Human Health Risk Assessment from Lead Exposure through Consumption of Raw Cow Milk from Free-Range Cattle Reared in the Vicinity of a Lead–Zinc Mine in Kabwe
Abstract
1. Introduction
2. Methods and Materials
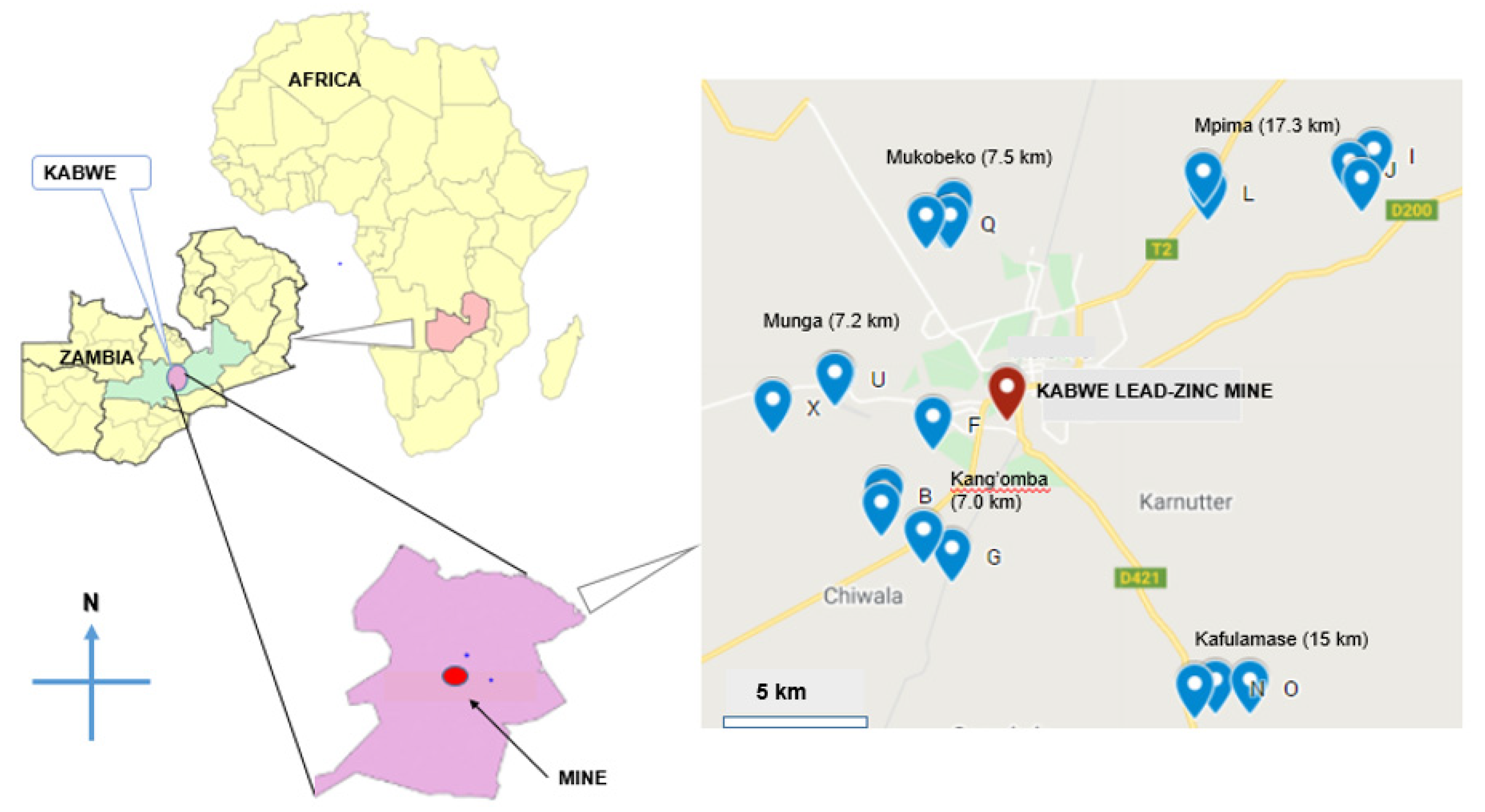
2.1. Location of the Study Area and Study Design
2.2. Milk Sampling
2.3. Sample Preparation and Microwave Acid Digestion
2.4. Lead Determination in Milk
2.5. Quality Assurance
2.6. Data Analysis
2.7. Probabilistic Health Risk Assessment of Lead in Milk
2.7.1. Chronic Daily Intake
2.7.2. Risk Characterization
Non-Carcinogenic Risk
Carcinogenic Risk
3. Results and Discussion
3.1. Lead Concentrations in Cow Milk
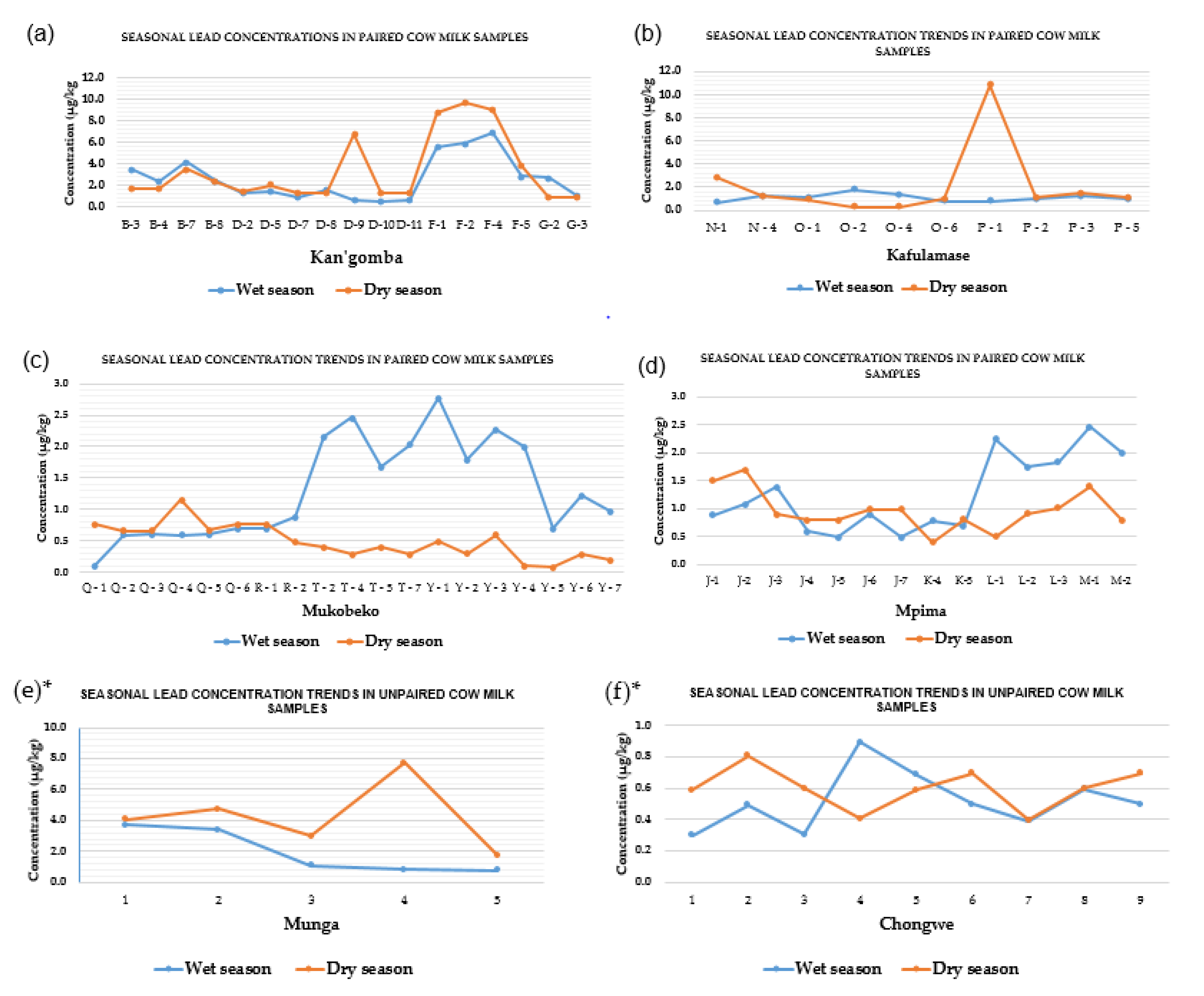
3.2. Seasonal Variations of Lead Concentration Trends in Milk
3.3. Health Risk Assessment
3.3.1. Chronic Daily Intake of Lead Metal in Milk
3.3.2. Evaluation of Non-Cancer Risk Assessment for the Kabwe Population
3.3.3. Evaluation of Carcinogenic Risk Assessment for the Kabwe Population
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miclean, M.; Cadar, O.; Levei, E.A.; Roman, R.; Ozunu, A.; Levei, L. Metal (Pb, Cu, Cd, and Zn) Transfer along Food Chain and Health Risk Assessment through Raw Milk Consumption from Free-Range Cows. Int. J. Environ. Res. Public Health 2019, 16, 4064. [Google Scholar] [CrossRef] [PubMed]
- Science Communication Unit, University of the West of England (UWE). Science for Environment Policy In-Depth Report, Soil Contamination: Impacts on Human Health. Science for Environment Policy. 2013. Available online: http://ec.europa.eu/science-environment-policy (accessed on 3 April 2022).
- World Health Organization. Exposure to Lead: A Major Public Health Concern, 2nd ed.; World Health Organization: Geneva, Switzerland, 2021; pp. 1–6. Available online: https://www.who.int/publications/i/item/9789240037656 (accessed on 1 April 2022).
- Oba, S.S.; Mumuni, A.; Mynepalli, S.; Chandra, K. Blood lead, zinc and calcium levels, and dietary intake habits among petrol station attendants and nomadic cattle rearers in Ilorin, North Central Nigeria. J. Environ. Pollut. Hum. Health 2018, 6, 165–173. [Google Scholar] [CrossRef]
- Zadnik, T. Monitoring of lead in topsoil, forage, blood, liver, and kidneys in cows in a lead-polluted area in Slovenia (1975–2002) and a Case of Lead Poisoning (1993). Int. J. Chem. Eng. 2010, 2010, 940206. [Google Scholar] [CrossRef]
- Oskarsson, A.; Jorhem, L.; Sundberg, J.; Nilsson, N.G.; Albanus, L. Lead poisoning in cattle—Transfer of lead to milk. Sci. Total Environ. 1992, 111, 83–94. [Google Scholar] [CrossRef]
- Patra, R.C.; Swarup, D.; Kumar, P.; Nandi, D.; Naresh, R.; Ali, S.L. Milk trace elements in lactating cows environmentally exposed to higher level of lead and cadmium around different industrial units. Sci. Total Environ. 2008, 404, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Aslani, M.R.; Heidarpour, M.; Najarnezhad, V.; Mostafavi, M.; Khorasani, Y. Lead poisoning in cattle associated with batteries recycling: High lead levels in milk of nonsymptomatic exposed cattle. Iran. J. Vet. Sci. Technol. 2012, 4, 47–52. [Google Scholar]
- Bischoff, K.; Higgins, W.; Thompson, B.; Ebel, J.G. Lead excretion in milk of accidentally exposed dairy cattle. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2014, 31, 839–844. [Google Scholar] [CrossRef]
- Galey, F.D.; Slenning, B.D.; Anderson, M.L.; Breneman, P.C.; Littlefield, E.S.; Melton, L.A.; Tracy, M.L.; Anderson, M.L.; Breneman, P.C.; Littlefield, E.S.; et al. Lead concentrations in blood and milk from periparturient dairy heifers seven months after an episode of acute lead toxicosis. J. Vet. Diagnostic Investig. 1990, 2, 222–226. [Google Scholar] [CrossRef]
- Licata, P.; Trombetta, D.; Cristani, M.; Giofrè, F.; Martino, D.; Calò, M.; Naccari, F. Levels of “toxic” and “essential” metals in samples of bovine milk from various dairy farms in Calabria, Italy. Environ. Int. 2004, 30, 1–6. [Google Scholar] [CrossRef]
- Swarup, D.; Patra, R.C.; Naresh, R.; Kumar, P.; Shekhar, P. Blood lead levels in lactating cows reared around polluted localities; Transfer of lead into milk. Sci. Total Environ. 2005, 349, 67–71. [Google Scholar] [CrossRef]
- Sharpe, R.T.; Livesey, C.T. Lead poisoning in cattle and its implications for food safety. Vet. Rec. 2006, 159, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Derakhshesh, S.M.; Rahimi, E.; Derakhshesh, S.M. Determination of lead residue in raw cow milk from different regions of iran by flameless atomic absorption spectrometry. Am. J. Toxicol. Sci. 2012, 4, 16–19. [Google Scholar] [CrossRef]
- El-Ansary, M.; El Leboudy, A. levels of cadmium and lead in raw cow and buffalos milk samples collected from local markets of El-Behera Governorate. Alex. J. Vet. Sci. 2015, 47, 129. [Google Scholar] [CrossRef]
- Nachiyunde, K.; Ikeda, H.; Okuda, T.; Nishijima, W. Assessment of dissolved heavy metal pollution in five Provinces of Zambia. J. Environ. Prot. 2013, 04, 80–85. [Google Scholar] [CrossRef]
- Younus, M.; Abbas, T.; Zafar, M.; Raza, S.; Khan, A.; Saleem, A.H.; Idrees, M.A.; Nisa, Q.U.; Akhtar, R.; Saleem, G. Assessment of heavy metal contamination in raw milk for human consumption. S. Afr. J. Anim. Sci. 2016, 46, 166–169. [Google Scholar] [CrossRef]
- Koyashiki, G.A.K.; Paoliello, M.M.B.; Tchounwou, P.B. Lead levels in human milk and children’s health risk: A systematic review. Rev. Environ. Health 2010, 25, 243–253. [Google Scholar] [CrossRef]
- Girma, K.; Tilahun, Z.; Haimanot, D. Review on milk safety with emphasis on its public health. World J. Dairy Food Sci. 2014, 9, 166–183. [Google Scholar] [CrossRef]
- Muhib, M.I.; Chowdhury, M.A.Z.; Easha, N.J.; Rahman, M.M.; Shammi, M.; Fardous, Z.; Bari, M.L.; Uddin, M.K.; Kurasaki, M.; Alam, M.K. Investigation of heavy metal contents in cow milk samples from area of Dhaka, Bangladesh. Int. J. Food Contam. 2016, 3, 16. [Google Scholar] [CrossRef]
- Tripathi, R.M.; Raghunath, R.; Sastry, V.N.; Krishnamoorthy, T.M. Daily intake of heavy metals by infants through milk and milk products. Sci. Total Environ. 1999, 227, 229–235. [Google Scholar] [CrossRef]
- Tong, S.; Von Schirnding, Y.E.; Prapamontol, T. Environmental lead exposure: A public health problem with global dimensions. Bull. World Health Organ. 2000, 49, 35–43. [Google Scholar]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liang, B.; Zhang, W.; Chen, K.; Zhang, Y.; Zhou, H.; Cheng, Y.; Liu, H.; Zhong, X.; Li, Y.; et al. Dietary lead exposure and associated health risks in Guangzhou, China. Int. J. Environ. Res. Public Health 2019, 16, 1417. [Google Scholar] [CrossRef] [PubMed]
- Tembo, B.D.; Sichilongo, K.; Cernak, J. Distribution of copper, lead, cadmium and zinc concentrations in soils around Kabwe town in Zambia. Chemosphere 2006, 63, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Yabe, J.; Nakayama, S.M.M.M.; Ikenaka, Y.; Muzandu, K.; Choongo, K.; Mainda, G.; Kabeta, M.; Ishizuka, M.; Umemura, T. Metal distribution in tissues of free-range chickens near a lead-zinc mine in Kabwe, Zambia. Environ. Toxicol. Chem. 2013, 32, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Toyomaki, H.; Yabe, J.; Nakayama, S.M.M.; Yohannes, Y.B.; Muzandu, K.; Liazambi, A.; Ikenaka, Y.; Kuritani, T.; Nakagawa, M.; Ishizuka, M. Factors associated with lead (Pb) exposure on dogs around a Pb mining area, Kabwe, Zambia. Chemosphere 2020, 247, 125884. [Google Scholar] [CrossRef]
- Kataba, A.; Nakayama, S.M.M.; Nakata, H.; Toyomaki, H.; Yohannes, Y.B.; Yabe, J.; Muzandu, K.; Zyambo, G.; Kubota, A.; Matsukawa, T.; et al. An investigation of the wild rat crown incisor as an indicator of lead (Pb) exposure using inductively couple plasma mass spectrometry (ICP-MS) and laser ablation ICP-MS. Int. J. Environ. Res. Public Health 2021, 18, 767. [Google Scholar] [CrossRef]
- Doya, R.; Nakayama, S.M.M.; Nakata, H.; Toyomaki, H.; Yabe, J.; Muzandu, K.; Yohannes, Y.B.; Kataba, A.; Zyambo, G.; Ogawa, T.; et al. Land use in habitats affects metal concentrations in wild lizards around a former lead mining Site. Environ. Sci. Technol. 2020, 54, 14474–14481. [Google Scholar] [CrossRef]
- Ikenaka, Y.; Nakayama, S.M.M.; Muroya, T.; Yabe, J.; Konnai, S.; Darwish, W.S.; Muzandu, K.; Choongo, K.; Mainda, G.; Teraoka, H.; et al. Effects of environmental lead contamination on cattle in a lead/zinc mining area: Changes in cattle immune systems on exposure to lead in vivo and in vitro. Environ. Toxicol. Chem. 2012, 31, 2300–2305. [Google Scholar] [CrossRef]
- Yabe, J.; Nakayama, S.M.M.; Ikenaka, Y.; Muzandu, K.; Ikenaka, Y.; Muzandu, K.; Ishizuka, M.; Umemura, T. Uptake of lead, cadmium, and other metals in the liver and kidneys of cattle near a lead-zinc mine in Kabwe, Zambia. Environ. Toxicol. Chem. 2011, 30, 1892–1897. [Google Scholar] [CrossRef]
- Nakata, H.; Nakayama, S.M.M.; Yabe, J.; Liazambi, A.; Mizukawa, H.; Darwish, W.S.; Ikenaka, Y.; Ishizuka, M. Reliability of stable Pb isotopes to identify Pb sources and verifying biological fractionation of Pb isotopes in goats and chickens. Environ. Pollut. 2016, 208, 395–403. [Google Scholar] [CrossRef]
- Yabe, J.; Nakayama, S.M.M.; Ikenaka, Y.; Yohannes, Y.B.; Bortey-sam, N.; Oroszlany, B.; Muzandu, K.; Choongo, K.; Nketani, A.; Ntapisha, J.; et al. Lead poisoning in children from townships in the vicinity of a lead—Zinc mine in Kabwe, Zambia. Chemosphere 2015, 119, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Mumba, C.; Samui, K.L.; Pandey, G.S.; Hang’ombe, B.M.; Simuunza, M.; Tembo, G.; Muliokela, S.W. Economic analysis of the viability of smallholder dairy farming in Zambia. Livest Res. Rural Dev. 2011, 23, 137. [Google Scholar]
- Mwaanga, P.; Silondwa, M.; Kasali, G.; Banda, P.M. Preliminary review of mine air pollution in Zambia. Heliyon 2019, 5, e02485. [Google Scholar] [CrossRef] [PubMed]
- Castro Gonzalez, N.P.; Moreno-Rojas, R.; Calderón Sánchez, F.; Moreno Ortega, A.; Juarez Meneses, M. Assessment risk to children’s health due to consumption of cow’s milk in polluted areas in Puebla and Tlaxcala, Mexico. Food Addit. Contam. Part B Surveill. 2017, 10, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Belete, T.; Hussen, A.; Rao, V.M. Determination of concentrations of selected heavy metals in cow’s milk: Borena Zone, Ethiopia. J. Health Sci. 2014, 4, 105–112. [Google Scholar] [CrossRef]
- Oana, C.; Tănaselia, C.; Miclean, M.; Levei, E.; Șenilă, M. Analysis of minor and trace elements in cow, goat and sheep milk in the NW Part of Romania. ProEnvironment/ProMediu 2016, 9, 87–90. [Google Scholar]
- Ataro, A.; McCrindle, R.I.; Botha, B.M.; McCrindle, C.M.E.; Ndibewu, P.P. Quantification of trace elements in raw cow’s milk by inductively coupled plasma mass spectrometry (ICP-MS). Food Chem. 2008, 111, 243–248. [Google Scholar] [CrossRef]
- USEPA (United States Environmental Protection Agency). Human Health Risk Assessment Guidance; United States Environmental Protection Agency: Washington, DC, USA, 2010; p. 117. Available online: https://www.oregon.gov/deq/filterdocs/humanhealthriskassessmentguidance.pdf (accessed on 1 April 2022).
- Haakonde, T.; Yabe, J.; Choongo, K.; Chongwe, G.; Nchima, G.; Islam, M.S. Uranium contamination of milk from cattle in the uranium-mining area in Siavonga District of Zambia: A preliminary human health risk assessment. Bull. Natl. Res. Cent. 2021, 45, 96. [Google Scholar] [CrossRef]
- Zambia Food Consumption, Quantities 1992–2007. Available online: https://knoema.com/bxfxff/food-consumption-quantities (accessed on 18 February 2022).
- Liang, Y.; Yi, X.; Dang, Z.; Wang, Q.; Luo, H.; Tang, J. Heavy metal contamination and health risk assessment in the vicinity of a tailing pond in Guangdong, China. Int. J. Environ. Res. Public Health 2017, 14, 1557. [Google Scholar] [CrossRef]
- Kasozi, K.I.; Natabo, P.C.; Namubiru, S.; Tayebwa, D.S.; Tamale, A.; Bamaiyi, P.H. food safety analysis of milk and beef in Southwestern Uganda. J. Environ. Public Health 2018, 7, 1627180. [Google Scholar] [CrossRef]
- Siddiqui, M.; Rajurkar, G.R. Lead—An emerging threat to livestock. Vet. World 2008, 1, 213–216. [Google Scholar]
- Sarsembayeva, N.B.; Abdigaliyeva, T.B.; Utepova, Z.A.; Biltebay, A.N.; Zhumagulova, S.Z. Heavy metal levels in milk and fermented milk products produced in the Almaty region, Kazakhstan. Vet. World 2020, 13, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Pšenková, M.; Toman, R.; Tančin, V. Concentrations of toxic metals and essential elements in raw cow milk from areas with potentially undisturbed and highly disturbed environment in Slovakia. Environ. Sci. Pollut. Res. 2020, 27, 26763–26772. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cai, L.M.; Wen, H.H.; Luo, J.; Wang, Q.S.; Liu, X. Spatial distribution and source apportionment of heavy metals in soil from a typical county-level city of Guangdong Province, China. Sci. Total Environ. 2019, 655, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Sobhanardakani, S. Human health risk assessment of Cd, Cu, Pb and Zn through consumption of raw and pasteurized cow’s milk. Iran. J. Public Health 2018, 47, 1172–1180. [Google Scholar]
- Hassan Elsaim, M.; Ali, Y. Evaluation of some heavy metals in fresh cow’s milk from different regions of Sudan. Am. J. Appl. Ind. Chem. 2018, 2, 8–14. [Google Scholar] [CrossRef]
- Norouzirad, R.; González-Montaña, J.R.; Martínez-Pastor, F.; Hosseini, H.; Shahrouzian, A.; Khabazkhoob, M.; Ali Malayeri, F.; Moallem Bandani, H.; Paknejad, M.; Foroughi-nia, B.; et al. Lead and cadmium levels in raw bovine milk and dietary risk assessment in areas near petroleum extraction industries. Sci. Total Environ. 2018, 635, 308–314. [Google Scholar] [CrossRef]
- Patel, H.B.; Singh, R.D.; Desai, R.R.; Mody, S.K.; Gondaliya, S.B. Determination of lead and cadmium level in milk of dairy animals in Mehsana district of Gujarat using ICP-AES. Bull. Environ. Pharmacol. Life Sci. 2017, 6, 54–58. [Google Scholar]
- Ismail, A.; Riaz, M.; Akhtar, S.; Amjad, F.; Shahzad, M.A.; Mujtaba, A.; Akhtar, S.; Amjad, F.; Ismail, A.; Riaz, M.; et al. Intake of heavy metals through milk and toxicity assessment. Pak. J. Zool. 2017, 49, 1413–1419. [Google Scholar] [CrossRef]
- El-Ansary, M.A. Determination of heavy metals content (cadmium and lead) in raw cow’s and buffalo’s milk. Int. J. Curr. Res. Biosci. Plant Biol. 2017, 4, 116–120. [Google Scholar] [CrossRef][Green Version]
- Kumar, V. Assessment of lead and cadmium status in feedstuffs, milk and water samples in Haryana State of India. Int. J. Livest. Res. 2019, 9, 129–137. [Google Scholar] [CrossRef]
- Alimentarius, C. Joint FAO/WHO Food Standards Programme Codex Alimentarius Committee on Contaminants in Foods, 5th ed.; Discussion Paper on Furan (Prepared by the Electronic Working Group Led by the United States of America); FAO: The Hague, The Netherlands, 2011. [Google Scholar]
- European Food Safety Authority (EFSA). Lead dietary exposure in the European population. EFSA J. 2012, 10, 2831. [Google Scholar] [CrossRef]
- Boudebbouz, A.; Boudalia, S.; Bousbia, A.; Habila, S.; Boussadia, M.I.; Gueroui, Y. Heavy metals levels in raw cow milk and health risk assessment across the globe: A systematic review. Sci. Total Environ. 2021, 751, 141830. [Google Scholar] [CrossRef] [PubMed]
- Cobbina, J. Non-cancer risk assessment from exposure to mercury (Hg), cadmium (Cd), arsenic (As), copper (Cu) and lead (Pb) in boreholes and surface water in Tinga, in the Bole-Bamboi District, Ghana. J. Toxicol. Environ. Health Sci. 2013, 5, 29–36. [Google Scholar] [CrossRef]
- Joint, Food Agricultural Organization of the United Nations/World Health Organization (FAO/WHO); WHO Expert Committee on Food Additives. Evaluation of Certain Food Additives and Contaminants: Seventy-Third Report of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); World Health Organization: Geneva, Switzerland, 2010; Available online: https://www.fao.org/documents/card/en/c/fbf01686-b940-44c2-a077-1052740ad1c6/ (accessed on 3 April 2022).
- Salah, F.A.A.E.; Esmat, I.A.; Bayoumi, M.A. Heavy metals residues and trace elements in milk powder marketed in Dakahlia Governorate. Int. Food Res. J. 2013, 20, 1807–1812. [Google Scholar]
- Meshref, A.M.S.; Moselhy, W.A.; Hassan, N.E.H.Y. Heavy metals and trace elements levels in milk and milk products. J. Food Meas. Charact. 2014, 8, 381–388. [Google Scholar] [CrossRef]
- Pilarczyk, R.; Wójcik, J.; Czerniak, P.; Sablik, P.; Pilarczyk, B.; Tomza-Marciniak, A. Concentrations of toxic heavy metals and trace elements in raw milk of Simmental and Holstein-Friesian cows from organic farm. Environ. Monit. Assess. 2013, 185, 8383–8392. [Google Scholar] [CrossRef]
- Loutfy, N.; Fuerhacker, M.; Tundo, P.; Raccanelli, S.; El Dien, A.G.; Ahmed, M.T.; El Dien, A.G.; Ahmed, M.T.; El Dien, A.G.; Ahmed, M.T. Dietary intake of dioxins and dioxin-like PCBs, due to the consumption of dairy products, fish/seafood and meat from Ismailia city, Egypt. Sci. Total Environ. 2006, 370, 1–8. [Google Scholar] [CrossRef]
- Abedi, A.S.; Nasseri, E.; Esfarjani, F.; Mohammadi-Nasrabadi, F.; Hashemi Moosavi, M.; Hoseini, H. A systematic review and meta-analysis of lead and cadmium concentrations in cow milk in Iran and human health risk assessment. Environ. Sci. Pollut. Res. 2020, 27, 10147–10159. [Google Scholar] [CrossRef]
- Satarug, S.; Gobe, G.C.; Vesey, D.A.; Phelps, K.R. Cadmium and lead exposure, nephrotoxicity, and mortality. Toxics 2020, 8, 86. [Google Scholar] [CrossRef]
- Bilandžić, N.; Sedak, M.; Čalopek, B.; Luburić, Đ.B.; Solomun Kolanović, B.; Varenina, I.; Đokić, M.; Kmetič, I.; Murati, T. Lead concentrations in raw cow and goat milk collected in rural areas of Croatia from 2010 to 2014. Bull. Environ. Contam. Toxicol. 2016, 96, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Proshad, R.; Islam, M.S.; Kormoker, T.; Bhuyan, B.M.; Hanif, M.A.; Hossain, N.; Roy, R.; Sharma, S.A. Contamination of heavy metals in agricultural soils: Ecological and health risk assessment. SF J. Nanochem. Nanotechnol. 2019, 2, 1012. [Google Scholar]
- Ismail, S.A.; Abolghait, S.K. Estimation of lead and cadmium residual levels in chicken giblets at retail markets in Ismailia city, Egypt. Int. J. Vet. Sci. Med. 2013, 1, 109–112. [Google Scholar] [CrossRef]
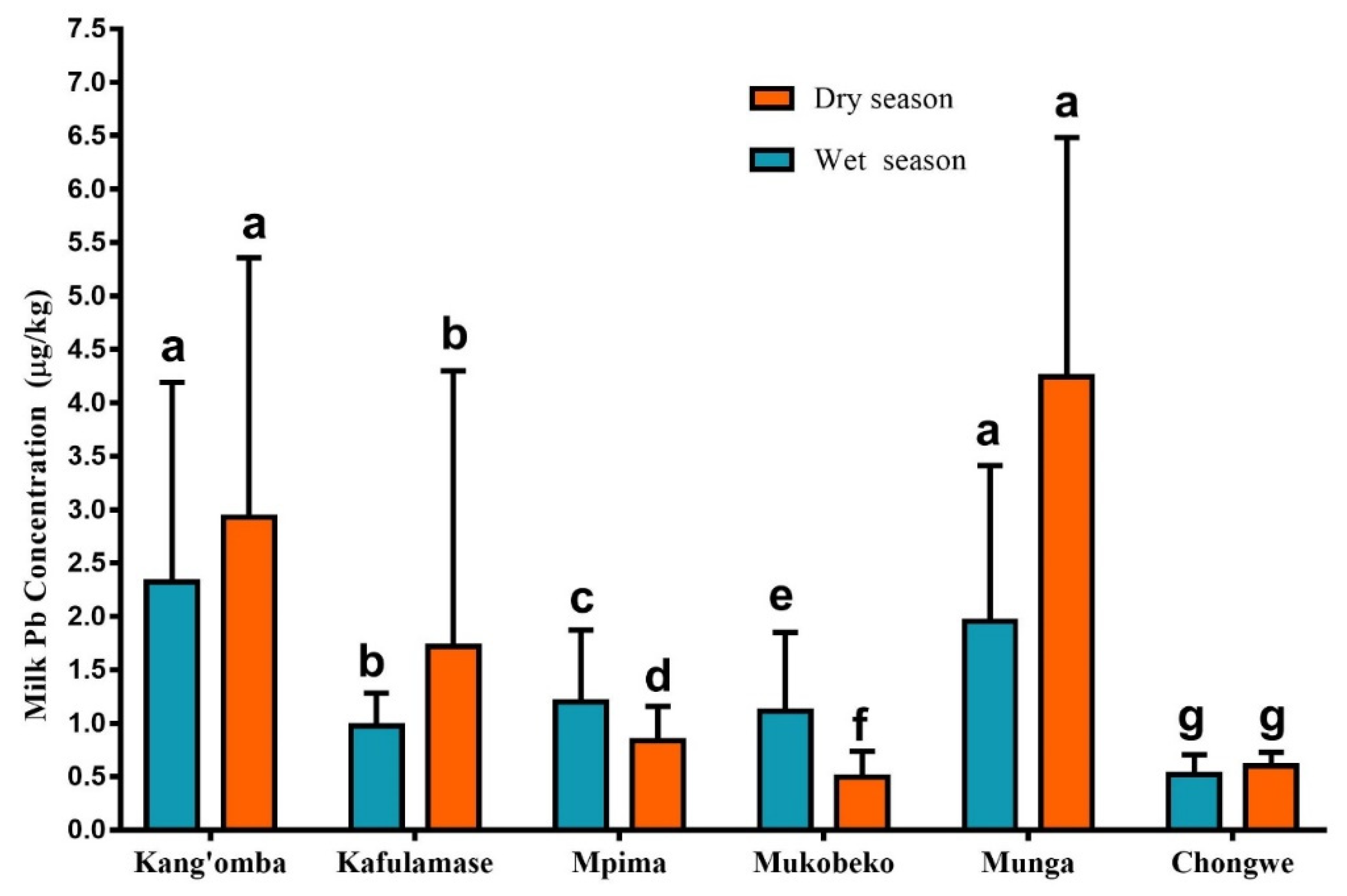
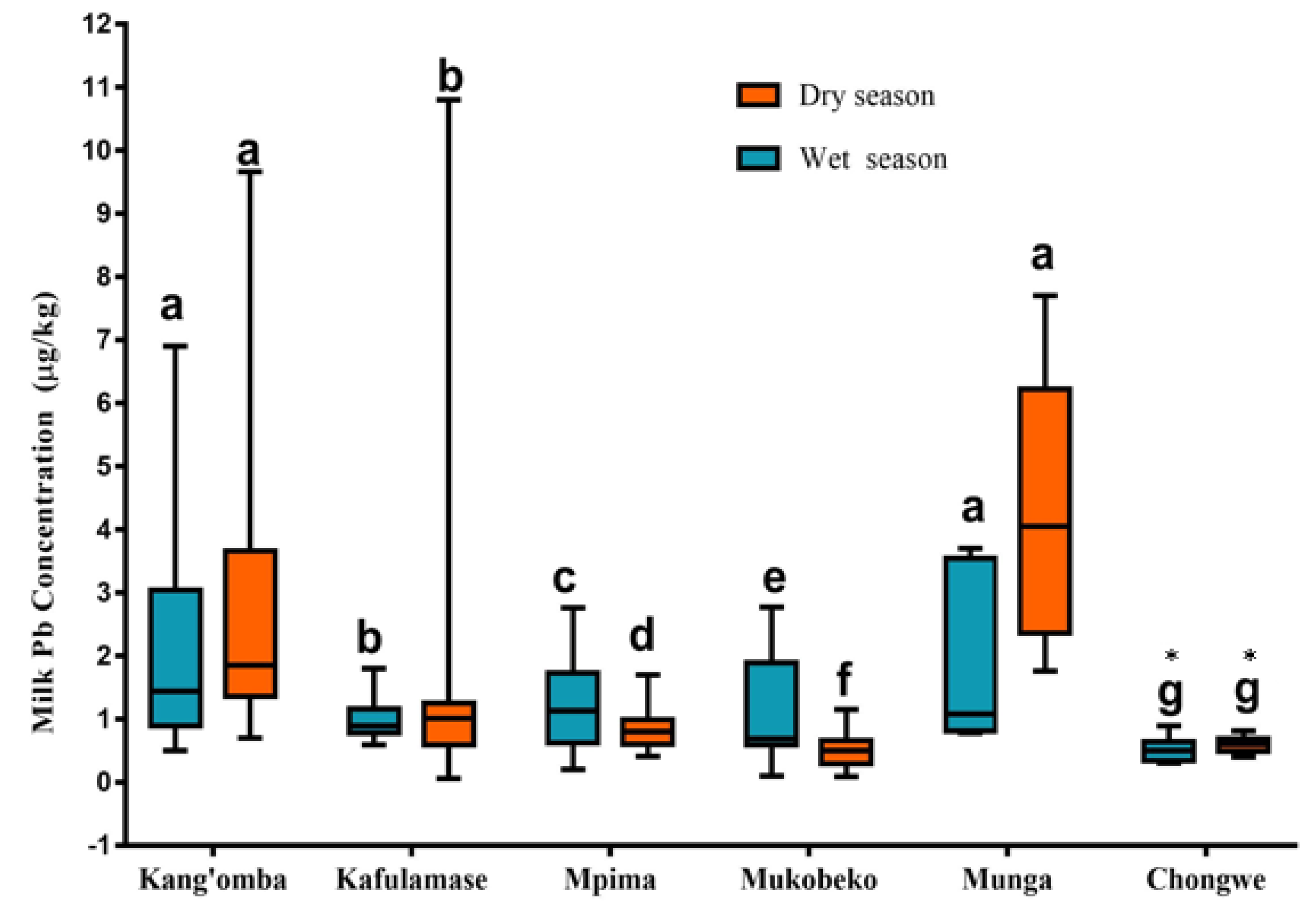
| Season | Region | No. of Farms | Samples (n) | Mean (m) | Standard Deviation (SD±) | Min-Max (µg/kg) |
|---|---|---|---|---|---|---|
| Kan’gomba | 5 | 22 | 2.32 | 1.87 | 0.05–6.90 | |
| Wet | Kafulamase | 3 | 16 | 0.98 | 0.30 | 0.59–1.80 |
| season | Mpima | 5 | 28 | 1.20 | 0.67 | 0.20–2.76 |
| Mukobeko | 5 | 29 | 1.11 | 0.74 | 0.10–2.77 | |
| Munga | 3 | 5 | 1.96 | 1.46 | 0.78–3.70 | |
| Chongwe | 3 | 9 | 0.60 | 0.19 | 0.89–4.66 | |
| Average | 1.36 | 0.88 | 0.05–6.90 | |||
| Kan’gomba | 4 | 34 | 2.93 | 2.43 | 0.70–9.66 | |
| Kafulamase | 3 | 19 | 1.72 | 2.58 | 0.06–10.8 | |
| Dry | Mpima | 5 | 26 | 0.84 | 0.32 | 0.41–1.70 |
| season | Mukobeko | 5 | 30 | 0.50 | 0.24 | 0.09–1.15 |
| Munga | 3 | 5 | 4.24 | 2.24 | 1.76–7.70 | |
| Chongwe | 3 | 9 | 0.51 | 0.13 | 0.40–0.81 | |
| Average | 1.79 | 1.32 | 0.06–10.8 |
| Year | Country | Source of Milk | N | Method Used | Mean (µg/kg) | Range | Reference |
|---|---|---|---|---|---|---|---|
| 2021 | Zambia | Farms near the mining area | 233 | GFAAS | 2.22 ± 1.89 | LOD-9.66 | Present study |
| 2020 | Kazakhstan | Farms | 120 | AAS | 11.6 ± 10.0 | - | [46] |
| 2020 | Slovakia | Farms | 40 | ETA-AAS | 10.0 | - | [47] |
| 2019 | China | Wholesale markets and stores | 208 | ICP-MS | 3.6 ± 2.30 | - | [48] |
| 2019 | Romania | Small cattle farms | 10 | GFAAS | 24. ± 15.0 | 0.010–0.048 | [1] |
| 2018 | Iran | Dairies and markets | 36 | ICP-OES | 32.83 ± 20.80 | 15.70–68.0 | [49] |
| 2018 | Sudan | Farms | 9 | AAS | <LOD | - | [50] |
| 2018 | Iran | Dairy, industrial and traditional farms | 118 | GFAAS | 47.0 ± 3.9 | N.D-250 | [51] |
| 2018 | Uganda | District | 20 | AAS | 10.48 ± 1.82 | 6.62–14.34 | [44] |
| 2017 | India | Farms | 30 | ICP-AES | 124.0 | 0.016–0.356 | [52] |
| 2017 | Pakistan | Farms | 240 | AAS | 0.021 | 0.007–0.041 | [53] |
| 2017 | Egypt | Dairy shops | 18 | AAS | 93.4 ± 18.8 | 0.007–0.341 | [54] |
| 2017 | Mexico | Sub-basin of industrial and urban region | 40 | ICP-OES | 46.0± 28. | 0.039–0.05 | [36] |
| 2016 | Bangladesh | Branded, dairy farm and household farmers | 27 | FAAS | 0.012 ± 0.001 | - | [20] |
| 2016 | Romania | Rural area | 19 | ICP-MS | 15.8 ± 5.45 | 0.01–0.48 | [38] |
| 2015 | Egypt | Dairy shops and groceries | 30 | GFAAS | 414.0 ± 34.2 | 0.09–0.52 | [15] |
| Wet Season CDI (µg/kg/day) | Dray Season CDI (µg/kg/day) | |||
|---|---|---|---|---|
| Region | Children | Adult | Children | Adult |
| Kan’gomba | 3.77 × 10−6 | 6.66 × 10−7 | 4.98 × 10−6 | 8.79 × 10−7 |
| Kafulamase | 8.33 × 10−7 | 3.43 × 10−7 | 1.46 × 10−6 | 6.02 × 10−7 |
| Mpima | 1.02 × 10−6 | 4.20 × 10−7 | 1.02 × 10−6 | 4.20 × 10−7 |
| Mukobeko | 9.44 × 10−7 | 3.89 × 10−7 | 4.25 × 10−7 | 1.75 × 10−7 |
| Munga | 1.67 × 10−6 | 6.86 × 10−7 | 3.60 × 10−6 | 1.48 × 10−6 |
| Chongwe * | 5.10 × 10−7 | 2.10 × 10−7 | 4.34 × 10−7 | 1.79 × 10−7 |
| Average | 8.75 × 10−6 | 2.71 × 10−6 | 1.19 × 10−5 | 3.74 × 10−6 |
| Wet Season THQ | Dry Season THQ | |||
|---|---|---|---|---|
| Region | Children | Adult | Children | Adult |
| Kan’gomba | 1.08 × 10−3 | 1.90 × 10−4 | 1.42 × 10−3 | 2.51 × 10−4 |
| Kafulamase | 2.38 × 10−4 | 9.80 × 10−5 | 4.18 × 10−4 | 1.72 × 10−4 |
| Mpima | 2.91 × 10−4 | 1.20 × 10−4 | 2.91 × 10−4 | 1.20 × 10−4 |
| Mukobeko | 2.70 × 10−4 | 1.11 × 10−4 | 1.21 × 10−4 | 5.00 × 10−5 |
| Munga | 4.76 × 10−4 | 1.96 × 10−4 | 1.03 × 10−3 | 4.24 × 10−4 |
| Chongwe * | 1.46 × 10−4 | 6.00 × 10−5 | 1.24 × 10−4 | 5.10 × 10−5 |
| Average | 2.50 × 10−3 | 7.75 × 10−4 | 3.41 × 10−3 | 1.07 × 10−3 |
| Wet Season ILCR | Dry Season ILCR | |||
|---|---|---|---|---|
| Region | Children | Adult | Children | Adult |
| Kan’gomba | 3.21 × 10−8 | 5.66 × 10−9 | 4.23 × 10−8 | 7.47 × 10−9 |
| Kafulamase | 7.08 × 10−9 | 2.92 × 10−9 | 1.24 × 10−8 | 5.12 × 10−9 |
| Mpima | 8.67 × 10−9 | 3.57 × 10−9 | 8.67 × 10−9 | 3.57 × 10−9 |
| Mukobeko | 8.02 × 10−9 | 3.30 × 10−9 | 3.61 × 10−9 | 1.49 × 10−9 |
| Munga | 1.42 × 10−8 | 5.83 × 10−9 | 3.06 × 10−8 | 1.26 × 10−8 |
| Chongwe | 4.34 × 10−9 | 1.79 × 10−9 | 3.68 × 10−9 | 1.52 × 10−9 |
| Average | 7.43 × 10−8 | 2.31 × 10−8 | 1.01 × 10−7 | 3.18 × 10−8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zyambo, G.; Yabe, J.; Muzandu, K.; M’kandawire, E.; Choongo, K.; Kataba, A.; Chawinga, K.; Liazambi, A.; Nakayama, S.M.M.; Nakata, H.; et al. Human Health Risk Assessment from Lead Exposure through Consumption of Raw Cow Milk from Free-Range Cattle Reared in the Vicinity of a Lead–Zinc Mine in Kabwe. Int. J. Environ. Res. Public Health 2022, 19, 4757. https://doi.org/10.3390/ijerph19084757
Zyambo G, Yabe J, Muzandu K, M’kandawire E, Choongo K, Kataba A, Chawinga K, Liazambi A, Nakayama SMM, Nakata H, et al. Human Health Risk Assessment from Lead Exposure through Consumption of Raw Cow Milk from Free-Range Cattle Reared in the Vicinity of a Lead–Zinc Mine in Kabwe. International Journal of Environmental Research and Public Health. 2022; 19(8):4757. https://doi.org/10.3390/ijerph19084757
Chicago/Turabian StyleZyambo, Golden, John Yabe, Kaampwe Muzandu, Ethel M’kandawire, Kennedy Choongo, Andrew Kataba, Kenneth Chawinga, Allan Liazambi, Shouta M. M Nakayama, Hokuto Nakata, and et al. 2022. "Human Health Risk Assessment from Lead Exposure through Consumption of Raw Cow Milk from Free-Range Cattle Reared in the Vicinity of a Lead–Zinc Mine in Kabwe" International Journal of Environmental Research and Public Health 19, no. 8: 4757. https://doi.org/10.3390/ijerph19084757
APA StyleZyambo, G., Yabe, J., Muzandu, K., M’kandawire, E., Choongo, K., Kataba, A., Chawinga, K., Liazambi, A., Nakayama, S. M. M., Nakata, H., & Ishizuka, M. (2022). Human Health Risk Assessment from Lead Exposure through Consumption of Raw Cow Milk from Free-Range Cattle Reared in the Vicinity of a Lead–Zinc Mine in Kabwe. International Journal of Environmental Research and Public Health, 19(8), 4757. https://doi.org/10.3390/ijerph19084757






