Human Exposure to Chlorinated Organophosphate Ester Flame Retardants and Plasticizers in an Industrial Area of Shenzhen, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Standards and Reagents
2.3. Sample Pretreatment and Instrumental Analysis
2.4. Quality Assurance and Quality Control
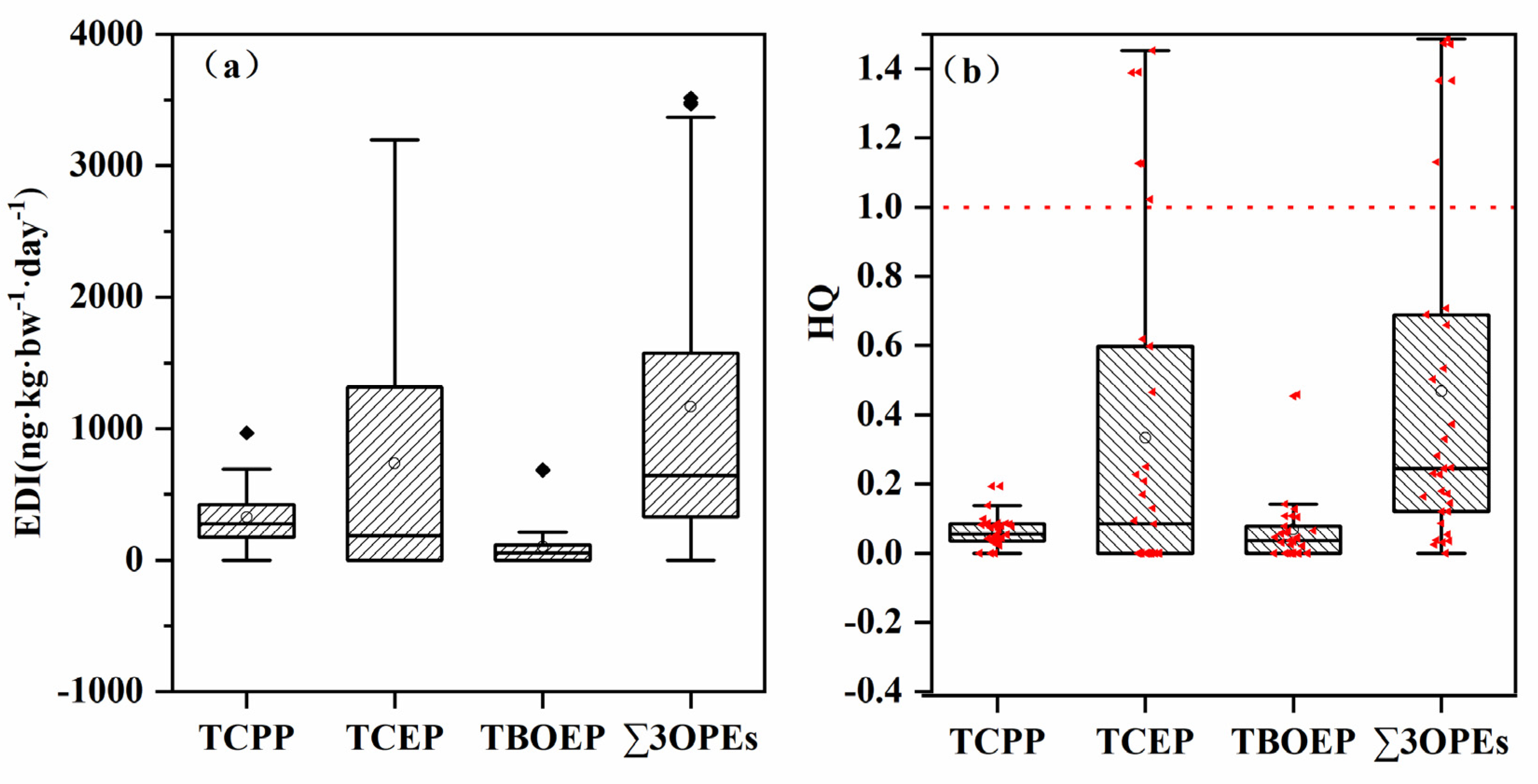
2.5. Heath Risk Assessment
2.6. Data Analyses
3. Results and Discussion
3.1. Concentrations and Profiles of OPEs in Urine and Plasma Sample
3.2. MOPEs in Plasma and Recommendations for Biomarkers
3.3. Global and Regional Comparison of OPEs and Health Risk Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
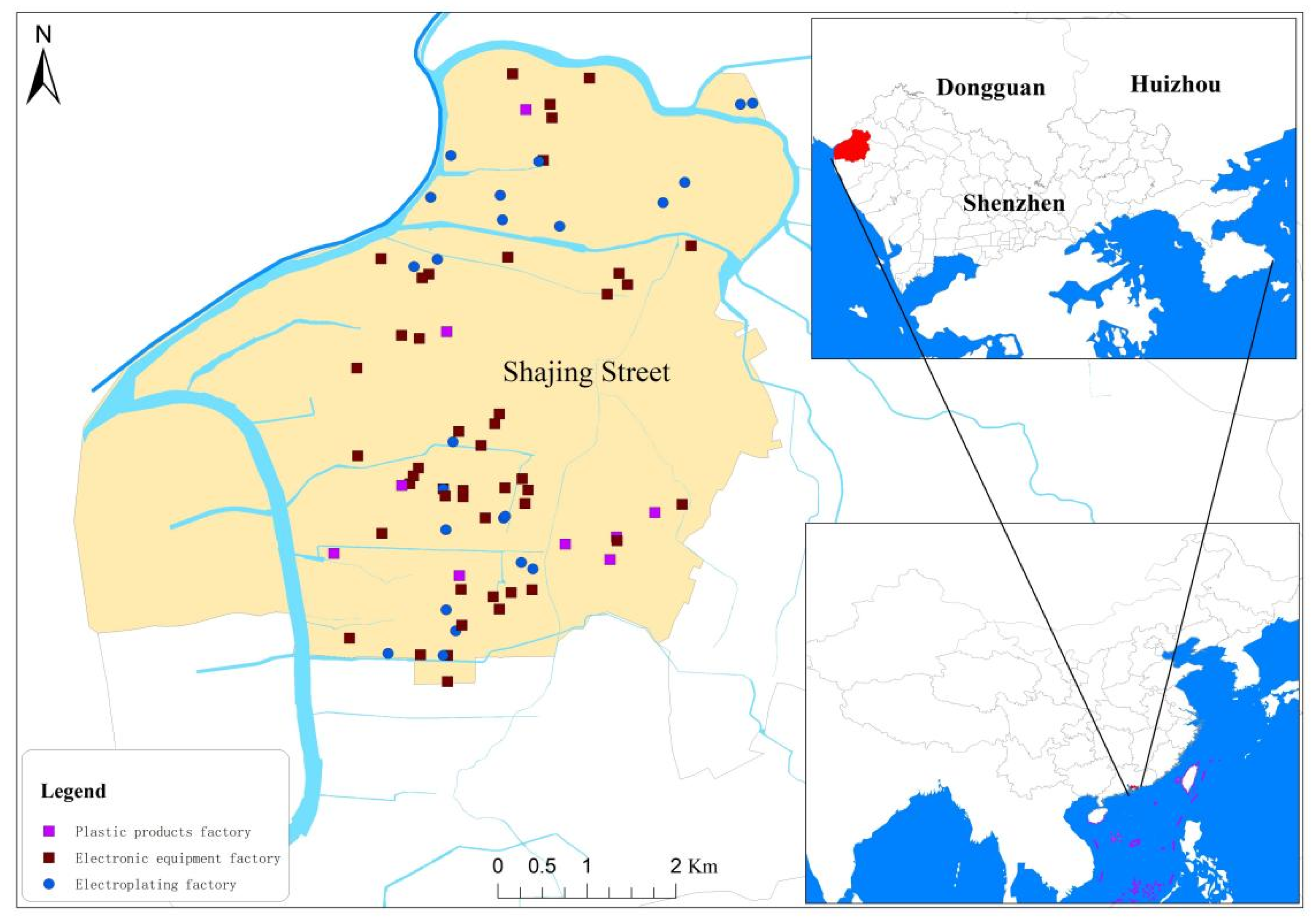
| Category | Number (Percentage) | |
|---|---|---|
| Age | <18 | 6 (11.76%) |
| 18–44 | 37 (72.55%) | |
| 45–59 | 7 (13.73%) | |
| >59 | 1 (1.96%) | |
| Gender | Male | 12 (23.53%) |
| Female | 39 (76.47%) | |
| No. | Compound | Abbreviation | Molecular Formula | CAS No. | Manufacturers |
|---|---|---|---|---|---|
| 1 | Tris(2-butoxyethyl) phosphate | TBOEP | C18H39O7P | 78-51-3 | Dr. Ehrenstorfer GmbH (Augsburg, Germany) |
| 2 | Trimethyl phosphate | TMP | C3H9O4P | 512-56-1 | AccuStandard (New Haven, CT, USA) |
| 3 | Tri-n-butyl phosphate | TnBP | C12H27O4P | 126-73-8 | Dr. Ehrenstorfer GmbH (Augsburg, Germany) |
| 4 | 2-Ethylhexyl diphenyl phosphate | EHDPP | C20H27O4P | 1241-94-7 | |
| 5 | Tri-iso-butyl phosphate | TIBP | C12H27O4P | 126-71-6 | ANPEL Laboratory Technologies Inc. (Shanghai, China) |
| 6 | Triethyl phosphate | TEP | C6H15O4P | 78-40-0 | AccuStandard (New Haven, CT, USA) |
| 7 | Tetraphenyl m-phenylene bis(phosphate) | RDP | C30H20O8P2 | 57583-54-7 | |
| 8 | Tripentyl phosphate | TNP | C15H33O4P | 2528-38-3 | |
| 9 | Tripropyl phosphate | TPrP | C9H21O4P | 513-08-6 | Dr. Ehrenstorfer GmbH (Augsburg, Germany) |
| 10 | Triphenyl phosphate | TPHP | C18H15O4P | 115-86-6 | |
| 11 | Triphenylphosphine Oxide | TPPO | C18H15OP | 791-28-6 | Toronto Research Chemicals Inc. (Toronto, ON, Canada) |
| 12 | Cresyl Diphenyl Phosphate | CDP | C19H17O4P | 26444-49-5 | Dr. Ehrenstorfer GmbH (Augsburg, Germany) |
| 13 | Tris(2-ethylhexyl) phosphate | TEHP | C24H51O4P | 78-42-2 | |
| 14 | Tris(2-chloropropyl) phosphate | TCPP | C9H18CL3PO4 | 13674-84-5 | AccuStandard (New Heavn, CT, USA) |
| 15 | Tris(1,3-dichloro-2-propyl) phosphate | TDCIPP | C9H15Cl6O4P | 13674-87-8 | |
| 16 | Tris(2-chloroethyl) phosphate | TCEP | C6H12Cl3O4P | 115-96-8 | Dr. Ehrenstorfer GmbH (Augsburg, Germany) |
| 17 | Triisopropyl phosphate | TiPrP | C9H21O4P | 513-02-0 | AccuStandard (New Heavn, CT, USA) |
| 18 | Diethyl phosphate | DEP | C4H11O4P | 598-02-7 | |
| 19 | Di-n-butyl phosphate | DnBP | C8H19O4P | 107-66-4 | Dr. Ehrenstorfer GmbH (Augsburg, Germany) |
| 20 | Bis(2-butoxyethyl)2-hydroxyethyl phosphate triester | BBOEHP | C14H31O7P | 1477494-86-2 | Toronto Research Chemicals Inc. (Toronto, ON, Canada) |
| 21 | Bis(butoxyethyl)phosphate | BBOEP | C12H27O6P | 14260-97-0 | |
| 22 | Bis(2-butoxyethyl)2-(3-hydroxybutoxy)ethyl phosphate ttriester | 3-OH-TBOEP | C18H39O8P | 1477494-87-3 |
| Time (min) | Mobile Phase A (%) | Mobile Phase B (%) |
|---|---|---|
| 0 | 30 | 70 |
| 2 | 60 | 40 |
| 22 | 85 | 15 |
| 24 | 98 | 2 |
| 25 | 98 | 2 |
| 30 | 30 | 70 |
| Analyte | Precursor Ion (m/z) | Fragment (V) | Product Ion (m/z) | CE (V) |
|---|---|---|---|---|
| TBOEP | 399 | 124 | 45/57 | 25/37 |
| TMP | 141 | 96 | 109/47 | 17/29 |
| TnBP | 267 | 81 | 99/211 | 13/5 |
| EHDPP | 251 | 117 | 51/77 | 69/33 |
| TIBP | 267 | 81 | 99/81 | 13/61 |
| TEP | 183 | 61 | 99/81 | 17/49 |
| RDP | 575 | 210 | 77/152 | 85/70 |
| TNP | 309 | 101 | 99/81 | 17/77 |
| TPrP | 225 | 76 | 99/141 | 13/5 |
| TPHP | 327 | 157 | 77/152 | 49/45 |
| TPPO | 279 | 170 | 201/77 | 25/53 |
| CDP | 341 | 175 | 91/65 | 37/73 |
| TEHP | 434 | 130 | 99/113 | 40/10 |
| TCPP | 327 | 100 | 99/175 | 18/10 |
| TDCIPP | 431 | 110 | 99/209 | 15/12 |
| TCEP | 285 | 85 | 99/63 | 20/26 |
| TiPrP | 225 | 64 | 99/81 | 13/57 |
| DEP | 155 | 75 | 99/127 | 13/5 |
| DnBP | 211 | 75 | 99/63 | 9/89 |
| BBOEHP | 343 | 95 | 45/243 | 17/9 |
| BBOEP | 299 | 95 | 45/199 | 17/9 |
| 3-OH-TBOEP | 415 | 105 | 45/55 | 25/37 |
| TBOEP-d27 | 426 | 105 | 46/66 | 25/37 |
| TMP-C13 | 144 | 90 | 111/80 | 20/40 |
| TnBP-d27 | 294 | 95 | 102/83 | 17/77 |
| Analyte | Recoveries | Urine | Plasma | |||
|---|---|---|---|---|---|---|
| Urine | Plasma | MDLs | MQLs | MDLs | MQLs | |
| TBOEP | 85.4 ± 20.3 | 86.9 ± 14.4 | 0.05 | 0.2 | 0.2 | 0.8 |
| TMP | 82.3 ± 17.4 | 84.3 ± 12.1 | 0.05 | 0.2 | 0.2 | 0.8 |
| TnBP | 76.5 ± 10.4 | 86.1 ± 15.3 | 0.05 | 0.2 | 0.2 | 0.8 |
| EHDPP | 91.0 ± 22.6 | 79.6 ± 13.4 | 0.1 | 0.4 | 0.4 | 1.5 |
| TIBP | 84.8 ± 17.4 | 84.5 ± 16.7 | 0.05 | 0.2 | 0.2 | 0.8 |
| TEP | 93.1 ± 20.2 | 83.1 ± 18.3 | 0.1 | 0.4 | 0.4 | 1.5 |
| RDP | 85.6 ± 18.8 | 89.2 ± 22.6 | 0.2 | 0.8 | 0.8 | 3 |
| TNP | 79.5 ± 12.9 | 76.8 ± 12.6 | 0.1 | 0.4 | 0.4 | 1.5 |
| TPrP | 86.7 ± 21.3 | 87.9 ± 23.7 | 0.02 | 0.08 | 0.1 | 0.4 |
| TPHP | 89.6 ± 20.8 | 89.7 ± 19.5 | 0.02 | 0.08 | 0.1 | 0.4 |
| TPPO | 68.3 ± 10.1 | 86.1 ± 15.3 | 0.05 | 0.2 | 0.2 | 0.8 |
| CDP | 78.8 ± 15.5 | 90.3 ± 23.2 | 0.2 | 0.8 | 0.8 | 3 |
| TEHP | 73.8 ± 16.2 | 86.8 ± 16.2 | 0.1 | 0.4 | 0.4 | 1.5 |
| TCPP | 76.2 ± 8.6 | 91.8 ± 21.3 | 0.2 | 0.8 | 0.8 | 3 |
| TDCIPP | 81.1 ± 15.2 | 103.2 ± 16.8 | 0.3 | 1 | 1 | 4 |
| TCEP | 103.1 ± 23.2 | 107.3 ± 24.0 | 0.3 | 1 | 1 | 4 |
| TiPrP | 79.2 ± 16.6 | 84.9 ± 14.3 | 0.02 | 0.08 | 0.08 | 0.4 |
| DEP | 89.3 ± 13.3 | 89.1 ± 8.3 | 0.08 | 0.3 | 0.3 | 1 |
| DnBP | 76.3 ± 5.6 | 79.3 ± 5.4 | 0.05 | 0.2 | 0.2 | 0.8 |
| BBOEHP | 87.2 ± 7.9 | 76.8.4 ± 3.6 | 0.05 | 0.2 | 0.2 | 0.8 |
| BBOEP | 78.4 ± 14.3 | 82.6 ± 11.3 | 0.05 | 0.2 | 0.2 | 0.8 |
| 3-OH-TBOEP | 84.5 ± 15.2 | 82.9 ± 12.6 | 0.04 | 0.15 | 0.15 | 0.6 |
References
- Chokwe, T.B.; Abafe, O.A.; Mbelu, S.P.; Okonkwo, J.O.; Sibali, L.L. A review of sources, fate, levels, toxicity, exposure and transformations of organophosphorus flame-retardants and plasticizers in the environment. Emerg. Contam. 2020, 6, 345–366. [Google Scholar] [CrossRef]
- He, J.; Wang, Z.; Zhao, L.; Ma, H.; Huang, J.; Li, H.; Mao, X.; Huang, T.; Gao, H.; Ma, J. Gridded emission inventory of organophosphorus flame retardants in China and inventory validation. Environ. Pollut. 2021, 290, 118071. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Li, H.; Xu, S.; Zhou, Q.; Jin, M.; Tang, J. A review of organophosphorus flame retardants (OPFRs): Occurrence, bioaccumulation, toxicity, and organism exposure. Environ. Sci. Pollut. Res. 2019, 26, 22126–22136. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-X.; Sun, Y.-X.; Li, X.; Xu, W.-H.; Zhang, Y.; Luo, X.-J.; Dai, S.-H.; Xu, X.-R.; Mai, B.-X. Organophosphorus flame retardants in mangrove sediments from the Pearl River Estuary, South China. Chemosphere 2017, 181, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.Y.; Mao, L.S.; Chen, Y.H.; Liu, G.H.; Luo, X.U.; Jang, J.; Guan, Y.F. Distribution characteristics and health risk assessment of five organophosphorus flame retardants in indoor dust in Shenzhen. J. Environ. Health 2019, 36, 1074–1077. (In Chinese) [Google Scholar] [CrossRef]
- Chen, Y.; Fang, J.; Ren, L.; Fan, R.; Zhang, J.; Liu, G.; Zhou, L.; Chen, D.; Yu, Y.; Lu, S. Urinary metabolites of organophosphate esters in children in South China: Concentrations, profiles and estimated daily intake. Environ. Pollut. 2018, 235, 358–364. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, L.; Lu, S.; Kang, L.; Luo, X.; Liu, G.; Cui, X.; Yu, Y. Organophosphate ester and phthalate ester metabolites in urine from primiparas in Shenzhen, China: Implications for health risks. Environ. Pollut. 2019, 247, 944–952. [Google Scholar] [CrossRef]
- Zhao, F.; Wan, Y.; Zhao, H.; Hu, W.; Mu, D.; Webster, T.F.; Hu, J. Levels of Blood Organophosphorus Flame Retardants and Association with Changes in Human Sphingolipid Homeostasis. Environ. Sci. Technol. 2016, 50, 8896–8903. [Google Scholar] [CrossRef]
- Luo, K.; Zhang, R.; Aimuzi, R.; Wang, Y.; Nian, M.; Zhang, J. Exposure to Organophosphate esters and metabolic syndrome in adults. Environ. Int. 2020, 143, 105941. [Google Scholar] [CrossRef]
- Ingle, M.E.; Watkins, D.; Rosario, Z.; Vélezvega, C.M.; Calafat, A.M.; Ospina, M.; Ferguson, K.; Cordero, J.F.; Alshawabkeh, A.; Meeker, J.D. An exploratory analysis of urinary organophosphate ester metabolites and oxidative stress among pregnant women in Puerto Rico. Sci. Total Environ. 2020, 703, 134798. [Google Scholar] [CrossRef]
- Yao, Y.; Li, M.; Pan, L.; Duan, Y.; Duan, X.; Li, Y.; Sun, H. Exposure to organophosphate ester flame retardants and plasticizers during pregnancy: Thyroid endocrine disruption and mediation role of oxidative stress. Environ. Int. 2021, 146, 106215. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Luo, D.; Xia, W.; Tao, Y.; Wang, L.; Yu, M.; Hu, L.; Zhou, A.; Covaci, A.; Lin, C.; et al. Prenatal exposure to halogenated, aryl, and alkyl organophosphate esters and child neurodevelopment at two years of age. J. Hazard. Mater. 2021, 408, 124856. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Dong, S.; Han, L.; Guo, R.; Fu, Y.; Zhang, S.; Chen, J. The risk and impact of organophosphate esters on the development of female-specific cancers: Comparative analysis of patients with benign and malignant tumors. J. Hazard. Mater. 2021, 404, 124020. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Shi, Y.; Jin, Q.; Cai, Y. Organophosphate esters and their metabolites in paired human whole blood, serum, and urine as biomarkers of exposure. Environ. Int. 2020, 139, 105698. [Google Scholar] [CrossRef]
- Zhang, T.; Bai, X.-Y.; Lu, S.-Y.; Zhang, B.; Xie, L.; Zheng, H.-C.; Jiang, Y.-C.; Zhou, M.-Z.; Zhou, Z.-Q.; Song, S.-M.; et al. Urinary metabolites of organophosphate flame retardants in China: Health risk from tris(2-chloroethyl) phosphate (TCEP) exposure. Environ. Int. 2018, 121, 1363–1371. [Google Scholar] [CrossRef]
- Van der Veen, I.; de Boer, J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 2012, 88, 1119–1153. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Martinez-Moral, M.-P.; Sun, H.; Kannan, K. Metabolites of organophosphate esters in urine from the United States: Concentrations, temporal variability, and exposure assessment. Environ. Int. 2019, 122, 213–221. [Google Scholar] [CrossRef]
- Chen, M.-H.; Ma, W.-L. A review on the occurrence of organophosphate flame retardants in the aquatic environment in China and implications for risk assessment. Sci. Total Environ. 2021, 783, 147064. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Gao, X.; Liu, L.; Shen, M.; Chen, H.; Zhu, M.; Zeng, L.; Zeng, E.Y. Occurrence of multiple classes of emerging photoinitiators in indoor dust from E-waste recycling facilities and adjacent communities in South China and implications for human exposure. Environ. Int. 2020, 136, 105462. [Google Scholar] [CrossRef]
- Luo, D.; Liu, W.; Wu, W.; Tao, Y.; Hu, L.; Wang, L.; Yu, M.; Zhou, A.; Covaci, A.; Xia, W.; et al. Trimester-specific effects of maternal exposure to organophosphate flame retardants on offspring size at birth: A prospective cohort study in China. J. Hazard. Mater. 2021, 406, 124754. [Google Scholar] [CrossRef]
- Iqbal, M.; Syed, J.H.; Katsoyiannis, A.; Malik, R.N.; Farooqi, A.; Butt, A.; Li, J.; Zhang, G.; Cincinelli, A.; Jones, K.C. Legacy and emerging flame retardants (FRs) in the freshwater ecosystem: A review. Environ. Res. 2017, 152, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Zhao, F.R.; Hu, J.Y. Exposure assessment and health risk of organophosphate flame retardants in general population in China. Asian J. Ecotoxicol. 2021, 16, 155–165. (In Chinese) [Google Scholar] [CrossRef]
- Quintana, J.B.; Rodil, R.; Reemtsma, T.; García-López, M.; Rodríguez, I. Organophosphorus flame retardants and plasticizers in water and air II. Analytical methodology. TrAC Trends Anal. Chem. 2008, 27, 904–915. [Google Scholar] [CrossRef]
- Hou, M.; Fang, J.; Shi, Y.; Tang, S.; Dong, H.; Liu, Y.; Deng, F.; Giesy, J.P.; Pollitt, K.J.G.; Cai, Y.; et al. Exposure to organophosphate esters in elderly people: Relationships of OPE body burdens with indoor air and dust concentrations and food consumption. Environ. Int. 2021, 157, 106803. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Hu, Q.; Liu, J.; Liu, S.; Liu, C.; Deng, Q.; Zeng, X.; Yu, Z. Occurrence of organophosphate esters and their diesters degradation products in industrial wastewater treatment plants in China: Implication for the usage and potential degradation during production processing. Environ. Pollut. 2019, 250, 559–566. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, B.; Hu, Q.; Liu, Y.; Shang, T.; Zeng, X.; Yu, Z. Occurrence and spatio-seasonal distribution of organophosphate tri- and di-esters in surface water from Dongting Lake and their potential biological risk. Environ. Pollut. 2021, 282, 117031. [Google Scholar] [CrossRef]
- Wang, C.; Chen, H.; Li, H.; Yu, J.; Wang, X.; Liu, Y. Review of emerging contaminant tris(1,3-dichloro-2-propyl)phosphate: Environmental occurrence, exposure, and risks to organisms and human health. Environ. Int. 2020, 143, 105946. [Google Scholar] [CrossRef]
- Li, N.; Ho, W.; Wu, R.S.S.; Ying, G.-G.; Wang, Z.; Jones, K.C.; Deng, W.-J. Organophosphate flame retardants and bisphenol A in children’s urine in Hong Kong: Has the burden been underestimated? Ecotoxicol. Environ. Saf. 2019, 183, 109502. [Google Scholar] [CrossRef]
- He, C.; English, K.; Baduel, C.; Thai, P.; Jagals, P.; Ware, R.; Li, Y.; Wang, X.; Sly, P.; Mueller, J. Concentrations of organophosphate flame retardants and plasticizers in urine from young children in Queensland, Australia and associations with environmental and behavioural factors. Environ. Res. 2018, 164, 262–270. [Google Scholar] [CrossRef]
- He, C.; Toms, L.-M.; Thai, P.; Eede, N.V.D.; Wang, X.; Li, Y.; Baduel, C.; Harden, F.A.; Heffernan, A.; Hobson, P.; et al. Urinary metabolites of organophosphate esters: Concentrations and age trends in Australian children. Environ. Int. 2018, 111, 124–130. [Google Scholar] [CrossRef]
- Wang, X.; Shan, G.; Zhu, L. Estimation of internal human daily intakes of organophosphate esters using one-compartment toxicokinetic model in the whole blood from Hebei Province, China. Environ. Res. 2020, 186, 109493. [Google Scholar] [CrossRef] [PubMed]
- Ya, M.; Yu, N.; Zhang, Y.; Su, H.; Tang, S.; Su, G. Biomonitoring of organophosphate triesters and diesters in human blood in Jiangsu Province, eastern China: Occurrences, associations, and suspect screening of novel metabolites. Environ. Int. 2019, 131, 105056. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Yang, J.; Bekele, T.G.; Zhao, S.; Zhao, H.; Li, J.; Wang, M.; Zhao, H. Organophosphate esters in human serum in Bohai Bay, North China. Environ. Sci. Pollut. Res. 2020, 27, 2721–2729. [Google Scholar] [CrossRef] [PubMed]
- Henríquez-Hernández, L.A.; Carretón, E.; Camacho, M.; Montoya-Alonso, J.A.; Boada, L.D.; Martín, V.B.; Cordón, Y.F.; Cordón, S.F.; Zumbado, M.; Luzardo, O.P. Potential role of pet cats as a sentinel species for human exposure to flame retardants. Front. Vet. Sci. 2017, 4, 79. [Google Scholar] [CrossRef] [Green Version]
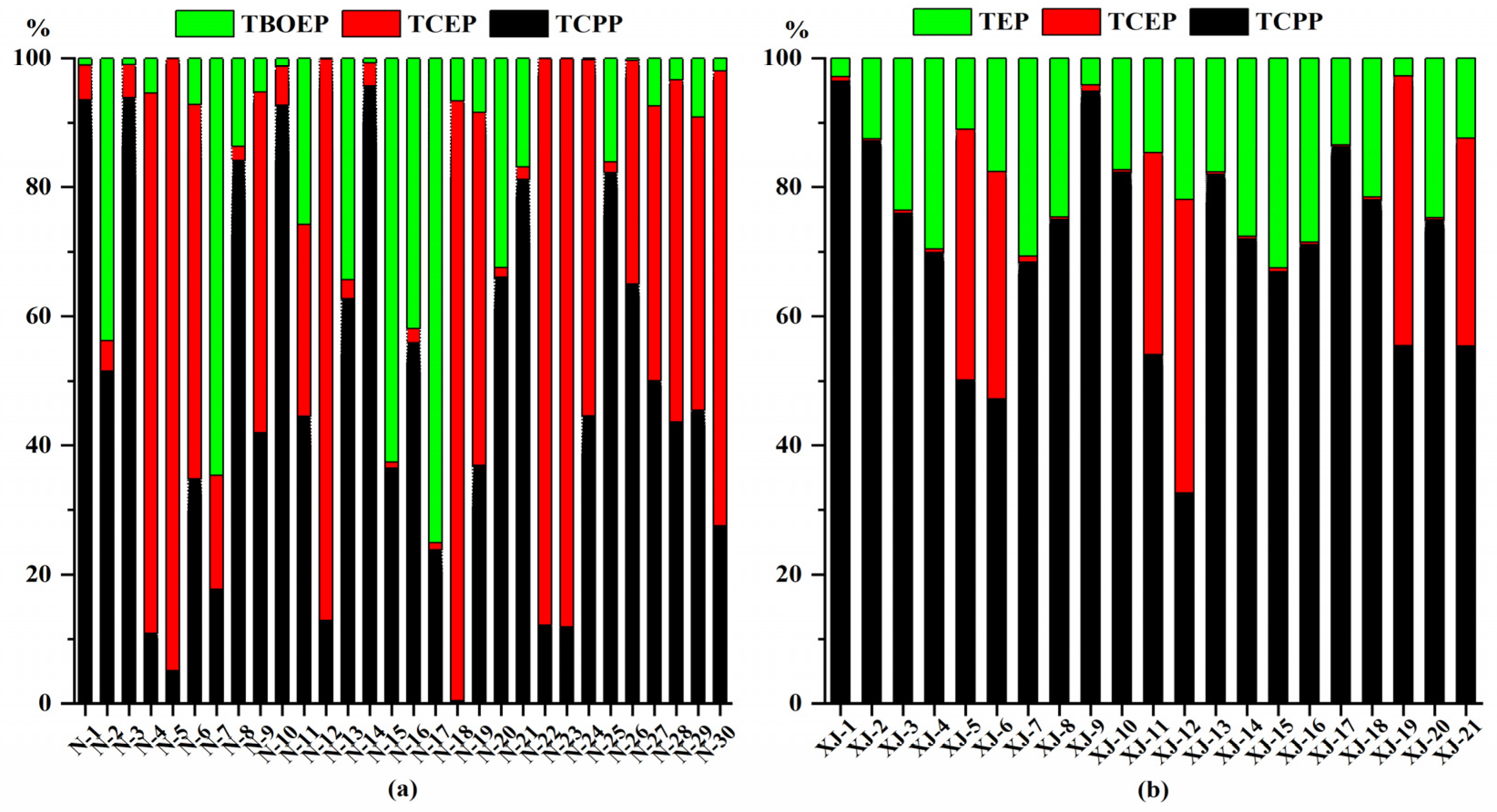
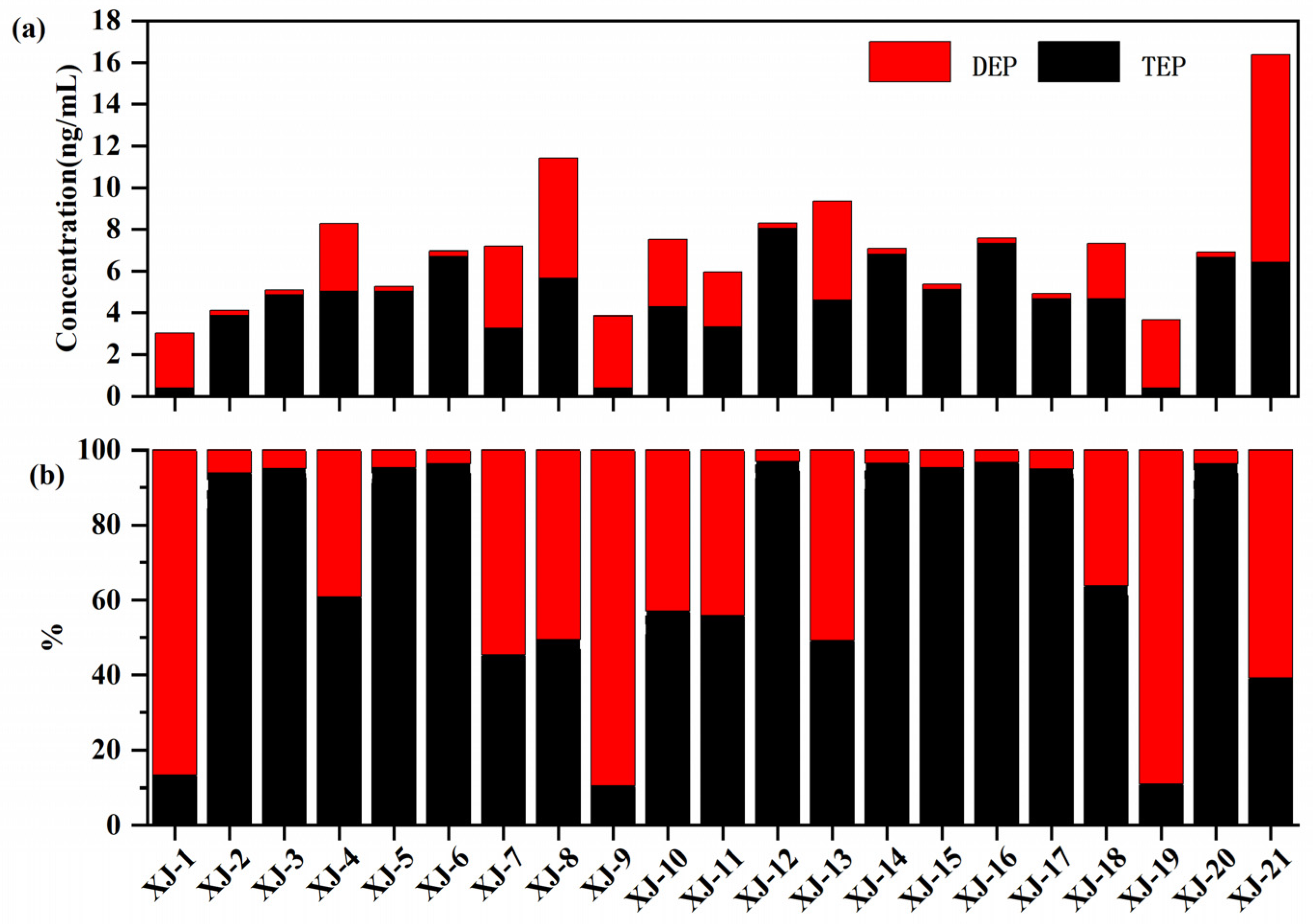
| DF(%) | Median | Mean | Geometric Mean | Standard Deviation | Min | Max | |
|---|---|---|---|---|---|---|---|
| Urine Samples (n = 30) | |||||||
| TCPP | 90 | 1.35 | 1.52 | 1.07 | 0.99 | n.d. | 4.79 |
| TCEP | 50 | n.d. | 3.37 | 0.47 | 5.19 | n.d. | 15.82 |
| TBOEP | 63.33 | 0.26 | 0.51 | 0.12 | 0.84 | n.d. | 3.41 |
| EHDPP | 6.67 | n.d. | 0.60 | n.d. | 0.1 | n.d. | 0.67 |
| Plasma Samples (n = 21) | |||||||
| TCPP | 100 | 17.21 | 17.09 | 15.89 | 6.49 | 7.27 | 29.92 |
| TCEP | 28.57 | n.d. | 3.79 | n.d. | 6.5 | n.d. | 17.77 |
| TEP | 85.71 | 4.85 | 4.64 | 3.59 | 2.18 | n.d. | 8.04 |
| TPPO | 4.76 | n.d. | n.d. | n.d. | - | n.d. | 12.68 |
| Parent OPEs | DF (%) | Mean (Min–Max) | mOPEs | Properties | DF (%) | Mean (Min–Max) |
|---|---|---|---|---|---|---|
| TnBP | 0 | n.d. | Di-n-butyl phosphate(DnBP) | Dialkyl metabolites | 85.71 | 8.22 (n.d.–13.78) |
| TEP | 85.71 | 4.64 (n.d.–8.04) | Diethyl phosphate(DEP) | 52.38 | 2.29 (n.d.–9.96) | |
| TBOEP | 0 | n.d. | Bis(2-butoxyethyl) phosphate (BBOEP) | Dialkyl metabolites | 0 | n.d. |
| Bis(2-butoxyethyl)2-hydroxyethyl phosphate trimester (BBOEHP) | Hydroxylated metabolites | 0 | n.d. | |||
| Bis(2-butoxyethyl)2-(3-hydroxybutoxy)ethyl phosphate trimester (3-OH-TBOEP) | 0 | n.d. |
| Region | Time | TCEP | TCIPP | TDCPP | TPHP | TEP | TBOEP | EHDPP | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Urine (Geometric Mean, ng/mL) | |||||||||
| Shenzhen, China | 2020 | 0.47 | 1.07 | n.d | n.d | n.d | 0.12 | n.d | This study |
| Beijing, China | 2018 | n.d | n.d | n.d | n.d | 0.075 | 0.038 | n.d | [14] |
| Hongkong, China | 2016 | 0.015 | 0.021 | 0.06 | 0.46 | - | - | 0.54 | [28] |
| Jinan, China | 2018 | 0.298 | 0.743 | - | 0.4 | - | - | 0.209 | [24] |
| Australia | 2014 | n.d | n.d | n.d | n.d | n.d | n.d | n.d | [29] |
| Australia | 2015 | n.d | n.d | 0.024 | - | - | 0.59 | n.d | [30] |
| Blood/Serum or Plasma (Median, ng/mL) | |||||||||
| Shenzhen, China | 2020 | n.d | 17.21 | n.d | n.d | 4.85 | n.d | n.d | This study |
| Shenzhen, China | 2012 | n.d | 0.71 | n.d. | 0.43 | 0.49 | 0.54 | 1.22 | [5] |
| Hebei, China | 2017 | 0.18 | 0.25 | n.d | 0.46 | - | n.d. | 0.78 | [31] |
| Jinan, China | 2018 | 0.3 | 0.74 | 0.11 | 0.4 | 0.14 | - | 0.21 | [24] |
| Beijing, China | 2018 | n.d | n.d | n.d | 0.37 | 0.43 | 0.16 | 1.1 | [14] |
| Jiangsu, China | 2013 | 0.1 | 0.05 | n.d | 0.35 | 0.15 | 0.05 | 0.85 | [32] |
| Shandong, China | 2018 | 214 | n.d. | - | - | n.d. | - | 7.2 | [33] |
| Spain | 2016 | 3.69 | 93.9 | n.d. | 22.7 | n.d. | 56.4 | 425.8 | [34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhu, T.; Xie, Z.; Deng, C.; Qi, X.; Hu, R.; Wang, J.; Chen, J. Human Exposure to Chlorinated Organophosphate Ester Flame Retardants and Plasticizers in an Industrial Area of Shenzhen, China. Int. J. Environ. Res. Public Health 2022, 19, 3126. https://doi.org/10.3390/ijerph19053126
Liu Y, Zhu T, Xie Z, Deng C, Qi X, Hu R, Wang J, Chen J. Human Exposure to Chlorinated Organophosphate Ester Flame Retardants and Plasticizers in an Industrial Area of Shenzhen, China. International Journal of Environmental Research and Public Health. 2022; 19(5):3126. https://doi.org/10.3390/ijerph19053126
Chicago/Turabian StyleLiu, Yunlang, Tingting Zhu, Zuoming Xie, Chen Deng, Xiujuan Qi, Rong Hu, Jinglin Wang, and Jianyi Chen. 2022. "Human Exposure to Chlorinated Organophosphate Ester Flame Retardants and Plasticizers in an Industrial Area of Shenzhen, China" International Journal of Environmental Research and Public Health 19, no. 5: 3126. https://doi.org/10.3390/ijerph19053126
APA StyleLiu, Y., Zhu, T., Xie, Z., Deng, C., Qi, X., Hu, R., Wang, J., & Chen, J. (2022). Human Exposure to Chlorinated Organophosphate Ester Flame Retardants and Plasticizers in an Industrial Area of Shenzhen, China. International Journal of Environmental Research and Public Health, 19(5), 3126. https://doi.org/10.3390/ijerph19053126






