Scholar] [CrossRef]
- WHO. Noncommunicable Diseases Progress Monitor 2017; World Health Organization: Geneva, Switzerland, 2017.
- WHO. Noncommunicable Diseases Country Profiles 2018; World Health Organization: Geneva, Switzerland, 2018.
- WHO. Global Status Report on Noncommunicable Diseases 2014; World Health Organization: Geneva, Switzerland, 2014.
- Brooks-Worrell, B.; Narla, R.; Palmer, J.P. Biomarkers and immune-modulating therapies for type 2 diabetes. Trends Immunol. 2012, 33, 546–553. [Google Scholar] [CrossRef]
- Soehnlein, O.; Libby, P. Targeting inflammation in atherosclerosis—From experimental insights to the clinic. Nat. Rev. Drug Discov. 2021, 20, 589–610. [Google Scholar] [CrossRef]
- Lebovitz, H.E. Thiazolidinediones: The forgotten diabetes medications. Curr. Diabetes Rep. 2019, 19, 151. [Google Scholar] [CrossRef]
- Bortolini, M.; Wright, M.B.; Bopst, M.; Balas, B. Examining the safety of PPAR agonists—Current trends and future prospects. Expert Opin. Drug Saf. 2013, 12, 65–79. [Google Scholar] [CrossRef]
- Tabas, I.; Glass, C.K. Anti-inflammatory therapy in chronic disease: Challenges and opportunities. Science 2013, 339, 166–172. [Google Scholar] [CrossRef]
- Fitton, J.H. Brown marine algae: A survey of therapeutic potentials. Altern. Complement. Ther. 2003, 9, 29–33. [Google Scholar] [CrossRef]
- Casas, R.; Castro-Barquero, S.; Estruch, R.; Sacanella, E. Nutrition and cardiovascular health. Int. J. Mol. Sci. 2018, 19, 3988. [Google Scholar] [CrossRef] [PubMed]
- Parolini, C. Effects of fish n-3 PUFAs on intestinal microbiota and immune system. Mar. Drugs 2019, 17, 374. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.; Caldas, A.; Oliveira, L.; Bressan, J.; Hermsdorff, H. Saturated fatty acids trigger TLR4-mediated inflammatory response. Atherosclerosis 2016, 244, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Rogero, M.M.; Calder, P.C. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef]
- Karakuła-Juchnowicz, H.; Rog, J.; Juchnowicz, D.; Morylowska-Topolska, J. GPR120: Mechanism of action, role and potential for medical applications. Postep. Hig. Med. Dosw. 2017, 71, 942–953. [Google Scholar] [CrossRef]
- Darwesh, A.M.; Sosnowski, D.K.; Lee, T.Y.; Keshavarz-Bahaghighat, H.; Seubert, J.M. Insights into the cardioprotective properties of n-3 PUFAs against ischemic heart disease via modulation of the innate immune system. Chem. Biol. Interact. 2019, 308, 20–44. [Google Scholar] [CrossRef]
- Li, K.; Huang, T.; Zheng, J.; Wu, K.; Li, D. Effect of marine-derived n-3 polyunsaturated fatty acids on C-reactive protein, interleukin 6 and tumor necrosis factor α: A meta-analysis. PLoS ONE 2014, 9, e88103. [Google Scholar]
- de Groot, R.H.; Emmett, R.; Meyer, B.J. Non-dietary factors associated with n-3 long-chain PUFA levels in humans–a systematic literature review. Br. J. Nutr. 2019, 121, 793–808. [Google Scholar] [CrossRef]
- Burdge, G.C.; Tan, S.-Y.; Henry, C.J. Long-chain n-3 PUFA in vegetarian women: A metabolic perspective. J. Nutr. Sci. 2017, 6, E58. [Google Scholar] [CrossRef]
- Rosell, M.S.; Lloyd-Wright, Z.; Appleby, P.N.; Sanders, T.A.; Allen, N.E.; Key, T.J. Long-chain n–3 polyunsaturated fatty acids in plasma in British meat-eating, vegetarian, and vegan men. Am. J. Clin. Nutr. 2005, 82, 327–334. [Google Scholar] [CrossRef]
- Salem Jr, N.; Eggersdorfer, M. Is the world supply of omega-3 fatty acids adequate for optimal human nutrition? Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Rohani-Ghadikolaei, K.; Abdulalian, E.; Ng, W.K. Evaluation of the proximate, fatty acid and mineral composition of representative green, brown and red seaweeds from the Persian Gulf of Iran as potential food and feed resources. J. Food Sci. Technol. 2012, 49, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Tabarsa, M.; Rezaei, M.; Ramezanpour, Z.; Robert Waaland, J.; Rabiei, R. Fatty acids, amino acids, mineral contents, and proximate composition of some brown seaweeds 1. J. Phycol. 2012, 48, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.; Ripol, A.; Afonso, C.; Freire, M.; Varela, J.; Quental-Ferreira, H.; Pousão-Ferreira, P.; Bandarra, N. Fatty acid profiles of the main lipid classes of green seaweeds from fish pond aquaculture. Food Sci. Nutr. 2017, 5, 1186–1194. [Google Scholar] [CrossRef]
- Collins, K.G.; Fitzgerald, G.F.; Stanton, C.; Ross, R.P. Looking beyond the terrestrial: The potential of seaweed derived bioactives to treat non-communicable diseases. Mar. Drugs 2016, 14, 60. [Google Scholar] [CrossRef]
- Peñalver, R.; Lorenzo, J.M.; Ros, G.; Amarowicz, R.; Pateiro, M.; Nieto, G. Seaweeds as a functional ingredient for a healthy diet. Mar. Drugs 2020, 18, 301. [Google Scholar] [CrossRef]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef]
- Palmieri, N.; Forleo, M.B. The potential of edible seaweed within the western diet. A segmentation of Italian consumers. Int. J. Gastron. Food Sci. 2020, 20, 100202. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Seaweeds as nutraceuticals for health and nutrition. Phycologia 2019, 58, 563–577. [Google Scholar] [CrossRef]
- Rengasamy, K.R.; Mahomoodally, M.F.; Aumeeruddy, M.Z.; Zengin, G.; Xiao, J.; Kim, D.H. Bioactive compounds in seaweeds: An overview of their biological properties and safety. Food Chem. Toxicol. 2020, 135, 111013. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, A.A.; Mohamed, H.I.; Ramadan, K.M.; Barqawi, A.A.; Mansour, A.T. Phytochemical and potential properties of seaweeds and their recent applications: A review. Mar. Drugs 2022, 20, 342. [Google Scholar] [CrossRef] [PubMed]
- Plouguerné, E.; da Gama, B.A.; Pereira, R.C.; Barreto-Bergter, E. Glycolipids from seaweeds and their potential biotechnological applications. Front. Cell. Infect. Microbiol. 2014, 4, 174. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Seca, A.M.; Pinto, D.C.; Michalak, I.; Trincone, A.; Mishra, A.P.; Nigam, M.; Zam, W.; Martins, N. Current trends on seaweeds: Looking at chemical composition, phytopharmacology, and cosmetic applications. Molecules 2019, 24, 4182. [Google Scholar] [CrossRef] [PubMed]
- Rindi, F.; Soler-Vila, A.; Guiry, M.D. Taxonomy of marine macroalgae used as sources of bioactive compounds. In Marine Bioactive Compounds; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–53. [Google Scholar]
- Mac Monagail, M.; Cornish, L.; Morrison, L.; Araújo, R.; Critchley, A.T. Sustainable harvesting of wild seaweed resources. Eur. J. Phycol. 2017, 52, 371–390. [Google Scholar] [CrossRef]
- Gomes, L.; Monteiro, P.; Cotas, J.; Gonçalves, A.M.; Fernandes, C.; Gonçalves, T.; Pereira, L. Seaweeds’ pigments and phenolic compounds with antimicrobial potential. Biomol. Concepts 2022, 13, 89–102. [Google Scholar] [CrossRef]
- Fish, J.D.; Fish, S. A Student’s Guide to the Seashore; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Morais, T.; Inácio, A.; Coutinho, T.; Ministro, M.; Cotas, J.; Pereira, L.; Bahcevandziev, K. Seaweed potential in the animal feed: A review. J. Mar. Sci. Eng. 2020, 8, 559. [Google Scholar] [CrossRef]
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M. Seaweed’s bioactive candidate compounds to food industry and global food security. Life 2020, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Agregán, R.; Munekata, P.E.; Franco, D.; Carballo, J.; Şahin, S.; Lacomba, R.; Barba, F.J. Proximate composition and nutritional value of three macroalgae: Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata. Mar. Drugs 2017, 15, 360. [Google Scholar] [CrossRef]
- Mohammed, H.; O’Grady, M.; O’Sullivan, M.; Hamill, R.; Kilcawley, K. An Assessment of Selected Nutritional, Bioactive, Thermal and Technological Properties of Brown and Red Irish Seaweed Species. Foods 2021, 10, 2784. [Google Scholar] [CrossRef]
- Cheney, D. Toxic and harmful seaweeds. In Seaweed in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2016; pp. 407–421. [Google Scholar]
- Afonso, N.C.; Catarino, M.D.; Silva, A.M.; Cardoso, S.M. Brown macroalgae as valuable food ingredients. Antioxidants 2019, 8, 365. [Google Scholar] [CrossRef]
- Rupérez, P. Mineral content of edible marine seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Qin, Y. Seaweed hydrocolloids as thickening, gelling, and emulsifying agents in functional food products. In Bioactive Seaweeds for Food Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 135–152. [Google Scholar]
- Lomartire, S.; Marques, J.C.; Gonçalves, A.M. An overview to the health benefits of seaweeds consumption. Mar. Drugs 2021, 19, 341. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Wieczorek, P.P.; Schroeder, G.; Michalak, I. Algae Biomass: Characteristics and Applications: Towards Algae-Based Products; Springer: Berlin/Heidelberg, Germany, 2018; Volume 8. [Google Scholar]
- Shah, M.D.; Venmathi Maran, B.A.; Shaleh, S.R.M.; Zuldin, W.H.; Gnanaraj, C.; Yong, Y.S. Therapeutic Potential and Nutraceutical Profiling of North Bornean Seaweeds: A Review. Mar. Drugs 2022, 20, 101. [Google Scholar] [CrossRef] [PubMed]
- Murai, U.; Yamagishi, K.; Kishida, R.; Iso, H. Impact of seaweed intake on health. Eur. J. Clin. Nutr. 2021, 75, 877–889. [Google Scholar] [CrossRef]
- Nelson, S.M.; Gao, Y.-T.; Nogueira, L.M.; Shen, M.-C.; Wang, B.; Rashid, A.; Hsing, A.W.; Koshiol, J. Diet and biliary tract cancer risk in Shanghai, China. PLoS ONE 2017, 12, e0173935. [Google Scholar] [CrossRef]
- Iso, H. Lifestyle and cardiovascular disease in Japan. J. Atheroscler. Thromb. 2011, 18, 83. [Google Scholar] [CrossRef]
- Bouga, M.; Combet, E. Emergence of seaweed and seaweed-containing foods in the UK: Focus on labeling, iodine content, toxicity and nutrition. Foods 2015, 4, 240–253. [Google Scholar] [CrossRef]
- Vo, T.-S.; Ngo, D.-H.; Kim, S.-K. Marine algae as a potential pharmaceutical source for anti-allergic therapeutics. Process Biochem. 2012, 47, 386–394. [Google Scholar] [CrossRef]
- Liu, F.T.; Yang, R.Y.; Hsu, D.K. Galectins in acute and chronic inflammation. Ann. N. Y. Acad. Sci. 2012, 1253, 80–91. [Google Scholar] [CrossRef]
- Santos, F.; Monteiro, J.P.; Duarte, D.; Melo, T.; Lopes, D.; da Costa, E.; Domingues, M.R. Unraveling the lipidome and antioxidant activity of native Bifurcaria bifurcata and invasive Sargassum muticum seaweeds: A lipid perspective on how systemic intrusion may present an opportunity. Antioxidants 2020, 9, 642. [Google Scholar] [CrossRef]
- Kendel, M.; Wielgosz-Collin, G.; Bertrand, S.; Roussakis, C.; Bourgougnon, N.; Bedoux, G. Lipid composition, fatty acids and sterols in the seaweeds Ulva armoricana, and Solieria chordalis from Brittany (France): An analysis from nutritional, chemotaxonomic, and antiproliferative activity perspectives. Mar. Drugs 2015, 13, 5606–5628. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Guihéneuf, F.; Fleming, G.; Chow, F.; Stengel, D. Temporal stability in lipid classes and fatty acid profiles of three seaweed species from the north-eastern coast of Brazil. Algal Res. 2019, 41, 101572. [Google Scholar] [CrossRef]
- Agregan, R.; Munekata, P.E.; Franco, D.; Dominguez, R.; Carballo, J.; Lorenzo, J.M. Phenolic compounds from three brown seaweed species using LC-DAD–ESI-MS/MS. Food Res. Int. 2017, 99, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.P.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M. Seaweeds as valuable sources of essential fatty acids for human nutrition. Int. J. Environ. Res. Public Health 2021, 18, 4968. [Google Scholar] [CrossRef]
- Van Ginneken, V.J.; Helsper, J.P.; de Visser, W.; van Keulen, H.; Brandenburg, W.A. Polyunsaturated fatty acids in various macroalgal species from north Atlantic and tropical seas. Lipids Health Dis. 2011, 10, 104. [Google Scholar] [CrossRef]
- Harwood, J.L. Algae: Critical sources of very long-chain polyunsaturated fatty acids. Biomolecules 2019, 9, 708. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Hannan, M.A.; Sohag, A.A.M.; Dash, R.; Haque, M.N.; Mohibbullah, M.; Oktaviani, D.F.; Hossain, M.T.; Choi, H.J.; Moon, I.S. Phytosterols of marine algae: Insights into the potential health benefits and molecular pharmacology. Phytomedicine 2020, 69, 153201. [Google Scholar] [CrossRef]
- Feriedoon, S.; Md. Jiaur, R. Bioactives in seaweeds, algae, and fungi and their role in health promotion. J. Food Bioact. 2018, 2, 58–81. [Google Scholar]
- Susanto, E.; Fahmi, A.S.; Hosokawa, M.; Miyashita, K. Variation in lipid components from 15 species of tropical and temperate seaweeds. Mar. Drugs 2019, 17, 630. [Google Scholar] [CrossRef]
- Pangestuti, R.; Haq, M.; Rahmadi, P.; Chun, B.S. Nutritional value and biofunctionalities of two edible green seaweeds (Ulva lactuca and Caulerpa racemosa) from Indonesia by subcritical water hydrolysis. Mar. Drugs 2021, 19, 578. [Google Scholar] [CrossRef] [PubMed]
- Magdugo, R.P.; Terme, N.; Lang, M.; Pliego-Cortés, H.; Marty, C.; Hurtado, A.Q.; Bedoux, G.; Bourgougnon, N. An analysis of the nutritional and health values of Caulerpa racemosa (Forsskål) and Ulva fasciata (Delile)—Two chlorophyta collected from the Philippines. Molecules 2020, 25, 2901. [Google Scholar] [CrossRef] [PubMed]
- Khotimchenko, S.; Vaskovsky, V.; Titlyanova, T. Fatty acids of marine algae from the Pacific coast of North California. Bot. Mar. 2002, 45, 17–22. [Google Scholar] [CrossRef]
- Pereira, H.; Barreira, L.; Figueiredo, F.; Custódio, L.; Vizetto-Duarte, C.; Polo, C.; Rešek, E.; Engelen, A.; Varela, J. Polyunsaturated fatty acids of marine macroalgae: Potential for nutritional and pharmaceutical applications. Mar. Drugs 2012, 10, 1920–1935. [Google Scholar] [CrossRef] [PubMed]
- Mæhre, H.K.; Malde, M.K.; Eilertsen, K.E.; Elvevoll, E.O. Characterization of protein, lipid and mineral contents in common Norwegian seaweeds and evaluation of their potential as food and feed. J. Sci. Food Agric. 2014, 94, 3281–3290. [Google Scholar] [CrossRef]
- Mandalka, A.; Cavalcanti, M.I.L.G.; Harb, T.B.; Toyota Fujii, M.; Eisner, P.; Schweiggert-Weisz, U.; Chow, F. Nutritional composition of beach-cast marine algae from the Brazilian coast: Added value for algal biomass considered as waste. Foods 2022, 11, 1201. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.; Melo, T.; Rey, F.; Meneses, J.; Monteiro, F.L.; Helguero, L.A.; Abreu, M.H.; Lillebø, A.I.; Calado, R.; Domingues, M.R. Valuing bioactive lipids from green, red and brown macroalgae from aquaculture, to foster functionality and biotechnological applications. Molecules 2020, 25, 3883. [Google Scholar] [CrossRef] [PubMed]
- Al-Adilah, H.; Al-Sharrah, T.K.; Al-Bader, D.; Ebel, R.; Küpper, F.C.; Kumari, P. Assessment of arabian gulf seaweeds from kuwait as sources of nutritionally important polyunsaturated fatty acids (Pufas). Foods 2021, 10, 2442. [Google Scholar] [CrossRef]
- Kinyuru, J.; Muraguri, E.; Wakibia, J. Chemical Composition and Functional Properties of Selected Seaweeds from the Kenya Coast. J. Food Res. 2016, 5, 114–123. [Google Scholar]
- Tabarsa, M.; Rezaei, M.; Ramezanpour, Z.; Waaland, J.R. Chemical compositions of the marine algae Gracilaria salicornia (Rhodophyta) and Ulva lactuca (Chlorophyta) as a potential food source. J. Sci. Food Agric. 2012, 92, 2500–2506. [Google Scholar] [CrossRef]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- McCauley, J.I.; Meyer, B.J.; Winberg, P.C.; Ranson, M.; Skropeta, D. Selecting Australian marine macroalgae based on the fatty acid composition and anti-inflammatory activity. J. Appl. Phycol. 2015, 27, 2111–2121. [Google Scholar] [CrossRef]
- Polat, S.; Ozogul, Y. Seasonal proximate and fatty acid variations of some seaweeds from the northeastern Mediterranean coast. Oceanologia 2013, 55, 375–391. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.; López-Cervantes, J.; Lopez-Hernandez, J.; Paseiro-Losada, P. Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chem. 2004, 85, 439–444. [Google Scholar] [CrossRef]
- Schmid, M.; Guihéneuf, F.; Stengel, D.B. Evaluation of food grade solvents for lipid extraction and impact of storage temperature on fatty acid composition of edible seaweeds Laminaria digitata (Phaeophyceae) and Palmaria palmata (Rhodophyta). Food Chem. 2016, 208, 161–168. [Google Scholar] [CrossRef]
- Susanto, E.; Fahmi, A.S.; Abe, M.; Hosokawa, M.; Miyashita, K. Lipids, fatty acids, and fucoxanthin content from temperate and tropical brown seaweeds. Aquat. Procedia 2016, 7, 66–75. [Google Scholar] [CrossRef]
- Conde, T.A.; Neves, B.F.; Couto, D.; Melo, T.; Neves, B.; Costa, M.; Silva, J.; Domingues, P.; Domingues, M.R. Microalgae as sustainable bio-factories of healthy lipids: Evaluating fatty acid content and antioxidant activity. Mar. Drugs 2021, 19, 357. [Google Scholar] [CrossRef]
- Gerasimenko, N.; Logvinov, S. Seasonal composition of lipids, fatty acids pigments in the brown alga Sargassum pallidum: The potential for health. Open J. Mar. Sci. 2016, 6, 498. [Google Scholar] [CrossRef]
- Cian, R.E.; Fajardo, M.A.; Alaiz, M.; Vioque, J.; González, R.J.; Drago, S.R. Chemical composition, nutritional and antioxidant properties of the red edible seaweed Porphyra columbina. Int. J. Food Sci. Nutr. 2014, 65, 299–305. [Google Scholar] [CrossRef]
- Robertson, R.C.; Guihéneuf, F.; Bahar, B.; Schmid, M.; Stengel, D.B.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. The anti-inflammatory effect of algae-derived lipid extracts on lipopolysaccharide (LPS)-stimulated human THP-1 macrophages. Mar. Drugs 2015, 13, 5402–5424. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Pechenkina-Shubina, E.E.; Rozentsvet, O.A. Glycolipids and fatty acids of some seaweeds and marine grasses from the Black Sea. Phytochemistry 1991, 30, 2279–2283. [Google Scholar] [CrossRef]
- da Costa, E.; Melo, T.; Reis, M.; Domingues, P.; Calado, R.; Abreu, M.H.; Domingues, M.R. Polar lipids composition, antioxidant and anti-inflammatory activities of the atlantic red seaweed Grateloupia turuturu. Mar. Drugs 2021, 19, 414. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.T.; Matanjun, P. Chemical composition and physicochemical properties of tropical red seaweed, Gracilaria changii. Food Chem. 2017, 221, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, N.; Kinami, T.; Miyashita, K.; Park, S.-B.; Endo, Y.; Fujimoto, K. Occurrence of conjugated polyenoic fatty acids in seaweeds from the Indian Ocean. Z. Naturforsch. C J. Biosci. 2004, 59, 310–314. [Google Scholar] [CrossRef]
- Stabili, L.; Acquaviva, M.; Biandolino, F.; Cavallo, R.; De Pascali, S.; Fanizzi, F.; Narracci, M.; Petrocelli, A.; Cecere, E. The lipidic extract of the seaweed Gracilariopsis longissima (Rhodophyta, Gracilariales): A potential resource for biotechnological purposes? New Biotechnol. 2012, 29, 443–450. [Google Scholar] [CrossRef]
- Lopes, D.; Melo, T.; Meneses, J.; Abreu, M.H.; Pereira, R.; Domingues, P.; Lillebø, A.I.; Calado, R.; Domingues, M.R. A new look for the red macroalga Palmaria palmata: A seafood with polar lipids rich in EPA and with antioxidant properties. Mar. Drugs 2019, 17, 533. [Google Scholar] [CrossRef]
- Gressler, V.; Fujii, M.T.; Martins, A.P.; Colepicolo, P.; Mancini-Filho, J.; Pinto, E. Biochemical composition of two red seaweed species grown on the Brazilian coast. J. Sci. Food Agric. 2011, 91, 1687–1692. [Google Scholar] [CrossRef]
- Giriwono, P.E.; Iskandriati, D.; Andarwulan, N. Screening of In-Vitro Anti-Inflammatory and Antioxidant Activity of Sargassum ilicifolium Crude Lipid Extracts from Different Coastal Areas in Indonesia. Mar. Drugs 2021, 19, 252. [Google Scholar]
- Kok, J.M.L.; Dowd, G.; Cabral, J.; Wise, L. Time-Dependent Anti-inflammatory Effects of a Lipid Extract from Macrocystis pyrifera on Toll-Like Receptor 2 Signaling in Human THP-1 Monocytes. Planta Med. Int. Open 2022, 9, e80–e89. [Google Scholar] [CrossRef]
- Banskota, A.H.; Stefanova, R.; Sperker, S.; Lall, S.; Craigie, J.S.; Hafting, J.T.; Citchley, A.T. Polar lipids from the marine macroalga Palmaria palmata inhibit lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophage cells. Pchtochemistry 2014, 101, 101–108. [Google Scholar] [CrossRef]
- Pham, T.H.; Nguyen, V.T.A.; Do, T.T.T.; Do, A.D.; Dam, D.T.; Tran, T.T.V.; Pham, Q.L.; Le, T.T. Lipidomics and Anti-Inflammation Activity of Brown Algae, Lobophora sp., in Vietnam. J. Chem. 2020, 2020, 8829054. [Google Scholar] [CrossRef]
- Banskota, A.H.; Stefanova, R.; Sperker, S.; Lall, S.; Craigie, J.S.; Hafting, J.T. Lipids isolated from the cultivated red alga Chondrus crispus inhibit nitric oxide production. J. Appl. Phycol. 2014, 26, 1565–1571. [Google Scholar] [CrossRef]
- Da Costa, E.; Melo, T.; Moreira, A.S.; Bernardo, C.; Helguero, L.; Ferreira, I.; Cruz, M.T.; Rego, A.M.; Domingues, P.; Calado, R. Valorization of lipids from Gracilaria sp. through lipidomics and decoding of antiproliferative and anti-inflammatory activity. Mar. Drugs 2017, 15, 62. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Daletos, G.; Proksch, P.; Andrade, p.B.; Valentão, P. Anti-inflammatory potential of monogalactosyl diacylglycerols and a monoacylglycerol from the edible brown seaweed Fucus spiralis Linnaeus. Mar. Drugs 2014, 12, 1406–1418. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.A.; Cho, J.-Y.; Lee, M.-C.; Kang, J.-Y.; Park, N.G.; Fujii, H.; Hong, Y.-K. Isolation of two anti-inflammatory and one pro-inflammatory polyunsaturated fatty acids from the brown seaweed Undaria pinnatifida. J. Agric. Food Chem. 2007, 55, 6984–6988. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Gyawali, Y.P.; Ahn, S.H.; Khan, M.N.; Kong, I.S.; Hong, Y.K. A methoxylated fatty acid isolated from the brown seaweed Ishige okamurae inhibits bacterial phospholipase A2. Phytother. Res. 2008, 22, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Attiq, A.; Jalil, J.; Husain, K.; Ahmad, W. Raging the war against inflammation with natural products. Front. Pharmacol. 2018, 9, 976. [Google Scholar] [CrossRef]
- Gandhi, J.; Khera, L.; Gaur, N.; Paul, C.; Kaul, R. Role of modulator of inflammation cyclooxygenase-2 in gammaherpesvirus mediated tumorigenesis. Front. Microbiol. 2017, 8, 538. [Google Scholar] [CrossRef]
- King, C.T. (Ed.) Inflammation, Inflammatory Mediators, and Immune-Mediated Disease. In Integrated Pathology; Elsevier: Amsterdam, The Netherlands, 2007; pp. 21–57. [Google Scholar]
- Tripathi, P.; Tripathi, P.; Kashyap, L.; Singh, V. The role of nitric oxide in inflammatory reactions. FEMS Immunol. Med. Microbiol. 2007, 51, 443–452. [Google Scholar] [CrossRef]
- Sharma, J.; Al-Omran, A.; Parvathy, S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Zamora, R.; Vodovotz, Y.; Billiar, T.R. Inducible nitric oxide synthase and inflammatory diseases. Mol. Med. 2000, 6, 347–373. [Google Scholar] [CrossRef] [PubMed]
- Banskota, A.H.; Gallant, P.; Stefanova, R.; Melanson, R.; O’Leary, S.J. Monogalactosyldiacylglycerols, potent nitric oxide inhibitors from the marine microalga Tetraselmis chui. Nat. Prod. Res. 2013, 27, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From clinical significance to quantification. Adv. Sci. 2021, 8, 2004433. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.N.; Siddiqui, S.A.; Ibrahim, M.; Hakim, M.L.; Ahammed, M.S.; Kabir, A.; Sultana, F. Inflammatory cytokines in the pathogenesis of cardiovascular disease and cancer. SAGE Open Med. 2020, 8, 2050312120965752. [Google Scholar] [CrossRef] [PubMed]
- Balsinde, J.; Winstead, M.V.; Dennis, E.A. Phospholipase A2 regulation of arachidonic acid mobilization. FEBS Lett. 2002, 531, 2–6. [Google Scholar] [CrossRef]
- Stromsnes, K.; Correas, A.G.; Lehmann, J.; Gambini, J.; Olaso-Gonzalez, G. Anti-Inflammatory properties of diet: Role in healthy aging. Biomedicines 2021, 9, 922. [Google Scholar] [CrossRef]
- Roohinejada, S.; Koubaab, M.; Barba, F.J.; Saljoughi, S.; Amide, M.; Greinera, R. Application of seaweeds to develop new food products with enhanced shelf-life, quality and health-related beneficial properties. Food Res. Int. 2017, 99, 1066–1083. [Google Scholar] [CrossRef]
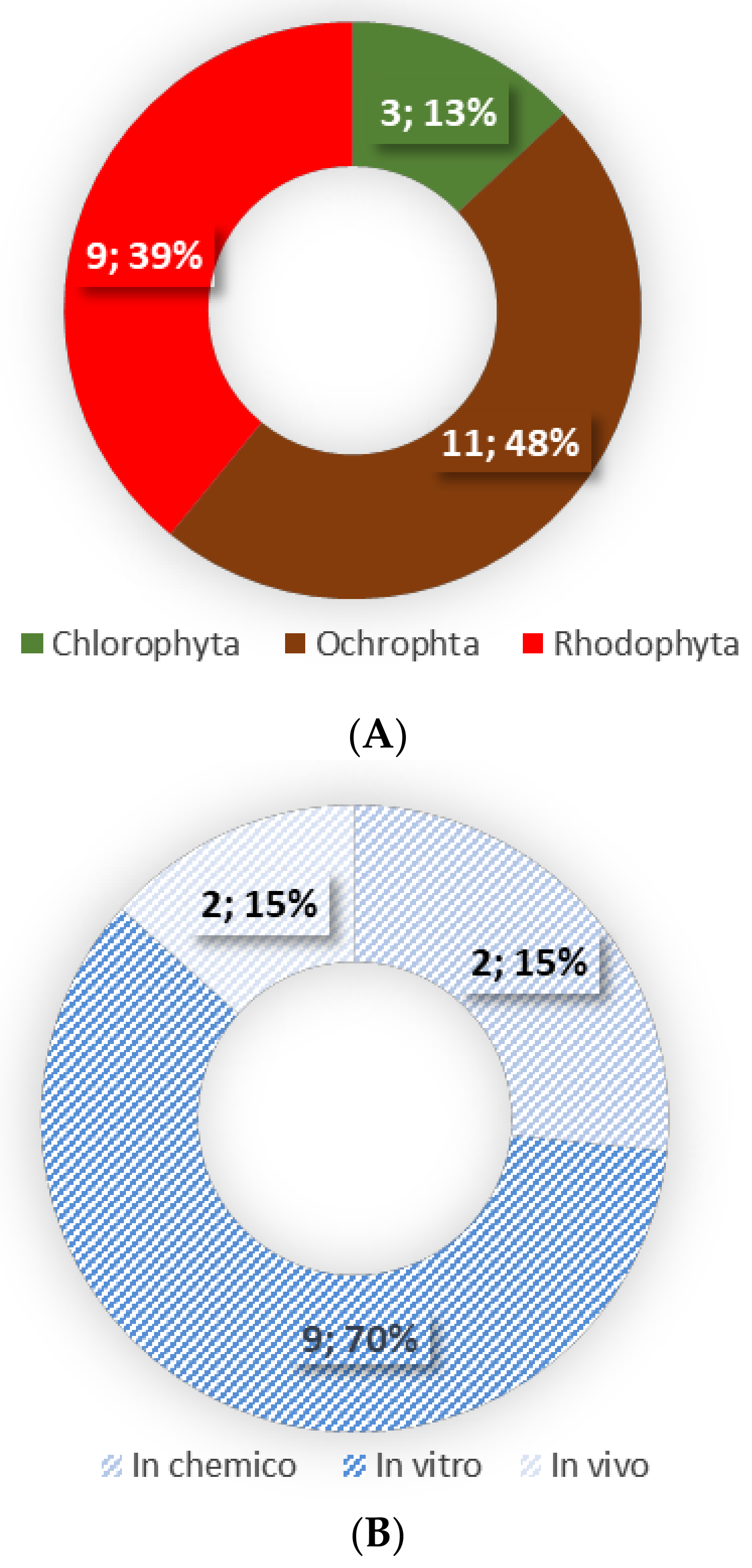
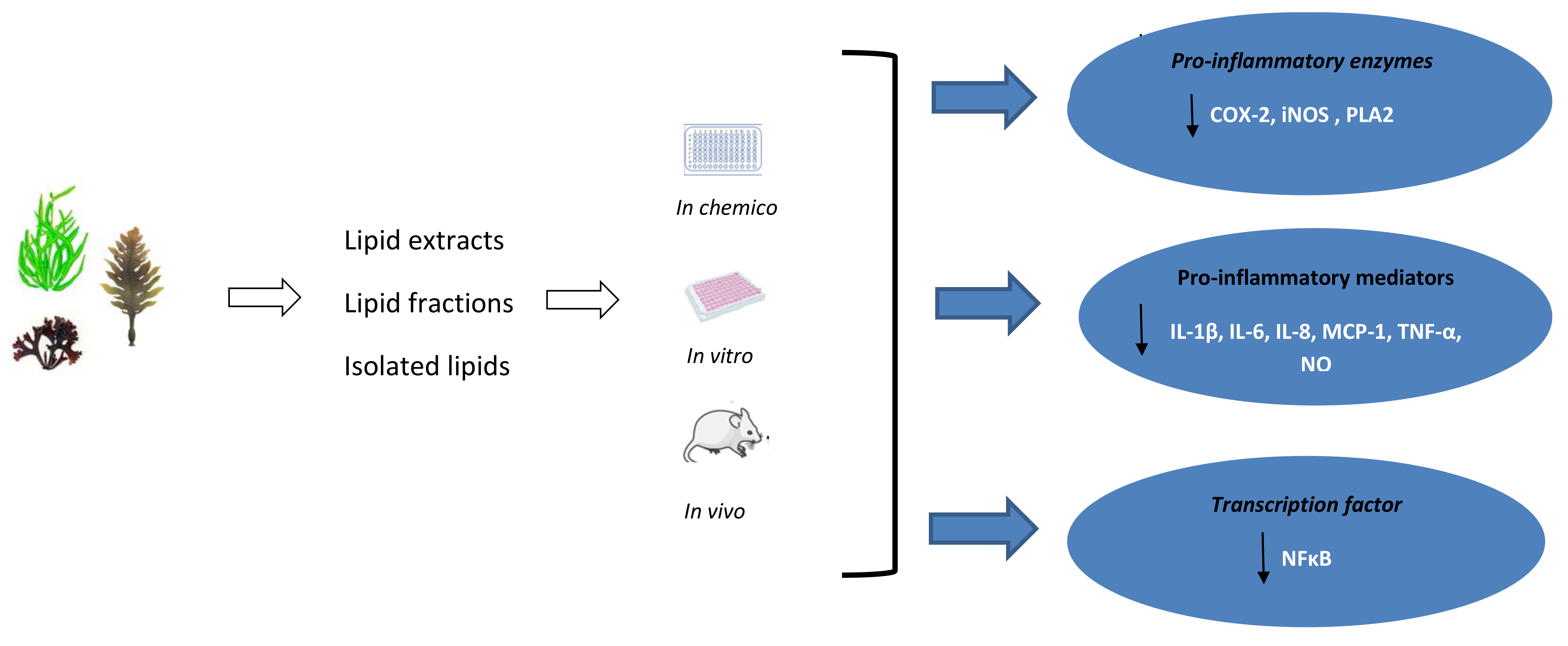
| Seaweed Species | Phylum | Type of Lipids/Lipid Extract | Model | Results | Reference |
|---|---|---|---|---|---|
| Sargassum ilicifolium | Ochrophyta | Crude lipid extract Extraction: methanol:chloroform 2:1 | Murine macrophage RAW 264.7 cells NO inhibition | S. ilicifocum from Ujung Genteng Beach–Sukabumi region: NO inhibition ranged from 83.21% to 26.10% in pre-incubation model, and from 28.07 to 61.81% in co-incubation model S. ilicifocum from Awur Bay–Jepara region: NO inhibition ranged from 11.29 to 65.76% in pre-incubated cell culture model and 13.44–41.80% in co-incubation model No significant effect was observed for SHB treatment | [106] |
| Porphyra dioica Palmaria palmate Chondrus crispus | Rhodphyta | Crude lipid extract Extraction: methanol:chloroform 1:1 | Human THP-1 monocytic cell line Production of pro-inflammatory cytokines Expression of genes linked to inflammatory signaling | Downregulation of TLR4, STAT3; upregulation of PTGRE1, Downregulated production of IL-6 and IL-8; upregulation of NOS2 Upregulated production of TNF-α All species downregulated TLR1, TLR8, TRAF5 | [98] |
| Macrocystis pyrifera | Ochrophyta | Crude lipid extract Extraction: methanol:chloroform 2:1 | Human THP-1 monocytic cell line Production of pro-inflammatory cytokines NFκΒ pathway | Reduced production of MCP-1, IL-8 and IL-1β Reduced expression of mRNA of MCP-1, IL-8, and IL-1β Reduced levels of MyD88 and NFκΒ2/p100 protein | [107] |
| Ulva rigida Codium tomentosum | Chlorophyta | Crude lipids extract Extraction: methanol:chloroform 2:1 | Cyclooxygenase Inhibition Assay | COX-2 inhibition of 87.9 ± 0.1% COX-2 inhibition of 82.3 ± 2.2% | [85] |
| Gracilaria gracilis Palmaria palmate Porphyra dioica | Rhodophyta | Crude lipids extract Extraction: methanol:chloroform 2:1 | Cyclooxygenase Inhibition Assay | No inhibition of COX-2 COX-2 inhibition of 89.5 ± 0.9%, COX-2 inhibition of 83.6 ± 8.1% | [85] |
| Fucus vesiculosus | Ochrophyta | Crude lipids extract Extraction: methanol:chloroform 2:1 | Cyclooxygenase Inhibition Assay | COX-2 inhibition of 34.6 ± 7.1% | [85] |
| Grateloupia turuturu | Rhodophyta | Crude lipids extract Extraction: methanol:chloroform 2:1 | Cyclooxygenase Inhibition Assay | COX-2 inhibition of 50% at the concentration of lipid extract of 33 µg mL−1 | [100] |
| Palmaria palmata | Rhodophyta | Crude lipids extract Extraction: methanol:chloroform 1:1 The extract partitioned with EtAOc and the EtAOc fraction, and next fractioned by silica gel column chromatography into three subfractions. 10 compounds were identified (2S)-1-O-eicosapentaenoyl-2-O-myristoyl-3-O-(6-sulfo-α-d-quinovopyranosyl)-glycerol (1), (2S)-1-O-eicosapentaenoyl-2-O-palmitoyl-3-O-(6-sulfo-α-d-quinovopyranosyl)-glycerol (2), 1-O-eicosapentaenoyl-2-O-trans-3-hexadecenoyl-3-phospho-(1′-glycerol)-glycerol (3), 1-O-eicosapentaenoyl-2-O-palmitoyl-3-phospho-(1′-glycerol)-glycerol (4), 1,2-Dieicosapentanoyl-glycero-3-phophocholine (5), (2S)-1,2-bis-O-eicosapentaenoyl-3-O-β-d-galactopyranosylglycerol (6), (2S)-1-O-eicosapentaenoyl-2-O-palmitoyl-3-O-β-d-galactopyranosylglycerol (7), (2S)-1,2-bis-O-eicosapentaenoyl-3-O-(β-d-galactopyranosyl-6-1α-d-galactopyranosyl)-glycerol (8), (2S)-1-O-eicosapentaenoyl-2-O-myristoyl-3-O-(β-d-galactopyranosyl-6-1α-d-galactopyranosyl)-glycerol (9) and (2S)-1-O-eicosapentaenoyl-2-O-palmitoyl-3-O-(β-d-galactopyranosyl-6-1α-d-galactopyranosyl)-glycerol (10) | Murine macrophage RAW 264.7 cell line NO production iNOS expression | The methanolic extract showed no effect on the NO production. The EtOAc fraction showed significant does-depended NO inhibition; at 100 μg/mL, the EtOAc fraction inhibited 89.4% of NO. All isolated lipids showed dose depended NO inhibitory activity and reduced iNOS expression. | [108] |
| Ecklonia radiata Hormosira banksia Myriogloea sciurus Phyllospora comosa Solieria robusta Ulva sp. | Ochrophyta Rhodophyta Chlorophyta | Crude lipid extract. Extraction: dichloromethane: methanol1:1, The crude extract was partitioned into DCM, EtOAc and BuOH. | Murine macrophage RAW 264.7 cell line NO production | Reduction in NO production across almost all extracts. The NO inhibitory activity was greatest in the nonpolar, lipid-rich DCM extracts (>76% activity for all species), followed by the intermediate polarity ethyl acetate (EtOAc) extracts (>50% activity for all species except H. banksia), with the lowest activity observed in the polar butanol (BuOH) extracts. | [90] |
| Lobophora | Ochrophyta | Crude lipids extract Extraction: methanol:chloroform 2:1 followed by the separation to polar and non-polar lipid fractions. | Murine macrophage RAW 264.7 cell line NO production | Non-polar lipid fraction displayed the highest NO inhibitory activity followed by crude lipid extract and polar lipid. | [109] |
| Chondrus crispus | Rhodophyta | Methanolic extract partitioned against EtOAc. The EtOAc fraction was subjected to solid phase extraction, followed by elution with hexane/EtOAc, CHCl3, and MeOH gradients to obtain three subfractions. 11 metabolites were identified: eicosapentaenoic (EPA, 1), arachidonic acids (AA, 2) and lutein (3), (2S)-1,2-Bis-O-eicosapentaenoyl-3-O-(β-D-galactopyranosylglycerol (4) (2S)-1-O-eicosapentaenoyl-2-O-arachidonoyl-3-O-β-D-galactopyranosylglycerol (5), (2S)-1-O-(6Z,9Z,12Z,15Z-octadecatetranoyl)-2-O-palmitoyl-3-O-β-D-galactopyranosylglycerol (6), (2S)-1-O-eicosapentaenoyl-2-O-palmitoyl-3-O-β-D-galactopyranosylglycerol (7), (2S)-1,2-bis-O-arachidonoyl-3-O-β-D-galactopyranosylglycerol (8), (2S)-1-O-arachidonoyl-2-O-palmitoyl-3-O-β-D-galactopyranosylglycerol (9), (2S)-1-O-eicosapentaenoyl-2-O-palmitoyl-3-O-(β-D-galactopyranosyl-6-1α-D-galactopyranosyl)-glycerol (10), and (2S)-1-O-arachidonoyl2-O-palmitoyl-3-O-(β-D-galactopyranosyl-6-1α-D-galactopyranosyl)-glycerol (11) | Murine macrophage RAW 264.7 cell line NO production | The methanolic extract showed does-dependent NO inhibition reduction by 15.6% of NO production at 100 μg mL−1 concentration. EtOAc fraction showed a fourfold increase in activity by inhibiting 64.6% NO production at 100 μg mL−1 concentration. Subfraction 1 (metabolites 1, 2, and 3) was relatively weaker in NO inhibitory activity, as compared to the remaining two polar fractions. Lutein and galactolipids showed the strongest NO inhibitory activity as compared to both free polyunsaturated fatty acids, EPA) and AA. | [110] |
| Gracilaria sp. | Rhodophyta | Crude lipid extract. Extraction: methanol:chloroform 2:1, followed by the fractionation of lipid extract to polar lipids. | Murine macrophage RAW 264.7 cell line NO production | Polar lipids showed a dose-dependent NO inhibition of 35% attained at the concentration of 100 µg/mL. The extract at concentrations lower than 50 μg/mL had no significant inhibitory effect on NO production. | [111] |
| Fucus spiralis | Ochrophyta | Methanolic extract was subjected to partitioning to give n-hexane, EtOAc and n-BuOH fractions. The EtOAc fraction was partitioned to: - monoacylglycerol featuring oleic acid (Compound (1)) - 1:1 mixture of two MGDGs containing eicosapentaenoic acid combined with octadecatetraenoic acid (Compound (2)) - 1:1 mixture of two MGDGs containing eicosapentaenoic acid combined with linolenic acid (Compound (3)) | Murine macrophage RAW 264.7 cell line NO production | All isolated compounds showed dose dependent NO inhibitory activity. The fraction consisting of compounds (2) and (3), in a ratio of 1:1, was slightly more effective than compound 1. | [112] |
| Undaria pinnatifida | Ochrophyta | Acetonitrile extract fractioned by silica gel column chromatography. Three fatty acids were isolated: stearidonic acid (SA), eicosapentanoic acid (EPA), arachidonic acid (AA), at a flow rate of 2 mL/min. | Inflammatory bioassay: BALB/c mice; phorbol 12-myristate 13-acetate (PMA) and various concentrations of SA, EPA and AA were applied topically to the mouse ears. Ear oedema, erythema and blood flow were measured 10 h later. | SA: IC50 values of 160, 314, and 235 µg per ear for edema, erythema, and blood flow, respectively. EPA: IC50 values of 230, 462, and 236 µg per ear, edema, erythema and blood flow, respectively. AA: low concentrations showed anti-inflammatory activities, but doses of more than 243 µg per ear induced inflammatory symptoms. | [113] |
| Ishige okamurae | Ochrophyta | Methanolic extract fractioned by polarity. The moderately polar chloroform extract was further fractionated on a Sephadex LH-20 column. The most active fraction was further fractionated on a silica gel column with n-hexane. The late n-hexane eluant was applied to a reverse-phase HPLC. The most active peak was eluted at 25% acetonitrile and analysed with GS-MS. The isolated compound was 7-methoxy-9-methylhexadeca-4,8-dienoic (MMHDA) | Phospholipase A2 (PLA2) inhibition Inflammatory bioassay: BALB/c mice; phorbol 12-myristate 13-acetate (PMA) and MMHDA were applied topically to the mouse ears. Ear oedema and erythema were measured 10 h later. | Inhibition of PLA2, oedema and erythema. PLA2 IC50 and MIC 1.9 µg/mL and 4.0 µg/mL, respectively. Oedema IC50 and MIC 3.6 and 5.2 mg/mL, respectively. Erythema IC50 and MIC 4.6 mg/mL and 9.1 mg/mL, respectively. | [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaworowska, A.; Murtaza, A. Seaweed Derived Lipids Are a Potential Anti-Inflammatory Agent: A Review. Int. J. Environ. Res. Public Health 2023, 20, 730. https://doi.org/10.3390/ijerph20010730
Jaworowska A, Murtaza A. Seaweed Derived Lipids Are a Potential Anti-Inflammatory Agent: A Review. International Journal of Environmental Research and Public Health. 2023; 20(1):730. https://doi.org/10.3390/ijerph20010730
Chicago/Turabian StyleJaworowska, Agnieszka, and Aliza Murtaza. 2023. "Seaweed Derived Lipids Are a Potential Anti-Inflammatory Agent: A Review" International Journal of Environmental Research and Public Health 20, no. 1: 730. https://doi.org/10.3390/ijerph20010730
APA StyleJaworowska, A., & Murtaza, A. (2023). Seaweed Derived Lipids Are a Potential Anti-Inflammatory Agent: A Review. International Journal of Environmental Research and Public Health, 20(1), 730. https://doi.org/10.3390/ijerph20010730






