Maternal Well-Being and Stage of Behaviour Change during Pregnancy: A Secondary Analysis of the PEARS Randomised Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Data Collection
2.4. Maternal Well-Being
2.5. Behaviour Change
2.6. Statistical Analysis
3. Results
3.1. Determinants of Behaviour-Change Stage
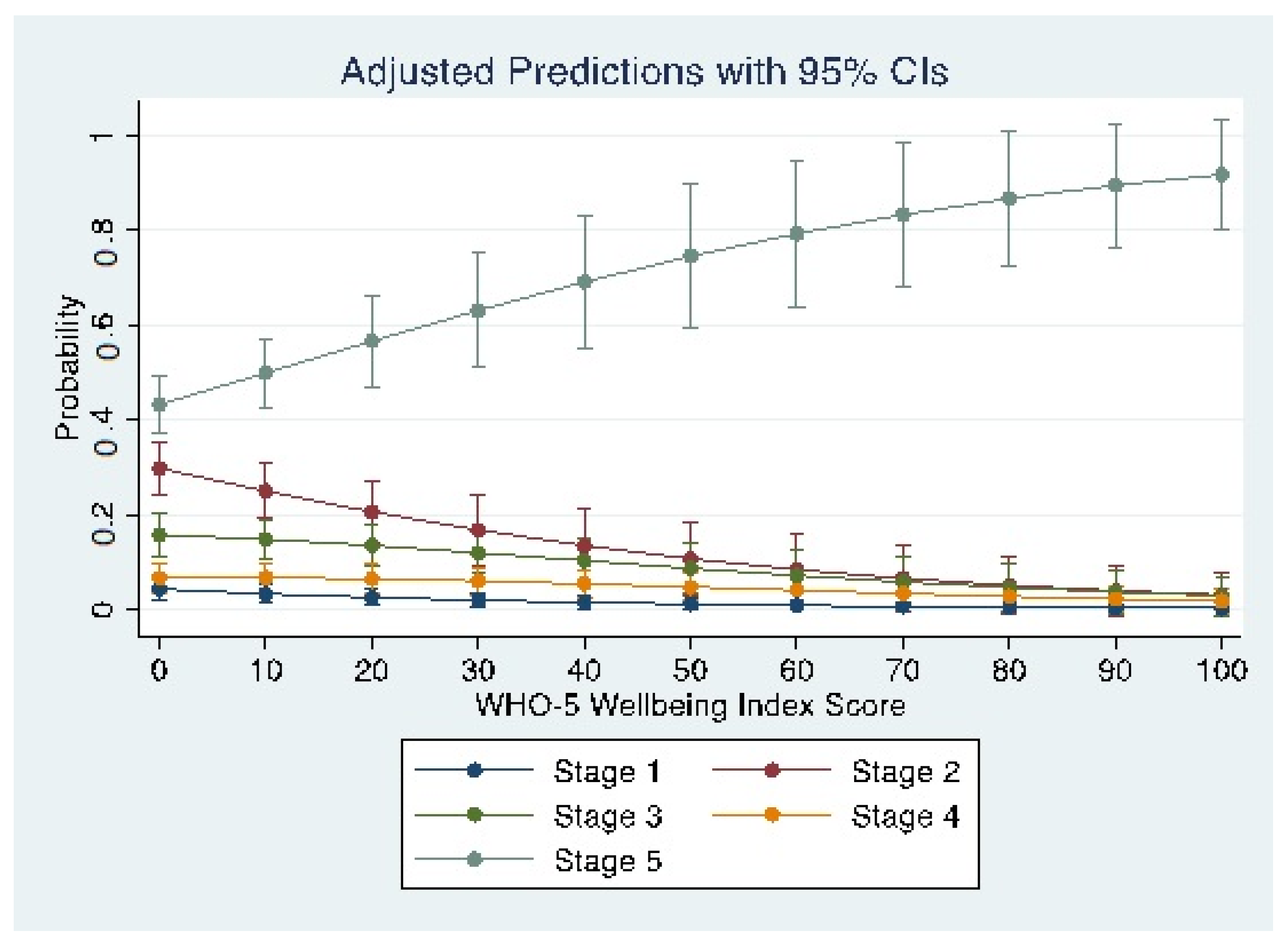
3.2. Maternal Well-Being and Stage of Behaviour Change
3.3. The PEARS RCT Intervention and Behaviour Change
4. Discussion
4.1. Principal Findings
4.2. Maternal Well-Being in Antenatal Care
4.3. Stage of Behaviour Change and Well-Being
4.4. Stages of Behaviour Change in Pregnancy
4.5. mHealth Intervention
4.6. Strengths and Limitations
4.7. Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Otchet, F.; Carey, M.S.; Adam, L. General health and psychological symptom status in pregnancy and the puerperium: What is normal? Obstet. Gynecol. 1999, 94, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, S.; Hatch, M.C. Depressive symptomatology during pregnancy: Evidence for an association with decreased fetal growth in pregnancies of lower social class women. Health Psychol. 2000, 19, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Della Vedova, A.M.; Ducceschi, B.; Cesana, B.M.; Imbasciati, A. Maternal bonding and risk of depression in late pregnancy: A survey of Italian nulliparous women. J. Reprod. Infant Psychol. 2011, 29, 208–222. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 1980. [Google Scholar]
- Bödecs, T.; Horváth, B.; Szilágyi, E.; Gonda, X.; Rihmer, Z.; Sándor, J. Effects of depression, anxiety, self-esteem, and health behaviour on neonatal outcomes in a population-based Hungarian sample. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 154, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Heron, J.; Francomb, H.; Oke, S.; Golding, J. Cohort study of depressed mood during pregnancy and after childbirth. BMJ 2001, 323, 257–260. [Google Scholar] [CrossRef]
- Guo, N.; Robakis, T.; Miller, C.; Butwick, A. Prevalence of depression among women of reproductive age in the United States. Obstet. Gynecol. 2018, 131, 671. [Google Scholar] [CrossRef]
- Fraga, A.C.S.A.; Theme-Filha, M.M. Pregestational overweight and obesity and symptoms of postpartum depression: Data from the Birth in Brazil Study. J. Affect. Disord. 2020, 277, 463–469. [Google Scholar] [CrossRef]
- Laraia, B.A.; Siega-Riz, A.M.; Dole, N.; London, E. Pregravid weight is associated with prior dietary restraint and psychosocial factors during pregnancy. Obesity 2009, 17, 550–558. [Google Scholar] [CrossRef]
- Rafferty, A.R.; Geraghty, A.A.; Kennelly, M.A.; O’Brien, E.C.; Reji, R.M.; Mehegan, J.; Segurado, R.; Smith, T.; Maguire, O.; Cronin, M.; et al. Limited impact of fetal sex and maternal body mass index on fetal and maternal insulin resistance and lipid metabolism: Findings from the PEARs study. Reprod. Sci. 2020, 27, 513–522. [Google Scholar] [CrossRef]
- McNamara, J.; Townsend, M.L.; Herbert, J.S. A systemic review of maternal wellbeing and its relationship with maternal fetal attachment and early postpartum bonding. PLoS ONE 2019, 14, e0220032. [Google Scholar] [CrossRef]
- Frey, B.S.; Stutzer, A. Happiness and Economics; Princeton University Press: Princeton, NJ, USA, 2010. [Google Scholar]
- Andrews, F.M.; Withey, S.B. Social Indicators of Well-Being: Americans’ Perceptions of Life Quality; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Dunn, H.L. High-level wellness for man and society. Am. J. Public Health Nations Health 1959, 49, 786–792. [Google Scholar] [CrossRef]
- Bech, P.; Gudex, C.; Johansen, K.S. The WHO (Ten) well-being index: Validation in diabetes. Psychother. Psychosom. 1996, 65, 183–190. [Google Scholar] [CrossRef]
- Topp, C.W.; Østergaard, S.D.; Søndergaard, S.; Bech, P. The WHO-5 Well-Being Index: A systematic review of the literature. Psychother. Psychosom. 2015, 84, 167–176. [Google Scholar] [CrossRef]
- Herrman, H.; Saxena, S.; Moodie, R. Promoting Mental Health: Concepts, Emerging Evidence, Practice; A Report of the World Health Organization, Department of Mental Health and Substance Abuse in Collaboration with the Victorian Health Promotion Foundation and the University of Melbourne; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Da Costa, D.; Larouche, J.; Dritsa, M.; Brender, W. Variations in stress levels over the course of pregnancy: Factors associated with elevated hassles, state anxiety and pregnancy-specific stress. J. Psychosom. Res. 1999, 47, 609–621. [Google Scholar] [CrossRef]
- Hernández-Valencia, M.; Montes, A.M.R.; López, C.V.; Angulo, J.A.P.; Girón, A.V.; Zárate, A. Catecholamines level variation during pregnancy in women with diabetes and preeclampsia. Ginecol. Obstet. Mex. 2007, 75, 454–458. [Google Scholar]
- Laatikainen, T.; Virtanen, T.; Kaaja, R.; Salminen-Lappalainen, K. Corticotropin-releasing hormone in maternal and cord plasma in pre-eclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 1991, 39, 19–24. [Google Scholar] [CrossRef]
- McAuliffe, F.M.; Killeen, S.L.; Jacob, C.M.; Hanson, M.A.; Hadar, E.; McIntyre, H.D.; Kapur, A.; Kihara, A.B.; Ma, R.C.; Divakar, H.; et al. Management of prepregnancy, pregnancy, and postpartum obesity from the FIGO Pregnancy and Non-Communicable Diseases Committee: A FIGO (International Federation of Gynecology and Obstetrics) guideline. Int. J. Gynaecol. Obstet. 2020, 151 (Suppl. S1), 16. [Google Scholar] [CrossRef]
- Levine, T.A.; Alderdice, F.A.; Grunau, R.E.; McAuliffe, F.M. Prenatal stress and hemodynamics in pregnancy: A systematic review. Arch. Women’s Ment. Health 2016, 19, 721–739. [Google Scholar] [CrossRef]
- Levine, T.A.; Grunau, R.E.; Segurado, R.; Daly, S.; Geary, M.P.; Kennelly, M.M.; O’Donoghue, K.; Hunter, A.; Morrison, J.J.; Burke, G.J.; et al. Pregnancy-specific stress, fetoplacental haemodynamics, and neonatal outcomes in women with small for gestational age pregnancies: A secondary analysis of the multicentre Prospective Observational Trial to Optimise Paediatric Health in Intrauterine Growth Restriction. BMJ Open 2017, 7, e015326. [Google Scholar]
- Gray, R.; Henderson, J. Review of the Fetal Effects of Prenatal Alcohol Exposure; University of Oxford: Oxford, UK, 2006. [Google Scholar]
- Shah, N.R.; Bracken, M.B. A systematic review and meta-analysis of prospective studies on the association between maternal cigarette smoking and preterm delivery. Am. J. Obstet. Gynecol. 2000, 182, 465–472. [Google Scholar] [CrossRef]
- Mikkelsen, T.B.; Osler, M.; Orozova-Bekkevold, I.; Knudsen, V.K.; Olsen, S.F. Association between fruit and vegetable consumption and birth weight: A prospective study among 43,585 Danish women. Scand. J. Public Health 2006, 34, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Rogers, I.; Emmett, P.; Baker, D.; Golding, J. Financial difficulties, smoking habits, composition of the diet and birthweight in a population of pregnant women in the South West of England. Eur. J. Clin. Nutr. 1998, 52, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, M.; Lindkvist, M.; Eurenius, E.; Persson, M.; Mogren, I. Change of lifestyle habits–motivation and ability reported by pregnant women in northern Sweden. Sex. Reprod. HealthCare 2017, 13, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Rockliffe, L.; Peters, S.; Heazell, A.E.; Smith, D.M. Factors influencing health behaviour change during pregnancy: A systematic review and meta-synthesis. Health Psychol. Rev. 2021, 15, 613–632. [Google Scholar] [CrossRef] [PubMed]
- Dodd, J.M.; Crowther, C.A.; Robinson, J.S. Dietary and lifestyle interventions to limit weight gain during pregnancy for obese or overweight women: A systematic review. Acta Obstet. Et Gynecol. Scand. 2008, 87, 702–706. [Google Scholar] [CrossRef]
- Barley, E.; Lawson, V. Using health psychology to help patients: Theories of behaviour change. Br. J. Nurs. 2016, 25, 924–927. [Google Scholar] [CrossRef]
- Poston, L.; Bell, R.; Croker, H.; Flynn, A.C.; Godfrey, K.M.; Goff, L.; Hayes, L.; Khazaezadeh, N.; Nelson, S.M.; Oteng-Ntim, E.; et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): A multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2015, 3, 767–777. [Google Scholar] [CrossRef]
- Hill, B.; Kothe, E.J.; Currie, S.; Danby, M.; Lang, A.Y.; Bailey, C.; Moran, L.J.; Teede, H.; North, M.; Bruce, L.J.; et al. A systematic mapping review of the associations between pregnancy intentions and health-related lifestyle behaviours or psychological wellbeing. Prev. Med. Rep. 2019, 14, 100869. [Google Scholar] [CrossRef]
- Elsenbruch, S.; Benson, S.; Rucke, M.; Rose, M.; Dudenhausen, J.; Pincus-Knackstedt, M.K.; Klapp, B.F.; Arck, P. Social support during pregnancy: Effects on maternal depressive symptoms, smoking and pregnancy outcome. Hum. Reprod. 2007, 22, 869–877. [Google Scholar] [CrossRef]
- Sheehan, T.J. Stress and low birth weight: A structural modeling approach using real life stressors. Soc. Sci. Med. 1998, 47, 1503–1512. [Google Scholar] [CrossRef]
- Bedaso, A.; Adams, J.; Peng, W.; Sibbritt, D. The relationship between social support and mental health problems during pregnancy: A systematic review and meta-analysis. Reprod. Health 2021, 18, 162. [Google Scholar] [CrossRef]
- Battulga, B.; Benjamin, M.R.; Chen, H.; Bat-Enkh, E. The impact of social support and pregnancy on subjective well-being: A systematic review. Front. Psychol. 2021, 12, 3321. [Google Scholar] [CrossRef]
- Wu, H.; Sun, W.; Huang, X.; Yu, S.; Wang, H.; Bi, X.; Sheng, J.; Chen, S.; Akinwunmi, B.; Zhang, C.J.P.; et al. Online Antenatal Care During the COVID-19 Pandemic: Opportunities and Challenges. J. Med. Internet Res. 2020, 22, e19916. [Google Scholar] [CrossRef]
- Ainscough, K.M.; O’Brien, E.C.; Lindsay, K.L.; Kennelly, M.A.; O’Sullivan, E.J.; O’Brien, O.A.; McCarthy, M.; De Vito, G.; McAuliffe, F.M. Nutrition, Behavior Change and Physical Activity Outcomes from the PEARS RCT—An mHealth-Supported, Lifestyle Intervention among Pregnant Women with Overweight and Obesity. Front. Endocrinol. 2019, 10, 938. [Google Scholar] [CrossRef]
- O’Sullivan, E.J.; Rokicki, S.; Kennelly, M.; Ainscough, K.; McAuliffe, F.M. Cost-effectiveness of a mobile health-supported lifestyle intervention for pregnant women with an elevated body mass index. Int. J. Obes. 2020, 44, 999–1010. [Google Scholar] [CrossRef]
- WHO. MHealth: New Horizons for Health through Mobile Technologies; Second Global Survey on eHealth; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Hughson, J.-A.P.; Daly, J.O.; Woodward-Kron, R.; Hajek, J.; Story, D. The rise of pregnancy apps and the implications for culturally and linguistically diverse women: Narrative review. JMIR Mhealth Uhealth 2018, 6, e189. [Google Scholar] [CrossRef]
- Beulen, Y.H.; Super, S.; de Vries, J.H.; Koelen, M.A.; Feskens, E.J.; Wagemakers, A. Dietary interventions for healthy pregnant women: A systematic review of tools to promote a healthy antenatal dietary intake. Nutrients 2020, 12, 1981. [Google Scholar] [CrossRef]
- Brown, H.M.; Bucher, T.; Collins, C.E.; Rollo, M.E. A review of pregnancy apps freely available in the Google Play Store. Health Promot. J. Aust. 2020, 31, 340–342. [Google Scholar] [CrossRef]
- Tassone, C.; Keshavjee, K.; Paglialonga, A.; Moreira, N.; Pinto, J.; Quintana, Y. Evaluation of mobile apps for treatment of patients at risk of developing gestational diabetes. Health Inform. J. 2020, 26, 1983–1994. [Google Scholar] [CrossRef]
- Chan, K.L.; Chen, M. Effects of social media and mobile health apps on pregnancy care: Meta-analysis. JMIR Mhealth Uhealth 2019, 7, e11836. [Google Scholar] [CrossRef]
- Kennelly, M.A.; Ainscough, K.; Lindsay, K.L.; O’Sullivan, E.; Gibney, E.R.; McCarthy, M.; Segurado, R.; DeVito, G.; Maguire, O.; Smith, T.; et al. Pregnancy Exercise and Nutrition With Smartphone Application Support: A Randomized Controlled Trial. Obstet. Gynecol. 2018, 131, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Kennelly, M.A.; Ainscough, K.; Lindsay, K.; Gibney, E.; Mc Carthy, M.; McAuliffe, F.M. Pregnancy, exercise and nutrition research study with smart phone app support (Pears): Study protocol of a randomized controlled trial. Contemp. Clin. Trials 2016, 46, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Kennelly, M.A.; Killeen, S.L.; Phillips, C.M.; Alberdi, G.; Lindsay, K.L.; Mehegan, J.; Cronin, M.; McAuliffe, F.M. Maternal C3 complement and C-reactive protein and pregnancy and fetal outcomes: A secondary analysis of the PEARS RCT-An mHealth-supported, lifestyle intervention among pregnant women with overweight and obesity. Cytokine 2022, 149, 155748. [Google Scholar] [CrossRef] [PubMed]
- Haase, T.; Pratschke, J. The 2011 Pobal HP Deprivation Index for Small Areas (SA): Introduction and Reference Tables; Poabl Dublin: Dublin, Ireland, 2012. [Google Scholar]
- Bech, P.; Olsen, L.R.; Kjoller, M.; Rasmussen, N.K. Measuring well-being rather than the absence of distress symptoms: A comparison of the SF-36 Mental Health subscale and the WHO-Five well-being scale. Int. J. Methods Psychiatr. Res. 2003, 12, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Marcus, B.H.; Forsyth, L.H. Motivating People to Be Physically Active; Human Kinetics: Champaign, IL, USA, 2008. [Google Scholar]
- Haakstad, L.A.H.; Voldner, N.; Bø, K. Stages of change model for participation in physical activity during pregnancy. J. Pregnancy 2013, 2013, 193170. [Google Scholar] [CrossRef]
- Prochaska, J.O.; Redding, C.A.; Evers, K.E. The transtheoretical model and stages of change. Health Behav. Theory Res. Pract. 2015, 97, 38–48. [Google Scholar]
- Meher, S.; Cuthbert, A.; Kirkham, J.J.; Williamson, P.; Abalos, E.; Aflaifel, N.; Bhutta, Z.; Bishop, A.; Blum, J.; Collins, P.; et al. Core outcome sets for prevention and treatment of postpartum haemorrhage: An international Delphi consensus study. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 83–93. [Google Scholar] [CrossRef]
- Emmanuel, E.N.; Sun, J. Health related quality of life across the perinatal period among Australian women. J. Clin. Nurs. 2014, 23, 1611–1619. [Google Scholar] [CrossRef]
- Fonseca, A.; Gorayeb, R.; Canavarro, M.C. Women’s help-seeking behaviours for depressive symptoms during the perinatal period: Socio-demographic and clinical correlates and perceived barriers to seeking professional help. Midwifery 2015, 31, 1177–1185. [Google Scholar] [CrossRef]
- O’Connor, E.; Rossom, R.C.; Henninger, M.; Groom, H.C.; Burda, B.U. Primary care screening for and treatment of depression in pregnant and postpartum women: Evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016, 315, 388–406. [Google Scholar] [CrossRef]
- Health NCCfM (Ed.) Antenatal and Postnatal Mental health: Clinical Management and Service Guidance: Updated Edition 2014; British Psychological Society: Leicester, UK, 2014. [Google Scholar]
- Guðmundsdóttir, H.B.; Ólason, D.Þ.; Guðmundsdóttir, D.G.; Sigurðsson, J.F. A psychometric evaluation of the Icelandic version of the WHO-5. Scand. J. Psychol. 2014, 55, 567–572. [Google Scholar] [CrossRef]
- Hajos, T.R.; Pouwer, F.; Skovlund, S.; Den Oudsten, B.L.; Geelhoed-Duijvestijn, P.; Tack, C.; Snoek, F.J. Psychometric and screening properties of the WHO-5 well-being index in adult outpatients with Type 1 or Type 2 diabetes mellitus. Diabet. Med. 2013, 30, e63–e69. [Google Scholar] [CrossRef]
- Furuya, M.; Hayashino, Y.; Tsujii, S.; Ishii, H.; Fukuhara, S. Comparative validity of the WHO-5 Well-Being Index and two-question instrument for screening depressive symptoms in patients with type 2 diabetes. Acta Diabetol. 2013, 50, 117–121. [Google Scholar] [CrossRef]
- Löwe, B.; Spitzer, R.L.; Gräfe, K.; Kroenke, K.; Quenter, A.; Zipfel, S.; Buchholz, C.; Witte, S.; Herzog, W. Comparative validity of three screening questionnaires for DSM-IV depressive disorders and physicians’ diagnoses. J. Affect. Disord. 2004, 78, 131–140. [Google Scholar] [CrossRef]
- Phelan, S. Pregnancy: A “teachable moment” for weight control and obesity prevention. Am. J. Obstet. Gynecol. 2010, 202, 135.e1–135.e8. [Google Scholar] [CrossRef]
- Crozier, S.R.; Robinson, S.M.; Borland, S.E.; Godfrey, K.M.; Cooper, C.; Inskip, H.M.; SWS Study Group. Do women change their health behaviours in pregnancy? Findings from the Southampton Women’s Survey. Paediatr. Perinat. Epidemiol. 2009, 23, 446–453. [Google Scholar] [CrossRef]
- Yelverton, C.A.; Rafferty, A.A.; Moore, R.L.; Byrne, D.F.; Mehegan, J.; Cotter, P.D.; Van Sinderen, D.; Murphy, E.F.; Killeen, S.L.; McAuliffe, F.M. Diet and mental health in pregnancy: Nutrients of importance based on large observational cohort data. Nutrition 2022, 96, 111582. [Google Scholar] [CrossRef]
- Alati, R.; Davey Smith, G.; Lewis, S.J.; Sayal, K.; Draper, E.S.; Golding, J.; Fraser, R.; Gray, R. Effect of prenatal alcohol exposure on childhood academic outcomes: Contrasting maternal and paternal associations in the ALSPAC study. PLoS ONE 2013, 8, e74844. [Google Scholar] [CrossRef]
- Fernandes, O.; Sabharwal, M.; Smiley, T.; Pastuszak, A.; Koren, G.; Einarson, T. Moderate to heavy caffeine consumption during pregnancy and relationship to spontaneous abortion and abnormal fetal growth: A meta-analysis. Reprod. Toxicol. 1998, 12, 435–444. [Google Scholar] [CrossRef]
- Yang, Z.; Phung, H.; Freebairn, L.; Sexton, R.; Raulli, A.; Kelly, P. Contribution of maternal overweight and obesity to the occurrence of adverse pregnancy outcomes. Aust. N. Z. J. Obstet. Gynaecol. 2019, 59, 367–374. [Google Scholar] [CrossRef]
- Bartholomew, L.K.; Mullen, P.D. Five roles for using theory and evidence in the design and testing of behavior change interventions. J. Public Health Dent. 2011, 71 (Suppl. S1), S20–S33. [Google Scholar] [CrossRef] [PubMed]
- Abalos, E.; Chamillard, M.; Diaz, V.; Tuncalp Gulmezoglu, A.M. Antenatal care for healthy pregnant women: A mapping of interventions from existing guidelines to inform the development of new WHO guidance on antenatal care. BJOG 2016, 123, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Wickberg, B.; Tjus, T.; Hwang, P. Using the EPDS in routine antenatal care in Sweden: A naturalistic study. J. Reprod. Infant Psychol. 2005, 23, 33–41. [Google Scholar] [CrossRef]
- Lupton, D. The use and value of digital media for information about pregnancy and early motherhood: A focus group study. BMC Pregnancy Childbirth 2016, 16, 171. [Google Scholar] [CrossRef] [PubMed]
- Greene, E.M.; O’Brien, E.C.; Kennelly, M.A.; O’Brien, O.A.; Lindsay, K.L.; McAuliffe, F.M. Acceptability of the pregnancy, exercise, and nutrition research study with smartphone app support (PEARS) and the use of mobile health in a mixed lifestyle intervention by pregnant obese and overweight women: Secondary analysis of a randomized controlled trial. JMIR Mhealth Uhealth 2021, 9, e17189. [Google Scholar]
- Ainscough, K.M.; Lindsay, K.L.; O’Sullivan, E.J.; Gibney, E.R.; McAuliffe, F.M. Behaviour change in overweight and obese pregnancy: A decision tree to support the development of antenatal lifestyle interventions. Public Health Nutr. 2017, 20, 2642–2648. [Google Scholar] [CrossRef]
- Currie, S.; Sinclair, M.; Murphy, M.H.; Madden, E.; Dunwoody, L.; Liddle, D. Reducing the decline in physical activity during pregnancy: A systematic review of behaviour change interventions. PLoS ONE 2013, 8, e66385. [Google Scholar] [CrossRef]
- Kusyanti, T.; Wirakusumah, F.F.; Rinawan, F.R.; Muhith, A.; Purbasari, A.; Mawardi, F.; Puspitasari, I.W.; Faza, A.; Stellata, A.G. Technology-Based (Mhealth) and Standard/Traditional Maternal Care for Pregnant Woman: A Systematic Literature Review. Healthcare 2022, 10, 1287. [Google Scholar] [CrossRef]
- Zhu, X.-H.; Tao, J.; Jiang, L.-Y.; Zhang, Z.-F. Role of usual healthcare combined with telemedicine in the management of high-risk pregnancy in Hangzhou, China. J. Healthc. Eng. 2019, 2019, 3815857. [Google Scholar] [CrossRef]
- Steinhubl, S.R.; Muse, E.D.; Topol, E.J. Can mobile health technologies transform health care? JAMA 2013, 310, 2395–2396. [Google Scholar] [CrossRef]
| Baseline Characteristics | Intervention (n = 140) | Control (n = 137) |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Age (y) | 32.60 (4.80) | 31.76 (4.04) |
| Height (m) | 1.64 (0.06) | 1.65 (0.07) |
| Weight (Kg) † | 77 (70, 84) | 78 (72, 85) |
| BMI (Kg/m2) † | 28 (27, 31) | 28 (27, 31) |
| Gestation (w) † | 15 (14, 16) | 15 (14, 16) |
| White * | 132 (94) | 122 (89) |
| Completed tertiary education * | 83 (59) | 107 (78) |
| Current smoker * | 5 (4) | 7 (5) |
| HP Pobal index Maternal well-being | 6.33 (10.90) 55 (15) | 6.13 (11.59) 58 (15) |
| Maternal Well-Being Scores (Mean (SD)) | |||
|---|---|---|---|
| Stage of Behaviour Change | Overall (n = 277) | Intervention (n = 140) | Control (n = 137) |
| Stage 1 (precontemplation) | 55 (11) | 52 (5) | 56 (4) |
| Stage 2 (contemplation) | 53 (15) | 55 (3) | 52 (2) |
| Stage 3 (preparation) | 55 (15) | 52 (2) | 57 (4) |
| Stage 4 (action) | 56 (13) | 59 (3) | 54 (4) |
| Stage 5 (maintenance) | 61 (14) | 62 (2) | 59 (2) |
| Overall Mean (SD) | 57 (1) | 58 (1) | 56 (1) |
| Stage of Behaviour Change | Intervention (n = 140) | Control (n = 137) | Percentage Difference | p-Value (CI) |
|---|---|---|---|---|
| Stage 1 (precontemplation) | 3% | 7% | −4% | <0.01 (−6.21, −1.06) |
| Stage 2 (contemplation) | 23% | 37% | −14% | <0.01 (−21.7, −6.11) |
| Stage 3 (preparation) | 14% | 16% | −2% | 0.03 (−3.29, −0.14) |
| Stage 4 (action) | 7% | 6% | <1% | 0.31 (−0.28, 0.86) |
| Stage 5 (maintenance) | 53% | 34% | +19% | <0.01 (8.65, 29.27) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roche, D.; Rafferty, A.; Holden, S.; Killeen, S.L.; Kennelly, M.; McAuliffe, F.M. Maternal Well-Being and Stage of Behaviour Change during Pregnancy: A Secondary Analysis of the PEARS Randomised Controlled Trial. Int. J. Environ. Res. Public Health 2023, 20, 34. https://doi.org/10.3390/ijerph20010034
Roche D, Rafferty A, Holden S, Killeen SL, Kennelly M, McAuliffe FM. Maternal Well-Being and Stage of Behaviour Change during Pregnancy: A Secondary Analysis of the PEARS Randomised Controlled Trial. International Journal of Environmental Research and Public Health. 2023; 20(1):34. https://doi.org/10.3390/ijerph20010034
Chicago/Turabian StyleRoche, Doireann, Anthony Rafferty, Sinead Holden, Sarah Louise Killeen, Maria Kennelly, and Fionnuala M. McAuliffe. 2023. "Maternal Well-Being and Stage of Behaviour Change during Pregnancy: A Secondary Analysis of the PEARS Randomised Controlled Trial" International Journal of Environmental Research and Public Health 20, no. 1: 34. https://doi.org/10.3390/ijerph20010034
APA StyleRoche, D., Rafferty, A., Holden, S., Killeen, S. L., Kennelly, M., & McAuliffe, F. M. (2023). Maternal Well-Being and Stage of Behaviour Change during Pregnancy: A Secondary Analysis of the PEARS Randomised Controlled Trial. International Journal of Environmental Research and Public Health, 20(1), 34. https://doi.org/10.3390/ijerph20010034







