Lifestyle, Insulin Resistance and Semen Quality as Co-Dependent Factors of Male Infertility
Abstract
1. Introduction
2. Sources of Articles, Search Strategies, and the Selection Process
2.1. Sources of Information
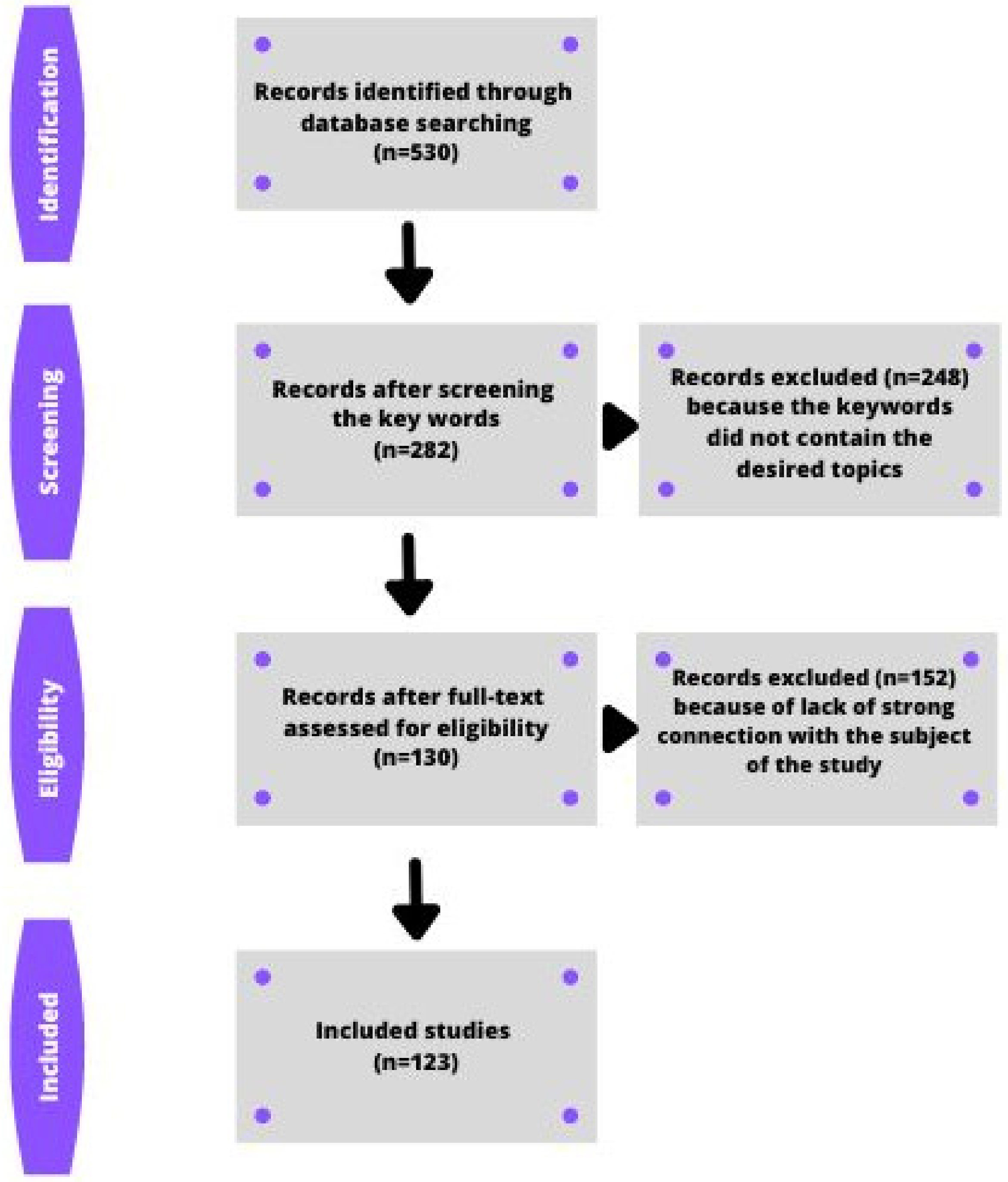
2.2. Search Strategy for the Identification of Studies
2.3. Inclusion/Exclusion Criteria: Data Management and Study Selection
2.4. Data Extraction
2.5. Quality Assessment of Studies
3. Lifestyle and Insulin Resistance
3.1. Diet
3.2. BMI
3.3. Physical Activity
3.4. Sleep
3.5. Stress
3.6. Psychoactive Substances
3.7. Sexual Abstinence
3.8. External Environment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koperwas, M.; Głowacka, M. Problem niepłodności wśród kobiet i mężczyzn—epidemiologia, czynniki ryzyka i świadomość społeczna. Asp. Zdrowia I Chor. 2017, 2, 31–49. [Google Scholar]
- Klimek, R. Niepłodność Uleczalna Czy Nie? Wydawnictwo Lekarskie PZWL: Warszawa, Poland, 1995; pp. 13, 19, 30, 31, 54, 57, 58, 60, 65. [Google Scholar]
- World Health Organization (WHO). WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Morrison, C.D.; Brannigan, R.E. Metabolic syndrome and infertility in men. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Pacey, A.A. Environmental and lifestyle factors associated with sperm DNA damage. Hum. Fertil. 2010, 13, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Han, R.-Y.; Mei, X.-A.; Qi, Y.-N.; Ma, J.-Y.; Liu, W.-J.; Wang, S.-S. Correlation of insulin resistance with male reproductive hormone levels and semen parameters. Zhonghua nan ke xue = Natl. J. Androl. 2018, 24, 695–699. [Google Scholar]
- Antoniolli, L.P.; Nedel, B.L.; Pazinato, T.C.; Mesquita, L.D.A.; Gerchman, F. Accuracy of insulin resistance indices for metabolic syndrome: A cross-sectional study in adults. Diabetol. Metab. Syndr. 2018, 10, 65. [Google Scholar] [CrossRef]
- Niemann, M.J.; Tucker, L.A.; Bailey, B.W.; Davidson, L.E. Strength Training and Insulin Resistance: The Mediating Role of Body Composition. J. Diabetes Res. 2020, 2020, 7694825. [Google Scholar] [CrossRef]
- Adnan, E.; Rahman, I.A.; Faridin, H. Relationship between insulin resistance, metabolic syndrome components and serum uric acid. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2158–2162. [Google Scholar] [CrossRef]
- Marotta, T.; Russo, B.F.; Ferrara, L.A. Triglyceride-to-HDL-cholesterol ratio an metabolic syndrome as contributors to cardiovas-cular risk in overweight patients. Obesity 2010, 18, 1608.e13. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Yang, S.-F.; Chen, Y.-J.; Chou, M.-Y.; Chang, Y.-C. The upregulation of insulin-like growth factor-1 in oral submucous fibrosis. Oral Oncol. 2005, 41, 940–946. [Google Scholar] [CrossRef]
- Jing, Y.; Wu, F.; Li, D.; Yang, L.; Li, Q.; Li, R. Metformin improves obesity-associated inflammation by altering macrophages polarization. Mol. Cell. Endocrinol. 2018, 461, 256–264. [Google Scholar] [CrossRef]
- Fushimi, H.; Horie, H.; Inoue, T.; Kameyama, M.; Kanao, K.; Ishihara, S.; Tsujimura, T.; Nunotani, H.; Minami, T.; Okazaki, Y.; et al. Low testosterone levels in diabetic men and animals: A possible role in testicular impotence. Diabetes Res. Clin. Pract. 1989, 6, 297–301. [Google Scholar] [CrossRef]
- Aquila, S.; Gentile, M.; Middea, E.; Catalano, S.; Andò, S. Autocrine regulation of insulin secretion in human ejaculated sper-matozoa. Endocrinology 2005, 146, 552–557. [Google Scholar] [CrossRef]
- Cappello, A.R.; Guido, C.; Santoro, A.; Santoro, M.; Capobianco, L.; Montanaro, D.; Madeo, M.; Ando, S.; Dolce, V.; Aquila, S. The mitochondrial citrate carrier (CIC) is present and regulates insulin secretion by human male gamete. Endocrinology 2012, 153, 1743–1754. [Google Scholar] [CrossRef]
- Szulińska, M.; Kujawska-Łuczak, M.; Bogdański, P.; Pupek-Muszalik, D. Wskaźnik insulinowrażliwości M i wskaźnik IRI/G w ocenie insulinooporności u pacjentów z nadciśnieniem tętniczym i otyłością. Arter. Hypertention 2010, 14, 142–150. [Google Scholar]
- Betancourt-Albrecht, M.; Cunningham, G.R. Hypogonadism and diabetes. Int. J. Impot. Res. 2003, 15, S14–S20. [Google Scholar] [CrossRef]
- Zubek, A.; Skikowska, M.; Słomińska, D.; Manikowska, K. Potential mechanisms responsible for the concurrence of diabetes type 2 and depressive symptoms in patients. Farm. Współczesna 2019, 12, 9–14. [Google Scholar]
- Sygit-Kowalkowska, E. The health behaviour of people in late adulthood -sociodemographic correlations and differences be-tween social environments. Ann. Acad. Med. Stetin. 2013, 59, 103–113. [Google Scholar]
- Zdrojewicz, Z.; Lelakowska, K. Rola stresu w problemach, zaburzeniach i preferencjach seksualnych. Seksuologia Pol. 2006, 4, 69–79. [Google Scholar]
- Povey, A.C.; Stocks, S.J. Epidemiology and trends in male subfertility. Hum. Fertil. 2010, 13, 182–188. [Google Scholar] [CrossRef]
- Danielewicz, A.; Morze, J.; Przybyłowicz, M.; Przybyłowicz, K.E. Association of the Dietary Approaches to Stop Hypertension, Physical Activity, and Their Combination with Semen Quality: A Cross-Sectional Study. Nutrients 2019, 12, 39. [Google Scholar] [CrossRef]
- Cutillas-Tolín, A.; Adoamnei, E.; Navarrete-Muñoz, E.M.; Vioque, J.; Moñino-García, M.; Jørgensen, N.; Chavarro, J.E.; Mendiola, J.; Torres-Cantero, A.M. Adherence to diet quality indices in relation to semen quality and reproductive hormones in young men. Hum. Reprod. 2019, 34, 1866–1875. [Google Scholar] [CrossRef]
- La, J.; Roberts, N.H.; Yafi, F.A. Diet and Men’s Sexual Health. Sex. Med. Rev. 2018, 6, 54–68. [Google Scholar] [CrossRef]
- Gołąbek, K.D.; Regulska-Ilow, B. Dietary support in insulin resistance: An overview of current scientific reports. Adv. Clin. Exp. Med. 2019, 28, 1577–1585. [Google Scholar] [CrossRef]
- Soliburska, J.; Kuśnierek, J. Nutritional and non-nutritional factors in development of insulin resistance. Forum Zaburzeń Met-Abolicznych 2010, 1, 177–183. [Google Scholar]
- Gaskins, A.J.; Colaci, D.S.; Mendiola, J.; Swan, S.H.; Chavarro, J.E. Dietary patterns and semen quality in young men. Hum. Reprod. 2012, 27, 2899–2907. [Google Scholar] [CrossRef]
- Oostingh, E.C.; Steegers-Theunissen, R.P.; de Vries, J.H.; Laven, J.S.; Koster, M.P. Strong adherence to a healthy dietary pattern is associated with better semen quality, especially in men with poor semen quality. Fertil. Steril. 2017, 107, 916–923. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, K.; Dai, Y.; Ou, J.; Zhang, X. The effect of seminal plasma antisperm on infertility male, and their semen parameters analysis. J. China Healthy Birth Genet. 2005, 13, 104–105. [Google Scholar]
- Marshall, J.A.; Bessesen, D.H.; Hamman, R.F. High saturated fat and low starch and fibre are associated with hyperinsulinaemia in a non-diabetic population: The San Luis Valley Diabetes Study. Diabetologia 1997, 49, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Feskens, E.J.; Loeber, J.G.; Kromhout, D. Diet and physical activity as determinants of hyperinsulinemia: The Zutphen Elderly Study. Am. J. Epidemiol. 1994, 140, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Afeiche, M.C.; Gaskins, A.J.; Williams, P.L.; Toth, T.L.; Wright, D.L.; Tanrikut, C.; Hauser, R.; Chavarro, J. Processed Meat Intake Is Unfavorably and Fish Intake Favorably Associated with Semen Quality Indicators among Men Attending a Fertility Clinic. J. Nutr. 2014, 144, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Afeiche, M.C.; Williams, P.L.; Gaskins, A.J.; Mendiola, J.; Jørgensen, N.; Swan, S.; Chavarro, J. Meat Intake and Reproductive Parameters Among Young Men. Epidemiology 2014, 25, 323–330. [Google Scholar] [CrossRef]
- Maldonado-Cárceles, A.B.; Mínguez-Alarcón, L.; Mendiola, J.; Vioque, J.; Jørgensen, N.; Árense-Gonzalo, J.J.; Torres-Cantero, A.M.; Chavarro, J. Meat intake in relation to semen quality and reproductive hormone levels among young men in Spain. Br. J. Nutr. 2019, 121, 451–460. [Google Scholar] [CrossRef]
- Jensen, T.K.; Heitmann, B.L.; Jensen, M.B.; Halldorsson, T.I.; Andersson, A.-M.; Skakkebaek, N.E.; Joensen, U.N.; Lauritsen, M.P.; Christiansen, P.; Lassen, T.H.; et al. High dietary intake of saturated fat is associated with reduced semen quality among 701 young Danish men from the general population. Am. J. Clin. Nutr. 2013, 97, 411–418. [Google Scholar] [CrossRef]
- Attaman, J.A.; Toth, T.L.; Furtado, J.; Campos, H.; Hauser, R.; Chavarro, J.E. Dietary fat and semen quality among men attending a fertility clinic. Hum. Reprod. 2012, 27, 1466–1474. [Google Scholar] [CrossRef]
- Chavarro, J.; Minguez-Alarcon, L.; Mendiola, J.; Cutillas-Tolin, A.; Lopez-Espin, J.J.; Torres-Cantero, A.M. Trans fatty acid intake is inversely related to total sperm count in young healthy men. Hum. Reprod. 2014, 29, 429–440. [Google Scholar] [CrossRef]
- Franzini, L.; Ardigò, D.; Zavaroni, I. Dietary antioxidants and glucose metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 471–476. [Google Scholar] [CrossRef]
- Fasching, P.; Ratheiser, K.; Waldhäusl, W.; Rohac, M.; Osterrode, W.; Nowotny, P.; Vierhapper, H. Metabolic effects of fish-oil supplementation in patients with impaired glucose tolerance. Diabetes 1991, 40, 583–589. [Google Scholar] [CrossRef]
- Salas-Huetos, A.; Bulló, M.; Salas-Salvadó, J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: A systematic review of observational studies. Hum. Reprod. Update 2017, 23, 371–389. [Google Scholar] [CrossRef]
- Salas-Huetos, A.; Rosique-Esteban, N.; Becerra-Tomás, N.; Vizmanos, B.; Bulló, M.; Salas-Salvadó, J. The Effect of Nutrients and Dietary Supplements on Sperm Quality Parameters: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Adv. Nutr. Int. Rev. J. 2018, 9, 833–848. [Google Scholar] [CrossRef]
- Giahi, L.; Mohammadmoradi, S.; Javidan, A.; Sadeghi, M.R. Nutritional modifications in male infertility: A systematic review covering 2 decades. Nutr. Rev. 2016, 74, 118–130. [Google Scholar] [CrossRef]
- Eslamian, G.; Amirjannati, N.; Rashidkhani, B.; Sadeghi, M.-R.; Baghestani, A.-R.; Hekmatdoost, A. Dietary fatty acid intakes and asthenozoospermia: A case-control study. Fertil. Steril. 2015, 103, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Mendiola, J.; Torres-Cantero, A.M.; Moreno-Grau, J.M.; Ten, J.; Roca, M.; Moreno-Grau, S.; Bernabeu, R. Food intake and its relationship with semen quality: A case-control study. Fertil. Steril. 2009, 91, 812–818. [Google Scholar] [CrossRef]
- Karayiannis, D.; Kontogianni, M.D.; Mendorou, C.; Douka, L.; Mastrominas, M.; Yiannakouris, N. Association between adherence to the Mediterranean diet and semen quality parameters in male partners of couples attempting fertility. Hum. Reprod. 2017, 32, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Cutillas-Tolín, A.; Mínguez-Alarcón, L.; Mendiola, J.; López-Espín, J.J.; Jørgensen, N.; Navarrete-Muñoz, E.M.; Torres-Cantero, A.M.; Chavarro, J.E. Mediterranean and western dietary patterns are related to markers of testicular function among healthy men. Hum. Reprod. 2015, 30, 2945–2955. [Google Scholar] [CrossRef] [PubMed]
- Rifai, L.; Silver, M.A. A Review of the DASH Diet as an Optimal Dietary Plan for Symptomatic Heart Failure. Prog. Cardiovasc. Dis. 2016, 58, 548–554. [Google Scholar] [CrossRef]
- Efrat, M.; Stein, A.; Pinkas, H.; Unger, R.; Birk, R. Dietary patterns are positively associated with semen quality. Fertil. Steril. 2018, 109, 809–816. [Google Scholar] [CrossRef]
- Shirani, F.; Salehi-Abargouei, A.; Azadbakht, L. Effects of Dietary Approaches to Stop Hypertension (DASH) diet on some risk for developing type 2 diabetes: A systematic review and meta-analysis on controlled clinical trials. Nutrition 2013, 29, 939–947. [Google Scholar] [CrossRef]
- Asemi, Z.; Samimi, M.; Tabassi, Z.; Shakeri, H.; Sabihi, S.-S.; Esmaillzadeh, A. Effects of DASH diet on lipid profiles and biomarkers of oxidative stress in overweight and obese women with polycystic ovary syndrome: A randomized clinical trial. Nutrition 2014, 30, 1287–1293. [Google Scholar] [CrossRef]
- Park, Y.-M.; Zhang, J.; Steck, S.E.; Fung, T.T.; Hazlett, L.J.; Han, K.; Ko, S.-H.; Merchant, A.T. Obesity Mediates the Association between Mediterranean Diet Consumption and Insulin Resistance and Inflammation in US Adults. J. Nutr. 2017, 147, 563–571. [Google Scholar] [CrossRef]
- Mattei, J.; Sotos-Prieto, M.; Bigornia, S.J.; Noel, S.E.; Tucker, K.L. The Mediterranean diet score is more strongly associated with fa-vorable cardiometabolic risk factors over 2 years than other diet quality indexes in Puerto Rican adults. J. Nutr. 2017, 147, 661–669. [Google Scholar] [CrossRef]
- Foroozanfard, F.; Rafiei, H.; Samimi, M.; Gilasi, H.R.; Gorjizadeh, R.; Heidar, Z.; Asemi, Z. The effects of dietary approaches to stop hypertension diet on weight loss, an-ti-Müllerian hormone and metabolic profiles in women with polycystic ovary syndrome: A randomized clinical trial. Clin. Endocrinol. 2017, 87, 51–58. [Google Scholar] [CrossRef]
- Asemi, Z.; Esmaillzadeh, A. DASH Diet, Insulin Resistance, and Serum hs-CRP in Polycystic Ovary Syndrome: A Randomized Controlled Clinical Trial. Horm. Metab. Res. 2015, 47, 232–238. [Google Scholar] [CrossRef]
- Hinderliter, A.L.; Babyak, M.A.; Sherwood, A.; Blumenthal, J.A. The DASH Diet and Insulin Sensitivity. Curr. Hypertens. Rep. 2011, 13, 67–73. [Google Scholar] [CrossRef]
- Allouche-Fitoussi, D.; Breitbart, H. The Role of Zinc in Male Fertility. Int. J. Mol. Sci. 2020, 21, 7796. [Google Scholar] [CrossRef]
- Gual-Frau, J.; Abad, C.; Amengual, M.J.; Hannaoui, N.; Checa, M.A.; Ribas-Maynou, J.; Lozano, I.; Nikolaou, A.; Benet, J.; García-Peiró, A.; et al. Oral antioxidant treatment partly improves integrity of human sperm DNA in infertile grade I varicocele patients. Hum. Fertil. 2015, 18, 225–229. [Google Scholar] [CrossRef]
- Agarwal, A.; Virk, G.; Ong, C.; Du Plessis, S.S. Effect of Oxidative Stress on Male Reproduction. World J. Men’s Health 2014, 32, 1–17. [Google Scholar] [CrossRef]
- Boisen, I.M.; Hansen, L.B.; Mortensen, L.J.; Lanske, B.; Juul, A.; Jensen, M.B. Possible influence of vitamin D on male reproduction. J. Steroid Biochem. Mol. Biol. 2017, 173, 215–222. [Google Scholar] [CrossRef]
- Szymczak-Pajor, I.; Śliwińska, A. Analysis of Association between Vitamin D Deficiency and Insulin Resistance. Nutrients 2019, 11, 794. [Google Scholar] [CrossRef]
- Wong, W.Y.; Merkus, H.M.W.M.; Thomas, C.M.G.; Menkveld, R.; Zielhuis, G.A.; Steegers-Theunissen, R.P.M. Effects of folic acid and zinc sulfate on male factor subfertility: A double-blind, randomized, placebo-controlled trial. Fertil. Steril. 2002, 77, 491–498. [Google Scholar] [CrossRef]
- Eisenberg, M.L.; Kim, S.; Chen, Z.; Sundaram, R.; Schisterman, E.F.; Louis, G.M.B. The relationship between male BMI and waist circumference on semen quality: Data from the LIFE study. Hum. Reprod. 2013, 29, 193–200. [Google Scholar] [CrossRef]
- Hammiche, F.; Laven, J.S.; Twigt, J.M.; Boellaard, W.P.; Steegers, E.A.; Steegers-Theunissen, R.P. Body mass index and central adiposity are associated with sperm quality in men of subfertile couples. Hum. Reprod. 2012, 27, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Fariello, R.M.; Pariz, J.R.; Spaine, D.M.; Cedenho, A.P.; Bertolla, R.P.; Fraietta, R. Association between obesity and alteration of sperm DNA integrity and mitochondrial activity. BJU Int. 2012, 110, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Romualdo, M.C.D.S.; de Nóbrega, F.J.; Escrivão, M.A.M.S. Insulin resistance in obese children and adolescents. J. De Pediatr. 2014, 90, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Mínguez-Alarcón, L.; Chavarro, J.E.; Mendiola, J.; Gaskins, A.J.; Torres-Cantero, A.M. Physical activity is not related to semen quality in young healthy men. Fertil. Steril. 2014, 102, 1103–1109. [Google Scholar] [CrossRef]
- Jóźków, P.; Rossato, M. The Impact of Intense Exercise on Semen Quality. Am. J. Men’s Health 2017, 11, 654–662. [Google Scholar] [CrossRef]
- Gaskins, A.; Afeiche, M.; Hauser, R.; Williams, P.; Gillman, M.; Tanrikut, C.; Petrozza, J.; Chavarro, J. Paternal physical and sedentary activities in relation to semen quality and reproductive outcomes among couples from a fertility center. Hum. Reprod. 2014, 29, 2575–2582. [Google Scholar] [CrossRef]
- Vaamonde, D.; Da Silva-Grigoletto, M.E.; García-Manso, J.M.; Barrera, N.; Vaamonde-Lemos, R. Physically active men show better semen parameters and hormone values than sedentary men. Eur. J. Appl. Physiol. 2012, 112, 3267–3273. [Google Scholar] [CrossRef]
- Maleki, B.H.; Tartibian, B. Resistance exercise modulates male factor infertility through anti-inflammatory and antioxidative mechanisms in infertile men: A RCT. Life Sci. 2018, 203, 150–160. [Google Scholar] [CrossRef]
- Marson, E.C.; Delevatti, R.S.; Prado, A.K.G.; Netto, N.; Kruel, L.F.M. Effects of aerobic, resistance, and combined exercise training on insulin resistance markers in overweight or obese children and adolescents: A systematic review and meta-analysis. Prev. Med. 2016, 93, 211–218. [Google Scholar] [CrossRef]
- Gebreegziabher, Y.; Marcos, E.; McKinon, W.; Rogers, G. Sperm Characteristics of Endurance Trained Cyclists. Endoscopy 2004, 25, 247–251. [Google Scholar] [CrossRef]
- Motahari-Tabari, N.; Shirvani, M.A.; Shirzad-E-Ahoodashty, M.; Yousefi-Abdolmaleki, E.; Teimourzadeh, M. The Effect of 8 Weeks Aerobic Exercise on Insulin Resistance in Type 2 Diabetes: A Randomized Clinical Trial. Glob. J. Health Sci. 2014, 7, p115. [Google Scholar] [CrossRef]
- Deng, N.; Kohn, T.P.; Lipshultz, L.I.; Pastuszak, A.W. The Relationship Between Shift Work and Men’s Health. Sex. Med. Rev. 2018, 6, 446–456. [Google Scholar] [CrossRef]
- Chen, H.-G.; Sun, B.; Chen, Y.-J.; Chavarro, J.E.; Hu, S.-H.; Xiong, C.-L.; Pan, A.; Meng, T.-Q.; Wang, Y.-X.; Messerlian, C. Sleep duration and quality in relation to semen quality in healthy men screened as potential sperm donors. Environ. Int. 2020, 135, 105368. [Google Scholar] [CrossRef]
- Viganò, P.; Chiaffarino, F.; Bonzi, V.; Salonia, A.; Ricci, E.; Papaleo, E.; Mauri, P.A.; Parazzini, F. Sleep disturbances and semen quality in an Italian cross sectional study. Basic Clin. Androl. 2017, 27, 16. [Google Scholar] [CrossRef]
- Du, C.-Q.; Yang, Y.-Y.; Chen, J.; Feng, L.; Lin, W.-Q. Association Between Sleep Quality and Semen Parameters and Reproductive Hormones: A Cross-Sectional Study in Zhejiang, China. Nat. Sci. Sleep 2020, 12, 11–18. [Google Scholar] [CrossRef]
- Ortiz, A.; Espino, J.; Bejarano, I.; Lozano, G.M.; Monllor, F.; García, J.F.; Pariente, J.A.; Rodríguez, A.B. High endogenous melatonin concentrations enhance sperm quality and short-term in vitro exposure to melatonin improves aspects of sperm motility. J. Pineal Res. 2010, 50, 132–139. [Google Scholar] [CrossRef]
- Luboshitzky, R.; Lavie, L.; Herer, P.; Shen-Orr, Z. Altered Luteinizing Hormone and Testosterone Secretion in Middle-Aged Obese Men with Obstructive Sleep Apnea. Obes. Res. 2005, 13, 780–786. [Google Scholar] [CrossRef]
- Lucassen, E.A.; Rother, K.I.; Cizza, G. Interacting epidemics? Sleep curtailment, insulin resistance, and obesity. Ann. N. Y. Acad. Sci. 2012, 1264, 110–134. [Google Scholar] [CrossRef]
- Sygit-Kowalkowska. Coping with stress as a health behavior—Psychological perspectives. Hygeia Public Health 2014, 49, 202–208. [Google Scholar]
- McGrady, A.V. Effects of Psychological Stress on Male Reproduction: A Review. Arch. Androl. 1984, 13, 1–7. [Google Scholar] [CrossRef]
- Gollenberg, A.L.; Hediger, M.L.; Mumford, S.L.; Whitcomb, B.W.; Hovey, K.M.; Wactawski-Wende, J.; Schisterman, E.F. Perceived Stress and Severity of Perimenstrual Symptoms: The BioCycle Study. J. Women’s Health 2010, 19, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Nuszkiewicz, J.; Kwiatkowska, A.; Majko, K.; Wesołowski, R.; Szewczyk-Golec, K. Oxidative stress, inflammation and development of obesity: Protective effect of melatonin. Probl. Hig. Epidemiol. 2017, 98, 226–232. [Google Scholar]
- Zou, P.; Sun, L.; Chen, Q.; Zhang, G.; Yang, W.; Zeng, Y.; Zhou, N.; Li, Y.; Liu, J.; Ao, L.; et al. Social support modifies an association between work stress and semen quality: Results from 384 Chinese male workers. J. Psychosom. Res. 2019, 117, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Janevic, T.; Kahn, L.G.; Landsbergis, P.; Cirillo, P.M.; Cohn, B.A.; Liu, X.; Factor-Litvak, P. Effects of work and life stress on semen quality. Fertil. Steril. 2014, 102, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Lindaman, M.L.A.; Pilsner, J.R.; Kroll-Desrosiers, A.R.; Haskell, S.; Brandt, C.A.; Mattocks, K.M. Semen Quality Parameters Among U.S. Veterans of Operation Enduring Freedom, Operation Iraqi Freedom, and Operation New Dawn. Mil. Med. 2017, 182, e1775–e1781. [Google Scholar] [CrossRef][Green Version]
- Chen, X.M.; Yue, H.X.; Lin, L.; Wu, Y.B.; Liu, B.; Jiang, M.; Ma, Y.X. Semen quality in adult male survivors 5 years after the 2008 Wenchuan earthquake. Andrologia 2016, 48, 1274–1280. [Google Scholar] [CrossRef]
- Fuller-Rowell, T.E.; Homandberg, L.; Curtis, D.; Tsenkova, V.K.; Williams, D.R.; Ryff, C.D. Disparities in insulin resistance between black and white adults in the United States: The role of lifespan stress exposure. Psychoneuroendocrinology 2019, 107, 1–8. [Google Scholar] [CrossRef]
- Asare-Anane, H.; Bannison, S.B.; Ofori, E.K.; Ateko, R.O.; Bawah, A.T.; Amanquah, S.D.; Oppong, S.Y.; Gandau, B.B.N.; Ziem, J.B. Tobacco smoking is associated with decreased semen quality. Reprod. Health 2016, 13, 90. [Google Scholar] [CrossRef]
- Joo, K.; Kwon, Y.; Myung, S.; Kim, T. The Effects of Smoking and Alcohol Intake on Sperm Quality: Light and Transmission Electron Microscopy Findings. J. Int. Med. Res. 2012, 40, 2327–2335. [Google Scholar] [CrossRef]
- Sharma, R.; Harlev, A.; Agarwal, A.; Esteves, S.C. Cigarette Smoking and Semen Quality: A New Meta-analysis Examining the Effect of the 2010 World Health Organization Laboratory Methods for the Examination of Human Semen. Eur. Urol. 2016, 70, 635–645. [Google Scholar] [CrossRef]
- Omolaoye, T.S.; El Shahawy, O.; Skosana, B.T.; Boillat, T.; Loney, T.; du Plessis, S.S. The mutagenic effect of tobacco smoke on male fertility. Environ. Sci. Pollut. Res. 2021, 29, 62055–62066. [Google Scholar] [CrossRef]
- Mouhamed, D.H.; Ezzaher, A.; Neffati, F.; Douki, W.; Gaha, L.; Najjar, M. Effect of cigarette smoking on insulin resistance risk. Ann. Cardiol. Angeiol. 2016, 65, 21–25. [Google Scholar] [CrossRef]
- Pourmasumi, S.; Sabeti, P.; Rahiminia, T.; Mangoli, E.; Tabibnejad, N.; Talebi, A.R. The etiologies of DNA abnormalities in male in-fertility: An assessment and review. Int. J. Reprod. Biomed. 2017, 15, 331–344. [Google Scholar]
- Condorelli, R.A.; Calogero, A.E.; Vicari, E.; La Vignera, S. Chronic consumption of alcohol and sperm parameters: Our experience and the main evidences. Andrologia 2015, 47, 368–379. [Google Scholar] [CrossRef]
- Teijón, M.L.; Garcia, F.; Serra, O.; Moragas, M.; Rabanal, A.; Olivares, R.; Alvarez, J. Semen quality in a population of volunteers from the province of Barcelona. Reprod. Biomed. Online 2007, 15, 434–444. [Google Scholar] [CrossRef]
- Yousefniapasha, Y.; Jorsaraei, G.; Gholinezhadchari, M.; Mahjoub, S.; Hajiahmadi, M.; Farsi, M. Nitric Oxide Levels and Total Antioxidant Capacity in The Seminal Plasma of Infertile Smoking Men. Cell J. 2015, 17, 129–136. [Google Scholar] [CrossRef]
- Hanson, B.M.; Aston, K.I.; Jenkins, T.G.; Carrell, D.T.; Hotaling, J.M. The impact of ejaculatory abstinence on semen analysis parameters: A systematic review. J. Assist. Reprod. Genet. 2018, 35, 213–220. [Google Scholar] [CrossRef]
- Rivaroli, M. Comparison between length of sexual abstinence and semen parameters in patients of an assisted reproduction center and a hospital from Porto Alegre—RS. J. Bras. Reprod. Assist. 2009, 13, 28–32. [Google Scholar]
- Meikle, A.W.; Reis, L.O.; Gibson, M.; Peterson, C.M.; Carrell, D.T.; Hammoud, A.O. Obesity and Male Infertility: A Practical Approach. Semin. Reprod. Med. 2012, 30, 486–495. [Google Scholar] [CrossRef]
- Huang, K.; Liang, F.; Yang, X.; Liu, F.; Li, J.; Xiao, Q.; Chen, J.; Liu, X.; Cao, J.; Shen, C.; et al. Long term exposure to ambient fine particulate matter and incidence of stroke: Prospective cohort study from the China-PAR project. BMJ 2019, 367, l6720. [Google Scholar] [CrossRef]
- Cai, H.; Zheng, W.; Zheng, P.; Wang, S.; Tan, H.; He, G.; Qu, W. Human urinary/seminal phthalates or their metabolite levels and semen quality: A meta-analysis. Environ. Res. 2015, 142, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Bao, H.; Liu, F.; Liu, L.; Zhu, Y.-G.; She, J.; Dong, S.; Cai, M.; Li, L.; Li, C.; et al. Environmental exposure to arsenic may reduce human semen quality: Associations derived from a Chinese cross-sectional study. Environ. Health 2012, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Tang, S.; Zhu, H.; Chen, Z.; Zang, Z.; Zhang, Y.; Niu, X.; Wang, X.; Yin, H.; Zeng, F.; et al. Biomonitoring PFAAs in blood and semen samples: Investigation of a potential link between PFAAs exposure and semen mobility in China. Environ. Int. 2018, 113, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, L.; Wu, Y.; Wang, X.; Luo, L.; Nan, B.; Zhang, J.; Tian, M.; Shen, H. Seminal plasma metabolites mediate the associations of multiple environmental pollutants with semen quality in Chinese men. Environ. Int. 2019, 132, 105066. [Google Scholar] [CrossRef] [PubMed]
- Lao, X.Q.; Zhang, Z.; Lau, A.K.H.; Chan, T.-C.; Chuang, Y.C.; Chan, J.; Lin, C.; Guo, C.; Jiang, W.K.; Tam, T.; et al. Exposure to ambient fine particulate matter and semen quality in Taiwan. Occup. Environ. Med. 2018, 75, 148–154. [Google Scholar] [CrossRef]
- Chen, Q.; Jiang, H.; Zhang, H.-T.; Zhang, Z.; Cao, J.; Tang, W.-H.; Zhang, H.-L.; Hong, K.; Lin, H.-C.; Wu, H. Ambient ozone pollution is associated with decreased semen quality: Longitudinal analysis of 8945 semen samples from 2015 to 2018 and during pollution-control period in Beijing, China. Asian J. Androl. 2019, 21, 501–507. [Google Scholar] [CrossRef]
- Manfo, F.P.T.; Jubendradass, R.; Nantia, E.A.; Moundipa, P.F.; Mathur, P.P. Adverse Effects of Bisphenol A on Male Reproductive Function. Rev. Environ. Contam. Toxicol. 2014, 228, 57–82. [Google Scholar] [CrossRef]
- Boberg, J.; Taxvig, C.; Christiansen, S.; Hass, U. Possible endocrine disrupting effects of parabens and their metabolites. Reprod. Toxicol. 2010, 30, 301–312. [Google Scholar] [CrossRef]
- Recio-Vega, R.; Ocampo-Gómez, G.; Borja-Aburto, V.H.; Moran-Martínez, J.; Cebrian-Garcia, M.E. Organophosphorus pesticide exposure decreases sperm quality: Association between sperm parameters and urinary pesticide levels. J. Appl. Toxicol. 2008, 28, 674–680. [Google Scholar] [CrossRef]
- Pant, N.; Kumar, G.; Upadhyay, A.D.; Gupta, Y.K.; Chaturvedi, P.K. Correlation between lead and cadmium concentration and semen quality. Andrologia 2015, 47, 887–891. [Google Scholar] [CrossRef]
- Barregard, L.; Bergström, G.; Fagerberg, B. Cadmium exposure in relation to insulin production, insulin sensitivity and type 2 diabetes: A cross-sectional and prospective study in women. Environ. Res. 2013, 121, 104–109. [Google Scholar] [CrossRef]
- Kim, J.H.; Hong, Y.-C. GSTM1, GSTT1, and GSTP1 Polymorphisms and Associations between Air Pollutants and Markers of Insulin Resistance in Elderly Koreans. Environ. Health Perspect. 2012, 120, 1378–1384. [Google Scholar] [CrossRef]
- Stahlhut, R.W.; Van Wijngaarden, E.; Dye, T.D.; Cook, S.; Swan, S.H. Concentrations of Urinary Phthalate Metabolites are Associated with Increased Waist Circumference and Insulin Resistance in Adult U.S. Males. Environ. Health Perspect. 2007, 115, 876–882. [Google Scholar] [CrossRef]
- Lee, D.-H.; Lee, I.-K.; Jin, S.-H.; Steffes, M.; Jacobs, D.R. Association between Serum Concentrations of Persistent Organic Pollutants and Insulin Resistance among Nondiabetic Adults. Diabetes Care 2007, 30, 622–628. [Google Scholar] [CrossRef]
- Radwan, M.; Jurewicz, J.; Merecz-Kot, D.; Sobala, W.; Radwan, P.; Bochenek, M.; Hanke, W. Sperm DNA damage—The effect of stress and everyday life factors. Int. J. Impot. Res. 2016, 28, 148–154. [Google Scholar] [CrossRef]
- Zilberlicht, A.; Wiener-Megnazi, Z.; Sheinfeld, Y.; Grach, B.; Lahav-Baratz, S.; Dirnfeld, M. Habits of cell phone usage and sperm quality—Does it warrant attention? Reprod. Biomed. Online 2015, 31, 421–426. [Google Scholar] [CrossRef]
- Pacey, A. Fertility issues in survivors from adolescent cancers. Cancer Treat. Rev. 2007, 33, 646–655. [Google Scholar] [CrossRef]
- Jung, A.; Schuppe, H.-C. Influence of genital heat stress on semen quality in humans. Andrologia 2007, 39, 203–215. [Google Scholar] [CrossRef]
- Zhou, Y.; Meng, T.; Wu, L.; Duan, Y.; Li, G.; Shi, C.; Zhang, H.; Peng, Z.; Fan, C.; Ma, J.; et al. Association between ambient temperature and semen quality: A longitudinal study of 10 802 men in China. Environ. Int. 2020, 135, 105364. [Google Scholar] [CrossRef]
- Xie, M.; Utzinger, K.S.; Blickenstorfer, K.; Leeners, B. Diurnal and seasonal changes in semen quality of men in subfertile partnerships. Chrono-Int. 2018, 35, 1375–1384. [Google Scholar] [CrossRef]
- Mao, H.; Feng, L.; Yang, W.-X. Environmental factors contributed to circannual rhythm of semen quality. Chrono-Int. 2017, 34, 411–425. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zańko, A.; Siewko, K.; Krętowski, A.J.; Milewski, R. Lifestyle, Insulin Resistance and Semen Quality as Co-Dependent Factors of Male Infertility. Int. J. Environ. Res. Public Health 2023, 20, 732. https://doi.org/10.3390/ijerph20010732
Zańko A, Siewko K, Krętowski AJ, Milewski R. Lifestyle, Insulin Resistance and Semen Quality as Co-Dependent Factors of Male Infertility. International Journal of Environmental Research and Public Health. 2023; 20(1):732. https://doi.org/10.3390/ijerph20010732
Chicago/Turabian StyleZańko, Adrianna, Katarzyna Siewko, Adam Jacek Krętowski, and Robert Milewski. 2023. "Lifestyle, Insulin Resistance and Semen Quality as Co-Dependent Factors of Male Infertility" International Journal of Environmental Research and Public Health 20, no. 1: 732. https://doi.org/10.3390/ijerph20010732
APA StyleZańko, A., Siewko, K., Krętowski, A. J., & Milewski, R. (2023). Lifestyle, Insulin Resistance and Semen Quality as Co-Dependent Factors of Male Infertility. International Journal of Environmental Research and Public Health, 20(1), 732. https://doi.org/10.3390/ijerph20010732






