Abstract
Background: The aim of this study was to assess risk factors for tooth loss in the population of the city of Bialystok, in north-eastern Poland, taking into account the entire population and different age groups. The study included 1138 subjects divided into three subgroups: 20–44 years, 45–64 years, and 65–79 years. Participants were classified according to the number of teeth lost (0–8 vs. 9–28). Socio-economic variables, smoking history, and dental habits were collected through a questionnaire. Medical examinations provided data on the body mass index and the fasting blood glucose level. Data were statistically analysed using Mann-Whitney U, Student’s t, chi2 tests, and binary logistic regression, p < 0.05. Results: For the general population, being female (OR 1.38, 1.07–1.79, p = 0.015), having secondary education (OR 4.18, Cl 2.97–5.87, p < 0.000), higher body mass index (OR 1.13, Cl 1.10–1.17, p < 0.000), higher fasting blood glucose level (OR 1.03 1.03–1.04, p < 0.000), being former smoker (OR 1.72, Cl 1.29–2.31, p < 0.000), ever smoker (OR 1.69, Cl 1.29–2.20, p < 0.000), current smoker (OR 1.62, Cl 1.15–2.29, p < 0.006), longer smoking period (OR 1.11, Cl 1.09–1.14, p < 0.000), last visit to the dentist over a year ago (OR 1.92, Cl 0.44–2.58, p < 0.000) and tooth brushing less than two times a day (OR 1.6, Cl 1.14–2.23, p < 0.006) were associated with losing more than 8 teeth. In the subgroup aged 20–44 years, only smoking duration was a risk factor for tooth loss (p = 0.02). For the middle-aged and oldest groups, education level (respectively p < 0.001, and p = 0.001), body mass index (respectively, p < 0.001, and p = 0.037), smoking status ever/former/current (respectively p < 0.001 and p = 0.002), smoking status never/ever (respectively p < 0.001 and p = 0.009), smoking duration (p < 0.001) were related to tooth loss. Additionally, in the elderly group, fasting blood glucose level (p = 0.044) and frequency of dental visits (p = 0.007) were related to tooth loss. We concluded that in the evaluated population, tooth loss was associated with socio-demographic, medical, and behavioural factors.
1. Introduction
According to the World Health Organization (WHO), a key aspect of oral health is the lifelong maintenance of functional dentition, understood as dentition consisting of no less than 20 teeth, without the need for tooth replacement [1,2]. Tooth loss results in functional, aesthetic, and social impairments, may decrease an individual’s quality of life and could be an effective determinant of population oral health [3,4]. The World Dental Federation (FDI) and WHO established Global Oral Health Goals for 2020, stipulating among other targets that the proportion of 35–44 and 65–74 year olds with functional dentition should increase in each population [5].
The prevalence of tooth loss has constantly decreased during the last decades, especially in developed countries [3,6], but disparities among countries and regions are may still be observed. The main reasons for missing teeth are dental caries, periodontal diseases, trauma, and orthodontic extractions [7,8,9]. Untreated dental caries is considered the main cause of tooth loss except for adults older than 80 years, and another leading reason is periodontitis [7,8,9,10,11]. Some reports indicate that causes of tooth extraction differ according to age and sex [12], and the distribution of missing teeth in dental arches depends on the cause. Several factors are associated with tooth loss: age, gender, socio-behavioural factors, oral health behaviours, availability, and quality of dental service [4]. There is still no clear evidence as to whether oral conditions or socio-behavioural factors should be considered as the most significant risk factors [6]. The association between tooth loss and non-communicable diseases (NCDs) was investigated, and its relation to cardiovascular disease, stroke, diabetes, metabolic syndrome, dementia, depression, and all-cause mortality was confirmed [13,14,15,16,17,18]. NCDs share common risk factors as dental conditions leading to dental extraction [19,20]. Poor general health (e.g., mental and physical disability, depression) and aging may also contribute, as a predisposing factor, to the neglect of oral hygiene and may increase the risk of severe caries and periodontal disease [2,21,22,23,24].
Previous studies on risk factors for tooth loss showed that the importance of factors related to the number of missing teeth varied according to the population assessed [4,25,26,27]. The majority of these studies, also conducted on the Polish population, concerned the elderly [28,29,30,31]. Learning about risk factors in younger populations may be important in reducing tooth loss over a lifetime. Therefore, the study aimed to assess risk factors for tooth loss among the population of the city of Bialystok, in north-eastern Poland, taking into account the entire population and different age groups.
2. Materials and Methods
2.1. Study Population
Based on the Mayor’s Office database, a random sampling was conducted to determine a study population. The data on age and gender distribution in the entire population are available from the Polish Statistical Office upon request [32]. The study was approved by the Ethics Committee of the University of Bialystok, Poland (R-i-002/108/2016) in conformity with the Declaration of Helsinki. The sample size was 3806, and was stratified according to age and gender distribution across the entire population (Figure 1). The inclusion criteria were ages between 20 and 79 and being a citizen of Bialystok, Poland.
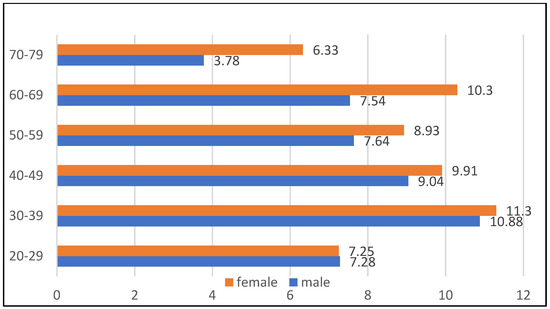
Figure 1.
The distribution of the population drawn for the survey by age and sex.
Between July 2017 and May 2021, 3246 subjects from the drawn group were invited to participate in the study. For the present study, the minimum sample size was determined using an online tool available at https://www.naukowiec.org/dobor.html (accessed on 17 August 2021) with a 383 person level. The calculation was based on the following criteria: in 2017, the average number of residents of Bialystok aged 20–79 years was 207,676, due to lack of epidemiological data on the prevalence of tooth loss in the entire population, the percentage of people with at least one missing tooth (the fraction size) was established at 50%, standard values for the confidence level −95% and the maximum error −5% were adopted [33].
2.2. Data Acquisition
The survey questionnaire was self-administered, printed, and prepared in Polish. It consisted of a broad range of closed and open questions concerning socio-economic situation, medical history, family history, health habits, and quality of life. For the present study, the following closed questions were included: age, gender, educational level, subject’s smoking history: being never/ever-smoker (NS/ES), never/former/current-smoker (NS/FS/CS), duration of smoking and their dental habits: frequency of tooth brushing and dental visits were collected using a questionnaire survey. Medical examinations provided data on body mass index (BMI, kg/m2). The fasting blood glucose level (FBG, mg/dL) measurement was a part of the general blood test. Medical and dental examinations were performed on the same day.
Dental examinations were conducted by four calibrated dentists under conditions of an epidemiological inquiry (with the use of artificial light and without the use of a saliva ejector or air jet). A full mouth oral examination was performed to estimate the number of lost teeth. Teeth missing due to dental caries, periodontal, and trauma reasons were counted as lost. Third molars, unerupted teeth, and teeth extracted due to orthodontic reasons were excluded from the analysis.
2.3. Statistical Analysis
Descriptive statistics for quantitative variables were presented as mean and standard deviations and as counts and frequencies for qualitative variables. Comparisons of variables between subgroups were conducted using the U Mann-Whitney test or t-Student test for quantitative variables and the chi2 test for qualitative variables. Statistical hypotheses were verified at a 0.05 significance level. A binary logistic regression analysis was performed to estimate the odds ratio (OR) for the association between risk factors and having more than 8 teeth lost. The Statistica 13.3. (StatSoft Polska Sp. z o.o., Cracow, Poland) was used for all calculations.
3. Results
Out of those invited to the survey, 1196 (36.85%) responded. A total of 58 individuals were excluded, either because they had refused to participate in the dental portion or there was another reason for the lack of data on missing teeth. As a result, 1138 individuals were included in the study group; the final response rate was 35.05%. The study group consisted of 503 (44.2%) men and 635 (55.8%) women, with a mean age of 48.8 ± 15.38 years. Participants were divided into three subgroups based on their age: aged 20 to 44 years (young group), 45 to 64 years (middle-aged group), and 65 years and older (elderly group). The number of participants in each group was respectively: 501, 409, and 228. For the analysis, participants were dichotomized into two groups according to the number of teeth lost due to caries, periodontal disease, and dental trauma (0–8 vs. 9–28).
Table 1 and Table 2 present the assessed risk factors for tooth loss for the whole population and subgroups by age. Age, gender, education level, BMI, FBG, cigarette smoking, duration of smoking, frequency of tooth brushing, and dental visits were factors related to tooth loss in the general population. In the 20–44 years subgroup, only duration of smoking was a risk factor for tooth loss. In the middle-aged group, variables favouring the preservation of more own teeth were lower BMI, no smoking history, and smoking for a shorter period. In the oldest group, factors associated with the number of lost teeth were higher BMI, higher FBG level, being ES and CS, long-term smoking, and irregular dental visits.

Table 1.
The tooth loss (0–8 vs. 9–28) and mean age, BMI, FBG, and duration of smoking. (p < 0.05, * U Mann–Whitney test, ** t–Student test) BMI—body mass index, FBG—fasting blood glucose level.

Table 2.
Prevalence of tooth loss according to demographic characteristics and smoking and oral habits. (p < 0.05, chi2 test) NS—never-smoker, FS—former smoker, CS—current smoker, ES—ever smoker.
In all groups, regardless of age and number of lost teeth, the mean BMI was above 25. The mean BMI in middle-aged and older participants who lost more than 8 teeth was 29.1 and was significantly higher compared to those who lost less than 8 teeth (27.3, p < 0.001 in the middle-aged subgroup and 27.6, p = 0.003 in the older subgroup, respectively).
In relation to the general population, subjects with a greater number of lost teeth had higher mean FBG levels (above normal range) compared to the other group, 109.4 ± 25.7 vs. 98.3 ± 16.4 (p < 0.001). In the youngest subjects, the mean FBG level was within normal limits (below 99 mg/dl) irrespective of the number of lost teeth. In other age subgroups, the mean parameter was above normal. However, only in the oldest subgroup, an association between mean FBG levels and the number of lost teeth was statistically confirmed (p = 0.044).
Smoking history was associated with a higher number of lost teeth in the general population (p < 0.001). ES accounted for 70.2% of those with severe tooth loss in the middle-aged subgroup (p < 0.001) and 62.6% in the oldest group (p = 0.009). Furthermore, in both groups, an association between being NS, FS, and CS and the chance of preserving dentition was observed.
Another risk factor for tooth loss was the duration of smoking. In the general population, this period was significantly shorter in subjects who lost less than 8 teeth compared to the other group (12.4 ± 8.9 vs. 27.5 ± 14, p < 0.001). Moreover, it was the only variable associated with the level of tooth loss in all age groups assessed (p = 0.02 for participants aged 20–44, and p < 0.001 for the other two subgroups).
Most subjects brushed their teeth at least twice a day (83.6%). Almost half of the participants (48.4%) declared their last dental visit within the last 6 months. Regarding these variables, only in the elderly group was there a statistical association between tooth loss and the frequency of dental visits. Only 25% of the participants in this age group who had more than twenty teeth preserved declared irregular visits to a dentist.
Table 3 presents an association between risk factors and having more than 8 teeth lost for the general population. It was shown that a lower education level had the most significant impact on the number of lost teeth (OR 4.18, Cl 2.97–5.87, p < 0.000), followed by dental visits less than once a year (OR 1.92, Cl 0.44–2.58, p < 0.000), having smoking habit: being a former smoker (OR 1.72, Cl 1.29–2.31, p < 0.000), ever smoker (OR 1.69, Cl 1.29–2.20, p < 0.000), current smoker (OR 1.62, Cl 1.15–2.29, p < 0.006) and brushing teeth less than two times a day (OR 1.6, Cl 1.14–2.23, p < 0.006). Moreover, for every unit of BMI, the odds of being in the 9–28 loss group increased by 1.13 times (Cl 1.10–1.17, p < 0.000), and for every additional unit of FBG, the odds of being in the group with tooth loss between 9 and 28 was 1.03 higher (1.03–1.04, p < 0.000). Each year of smoking increased the odds of losing more than 8 teeth by 1.11. (Cl 1.09–1.14, p < 0.000).

Table 3.
Association between risk factors and having more than 8 teeth lost (binary logistic regression, OR—odds ratio, Cl—confidence level).
4. Discussion
Demographic and socio-economic factors were evaluated as risk factors of tooth loss in several studies [3,25,26,27,28,29,30,31,34]. The association between tooth loss and participants’ age found in this study is consistent with previous reports [3,35]. A negative impact of caries and periodontal disease accumulating throughout life results in an increasing number of missing teeth with age, with the peak incidence in the seventh decade of life [3]. Carmen et al. [35] found that the average number of missing teeth increased by 5% for each year of the patient’s age. In this study, the evaluation of risk factors was conducted in three separate age groups: 20–44, 45–64, and 65–79 years. In the evaluated sample, the gender contributed as a tooth loss risk factor for the whole population (OR 1.38, Cl 1.07–1.97), but not for particular age groups, similarly to the Mexican population [35]. Kassebaum et al. [3] found that globally gender differences in tooth loss decreased between 1990 and 2010. In our study, having higher education reduced the risk of severe tooth loss by 41.8 times. A university level education contributed as a protective factor in the middle-aged and elderly groups, in accordance with de Miguel-Infante et al. [36] results that a university degree is associated with a lower risk of periodontitis. The Study of Health in Pomerania (SHIP) found that low education and low income were associated with tooth loss [27,37]. Kim and Kim [38], in their study on self-rated poor oral health conducted for the same age groups as in the present study, found that the educational level was a more important covariate among young and middle-aged people (20–44 and 45–64), and income played a greater role among adults aged 65 and over.
Many studies confirmed that obesity correlated with fewer teeth [39,40], however, there are reports of no such link [41]. In the study by Őstberg et al. [40], the association between tooth loss and general and abdominal obesity in people under 60 years was independent of age and gender, socio-economic factors, lifestyle, and co-morbidities. The relationship between oral health and obesity is multifactorial. Undoubtedly, high consumption of added sugars may lead to increased BMI and severe dental caries [42,43,44,45]. It was found that middle-aged Thai adults with frequent consumption of sugary snacks were more prone to tooth loss [46]. Increased consumption of sugar-sweetened beverages, a marker of fermentable sugars intake, was observed in young and elderly people with tooth loss [9,47]. Obesity and associated diseases were found to be risk factors for periodontitis [48,49]. The pathways of links between obesity and periodontitis remain unclear, with a likely bidirectional influence [49]. Meisel et al. [39] suggested sex-specific differences in the incidence of periodontitis and tooth loss in obese subjects related to different CRP levels. In the present group, higher BMI was observed in those who lost more than 8 teeth in the general population and in both the middle-aged and oldest age groups. We found that the chance of losing more than 8 teeth increased with every additional BMI unit by 1.13. The present study indicates that not only obesity but also BMI at the upper limit of overweight are variables associated with the risk of tooth loss. From a clinical perspective, however, there were no great differences in mean BMI, as it ranged between 25 and 29.9, indicating that Bialystok residents, in general, tended to be overweight.
Hyperglycaemia was observed in the middle-aged and the oldest groups. In elderly subjects, it was statistically proved that the prevalence of severe tooth loss was higher in those with higher levels of fasting glucose blood levels. A direct association between the number of missing teeth and diabetes was confirmed [9,16,50]. Liljestrand et al. [16] found missing more than 9 teeth associated with an incident of cardiovascular disease, diabetes, and death of any cause. A similar conclusion can be drawn from the National Health and Nutrition Examination Survey, 2003–2004, conducted in the USA population aged 50 and more. The mean number of missing teeth in people with diagnosed diabetes was 9.8 compared to 6.8 in those without the disease [51]. Moreover, according to that study, twenty percent of cases of edentulism in the USA were linked to diabetes. Greenblatt et al. [50] found that uncontrolled diabetes significantly increased the likelihood of missing >9 teeth, including edentulousness, especially in younger individuals (18–44 years). A possible connection between tooth loss and diabetes is via periodontal disease [52]. Data from a South Korean Nationwide Health Screening Program showed that the FBG correlated with periodontitis and tooth loss but not with dental caries [53]. In the study of de Miguel-Infante et al. [36], the subjects with diabetes had an increased ratio of periodontal disease by 22%. Diabetes mellitus is also one of the co-morbidities associated with obesity; links between tooth loss and increased BMI have been explained above.
The present study confirmed that cigarette smoking was an important risk factor regarding tooth loss. Habitual smokers made up the majority in the middle-age and oldest age groups with tooth loss of more than 8 teeth. Moreover, the number of years of smoking was strongly associated with a greater number of lost teeth in all age groups. The data from the literature are inconsistent [26,28,54]. Some studies align with our results [28,54], but other studies did not confirm the relationship between smoking status and tooth loss [26]. Similä et al. [54] confirmed that both intensity and duration of smoking contributed as risk factors of tooth loss. The modern oral health approach states that oral conditions share common risk factors with other non-communicable diseases, and tobacco smoking is one of such variables [20]. The correlation between tobacco use and an increased rate of dental caries was previously reported. However, the mechanism of promoting caries by nicotine products is still unclear [55]. Periodontal disease is more common, has a more severe course, and therefore presents a higher risk of treatment failure in smokers [56]. In addition, this relationship is dose-dependent [57]. There are several mechanisms of negative effects of smoking on periodontal tissues, and one of them is the alteration of alveolar bone metabolism. Increased alveolar bone resorption due to osteoclast formation and activation results in an increased tooth loss [57,58], and smoking cessation may improve periodontal health [56]. We found that with longer duration of smoking increased the chance of being in the group with 9–28 teeth lost. Surprisingly, our study revealed that being FS was associated with a higher risk of losing more than 8 teeth (higher than being CS (OR 1.72, Cl 1.29–2.31 and OR 1.62, Cl 1.15–2.29, respectively). This may be explained by the fact that we did not assess the number of pack-years, an indicator of the accurate exposure to smoking.
Our observation that people over 64 years of age brushed their teeth less frequently than young and middle-aged people and that they were prone to miss regular dental visits is consistent with Canadian data for the same age stratification [59]. In the present study, the prevalence of tooth loss was more often in participants who brushed teeth less than two times a day and were irregular dental attenders. However, with respect to the age subgroup, only the frequency of dental visits was significant for the oldest group. According to Tiwari et al. [26], regular visits to a dentist impacted tooth retention in older adults. However, in the Mexican population, the frequency of dental attendance did not influence the number of missing teeth, but brushing teeth less than every day was associated with it [35].
The strengths of this study include a random selection of participants and a study population that is three times the minimum sample size. An additional strength of the present study is the inclusion of adults from all life stages. Most studies on risk factors for tooth loss focused on middle-aged or elderly individuals only. The low response rate (35.05%) should be considered as a limitation, especially for a cross-sectional survey, as there is a possibility that non-respondents differed from respondents. Another limitation of cross-sectional studies constitutes the difficulties in distinguishing between association and causation in the exposure-outcome relationship. For data collected through the questionnaire, there may be a bias in responses regarding health habits and a ‘fatigue effect’ [60] due to the length of the survey tool. Another limitation is that only people living in the city were surveyed, so the data obtained cannot be directly transferred to the entire population living in north-eastern Poland.
5. Conclusions
In the study population, tooth loss was associated with socio-demographic, medical, and behavioural factors that varied depending on the age group. The duration of smoking was the only factor associated with tooth loss in all groups. Smoking cessation counselling should be an integral part of dental treatment and prevention, especially in young patients.
Author Contributions
Conceptualization: K.G., J.B. and K.A.K.; Methodology: K.G., J.B., E.R., I.K. and K.A.K.; Formal Analysis: W.Ł., Investigation: K.G., J.B., E.R., I.K., Z.S., M.D. and M.K.; Resources: K.A.K.; Data Curation: Z.S. and M.D.; Writing—Original Draft Preparation: K.G., J.B. and W.Ł.; Writing—Review & Editing: E.R., I.K., Z.S., M.D., M.K. and K.A.K.; Supervision K.A.K.; Project Administration: K.A.K.; Funding Acquisition J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grant No. N/ST/ZB/18/001/1191 and SUB/1/DN/20/001/1191 from the Medical University of Bialystok, Poland.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the University of Bialystok, Poland (R-i-002/108/2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available on request from the authors.
Acknowledgments
The study is a part of the Bialystok PLUS project. The authors would like to thank all participants who agreed to participate in the study. The authors thank Marek Bagiński for language corrections.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chalub, L.; Ferreira, R.C.; Vargas, A. Influence of functional dentition on satisfaction with oral health and impacts on daily performance among Brazilian adults: A population-based cross-sectional study. BMC Oral Health 2017, 17, 112. [Google Scholar] [CrossRef]
- Lamster, I.B.; Asadourian, L.; Del Carmen, T.; Friedman, P.K. The aging mouth: Differentiating normal aging from disease. Periodontology 2016, 72, 96–107. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of severe tooth loss: A systematic review and meta-analysis. J. Dent. Res. 2014, 3 (Suppl. S7), 20S–28S. [Google Scholar] [CrossRef]
- Silva, M.F., Jr.; Batista, M.J.; de Sousa, M.D.L.R. Risk factors for tooth loss in adults: A population-based prospective cohort study. PLoS ONE 2019, 14, e0219240. [Google Scholar]
- Hobdell, M.; Petersen, P.E.; Clarkson, J.; Johnson, N. Global goals for oral health 2020. Int. Dent. J. 2003, 53, 285–288. [Google Scholar] [CrossRef]
- Müller, F.; Naharro, M.; Carlsson, G.E. What are the prevalence and incidence of tooth loss in the adult and elderly population in Europe? Clin. Oral Implants Res. 2007, 18, 2–14. [Google Scholar] [CrossRef]
- Al-Shammari, K.F.; Al-Ansari, J.M.; Al-Melh, M.A.; Al-Khabbaz, A.K. Reasons for tooth extraction in Kuwait. Med. Princ. Pract. 2006, 15, 417–422. [Google Scholar] [CrossRef]
- Caldas, A.F., Jr. Reasons for tooth extraction in a Brazilian population. Int. Dent. J. 2000, 50, 267–273. [Google Scholar] [CrossRef]
- Wiener, R.C.; Shen, C.; Findley, P.A.; Sambamoorthi, U.; Tan, X. The association between diabetes mellitus, sugar-sweetened beverages, and tooth loss in adults: Evidence from 18 states. J. Am. Dent. Assoc. 2017, 148, 500–509. [Google Scholar] [CrossRef]
- Ong, G. Periodontal disease and tooth loss. Int. Dent. J. 1998, 48, 233–238. [Google Scholar] [CrossRef]
- Neely, A.L.; Holford, T.R.; Löe, H.; Anerud, A.; Boysen, H. The natural history of periodontal disease in humans: Risk factors for tooth loss in caries-free subjects receiving no oral health care. J. Clin. Periodontol. 2005, 32, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Aida, J.; Morita, M.; Akhter, R.; Aoyama, H.; Masui, M.; Ando, Y. Relationships between patient characteristics and reasons for tooth extraction in Japan. Community Dent. Health 2009, 26, 104–109. [Google Scholar] [PubMed]
- Peng, J.; Song, J.; Han, J.; Chen, Z.; Yin, Z.; Zhu, J.; Song, J. The relationship between tooth loss and mortality from all causes, cardiovascular diseases, and coronary heart disease in the general population: Systematic review and dose-response meta-analysis of prospective cohort studies. Biosci. Rep. 2019, 11, 39. [Google Scholar] [CrossRef]
- Vedin, O.; Hagström, E.; Östlund, O.; Avezum, A.; Budaj, A.; Flather, M.D.; Harrington, R.A.; Koenig, W.; Soffer, J.; Siegbahn, A.; et al. Associations between tooth loss and prognostic biomarkers and the risk for cardiovascular events in patients with stable coronary heart disease. Int. J. Cardiol. 2017, 15, 271–276. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheng, F.; Zhang, M.; Wang, Q.; Xu, H.; Dong, X.; Gao, Z.; Chen, J.; Wei, Y.; Qin, F. Tooth loss and risk of cardiovascular disease and stroke: A dose-response meta-analysis of prospective cohort studies. PLoS ONE 2018, 13, 0194563. [Google Scholar] [CrossRef] [PubMed]
- Liljestrand, J.M.; Havulinna, A.S.; Paju, S.; Männistö, S.; Salomaa, V.; Pussinen, P.J. Missing teeth predict incident cardiovascular events, diabetes and death. J. Dent. Res. 2015, 94, 1005–1062. [Google Scholar] [CrossRef]
- Yoo, J.J.; Yoon, J.H.; Kang, M.J.; Kim, M.; Oh, N. The effect of missing teeth on dementia in older people: A nationwide population-based cohort study in South Korea. BMC Oral Health 2019, 19, 61. [Google Scholar] [CrossRef]
- Schwahn, C.; Polzer, I.; Haring, R.; Dörr, M.; Wallaschofski, H.; Kocher, T.; Mundt, T.; Holtfreter, B.; Samietz, S.; Völzke, H.; et al. Missing, unreplaced teeth and risk of all-cause and cardiovascular mortality. Int. J. Cardiol. 2013, 167, 1430–1437. [Google Scholar] [CrossRef]
- Dörfer, C.; Benz, C.; Aida, J.; Campard, G. The relationship of oral health with general health and NCDs: A brief review. Int. Dent. J. 2017, 67, 14–18. [Google Scholar] [CrossRef]
- Wolf, T.G.; Cagetti, M.G.; Fisher, J.-M.; Seeberger, G.K.; Campus, G. Non-communicable diseases and oral health: An Overview. Front. Oral Health 2021, 2, 725460. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lim, K.C.; Kim, S.Y.; Paik, H.R.; Kim, Y.J.; Jin, B.H. Oral health status of the disabled compared with that of the non-disabled in Korea: A propensity score matching analysis. PLoS ONE 2019, 14, e0208246. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.M.; Cooper, S.A.; Hughes-McCormack, L.; Macpherson, L.; Kinnear, D. Oral health of adults with intellectual disabilities: A systematic review. J. Intellect. Disabil. Res. 2019, 63, 1359–1378. [Google Scholar] [CrossRef] [PubMed]
- Skośkiewicz-Malinowska, K.; Malicka, B.; Ziętek, M.; Kaczmarek, U. Oral health condition and occurrence of depression in the elderly. Medicine 2018, 97, e12490. [Google Scholar] [CrossRef]
- Kinasi, E.; Ayilavarapu, S.; Jones, J. The aging population: Demographics and the biology of aging. Periodontology 2016, 72, 13–18. [Google Scholar] [CrossRef]
- Gilbert, G.H.; Duncan, R.P.; Shelton, B.J. Social determinants of tooth loss. Health Serv. Res. 2003, 38, 1843–1862. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, T.; Scarbro, S.; Bryant, L.L.; Puma, J. Factors associated with tooth loss in older adults in Rural Colorado. J. Community Health 2016, 41, 476–481. [Google Scholar] [CrossRef]
- Mundt, T.; Polzer, I.; Samietz, S.; Grabe, H.J.; Dören, M.; Schwarz, S.; Kocher, T.; Biffar, R.; Schwahn, C. Gender-dependent associations between socioeconomic status and tooth loss in working age people in the study of health in Pomerania (SHIP), Germany. Community Dent. Oral Epidemiol. 2011, 39, 398–408. [Google Scholar] [CrossRef]
- Natto, Z.S.; Aladmawy, M.; Alasqah, M.; Papas, A. Factors contributing to tooth loss among the elderly: A cross sectional study. Singap. Dent. J. 2014, 35, 17–22. [Google Scholar] [CrossRef]
- Barbato, P.R.; Peres, K.G. Contextual socioeconomic determinants of tooth loss in adults and elderly: A systematic review. Rev. Bras. Epidemiol. 2015, 18, 357–371. [Google Scholar] [CrossRef]
- Głowacka, B.; Chrzęszczyk, D.; Konopka, T. Reasons and risk indicators tor tooth loss in the Polish cross-sectional gerodontological study. Prz. Epidemiol. 2019, 73, 531–547. [Google Scholar]
- Mehr, K.; Olszanecka-Glinianowicz, M.; Chudek, J.; Szybalska, A.; Mossakowska, M.; Zejda, J.; Wieczorowska-Tobis, K.; Grodzicki, T.; Piotrowski, P. Dental status in the Polish senior population and its correlates—Results of the national survey PolSenior. Gerodontology 2018, 35, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Polish Statistical Office. Bank Danych Lokalnych. Available online: https://bdl.stat.gov.pl/bdl/start (accessed on 24 January 2022).
- Lwangs, K.S.; Lemeshow, S. Sample Size Determination in Health Studies: A Practical Manual; World Health Organization: Geneva, Switzerland, 1991. [Google Scholar]
- Seerig, L.M.; Nascimento, G.G.; Peres, M.A.; Horta, B.L.; Demarco, F.F. Tooth loss in adults and income: Systematic review and meta-analysis. J. Dent. 2015, 43, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Fatima Del Carmen, A.D.; Aída, B.S.; Javier, F.H. Risk indicators of tooth loss among Mexican adult population: A cross-sectional study. Int. Dent. J. 2021, 71, 414–419. [Google Scholar] [CrossRef] [PubMed]
- de Miguel-Infante, A.; Martinez-Huedo, M.A.; Mora-Zamorano, E.; Hernandez-Barrera, V.; Jimenez-Trujillo, I.; de Burgos-Lunar, C.; Valladolid, J.C.; Jimenez-Garcia, R.; Lopez-de-Andres, A. Periodontal disease in adults with diabetes, prevalence and risk factors. Results of an observational study. Int. J. Clin. Pract. 2018, 73, e13294. [Google Scholar] [CrossRef]
- Buchwald, S.; Kocher, T.; Biffar, R.; Harb, A.; Holtfreter, B.; Meisel, P. Tooth loss and periodontitis by socio-economic status and inflammation in a longitudinal population-based study. J. Clin. Periodontol. 2013, 40, 203–211. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, N.H. Trends in self-rated poor oral health among all age populations in Korea from 2007 to 2015: Monitoring expansion of dental insurance. Int. Dent. J. 2021, 71, 76–84. [Google Scholar] [CrossRef]
- Meisel, P.; Holtfreter, B.; Völzke, H.; Kocher, T. Sex differences of tooth loss and obesity on systemic markers of inflammation. J. Dent. Res. 2014, 93, 774–779. [Google Scholar] [CrossRef]
- Östberg, A.-L.; Nyholm, M.; Gullberg, B.; Råstam, L.; Lindblad, U. Tooth loss and obesity in a defined Swedish population. Scand. J. Public Health 2009, 37, 427–433. [Google Scholar] [CrossRef]
- Aoyama, N.; Fujii, T.; Kida, S.; Nozawa, I.; Taniguchi, K.; Fujiwara, M.; Iwane, T.; Tamaki, K.; Minabe, M. Association of periodontal status, number of teeth, and obesity: A cross-sectional study in Japan. J. Clin. Med. 2021, 10, 208. [Google Scholar] [CrossRef]
- Burt, B.A.; Pai, S. Sugar consumption and caries risk: A systematic review. J. Dent. Educ. 2001, 65, 1017–1023. [Google Scholar] [CrossRef]
- Moynihan, P. Sugars and dental caries: Evidence for setting a recommended threshold for intake. Adv. Nutr. 2016, 7, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Steffen, L.M.; Zhou, X.; Harnack, L.; Luepker, R.V. Consistency between increasing trends in added-sugar intake and body mass index among adults: The Minnesota heart survey, 1980–1982 to 2007–2009. Am. J. Public Health 2013, 103, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Vorster, H.H.; Kruger, A.; Wentzel-Viljoen, E.; Kruger, H.S.; Margetts, B.M. Added sugar intake in South Africa: Findings from the adult prospective urban and rural epidemiology cohort study. Am. J. Clin. Nutr. 2014, 99, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Naorungroj, S. Sugary snack consumption and tooth retention among middle-aged thai adults. J. Int. Soc. Prev. Community Dent. 2020, 10, 394–401. [Google Scholar] [CrossRef]
- Kim, S.; Park, S.; Lin, M. Permanent tooth loss and sugar-sweetened beverage intake in U.S. young adults. J. Public Health Dent. 2017, 77, 148–154. [Google Scholar] [CrossRef]
- Nishida, N.; Tanaka, M.; Hayashi, N.; Nagata, H.; Takeshita, T.; Nakayama, K. Determination of smoking and obesity as periodontitis risk using the classification and regression tree method. Periodontology 2005, 76, 923–928. [Google Scholar] [CrossRef]
- Martinez-Herrera, M.; Silvestre-Rangil, J.; Silvestre, F.J. Association between obesity and periodontal disease. A systematic review of epidemiological studies and controlled clinical trials. Med. Oral Patol. Oral Cir. Bucal. 2017, 22, e708–e715. [Google Scholar] [CrossRef]
- Greenblatt, A.P.; Salazar, C.R.; Northridge, M.E.; Kaplan, R.C.; Taylor, G.W.; Finlayson, T.L.; Qi, Q.; Badner, V. Association of diabetes with tooth loss in Hispanic/Latino adults: Findings from the Hispanic community health study/study of Latinos. BMJ Open Diabetes Res. Care 2016, 4, e000211. [Google Scholar] [CrossRef]
- Patel, M.H.; Kumar, J.V.; Moss, M.E. Diabetes and tooth loss: An analysis of data from the national health and nutrition examination survey, 2003–2004. J. Am. Dent. Assoc. 2013, 144, 478–485. [Google Scholar] [CrossRef]
- Graziani, F.; Gennai, S.; Solini, A.; Petrini, M. A systematic review and meta-analysis of epidemiologic observational evidence on the effect of periodontitis on diabetes an update of the EFP-AAP review. J. Clin. Periodontol. 2018, 45, 167–187. [Google Scholar] [CrossRef]
- Song, T.-J.; Chang, Y.; Jeon, J.; Kim, J. Oral health and longitudinal changes in fasting glucose levels: A nationwide cohort study. PLoS ONE. 2021, 16, e0253769. [Google Scholar] [CrossRef] [PubMed]
- Similä, T.; Virtanen, J.I. Association between smoking intensity and duration and tooth loss among Finnish middle-aged adults: The northern Finland birth cohort 1966 project. BMC Public Health 2015, 15, 1141. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Jiang, X.; Wang, Y.; Huang, R. Correlation between tobacco smoking and dental caries: A systematic review and meta-analysis. Tob. Induc. Dis. 2019, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S. Cigarette smoking and periodontal diseases: Etiology and management of disease. Ann. Periodontol. 1998, 3, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, J.; He, B.; Huang, R.; Li, M. Effect of tobacco on periodontal disease and oral cancer. Tob. Induc. Dis. 2019, 17, 40. [Google Scholar] [CrossRef]
- Johnson, G.K.; Hill, M. Cigarette smoking and the periodontal patient. J. Periodontol. 2004, 75, 196–209. [Google Scholar] [CrossRef]
- Fitzerald, P.; Kharbouch, M.; Dawson, E. Oral Health in Peterborough; Peterborough Public Health: Peterborough, ON, Canada, 2019. [Google Scholar]
- Rodakowska, E.; Mierzyńska, K.; Bagińska, J.; Jamiołkowski, J. Quality of life measured by OHIP-14 and GOHAI in elderly people from Bialystok, north-east Poland. BMC Oral Health 2014, 14, 106. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).