International Travel as a Risk Factor for Carriage of Extended-Spectrum β-Lactamase-Producing Escherichia coli in a Large Sample of European Individuals—The AWARE Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Variables of Interest
Potential Risk Factors
2.3. Outcome of Interest
2.4. Statistical Analyses
3. Results
3.1. Study Population
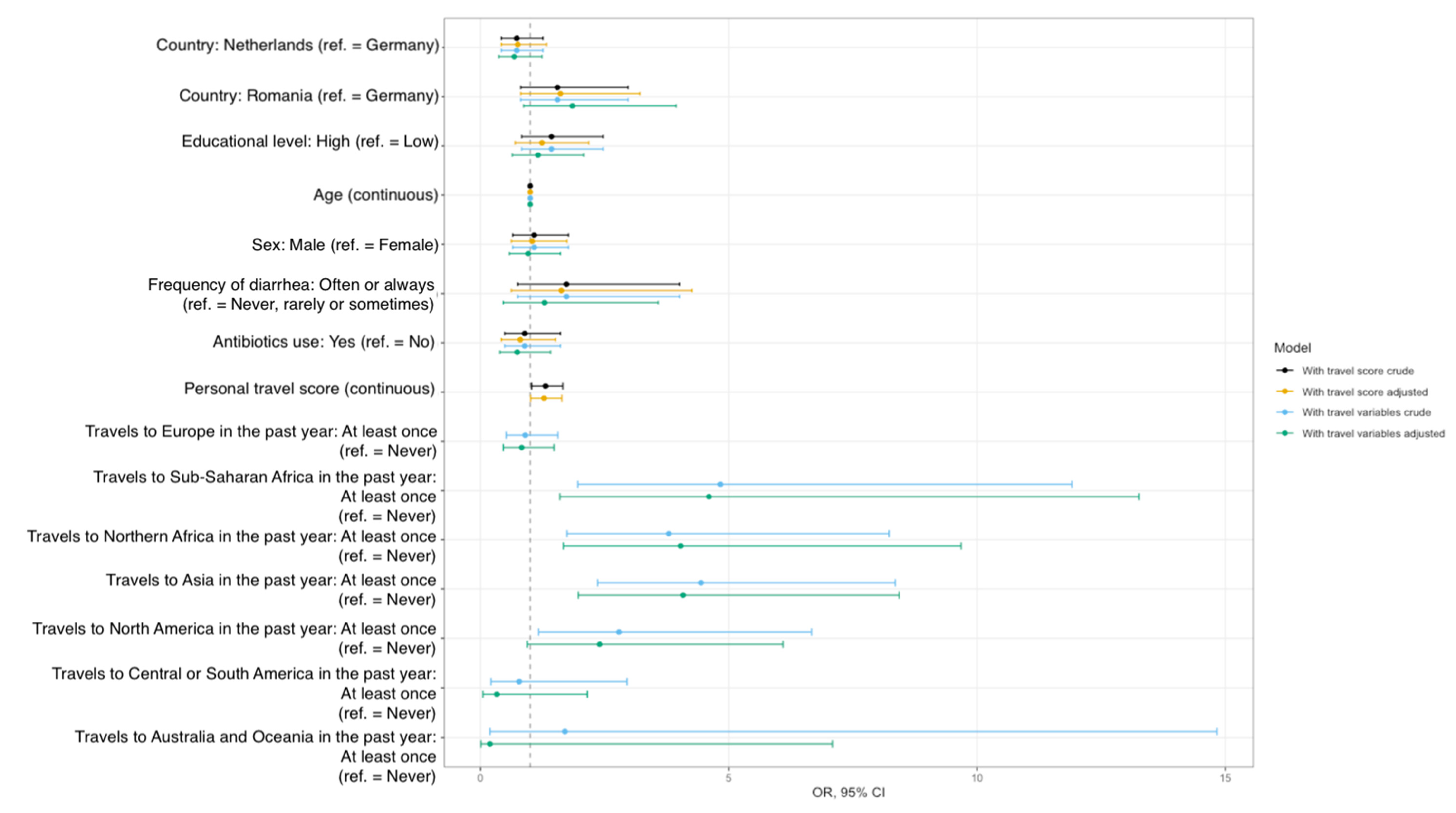
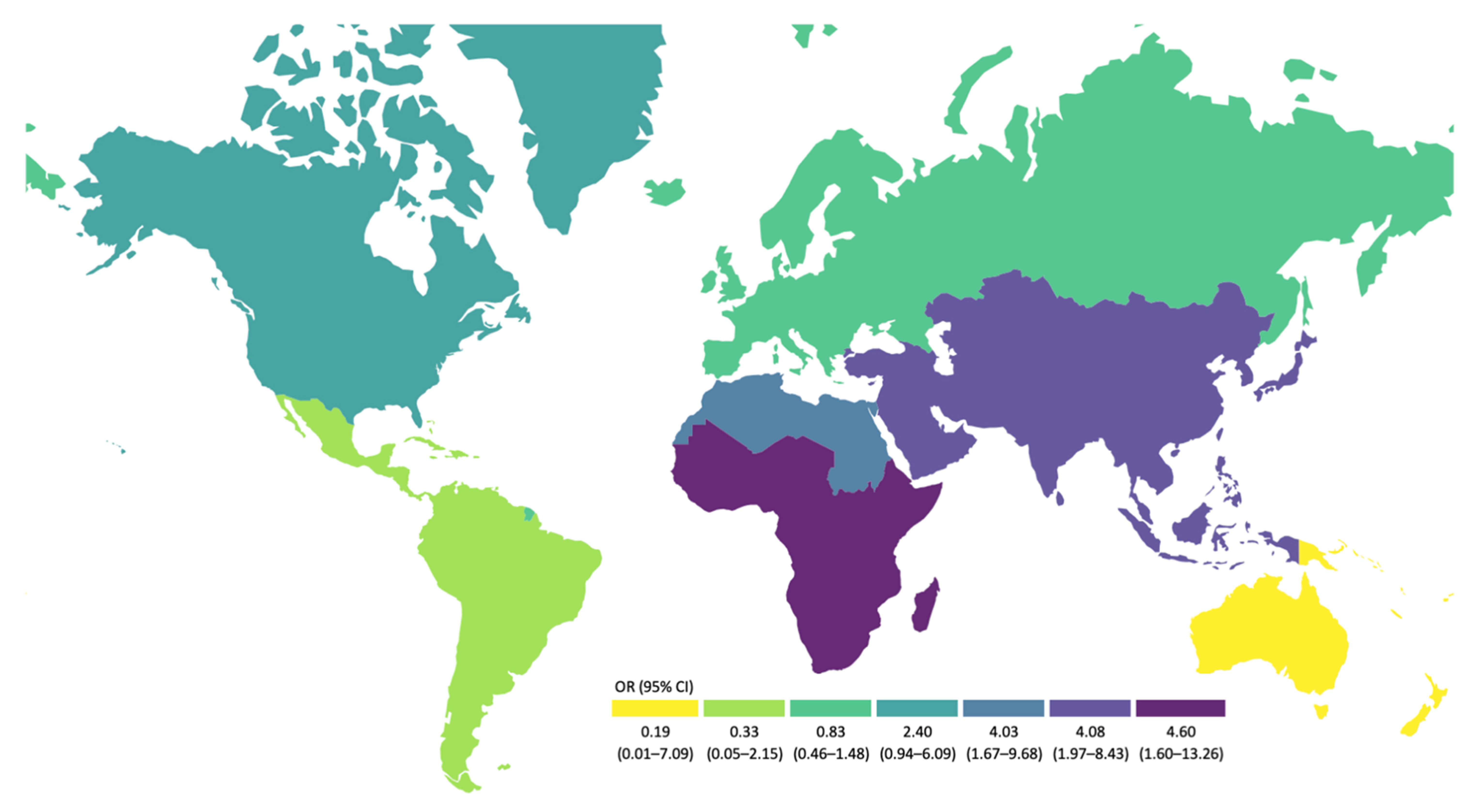
3.2. Risk Factors for ESBL-EC Carriage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Subramaniam, G.; Girish, M. Antibiotic Resistance—A Cause for Reemergence of Infections. Indian J. Pediatr. 2020, 87, 937–944. [Google Scholar] [CrossRef]
- Kamenshchikova, A.; Wolffs, P.F.G.; Hoebe, C.J.P.A.; Penders, J.; Park, H.Y.; Kambale, M.S.; Horstman, K. Combining stool and stories. Exploring antimicrobial resistance among a longitudinal cohort of international health students. BMC Infect. Dis. 2021, 21, 1008. [Google Scholar] [CrossRef]
- Polianciuc, S.I.; Gurzău, A.E.; Kiss, B.; Ştefan, M.G.; Loghin, F. Antibiotics in the environment: Causes and consequences. Med. Pharm. Rep. 2020, 93, 231–240. [Google Scholar] [CrossRef]
- Budhram, D.R.; Mac, S.; Bielecki, J.M.; Patel, S.N.; Sander, B. Health outcomes attributable to carbapenemase-producing Enterobacteriaceae infections: A systematic review and meta-analysis. Infect. Control Hosp. Epidemiol. 2020, 41, 37–43. [Google Scholar] [CrossRef]
- Bezabih, Y.M.; Sabiiti, W.; Alamneh, E.; Bezabih, A.; Peterson, G.M.; Bezabhe, W.M.; Roujeinikova, A. The global prevalence and trend of human intestinal carriage of ESBL-producing Escherichia coli in the community. J. Antimicrob. Chemother. 2021, 76, 22–29. [Google Scholar] [CrossRef]
- Rodríguez-Molina, D.; Berglund, F.; Blaak, H.; Flach, C.-F.; Kemper, M.; Marutescu, L.; Gradisteanu, G.P.; Popa, M.; Spießberger, B.; Weinmann, T.; et al. Carriage of ESBL-producing Enterobacterales in wastewater treatment plant workers and surrounding residents—The AWARE Study. Eur. J. Clin. Microbiol. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Andremont, A.; Bengtsson-Palme, J.; Brandt, K.K.; de Roda Husman, A.M.; Fagerstedt, P.; Fick, J.; Flach, C.-F.; Gaze, W.H.; Kuroda, M.; et al. Critical knowledge gaps and research needs related to the environmental dimensions of antibiotic resistance. Environ. Int. 2018, 117, 132–138. [Google Scholar] [CrossRef]
- Iwu, C.D.; Korsten, L.; Okoh, A.I. The incidence of antibiotic resistance within and beyond the agricultural ecosystem: A concern for public health. MicrobiologyOpen 2020, 9, e1035. [Google Scholar] [CrossRef]
- Arcilla, M.S.; Van Hattem, J.M.; Bootsma, M.C.; Van Genderen, P.J.; Goorhuis, A.; Schultsz, C.; E Stobberingh, E.; A Verbrugh, H.; De Jong, M.D.; Melles, D.C.; et al. The Carriage of Multiresistant Bacteria after Travel (COMBAT) prospective cohort study: Methodology and design. BMC Public Health 2014, 14, 410. [Google Scholar] [CrossRef] [Green Version]
- Kantele, A.; Lääveri, T.; Mero, S.; Vilkman, K.; Pakkanen, S.; Ollgren, J.; Antikainen, J.; Kirveskari, J. Antimicrobials Increase Travelers’ Risk of Colonization by Extended-Spectrum Betalactamase-Producing Enterobacteriaceae. Clin. Infect. Dis. 2015, 60, 837–846. [Google Scholar] [CrossRef]
- Ruppé, E.; Armand-Lefèvre, L.; Estellat, C.; Consigny, P.-H.; El Mniai, A.; Boussadia, Y.; Goujon, C.; Ralaimazava, P.; Campa, P.; Girard, P.-M.; et al. High Rate of Acquisition but Short Duration of Carriage of Multidrug-Resistant Enterobacteriaceae after Travel to the Tropics. Clin. Infect. Dis. 2015, 61, 593–600. [Google Scholar] [CrossRef] [Green Version]
- van Hattem, J.M.; Arcilla, M.S.; Bootsma, M.C.; van Genderen, P.J.; Goorhuis, A.; Grobusch, M.P.; Molhoek, N.; Lashof, A.M.O.; Schultsz, C.; E Stobberingh, E.; et al. Prolonged carriage and potential onward transmission of carbapenemase-producing Enterobacteriaceae in Dutch travelers. Future Microbiol. 2016, 11, 857–864. [Google Scholar] [CrossRef] [Green Version]
- Arcilla, M.S.; van Hattem, J.M.; Haverkate, M.R.; Bootsma, M.C.J.; van Genderen, P.J.J.; Goorhuis, A.; Grobusch, M.P.; Lashof, A.M.O.; Molhoek, N.; Schultsz, C.; et al. Import and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae by international travellers (COMBAT study): A prospective, multicentre cohort study. Lancet Infect. Dis. 2017, 17, 78–85. [Google Scholar] [CrossRef]
- Woerther, P.-L.; Andremont, A.; Kantele, A. Travel-acquired ESBL-producing Enterobacteriaceae: Impact of colonization at individual and community level. J. Travel Med. 2017, 24 (Suppl. 1), S29–S34. [Google Scholar] [CrossRef] [Green Version]
- Lorme, F.; Maataoui, N.; Rondinaud, E.; Esposito-Farèse, M.; Clermont, O.; Ruppe, E.; Arlet, G.; Genel, N.; Matheron, S.; Andremont, A.; et al. Acquisition of plasmid-mediated cephalosporinase producing Enterobacteriaceae after a travel to the tropics. PLoS ONE 2018, 13, e0206909. [Google Scholar] [CrossRef]
- Vilkman, K.; Lääveri, T.; Pakkanen, S.H.; Kantele, A. Stand-by antibiotics encourage unwarranted use of antibiotics for travelers’ diarrhea: A prospective study. Travel Med. Infect. Dis. 2019, 27, 64–71. [Google Scholar] [CrossRef]
- Arcilla, M.S.; Van Hattem, J.M.; Bootsma, M.C.; van Genderen, P.J.; Goorhuis, A.; Grobusch, M.P.; Klaassen, C.H.; Lashof, A.M.O.; Schultsz, C.; Stobberingh, E.E.; et al. Prevalence and risk factors for carriage of ESBL-producing Enterobacteriaceae in a population of Dutch travellers: A cross-sectional study. Travel Med. Infect. Dis. 2020, 33, 101547. [Google Scholar] [CrossRef]
- Dao, T.L.; Canard, N.; Hoang, V.T.; Ly, T.D.A.; Drali, T.; Ninove, L.; Fenollar, F.; Raoult, D.; Parola, P.; Marty, P.; et al. Risk factors for symptoms of infection and microbial carriage among French medical students abroad. Int. J. Infect. Dis. 2020, 100, 104–111. [Google Scholar] [CrossRef]
- Dao, T.L.; Hoang, V.T.; Ly, T.D.A.; Magmoun, A.; Canard, N.; Drali, T.; Fenollar, F.; Ninove, L.; Raoult, D.; Parola, P.; et al. Infectious disease symptoms and microbial carriage among French medical students travelling abroad: A prospective study. Travel Med. Infect. Dis. 2020, 34, 101548. [Google Scholar] [CrossRef]
- Mellon, G.; Turbett, S.E.; Worby, C.; Oliver, E.; Walker, A.T.; Walters, M.; Kelly, P.; Leung, D.; Knouse, M.; Hagmann, S.; et al. Acquisition of Antibiotic-Resistant Bacteria by U.S. International Travelers. N. Engl. J. Med. 2020, 382, 1372–1374. [Google Scholar] [CrossRef]
- Meurs, L.; Lempp, F.S.; Lippmann, N.; Trawinski, H.; Rodloff, A.C.; Eckardt, M.; Klingeberg, A.; Eckmanns, T.; Walter, J.; Lübbert, C.; et al. Intestinal colonization with extended-spectrum β-lactamase producing Enterobacterales (ESBL-PE) during long distance travel: A cohort study in a German travel clinic (2016–2017). Travel Med. Infect. Dis. 2020, 33, 101521. [Google Scholar] [CrossRef]
- Worby, C.J.; Earl, A.M.; Turbett, S.E.; Becker, M.; Rao, S.R.; Oliver, E.; Walker, A.T.; Walters, M.; Kelly, P.; Leung, D.T.; et al. Acquisition and Long-term Carriage of Multidrug-Resistant Organisms in US International Travelers. Open Forum Infect. Dis. 2020, 7, ofaa543. [Google Scholar] [CrossRef]
- Dao, T.L.; Hoang, V.T.; Magmoun, A.; Ly, T.D.A.; Baron, S.A.; Hadjadj, L.; Canard, N.; Drali, T.; Gouriet, F.; Raoult, D.; et al. Acquisition of multidrug-resistant bacteria and colistin resistance genes in French medical students on internships abroad. Travel Med. Infect. Dis. 2021, 39, 101940. [Google Scholar] [CrossRef]
- Kantele, A.; Lääveri, T. Extended-spectrum beta-lactamase-producing strains among diarrhoeagenic Escherichia coli—Prospective traveller study with literature review. J. Travel Med. 2021, 29, taab042. [Google Scholar] [CrossRef]
- Lääveri, T.; Antikainen, J.; Mero, S.; Pakkanen, S.H.; Kirveskari, J.; Roivainen, M.; Kantele, A. Bacterial, viral and parasitic pathogens analysed by qPCR: Findings from a prospective study of travellers’ diarrhoea. Travel Med. Infect. Dis. 2021, 40, 101957. [Google Scholar] [CrossRef]
- Sridhar, S.; Turbett, S.E.; Harris, J.B.; LaRocque, R.C. Antimicrobial-resistant bacteria in international travelers. Curr. Opin. Infect. Dis. 2021, 34, 423–431. [Google Scholar] [CrossRef]
- Tufic-Garutti, S.d.S.; Ramalho, J.V.A.R.; Longo, L.G.d.A.; de Oliveira, G.C.; Rocha, G.T.; Vilar, L.C.; da Costa, M.D.; Picão, R.C.; de Carvalho Girão, V.B.; Santoro-Lopes, G.; et al. Acquisition of antimicrobial resistance determinants in Enterobacterales by international travelers from a large urban setting in Brazil. Travel Med. Infect. Dis. 2021, 41, 102028. [Google Scholar] [CrossRef]
- Turunen, K.A.; Kantele, A.; Professor of Infectious Diseases. Revisiting travellers’ diarrhoea justifying antibiotic treatment: Prospective study. J. Travel Med. 2021, 28, taaa237. [Google Scholar] [CrossRef]
- Mulder, M.; Jong, J.K.-D.; Goessens, W.; de Visser, H.; Ikram, M.A.; Verbon, A.; Stricker, B. Diet as a risk factor for antimicrobial resistance in community-acquired urinary tract infections in a middle-aged and elderly population: A case–control study. Clin. Microbiol. Infect. 2019, 25, 613–619. [Google Scholar] [CrossRef]
- Mughini-Gras, L.; Dorado-García, A.; Van Duijkeren, E.; van Bunt, G.; van den Dierikx, C.M.; Bonten, M.J.M.; Bootsma, M.C.J.; Schmitt, H.; Hald, T.; Evers, E.G.; et al. Attributable sources of community-acquired carriage of Escherichia coli containing β-lactam antibiotic resistance genes: A population-based modelling study. Lancet Planet. Health 2019, 3, e357–e369. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, Y.; Kakizawa, H.; Baba, Y.; Ito, T.; Haremaki, Y.; Yonemichi, M.; Ikeda, T.; Kuroda, M.; Ohya, K.; Hara-Kudo, Y.; et al. Antimicrobial Resistance in Salmonella Isolated from Food Workers and Chicken Products in Japan. Antibiotics 2021, 10, 1541. [Google Scholar] [CrossRef] [PubMed]
- Van Gompel, L.; Dohmen, W.; Luiken, R.E.C.; Bouwknegt, M.; Heres, L.; Van Heijnsbergen, E.; Jongerius-Gortemaker, B.G.M.; Scherpenisse, P.; Greve, G.D.; Tersteeg-Zijderveld, M.H.G.; et al. Occupational Exposure and Carriage of Antimicrobial Resistance Genes (tetW, ermB) in Pig Slaughterhouse Workers. Ann. Work Expo. Health 2020, 64, 125–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Lyu, N.; Liu, F.; Liu, W.J.; Bi, Y.; Zhang, Z.; Ma, S.; Cao, J.; Song, X.; Wang, A.; et al. More diversified antibiotic resistance genes in chickens and workers of the live poultry markets. Environ Int. 2021, 153, 106534. [Google Scholar] [CrossRef] [PubMed]
- Talukder, S.; Hasan, M.; Mandal, A.K.; Tasmim, S.T.; Parvin, M.S.; Ali, Y.; Nahar, A.; Islam, Z.; Islam, T. Epidemiology and antimicrobial resistance profiles of Salmonella in chickens, sewage, and workers of broiler farms in selected areas of Bangladesh. J. Infect. Dev. Ctries 2021, 15, 1155–1166. [Google Scholar] [CrossRef]
- Momoh, A.H.; Kwaga, J.K.P.; Bello, M.; Sackey, A.K.B.; Larsen, A.R. Antibiotic resistance and molecular characteristics of Staphylococcus aureus isolated from backyard-raised pigs and pig workers. Trop. Anim. Health Prod. 2018, 50, 1565–1571. [Google Scholar] [CrossRef]
- Elhariri, M.; Elhelw, R.; Selim, S.; Ibrahim, M.; Hamza, D.; Hamza, E. Virulence and Antibiotic Resistance Patterns of Extended-Spectrum Beta-Lactamase-Producing Salmonella enterica serovar Heidelberg Isolated from Broiler Chickens and Poultry Workers: A Potential Hazard. Foodborne Pathog. Dis. 2020, 17, 373–381. [Google Scholar] [CrossRef]
- Zieliński, W.; Korzeniewska, E.; Harnisz, M.; Drzymała, J.; Felis, E.; Bajkacz, S. Wastewater treatment plants as a reservoir of integrase and antibiotic resistance genes—An epidemiological threat to workers and environment. Environ Int. 2021, 156, 106641. [Google Scholar] [CrossRef]
- Tamta, S.; Kumar, O.R.V.; Singh, S.V.; Pruthvishree, B.S.; Karthikeyan, R.; Rupner, R.; Sinha, D.K.; Singh, B.R. Antimicrobial resistance pattern of extended-spectrum β-lactamase-producing Escherichia coli isolated from fecal samples of piglets and pig farm workers of selected organized farms of India. Vet World 2020, 13, 360–363. [Google Scholar] [CrossRef] [Green Version]
- Ding, D.; Zhu, J.; Gao, Y.; Yang, F.; Ma, Y.; Cheng, X.; Li, J.; Dong, P.; Yang, H.; Chen, S. Effect of cattle farm exposure on oropharyngeal and gut microbial communities and antibiotic resistance genes in workers. Sci. Total Environ. 2022, 806, 150685. [Google Scholar] [CrossRef]
- Ymaña, B.; Luque, N.; Ruiz, J.; Pons, M.J. Worrying levels of antimicrobial resistance in Gram-negative bacteria isolated from cell phones and uniforms of Peruvian intensive care unit workers. Trans. R. Soc. Trop. Med. Hyg. 2022, trab186. [Google Scholar] [CrossRef]
- Chanchaithong, P.; Perreten, V.; Am-In, N.; Lugsomya, K.; Tummaruk, P.; Prapasarakul, N. Molecular Characterization and Antimicrobial Resistance of Livestock-Associated Methicillin-Resistant Staphylococcus aureus Isolates from Pigs and Swine Workers in Central Thailand. Microb. Drug Resist. 2019, 25, 1382–1389. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, W.; Guo, C.; Xiong, H.; Chen, X.; Jiao, X.; Su, J.; Mao, L.; Zhao, Z.; Li, Q. Prevalence, Serotypes, and Antimicrobial Resistance Profiles among Salmonella Isolated from Food Catering Workers in Nantong, China. Foodborne Pathog. Dis. 2019, 16, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Tahoun, A.B.M.B.; Abou Elez, R.M.M.; Abdelfatah, E.N.; Elsohaby, I.; El-Gedawy, A.A.; Elmoslemany, A.M. Listeria monocytogenes in raw milk, milking equipment and dairy workers: Molecular characterization and antimicrobial resistance patterns. J. Glob. Antimicrob. Resist. 2017, 10, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Huang, T.; Chen, C.; Cao, T.-T.; Cheng, K.; Liao, X.-P.; Liu, Y.-H. Comparison of Fecal Microbial Composition and Antibiotic Resistance Genes from Swine, Farm Workers and the Surrounding Villagers. Sci. Rep. 2017, 7, 4965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Malhotra, R.; Grover, P.; Bansal, R.; Galhotra, S.; Kaur, R.; Jindal, N. Antimicrobial resistance profile of Methicillin-resistant Staphylococcus aureus colonizing the anterior nares of health-care workers and outpatients attending the remotely located tertiary care hospital of North India. J. Lab. Physicians 2017, 9, 317–321. [Google Scholar] [CrossRef]
- Wang, H.-P.; Zhang, H.-J.; Liu, J.; Dong, Q.; Duan, S.; Ge, J.-Q.; Wang, Z.-H.; Zhang, Z. Antimicrobial resistance of 3 types of gram-negative bacteria isolated from hospital surfaces and the hands of health care workers. Am. J. Infect. Control 2017, 45, E143–E147. [Google Scholar] [CrossRef] [PubMed]
- Paltansing, S.; Vlot, J.A.; Kraakman, M.E.M.; Mesman, R.; Bruijning, M.L.; Bernards, A.T.; Visser, L.G.; Veldkamp, K.E. Extended-spectrum β-lactamase-producing enterobacteriaceae among travelers from the Netherlands. Emerg. Infect. Dis. 2013, 19, 1206–1213. [Google Scholar] [CrossRef]
- Moirongo, R.M.; Lorenz, E.; Ntinginya, N.E.; Dekker, D.; Fernandes, J.; Held, J.; Lamshöft, M.; Schaumburg, F.; Mangu, C.; Sudi, L.; et al. Regional Variation of Extended-Spectrum β-Lactamase (ESBL)-Producing Enterobacterales, Fluoroquinolone-Resistant Salmonella enterica and Methicillin-Resistant Staphylococcus aureus among Febrile Patients in Sub-Saharan Africa. Front. Microbiol. 2020, 11, 567235. Available online: https://www.frontiersin.org/article/10.3389/fmicb.2020.567235 (accessed on 16 February 2022). [CrossRef]
- Gashaw, M.; Berhane, M.; Bekele, S.; Kibru, G.; Teshager, L.; Yilma, Y.; Ahmed, Y.; Fentahun, N.; Assefa, H.; Wieser, A.; et al. Emergence of high drug resistant bacterial isolates from patients with health care associated infections at Jimma University medical center: A cross sectional study. Antimicrob. Resist. Infect Control. 2018, 7, 138. [Google Scholar] [CrossRef]
- Tham, J.; Odenholt, I.; Walder, M.; Andersson, L.; Melander, E. Risk factors for infections with extended-spectrum β-lactamase-producing Escherichia coli in a county of Southern Sweden. Infect. Drug Resist. 2013, 6, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Leonard, A.F.; Zhang, L.; Balfour, A.J.; Garside, R.; Hawkey, P.M.; Murray, A.K.; Ukoumunne, O.C.; Gaze, W.H. Exposure to and colonisation by antibiotic-resistant E. coli in UK coastal water users: Environmental surveillance, exposure assessment, and epidemiological study (Beach Bum Survey). Environ. Int. 2018, 114, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Schijven, J.F.; Blaak, H.; Schets, F.M.; de Roda Husman, A.M. Fate of Extended-Spectrum β-Lactamase-Producing Escherichia coli from Faecal Sources in Surface Water and Probability of Human Exposure through Swimming. Environ. Sci. Technol. 2015, 49, 11825–11833. [Google Scholar] [CrossRef] [PubMed]
- Dorado-García, A.; Smid, J.H.; van Pelt, W.; Bonten, M.J.M.; Fluit, A.C.; van den Bunt, G.; Wagenaar, J.A.; Hordijk, J.; Dierikx, C.M.; Veldman, K.T.; et al. Molecular relatedness of ESBL/AmpC-producing Escherichia coli from humans, animals, food and the environment: A pooled analysis. J. Antimicrob. Chemother. 2018, 73, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Wengenroth, L.; Berglund, F.; Blaak, H.; Chifiriuc, M.; Flach, C.-F.; Pircalabioru, G.; Larsson, D.; Marutescu, L.; van Passel, M.; Popa, M.; et al. Antibiotic Resistance in Wastewater Treatment Plants and Transmission Risks for Employees and Residents: The Concept of the AWARE Study. Antibiotics 2021, 10, 478. [Google Scholar] [CrossRef]
- ESRI. ArcGIS Desktop: Release 10; Environmental Systems Research Institute: Redlands, CA, USA, 2011. [Google Scholar]
- BMBF TD des. ISCED 2011—BMBF Datenportal. Datenportal des Bundesministeriums für Bildung und Forschung—BMBF. Available online: https://www.datenportal.bmbf.de/portal/de/glossary.html (accessed on 12 April 2022).
- Luijkx, R.; de Heus, M. The educational system of the Netherlands. In The International Standard Classification of Education (ISCED-97) An Evaluation of Content and Criterion Validity for 15 European Countries; Mannheimer Zentrum für Europäische Sozialforschung: Mannheim, Germany, 2008; pp. 47–75. [Google Scholar]
- Clasificarea Internațională Standard a Educației—ISCED. 2018. Available online: https://www.parintiicerschimbare.ro/clasificarea-internationala-standard-a-educatiei/ (accessed on 12 April 2022).
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- White, I.R.; Royston, P.; Wood, A.M. Multiple imputation using chained equations: Issues and guidance for practice. Stat. Med. 2011, 30, 377–399. [Google Scholar] [CrossRef]
- Haneuse, S.; Schildcrout, J.; Crane, P.; Sonnen, J.; Breitner, J.; Larson, E. Adjustment for selection bias in observational studies with application to the analysis of autopsy data. Neuroepidemiology 2009, 32, 229–239. [Google Scholar] [CrossRef]
- Cole, S.R.; Stuart, E.A. Generalizing Evidence From Randomized Clinical Trials to Target Populations: The ACTG 320 Trial. Am. J. Epidemiol. 2010, 172, 107–115. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 12 April 2022).
- Frost, I.; Van Boeckel, T.P.; Pires, J.; Craig, J.; Laxminarayan, R. Global geographic trends in antimicrobial resistance: The role of international travel. J. Travel Med. 2019, 26, taz036. [Google Scholar] [CrossRef]
- Bengtsson-Palme, J.; Angelin, M.; Huss, M.; Kjellqvist, S.; Kristiansson, E.; Palmgren, H.; Larsson, D.G.J.; Johansson, A. The Human Gut Microbiome as a Transporter of Antibiotic Resistance Genes between Continents. Antimicrob. Agents Chemother. 2015, 59, 6551–6560. [Google Scholar] [CrossRef] [Green Version]
- van den Bunt, G.; van Pelt, W.; Hidalgo, L.; Scharringa, J.; de Greeff, S.C.; Schürch, A.C.; Mughini-Gras, L.; Bonten, M.J.M.; Fluit, A.C. Prevalence, risk factors and genetic characterisation of extended-spectrum beta-lactamase and carbapenemase-producing Enterobacteriaceae (ESBL-E and CPE): A community-based cross-sectional study, the Netherlands, 2014 to 2016. Eurosurveillance 2019, 24, 1800594. [Google Scholar] [CrossRef] [Green Version]
- Critchley, I.A.; Cotroneo, N.; Pucci, M.J.; Mendes, R. The burden of antimicrobial resistance among urinary tract isolates of Escherichia coli in the United States in 2017. PLoS ONE 2019, 14, e0220265. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, R.S.; Munk, P.; Njage, P.; Van Bunnik, B.; McNally, L.; Lukjancenko, O.; Röder, T.; Nieuwenhuijse, D.; Pedersen, S.K.; Kjeldgaard, J.; et al. Global monitoring of antimicrobial resistance based on metagenomics analyses of urban sewage. Nat. Commun. 2019, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- Karkman, A.; Berglund, F.; Flach, C.-F.; Kristiansson, E.; Larsson, D.G.J. Predicting clinical resistance prevalence using sewage metagenomic data. Commun. Biol. 2020, 3, 711. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Musoke, D.; Namata, C.; Lubega, G.B.; Niyongabo, F.; Gonza, J.; Chidziwisano, K.; Nalinya, S.; Nuwematsiko, R.; Morse, T. The role of Environmental Health in preventing antimicrobial resistance in low- and middle-income countries. Environ. Health Prev. Med. 2021, 26, 100. [Google Scholar] [CrossRef]
- Ibrahim, R.A.; Teshal, A.M.; Dinku, S.F.; Abera, N.A.; Negeri, A.A.; Desta, F.G.; Seyum, E.T.; Gemeda, A.W.; Keficho, W.M. Antimicrobial resistance surveillance in Ethiopia: Implementation experiences and lessons learned. Afr. J. Lab. Med. 2018, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Opintan, J.A. Leveraging donor support to develop a national antimicrobial resistance policy and action plan: Ghana’s success story. Afr. J. Lab. Med. 2018, 7, 1–4. [Google Scholar] [CrossRef]
- Varma, J.K.; Oppong-Otoo, J.; Ondoa, P.; Perovic, O.; Park, B.J.; Laxminarayan, R.; Peeling, R.W.; Schultsz, C.; Li, H.; Ihekweazu, C.; et al. Africa Centres for Disease Control and Prevention’s framework for antimicrobial resistance control in Africa. Afr. J. Lab. Med. 2018, 7, 4. [Google Scholar] [CrossRef]
- Elton, L.; Thomason, M.J.; Tembo, J.; Velavan, T.P.; Pallerla, S.R.; Arruda, L.B.; Vairo, F.; Montaldo, C.; Ntoumi, F.; Hamid, M.M.A.; et al. Antimicrobial resistance preparedness in sub-Saharan African countries. Antimicrob. Resist. Infect. Control 2020, 9, 145. [Google Scholar] [CrossRef]
- Gandra, S.; Alvarez-Uria, G.; Turner, P.; Joshi, J.; Limmathurotsakul, D.; van Doorn, H.R. Antimicrobial Resistance Surveillance in Low- and Middle-Income Countries: Progress and Challenges in Eight South Asian and Southeast Asian Countries. Clin. Microbiol. Rev. 2022, 33, 33. Available online: https://journals.asm.org/doi/abs/10.1128/CMR.00048-19 (accessed on 16 February 2022). [CrossRef]
- Yam, E.L.Y.; Hsu, L.Y.; Yap, E.P.-H.; Yeo, T.W.; Lee, V.; Schlundt, J.; Lwin, M.O.; Limmathurotsakul, D.; Jit, M.; Dedon, P.; et al. Antimicrobial Resistance in the Asia Pacific region: A meeting report. Antimicrob. Resist. Infect. Control 2019, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, M.; Chatterjee, P.; Chauhan, A.S.; Grace, D.; Lindahl, J.; Beeche, A.; Jing, F.; Chotinan, S. Antimicrobial resistance in South East Asia: Time to ask the right questions. Glob. Health Action 2018, 11, 1483637. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report: 2021; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications-detail-redirect/9789240027336 (accessed on 2 March 2022).
| Overall, n = 1074 | Germany, n = 238 | The Netherlands, n = 686 | Romania, n = 150 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Missing Values for Stool Samples, n | 109 | 95 | 3 | 11 | ||||||
| Variable | Missing | Level | ESBL_EC+, n (%) | p | ESBL_EC+, n (%) | p | ESBL_EC+, n (%) | p | ESBL_EC+, n (%) | p |
| ESBL-EC positives | 81 (8) | 20 (8) | 42 (6) | 19 (13) | ||||||
| Sex | 4 | Female | 47 (7) | 0.814 | 12 (9) | 1.000 | 25 (6) | 0.871 | 19 (13) | 1.000 |
| Male | 34 (8) | 8 (8) | 17 (6) | 9 (13) | ||||||
| Highest educational level obtained a | 2 | Low | 19 (5) | 0.050 | 6 (10) | 0.602 | 13 (5) | 0.196 | 0 (0) | 0.217 |
| High | 62 (9) | 14 (8) | 29 (7) | 19 (14) | ||||||
| Work with animals in the past year | 35 | No | 75 (7) | 0.752 | 18 (8) | 1.000 | 40 (6) | 0.659 | 17 (12) | 0.555 |
| Yes | 3 (8) | 0 (0) | 2 (8) | 1 (17) | ||||||
| Work at a farm in the past year | 25 | No | 77 (7) | 0.197 | 18 (8) | 1.000 | 40 (6) | 0.104 | 19 (13) | NA |
| Yes | 2 (18) | 0 (0) | 2 (22) | --- (---) | ||||||
| Work at a slaughterhouse in the past year | 20 | No | 79 (7) | 1.000 | 18 (8) | NA | 42 (6) | 1.000 | 19 (13) | 1.000 |
| Yes | 0 (0) | --- (---) | 0 (0) | 0 (0) | ||||||
| Work with manure in the past year | 22 | No | 76 (7) | 1.000 | 18 (8) | 1.000 | 41 (6) | 1.000 | 17 (12) | 0.482 |
| Yes | 2 (6) | 0 (0) | 1 (5) | 1 (20) | ||||||
| Patient contact or work with human tissues in the past year b | 20 | No | 52 (7) | 1.000 | 12 (8) | 1.000 | 29 (7) | 0.738 | 11 (10) | 0.133 |
| Yes | 26 (7) | 6 (7) | 13 (6) | 7 (21) | ||||||
| Patient contact in the past year | 20 | Never | 58 (7) | 0.481 | 13 (8) | 0.552 | 31 (6) | 0.872 | 14 (12) | 0.672 |
| Rarely or sometimes | 11 (9) | 3 (12) | 5 (7) | 3 (18) | ||||||
| Often or always | 9 (6) | 2 (5) | 6 (5) | 1 (12) | ||||||
| Work with human tissues in the past year | 16 | Never | 57 (7) | 0.704 | 13 (8) | 1.000 | 32 (6) | 0.928 | 12 (10) | 0.097 |
| Rarely or sometimes | 13 (9) | 3 (8) | 6 (7) | 4 (25) | ||||||
| Often or always | 8 (7) | 2 (7) | 4 (5) | 2 (20) | ||||||
| Hospital visits as a patient in the past year | 0 | No | 76 (8) | 0.672 | 16 (8) | 0.517 | 41 (6) | 0.507 | 19 (13) | 0.597 |
| Yes | 5 (6) | 4 (11) | 1 (2) | 0 (0) | ||||||
| Hospital visits as a professional in the past year | 0 | No | 78 (8) | 0.761 | 19 (9) | 1.000 | 41 (6) | 1.000 | 18 (12) | 0.336 |
| Yes | 3 (8) | 1 (5) | 1 (7) | 1 (33) | ||||||
| Hospital visits as a visitor in the past year | 0 | No | 79 (8) | 0.690 | 18 (8) | 0.169 | 42 (6) | 1.000 | 19 (13) | 1.000 |
| Yes | 2 (9) | 2 (22) | 0 (0) | 0 (0) | ||||||
| Farm visits in the past year | 4 | No | 71 (7) | 0.578 | 17 (9) | 0.773 | 35 (6) | 0.097 | 19 (13) | 0.596 |
| Yes | 10 (9) | 3 (7) | 7 (11) | 0 (0) | ||||||
| Owning horses in the past year | 139 | No | 77 (8) | 0.722 | 20 (10) | 0.605 | 40 (7) | 1.000 | 17 (15) | 1.000 |
| Yes | 1 (4) | 0 (0) | 1 (7) | 0 (0) | ||||||
| Having dogs as pets in the past year | 70 | No | 70 (9) | 0.011 | 20 (11) | 0.084 | 34 (7) | 0.267 | 16 (16) | 0.156 |
| Yes | 9 (4) | 0 (0) | 7 (4) | 2 (6) | ||||||
| Having cats as pets in the past year | 75 | No | 65 (9) | 0.130 | 17 (10) | 0.418 | 34 (7) | 0.348 | 14 (15) | 0.558 |
| Yes | 13 (5) | 3 (5) | 7 (5) | 3 (9) | ||||||
| Use of antibiotics in the past year | 0 | No | 60 (7) | 0.685 | 13 (8) | 1.000 | 36 (6) | 0.544 | 11 (11) | 0.289 |
| Yes | 21 (8) | 7 (8) | 6 (5) | 8 (17) | ||||||
| Use of antacids in the past year | 2 | No | 64 (8) | 0.783 | 12 (7) | 0.297 | 36 (7) | 0.253 | 16 (13) | 1.000 |
| Yes | 17 (7) | 8 (12) | 6 (4) | 3 (11) | ||||||
| Surgeries in the past year | 1 | No | 80 (8) | 0.255 | 19 (9) | 1.000 | 42 (6) | 0.403 | 19 (13) | 1.000 |
| Yes | 1 (2) | 1 (6) | 0 (0) | 0 (0) | ||||||
| Self-reported frequency of diarrhea in the past year | 4 | Never, rarely or sometimes | 74 (7) | 0.223 | 17 (8) | 0.069 | 38 (6) | 0.347 | 19 (13) | 1.000 |
| Often or always | 7 (11) | 3 (25) | 4 (9) | 0 (0) | ||||||
| Self-reported health status in the past year | 5 | Good, very good or excellent | 69 (7) | 0.734 | 18 (8) | 0.365 | 34 (6) | 0.523 | 17 (13) | 1.000 |
| Fair or poor | 12 (8) | 2 (13) | 8 (7) | 2 (11) | ||||||
| Travel to high-risk areas for AR in the past year c | 8 | No | 36 (6) | 0.012 | 6 (6) | 0.336 | 17 (4) | 0.004 | 13 (13) | 0.791 |
| Yes | 42 (10) | 13 (10) | 24 (10) | 5 (10) | ||||||
| Travels to Europe in the past year | 5 | Never | 27 (9) | 0.498 | 5 (12) | 0.378 | 11 (6) | 0.718 | 11 (13) | 0.498 |
| Once | 18 (8) | 1 (2) | 12 (7) | 5 (18) | ||||||
| 2–3 times | 22 (6) | 8 (8) | 12 (5) | 2 (8) | ||||||
| More than 3 times | 12 (7) | 5 (10) | 7 (7) | 0 (0) | ||||||
| Travels to Bulgaria, Greece, Italy, or Slovenia in the past year | 7 | No | 59 (8) | 0.514 | 10 (8) | 1.000 | 34 (6) | 0.561 | 15 (15) | 0.182 |
| Yes | 19 (6) | 9 (8) | 7 (5) | 3 (6) | ||||||
| Travels to Sub-Saharan Africa in the past year | 5 | Never | 73 (7) | 0.010 | 19 (8) | 1.000 | 36 (5) | 0.002 | 18 (12) | NA |
| Once | 4 (19) | 0 (0) | 4 (22) | --- (---) | ||||||
| 2–3 times | 1 (33) | --- (---) | 1 (33) | --- (---) | ||||||
| More than 3 times | 1 (50) | --- (---) | 1 (50) | --- (---) | ||||||
| Travels to Northern Africa in the past year | 6 | Never | 69 (7) | 0.001 | 17 (7) | 0.013 | 35 (5) | 0.019 | 17 (12) | 0.324 |
| Once | 8 (20) | 2 (25) | 5 (17) | 1 (33) | ||||||
| 2–3 times | 2 (50) | 1 (100) | 1 (33) | --- (---) | ||||||
| More than 3 times | 0 (0) | --- (---) | 0 (0) | --- (---) | ||||||
| Travels to Asia in the past year | 4 | Never | 63 (6) | <0.001 | 15 (7) | 0.116 | 31 (5) | <0.001 | 17 (12) | 0.408 |
| Once | 13 (20) | 3 (16) | 9 (22) | 1 (25) | ||||||
| 2–3 times | 2 (18) | 1 (25) | 1 (14) | --- (---) | ||||||
| More than 3 times | 1 (50) | --- (---) | 1 (50) | --- (---) | ||||||
| Travels to North America in the past year | 4 | Never | 73 (7) | 0.036 | 17 (8) | 0.041 | 38 (6) | 0.146 | 18 (12) | NA |
| Once | 5 (17) | 2 (20) | 3 (16) | --- (---) | ||||||
| 2–3 times | 2 (25) | 1 (50) | 1 (17) | --- (---) | ||||||
| More than 3 times | 0 (0) | --- (---) | 0 (0) | --- (---) | ||||||
| Travels to Central America or Mexico in the past year | 6 | Never | 78 (7) | 0.190 | 19 (8) | 1.000 | 41 (6) | 0.171 | 18 (12) | NA |
| Once | 0 (0) | 0 (0) | 0 (0) | --- (---) | ||||||
| 2–3 times | 0 (0) | 0 (0) | --- (---) | --- (---) | ||||||
| More than 3 times | 1 (50) | --- (---) | 1 (50) | --- (---) | ||||||
| Travels to South America in the past year | 6 | Never | 77 (7) | 0.149 | 18 (8) | 0.287 | 41 (6) | 0.126 | 18 (12) | NA |
| Once | 1 (6) | 1 (25) | 0 (0) | --- (---) | ||||||
| 2–3 times | 0 (0) | --- (---) | 0 (0) | --- (---) | ||||||
| More than 3 times | 1 (100) | --- (---) | 1 (100) | --- (---) | ||||||
| Travels to Australia or Oceania in the past year | 6 | Never | 78 (7) | 0.572 | 19 (8) | 0.465 | 41 (6) | 1.000 | 18 (12) | NA |
| Once | 1 (10) | 1 (17) | 0 (0) | --- (---) | ||||||
| 2–3 times | 0 (0) | 0 (0) | --- (---) | --- (---) | ||||||
| More than 3 times | --- (---) | --- (---) | --- (---) | --- (---) | ||||||
| Overall, n = 1074 | Germany, n = 238 | The Netherlands, n = 686 | Romania, n = 151 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Missing Values for Stool Samples, n | 109 | 95 | 3 | 11 | |||||||||
| Variable | Missings | ESBL_EC+ | ESBL_EC− | p | ESBL_EC+ | ESBL_EC− | p | ESBL_EC+ | ESBL_EC− | p | ESBL_EC+ | ESBL_EC− | p |
| n | 81 | 993 | 20 | 218 | 42 | 644 | 19 | 131 | |||||
| Age, years (median [IQR]) | 0 | 47 [34, 57] | 51 [37, 60] | 0.172 | 38 [31, 50] | 49 [36, 58] | 0.146 | 55 [42, 61] | 54 [39, 61] | 0.964 | 39 [34, 44] | 40 [33, 50] | 0.739 |
| Travel score (mean ± SD, median [min, max]) a | 12 | 0.86 ± 1.60, 1 [0, 13] | 0.46 ± 0.79, 0 [0, 15] | 0.020 | 1 ± 0.94, 1 [0, 3] | 0.64 ± 0.74, 1 [0, 6] | 0.081 | 1.05 ± 2.07, 1 [0, 13] | 0.42 ± 0.84, 0 [0, 15] | 0.001 | 0.28 ± 0.46, 0 [0, 1] | 0.38 ± 0.56, 0 [0, 3] | 0.533 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Molina, D.; Berglund, F.; Blaak, H.; Flach, C.-F.; Kemper, M.; Marutescu, L.; Pircalabioru Gradisteanu, G.; Popa, M.; Spießberger, B.; Wengenroth, L.; et al. International Travel as a Risk Factor for Carriage of Extended-Spectrum β-Lactamase-Producing Escherichia coli in a Large Sample of European Individuals—The AWARE Study. Int. J. Environ. Res. Public Health 2022, 19, 4758. https://doi.org/10.3390/ijerph19084758
Rodríguez-Molina D, Berglund F, Blaak H, Flach C-F, Kemper M, Marutescu L, Pircalabioru Gradisteanu G, Popa M, Spießberger B, Wengenroth L, et al. International Travel as a Risk Factor for Carriage of Extended-Spectrum β-Lactamase-Producing Escherichia coli in a Large Sample of European Individuals—The AWARE Study. International Journal of Environmental Research and Public Health. 2022; 19(8):4758. https://doi.org/10.3390/ijerph19084758
Chicago/Turabian StyleRodríguez-Molina, Daloha, Fanny Berglund, Hetty Blaak, Carl-Fredrik Flach, Merel Kemper, Luminita Marutescu, Gratiela Pircalabioru Gradisteanu, Marcela Popa, Beate Spießberger, Laura Wengenroth, and et al. 2022. "International Travel as a Risk Factor for Carriage of Extended-Spectrum β-Lactamase-Producing Escherichia coli in a Large Sample of European Individuals—The AWARE Study" International Journal of Environmental Research and Public Health 19, no. 8: 4758. https://doi.org/10.3390/ijerph19084758
APA StyleRodríguez-Molina, D., Berglund, F., Blaak, H., Flach, C.-F., Kemper, M., Marutescu, L., Pircalabioru Gradisteanu, G., Popa, M., Spießberger, B., Wengenroth, L., Chifiriuc, M. C., Larsson, D. G. J., Nowak, D., Radon, K., de Roda Husman, A. M., Wieser, A., & Schmitt, H. (2022). International Travel as a Risk Factor for Carriage of Extended-Spectrum β-Lactamase-Producing Escherichia coli in a Large Sample of European Individuals—The AWARE Study. International Journal of Environmental Research and Public Health, 19(8), 4758. https://doi.org/10.3390/ijerph19084758









